Abstract
Streptococcus dysgalactiae is one of the most important bacterial species isolated from bovine mastitis. To identify potential virulence factors of this species we prepared chromosomal DNA from strain 8215 and constructed a phage display library. By affinity selection of the library against fibrinogen (Fg), we isolated and characterized a gene, called demA, encoding a protein with the molecular mass of ∼58 kDa, called DemA, displaying both plasma protein binding properties and sequence similarities with the M and M-like proteins of other streptococcal species. Purified recombinant DemA protein was found to completely inhibit Fg-binding to cells of S. dysgalactiae. A continued sequence analysis revealed that the demA gene was preceded by an open reading frame (dmgA) coding for a putative protein, called DmgA, with high similarities to the Mga proteins of Streptococcus pyogenes. By additional cloning, the corresponding dmgA and demA genes from another strain, called Epi9, were isolated and analyzed. These genes, called dmgB and demB, respectively, revealed a high degree of similarity to the corresponding genes in strain 8215. Increased binding of Fg by cells of strain Epi9, grown in an atmosphere with 10% CO2, was correlated to an enhanced transcription of the demB gene as shown in a Northern blot. Strain 8215 did not respond to CO2, which could be explained by a nonfunctional dmgA gene due to insertion of an insertion sequence element. Based on sequence similarities of the described proteins to Mga, M, and M-like proteins and the response to elevated level of CO2, we suggest that the dmg and dem genes are members of a regulon similar to the described mga regulon in S. pyogenes, which encodes several virulence factors in this species.
Streptococcus dysgalactiae, a Lancefield group C α-hemolytic streptococcus species, is a common pathogen in subclinical and clinical mastitis causing substantial economic losses in dairy herds (6, 41). The bacterium expresses various extracellular and cell surface bound proteins which specifically interact with plasma or connective tissue proteins of the host (21, 26, 35, 39) and are assumed to play an important role for the colonization and persistence of the pathogen in the host. Genes of various S. dysgalactiae isolates coding for proteins binding α2-macroglobulin (α2M), albumin, immunoglobulin G (IgG), and fibronectin have been cloned and characterized (15–17, 22, 43). The interaction between the α2M-protease complex and cells of streptococci reduces the phagocytosis of the bacteria by polymorphonuclear leukocytes (42). Binding of IgG from various animal species in a nonimmune fashion via the constant region of the molecule is considered to interfere in various ways with the recognition of the streptococcal cells by the host immune system (3, 9, 34). Recent publications on colonization and invasion of host epithelial cells by group A streptococci revealed the importance of the fibronectin-binding property and the M1 protein in this process (10, 31). The invasion of epithelial cells of bovine mammary glands by S. dysgalactiae has also been shown, although the bacterial factors involved have not been identified (1, 4, 5).
Searching for additional potential virulence factors in S. dysgalactiae, a phagemid library was made from strain 8215, the strain from which the mag gene (which encodes a protein binding α2M, albumin, and IgG) was cloned (16). Since fibrinogen (Fg) binding is common among mastitis isolates of S. dysgalactiae (26, 35) and is also a feature expressed by M or M-like proteins, the library was affinity selected against this protein. Phagemid clones binding to Fg were isolated, and the inserts of chromosomal DNA in the corresponding phagemid vectors were sequenced. A gene called demA, coding for both Fg- and IgG-binding capacity, was isolated and analyzed. By additional cloning, a gene similar to demA was isolated from another strain of S. dysgalactiae and named demB. Directly upstream of the respective dem genes we recognized genes, called dmgA and dmgB, which, when deduced, revealed similarities to Mga proteins in Streptococcus pyogenes known to be involved in the regulation of the expression of various virulence factors (7, 33). Using Northern blot assays, the transcription of the dmg and dem genes was studied under different growth conditions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. dysgalactiae strains isolated from bovine mastitis cases from different parts of Sweden were obtained from the National Veterinary Institute, Uppsala, Sweden. The strains were kept on blood agar plates and were cultured overnight in Todd-Hewitt broth (Oxoid, Basingstoke, Hampshire, United Kingdom) supplemented with 0.3% (wt/vol) yeast extract (Oxoid) under slow agitation at 37°C. In some experiments, the bacteria were grown in an incubator (Forma Scientific Inc., Marietta, Ohio) with an atmosphere enriched with 10% CO2. Escherichia coli TG1 [supE hsdΔ5 thi Δ(lac-proAB) F′ (traD36 proAB+ lacIq lacZΔM15)] was used for the construction of phagemid library and for the production of phage stocks. E. coli DH5α [supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used for the expression of recombinant proteins. The E. coli strains were grown in Luria-Bertani (LB) medium supplemented, when appropriate, with 100 μg of ampicillin per ml, and alternatively on LA plates (LB medium supplemented with 1.5% agar and 100 μg of ampicillin per ml). All incubations were at 37°C. The phagemid vector pG8H6 (14) was used to construct the phage display library. For additional cloning, the plasmid vectors pUC19 and pGEX-2T (Amersham Pharmacia Biotech, Uppsala, Sweden) were used.
Proteins and reagents.
Restriction endonucleases and Taq DNA polymerase were either from Amersham Pharmacia Biotech or MBI Fermentas (Vilnius, Lithuania). VentR DNA polymerase with 3′→5′ proofreading exonuclease activity was from New England Biolabs (Beverly, Mass.). Human Fg was obtained from IMCO Corporation Ltd. (Stockholm, Sweden) and rabbit anti-human Fg IgG conjugated to horseradish peroxidase (HRP) was purchased from Dakopatts A/S, Glostrup, Denmark. Rabbit anti-sheep IgG-HRP was the product of Southern Biotechnology Associates, Inc., Birmingham, Ala. Bovine serum albumin (BSA; fraction V) was from USB (Cleveland, Ohio). Reagent-grade human IgG was from Sigma Chemical Co. (St. Louis, Mo.). Human IgG Fab and Fc fragments were the products of Rockland (Gilbertsville, Pa.). Human Fg and the recombinant protein GDEMA8 were iodinated with 125I (Amersham, Little Chalfont, England) by the Iodo-Beads labelling method according to the description of the manufacturer (Pierce, Rockford, Ill.). Molecular weight markers used in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were obtained from Bio-Rad (Richmond, Calif.). Nitrocellulose (NC) filters, BA-S 85, and Hybond-C used for Western blotting were from Schleicher and Schuell (Dassel, Germany) and Amersham Pharmacia Biotech, respectively. Nylon membrane (Hybond-N+) used for Southern blotting was the product of Amersham Pharmacia Biotech. Sterile filters (Minisart N; pore size, 0.45 μm) were obtained from Sartorius AG (Göttingen, Germany).
Construction of phagemid libraries of S. dysgalactiae.
All DNA manipulations were performed by standard methods (37). The shotgun phage display library was constructed essentially as described by Jacobsson and Frykberg (13, 14). Short chromosomal DNA of S. dysgalactiae strain 8215 was prepared and fragmented by sonication. After different time intervals, aliquots were analyzed on an agarose gel. A sample containing a majority of DNA fragments with sizes of ∼1,000 bp and without prior size fractionation was made blunt ended with T4 DNA polymerase. The DNA fragments were ligated, with the Ready-To-Go T4 DNA ligase kit (Amersham Pharmacia Biotech), into an SmaI-digested and dephosphorylated pG8H6 vector. Electrotransformation of the ligated material into E. coli TG1 cells yielded ∼1.2 × 108 ampicillin-resistant transformants. A 5-ml sample of an overnight culture of the electroporated bacteria was infected with helper phage R408 at a multiplicity of infection (MOI) of 20, melted agar was added to 0.5%, and the mixture was distributed over 20 LA plates. After incubation overnight, the phage particles were eluted from the soft agar by vigorous shaking. The suspension was centrifuged (15,000 × g), followed by sterile filtration, and the titer of the phage display library was determined to be 3 × 1010 CFU/ml.
Panning of phagemid library and identification of Fg-binding clones.
Microtiter wells (Maxisorp Nunc, Copenhagen, Denmark) were coated overnight at 4°C with 200 μl of human Fg at a concentration of 100 μg/ml in 0.05 M NaHCO3 (pH 9.7). The wells were blocked by incubation with phosphate-buffered saline (PBS) and 0.05% Tween 20 (PBST) containing BSA (final concentration, 1 mg/ml) for 1 h at room temperature (RT). After washing with PBST, 600 μl of the phagemid library was added to each of three coated wells and the wells were incubated overnight at 4°C. Before elution, the wells were extensively washed with PBST at RT, and then they were eluted stepwise with 200-μl buffer solutions consisting of 50 mM Na citrate and 150 mM NaCl with decreasing pH (5.4, 3.4, and 1.9). The eluates were neutralized by the addition of 75 μl of 2 M Tris-HCl (pH 8.6). From the eluates, 50 μl was used to infect 20 μl of E. coli TG1 cells (overnight culture) and 100 μl of fresh LB was added. After a 20-min incubation at 37°C, the cells were spread on LA plates containing 2% glucose. The plates were incubated overnight, and colonies derived from fractions eluted at pH 3.4 and 1.9 were resuspended in LB medium and pooled. After infection with helper phage R408 at a MOI of 20, the sample was mixed with 5 ml of 0.5% LB soft agar and poured onto an LA plate. After incubation overnight, the phagemid particles were eluted and subjected to another round of panning as described earlier (13, 14). Finally, after the second panning, individual clones were grown in small scale for preparation of phagemid DNA in order to sequence the inserts.
Panning of selected phagemid particles against IgG and BSA.
Stocks from phagemid clones harboring fragments of different size of the demA gene were prepared and analyzed for the presence of additional binding capacity to human IgG, bovine IgG, and BSA. The phagemid stocks were prepared as follows. A volume of 500 μl of E. coli TG1 cells harboring the appropriate phagemid was infected with helper phage R408 (MOI of 20). After propagation in soft agar on an LA plate, the phagemid particles were eluted as described above. The generated phage stocks (3 × 107 CFU/ml) were panned in duplicate (200 μl/microtiter well) against immobilized human IgG, bovine IgG, and BSA, and as a positive control, they were panned against human Fg for 2 h at RT. Wells coated with hen egg ovalbumin served as a negative control. The wells were extensively washed with PBST and eluted with 200 μl of buffer solution containing 50 mM Na citrate–150 mM NaCl (pH 2). The eluate was immediately neutralized by addition of 15 μl of 1 M Tris-base (pH 11). Aliquots of the eluted phagemid particles were used to infect E. coli TG1 cells and plated on LA plates supplemented with 2% glucose to determine the CFU.
Production and purification of GST fusion proteins.
Two DNA fragments were PCR amplified from the pDEMA6 clone harboring the demA gene with a combination of primer pairs of O1 (5′-CGCGGATCCAAGGCTAATGACGATATTTAC-3′), O2 (5′-CCGAATTCCGCTATCCACAGTATTGAGAA-3′), and O3 (5′-CCGAATTCAACCTGTTGATGGTAATTGTA-3′). The underlined sequences of the primers hybridize to the following positions at the 5′ end of the demA gene: O1, 118 to 138; O2, 818 to 837; and O3, 1554 to 1535. The primers were designed in such a way that the forward primer included the BamHI site while the reverse ones contained the EcoRI restriction site in the 5′ end. The amplifications were made with VentR DNA polymerase in order to minimize the possibility of errors in the nucleotide sequence produced by the PCR. pGEX-2T was double digested with the BamHI and EcoRI restriction endonucleases and dephosphorylated with calf intestine alkaline phosphatase. The two PCR products were also cleaved with BamHI and EcoRI and then ligated into the linearized pGEX-2T vector, resulting in the plasmid constructs pGDEMA7 and pGDEMA8, respectively. The constructs were electroporated into E. coli DH5α cells, and positive colonies were identified by colony blotting with 125I-Fg. The production and purification of the two glutathione S-transferase (GST) fusion proteins from the isolated transformants were performed by following the manufacturer's recommendations. GST protein alone was prepared as a control.
SDS-PAGE and Western blot analysis.
The purity of the recombinant proteins was analyzed by SDS-PAGE, using precasted 8 to 25% gradient gels and the PhastSystem (Amersham Pharmacia Biotech). The gels were stained with Coomassie blue. To test the binding activities, four parallel sets with the two recombinant proteins (GDEMA7 and GDEMA8) and the control (GST protein alone) were run on gels and diffusion blotted to NC membranes for 30 min at 65°C. The membranes were cut in such a way that every strip contained all three proteins. The filter strips were subsequently blocked in PBS–0.5% (wt/vol) ovalbumin–0.05% Tween 20 for 2 h at RT. Each filter was then incubated for 2 h with a different ligand: HRP-conjugated rabbit IgG (1:1,000 dilution), HRP-conjugated human IgG (1:250 dilution), or 125I-labelled human Fg or unlabelled horse IgG (10 μg/ml). The interaction of the unlabelled horse IgG with the proteins was detected by additional incubation of the filters with HRP-conjugated Protein G (Amersham Pharmacia Biotech) for 2 h. After extensive washing, the bound HRP-labelled ligands were detected with 4-chloro-1-naphthol (Serva, Heidelberg, Germany) as substrate. The captured 125I-Fg was visualized by exposing the blot to Biomax MS (Kodak) film for 24 h at −70°C.
Binding of 125I-labelled GDEMA8 protein to separated chains of human Fg and to Fab and Fc fragments of human IgG was analyzed in a similar Western blot assay. After boiling Fg in a reducing sample buffer (62 mM Tris-HCl, pH 6.8, 9% glycerol, 2% SDS, 40 mM dithiothreitol, and 0.025% bromophenol blue), the resulting chains were separated on a 12.5% homogeneous gel. The IgG fragments were run under nonreducing conditions on the same gel. The proteins were transferred to an NC membrane and probed with 125I-GDEMA8 protein, and the membrane was exposed to Biomax MS (Kodak) film for 48 h at −70°C.
Comparative binding assays with S. dysgalactiae strains 8215 and Epi9.
Strains 8215 and Epi9 were cultured overnight in Todd-Hewitt broth–0.3% yeast extract in either normal or 10% CO2-enriched atmosphere. The cells were harvested by centrifugation, and the pellets were washed twice with ice cold PBS–1 mM NaN3. The cells were resuspended in PBS–0.1% (wt/vol) ovalbumin–0.1% Tween 20–1 mM NaN3, and the optical density (OD) of the suspensions at 600 nm (OD600) was adjusted to 0.6. Four hundred microliters of each cell suspension was mixed in Eppendorf tubes with 100 μl of 125I-Fg (specific activity, 113,000 cpm). The tubes were incubated on a rolling drum for 2 h at RT. The cells were pelletized by centrifugation and washed twice with PBS–0.1% Tween 20. The radioactivity associated with the cells was measured in a γ-counter. Streptococcus zooepidemicus ZV with high Fg-binding capacity (H. Lindmark, personal communication) was used as a positive control while E. coli TG1 served as a negative control in the assays.
Competitive binding assays with S. dysgalactiae strains 8215 and Epi9.
The capacity of the pGDEMA8-encoded fusion protein, called GDEMA8, and the control GST protein to inhibit the binding of 125I-Fg to cells of S. dysgalactiae strains was studied. The assays were performed similarly to the binding experiments, with only slight modifications. Shortly, cell suspensions of S. dysgalactiae 8215 and Epi9 were prepared in PBS–0.1% (wt/vol) ovalbumin–0.1% Tween 20–1 mM NaN3 from cultures grown overnight in ambient atmosphere. The OD600s of the cell suspensions were adjusted to 1.4 for strain 8215 and to 0.75 for strain Epi9. Serial dilutions of GDEMA8 and GST proteins were made in PBS–0.1% (wt/vol) ovalbumin–0.1% Tween 20–1 mM NaN3, and 100 μl of each dilution step was mixed with 300 μl of the respective cell suspension and 100 μl of 125I-Fg. The tubes were incubated on a rolling drum for 4 h at RT. After washing with PBS–0.1% Tween 20, the radioactivity associated to the pellets was determined as described above.
DNA sequencing and homology studies.
The nucleotide sequences were determined with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit and the ABI Model 373A DNA sequencer. Specific synthetic primers were purchased from Life Technologies (Rockville, Md.). Computer programs from PCGENE (Intelligenetics Inc., Mountain View, Calif.), a DNA and protein sequence analysis software package, were used to record and analyze the sequence data. The databases Genbank, EMBL, Swissprot, and PIR were screened for sequence homologies. Prediction of the coiled-coil secondary structure of the protein was made on the COIL web server (http://ulrec3.unil.ch/software/COIL_form.html) as described by Meehan et al. (29).
Detection of demA in strains of S. dysgalactiae.
Genomic DNA was prepared from mastitis isolates of S. dysgalactiae as described before (18). The DNA samples were digested with BamHI and EcoRI restriction endonucleases, and the cleavage products were separated by agarose gel electrophoresis. The fragments were vacuum blotted to a nylon membrane and probed with an [α-32P]CTP-labelled insert of pGDEMA8. The bands hybridizing with the probe were visualized by exposing the filter to Biomax MR (Kodak) film.
Cloning of the mga-like and demA-like genes from S. dysgalactiae strain Epi9.
PCRs were performed with chromosomal DNA of 10 mastitis isolates of S. dysgalactiae to identify strains which contain the complete mga-like gene, but not the ΔIS-dmgA junction. Primer pair O4 (5′-CAGATTATCCAACAGACTTGAATGAA-3′) and O5 (5′-CCTTAGACTCACCTACAACA-3′), complementary to nucleotide positions 28 to 52 and 402 to 421, respectively, in the insert of the pDEMA6 clone were used to amplify the ΔIS-dmgA junction. Primer pair O6 (5′-TGTTGTAGGTGAGTCTAAGG-3′) and O7 (5′-ATTGTCCCATTCTGATCCTA-3′), hybridizing to nucleotide positions 402 to 421 and 1209 to 1228, respectively, in the insert of the pDEMA6 clone (corresponding to nucleotides 126 to 145 and 933 to 952, respectively, in the dmgA gene) were selected to amplify the 3′ end of the dmgA gene.
Chromosomal DNA from strain Epi9 was double digested with restriction enzymes BamHI and EcoRI. After agarose gel separation of the fragments, the DNA band containing the dmgA-like gene was identified in a Southern blot assay with a radioactive probe. The probe was a product of PCR amplification from chromosomal DNA with the primer pair O6 and O7. DNA fragments with the appropriate size were purified from a preparative agarose gel and ligated into a BamHI- and EcoRI-cleaved and 5′ dephosphorylated pUC19 vector. Positive colonies were detected by colony hybridization with the same radioactive probe as before, and the inserts were sequenced. One clone, called pDMGB1, was selected for further studies.
A PCR fragment corresponding to the demB gene was generated from chromosomal DNA of strain Epi9 with primers O8 (5′-TATCTTAGGATCAGAATGG-3′) and O9 (5′-ACCTGTTGATGGTAATTGTA-3′), which hybridized to nucleotides 928 to 946 of the dmgA gene (corresponding to nucleotides 1204 to 1222 in the insert of pDEMA6) and to nucleotides 1535 to 1554 of the demA gene (corresponding to nucleotides 2942 to 2961 in the insert of pDEMA6), respectively. The generated PCR product was DNA sequenced. To obtain the 3′ end of the demB gene, an ∼3-kb chromosomal DNA fragment was cloned into the pUC19 vector and sequenced.
Detection of RNA transcripts of the dmg and dem genes in Northern blot assay.
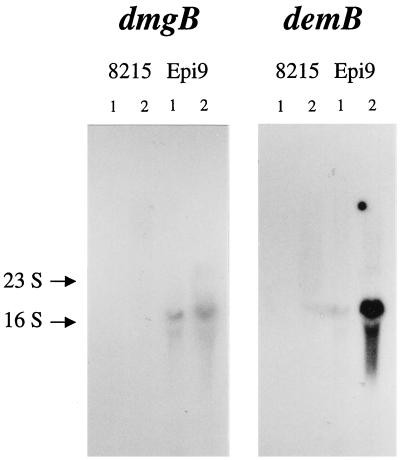
Cultures of S. dysgalactiae strains 8215 and Epi9 were grown to mid-log growth phase (OD600, ∼0.5) in ambient as well as in 10% CO2-enriched atmosphere, and total RNA was prepared from the cells with the FastRNA kit, blue (BIO 101, Inc., Vista, Calif.) according to the manufacturer's recommendation. Twenty micrograms of total RNA from each of the four samples in two parallel sets were separated on a 1% agarose gel as described by Kihlberg et al. (19) and vacuum blotted to a nylon membrane. One-half of the membrane with one set of samples was incubated in 6× SSC (1× is 0.15 M NaCl plus 0.015 M sodium citrate)–3× Denhart–0.5% SDS overnight at 65°C with a radioactive dmgB-derived probe (corresponding to nucleotides 654 to 1480) while the other half was hybridized with a demB-derived probe (corresponding to nucleotides 1107 to 1518). After repeated washes in 2× SSC–0.1% SDS at 65°C, the membranes were exposed to x-ray film for 72 h at −70°C. The dmgB- and demB-derived probes showed 94 and 99% nucleotide sequence identity, respectively, to the corresponding regions of the dmgA and demA genes of strain 8215.
Extraction of surface proteins from S. dysgalactiae isolates.
A mutanolysine extraction procedure was used to obtain bacterial surface proteins from overnight cultures of S. dysgalactiae strains 8215 and Epi9 essentially as described by Meehan et al. (29). The extracted material was subjected to SDS-PAGE analysis and used in Western blot assays for detection of Fg-binding activity.
Nucleotide sequence accession numbers.
The novel nucleotide sequences have been deposited in the EMBL sequence data bank and are available under accession no. AJ243529 (harboring the dmgA and demA genes of strain 8215) and AJ243530 (harboring the dmgB and demB genes of strain Epi9).
RESULTS
Identification of phagemid clones encoding specific Fg-binding activity.
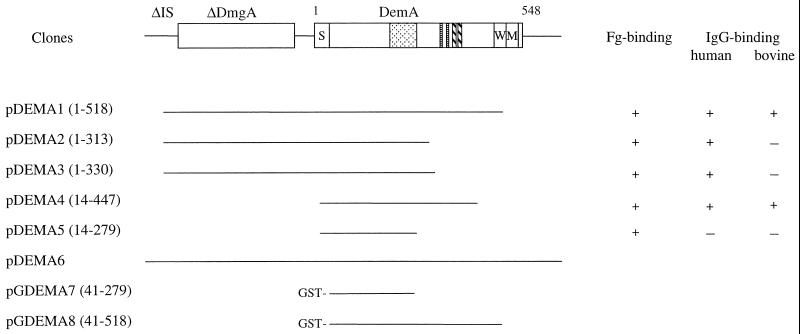
A shotgun phage display library was made with fragmented chromosomal DNA from S. dysgalactiae 8215 and affinity selected against Fg. Phagemid clones were chosen from the second cycle of affinity selection. DNA sequence analysis of the junctions between the inserts and the vector showed that several clones had overlapping inserts, which could be arranged into five groups represented by pDEMA1 to pDEMA5 (Fig. 1). Alignment of the nucleotide sequences of the various inserts revealed a common overlapping core region. By analysis of the sequence of the ∼2.8-kb insert of clone pDEMA1, two open reading frames (ORFs) were found. Alignment of the sequences of additional phagemid clones indicated that the Fg-binding activity was coded by the ORF located in the 3′ end of the insert in pDEMA1. Analysis of the deduced amino acid sequence revealed a C-terminally truncated protein. To isolate the 3′ end of the gene, Southern blot analysis was performed, in which chromosomal DNA from strain 8215 was digested with various restriction enzymes. The insert of phagemid clone pDEMA5 was used as a probe (data not shown). The result showed that the probe hybridized to a 3.5-kb BamHI fragment which was subsequently cloned into the vector pUC19, resulting in the clone pDEMA6. Additional sequencing of the insert in pDEMA6 revealed that the complete gene, called demA, is 1,644 nucleotides long, starting with an ATG codon at nucleotide position 1408 (amino acid residue 1 of DemA in Fig. 1) and ending with a TAA codon at position 3052 of the insert (accession no. AJ243529). The ORF is preceded by a sequence typical for a ribosome-binding site of gram-positive cocci and followed by sequences resembling a transcriptional termination signal. The gene codes for a protein, called DemA, consisting of 548 amino acid residues. The deduced amino acid sequence revealed a primary structure typical for cell surface bound proteins of gram-positive cocci (Fig. 2). The N-terminal part of the protein consists of a predicted signal sequence with a possible cleavage site between amino acid residues 41 and 42, resulting in a mature protein with 507 amino acids and a molecular mass of ∼54 kDa. At 128 amino acid residues downstream of the signal peptide there is a region, called A (amino acid positions 171 to 243), consisting of three complete repetitive sequences, A1, A2, and A3, and one incomplete repeat, A4. One A repeat unit contains 21 amino acids, and the truncated last unit, A4, contains only 10 amino acids. Comparisons of the individual repeats to A1 show that they are almost identical. In A2, there are three amino acids changed relative to A1, while A3 differs only in two amino acids. In both A2 and A3, one of the changes has a conserved site (amino acid residue 11). The second change in A3 is after the lysine residue at position 16, while in A2, both amino acids 15 and 17 are changed. In the C-terminal part of the protein there are also two short repetitive sequences (Fig. 2, B and C) consisting of the motifs SDLA and SEAKVA(E/K)L, which are repeated twice. The proposed cell wall spanning region, called W, is rich in serine residues and ends with the conserved cell wall anchoring motif LPSTG (38). This region is directly followed by a row of almost exclusively hydrophobic amino acids which corresponds to a cell membrane spanning region, called M. The protein ends with five charged residues in the C terminus. Secondary structure analysis of the deduced protein with the COIL program (23, 24) suggests that the protein possesses an extremely high probability (80 to 100%) of forming a coiled-coil structure, except for the first 82 residues and residues 494 to 548 within the C-terminal W and M regions.
FIG. 1.
Schematic presentation of deduced gene products encoded by the pDEMA6 clone derived from S. dysgalactiae 8215. pDEMA1 to pDEMA5 are phagemid clones isolated after panning a phage display library of strain 8215 against Fg. pGDEMA7 and pGDEMA8 are expression clones of the pGEX-2T vector with inserts corresponding to the phagemid clone pDEMA5 and a PCR fragment derived from pDEMA1 encoding amino acids 41 to 518 of DemA, respectively, yielding GST fusion proteins. The figures within parentheses after the names of the clones indicate the encoded amino acids of protein DemA. The DemA protein, including the signal peptide, consists of 548 amino acid residues as indicated above the schematic drawing. S, signal peptide, followed by the cell surface exposed part of the protein with repetitive sequences marked by different patterns; W, the cell wall spanning region; M, the membrane spanning region directly followed by five charged amino acids; ΔDmgA, a truncated protein highly homologous to the Mga regulatory protein from S. pyogenes (32); ΔIS, 276 bp homologous to an IS element. +, the phagemid clone can bind Fg, human IgG, and bovine IgG; −, no detectable binding.
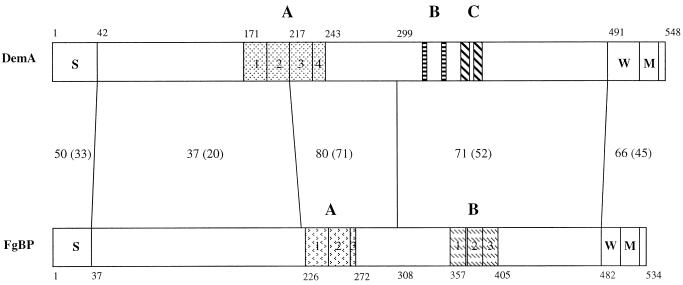
FIG. 2.
Schematic presentation of the alignment of the primary structures of the Fg-binding proteins DemA from S. dysgalactiae 8215 and FgBP from S. equi TW (29). The deduced amino acid sequences of the molecules were aligned by the PALIGNE program of PCGENE and are shown as schematic bars. Numbers along the bars indicate positions of amino acids. Numbers between the bars indicate the percentage of similar residues relative to the total number of amino acids within the compared domains, marked by connecting vertical lines. The percentages of identical residues were calculated in a similar way and are shown within parentheses. S, signal peptide; A, B, and C, various repeated sequences in the respective molecules, each marked with different patterns; W, wall spanning regions; M, membrane spanning regions directly followed by five charged amino acids.
Proteins similar to DemA.
Searching for homologies to the amino acid sequence of DemA in databases revealed various levels of similarity to the M and M-like proteins in other streptococci. The highest overall identity, 41%, was observed in comparing DemA to proteins FgBP and SeM, two almost-identical M proteins of Streptococcus equi differing in only six amino acids (29, 40). The similarity resides mainly in the middle and in the C-terminal parts of the molecules, where stretches of identical residues are found. However, the signal sequences and the following N-terminal parts of these proteins are divergent (Fig. 2). There are also repeats present in the FgBP and SeM proteins, as shown in Fig. 2. Alignment of repeats A1 to A4 of DemA to repeats A1 to A3 of FgBP reveals a stretch of amino acids, VSKDLADKL, present in all repeat units except for repeat A3 in FgBP, which contains only the LADKL sequence.
Additional binding activities of the DemA protein.
Phage stocks of clones pDEMA1 to pDEMA5 (Fig. 1) were additionally panned against human IgG, bovine IgG, and BSA in order to determine whether they, in addition to binding Fg, also bound these plasma components. Fg was used as a positive control and ovalbumin was used as a negative control in the assays. Figure 1 shows that clones pDEMA1 to pDEMA4 reacted with human IgG while clone pDEMA5, which represents the shortest Fg-binding clone coding for the N-terminal half of the DemA protein, showed no reactivity to human IgG. The interaction with bovine IgG was observed only for clones pDEMA1 and pDEMA4. Concerning the Fg-binding activity, there was no difference between the five phagemid clones, indicating that the Fg-binding region is more N terminal relative to the IgG-binding domain. The phages did not reveal any binding to BSA (data not shown).
Characterization of the recombinant proteins GDEMA7 and GDEMA8.
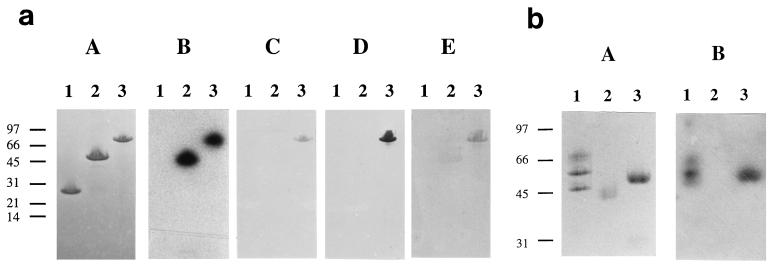
Recombinant proteins corresponding to the shortest phagemid clone (pDEMA5, amino acids 41 to 279) and to the mature DemA protein (amino acids 41 to 518) were expressed as fusions to GST (Fig. 1 and 3a, A). The two recombinant proteins, affinity purified on glutathione-Sepharose, bound 125I-labelled Fg in a Western blot assay (Fig. 3a, B). To further analyze the IgG-binding activity of DemA observed in the panning experiments with the phage particles (Fig. 1), we performed a Western blot assay with human, rabbit, and horse IgG. The result showed that the GDEMA8 protein, but not the GDEMA7 protein, bound to the different IgGs (Fig. 3a, C to E). Additional Western blot assays revealed that protein GDEMA8 interacts predominantly with the β-chain of Fg and bound the Fc fragment but not the Fab fragment of human IgG (Fig. 3b, B).
FIG. 3.
SDS-PAGE and Western blotting. (a) The affinity-purified GST fusion proteins coded by pGDEMA7 and pGDEMA8 clones derived from S. dysgalactiae strain 8215 were separated on an SDS-PAGE gel (8 to 25% polyacrylamide) (A) and blotted to an NC membrane. Binding of Fg by the recombinant proteins was tested by probing with 125I-Fg and visualized by exposing the strips to x-ray film (B). IgG binding by the recombinant proteins was probed with HRP-labelled human (C) and rabbit (D) IgG while HRP-labelled protein G was used to detect the bound unlabelled horse IgG (E). Lanes: 1, GST; 2, GDEMA7; 3, GDEMA8. (b) Human Fg in reducing sample buffer and Fab and Fc fragments of human IgG in nonreducing sample buffer were separated on a homogeneous SDS–12% PAGE gel (A), transferred to an NC membrane, and probed with 125I-GDEMA8 protein. The bound probe was detected by exposing the membrane to x-ray film (B). Lanes: 1, Fg (from the top: α-, β-, and γ-chains); 2, human IgG Fab fragment; 3, human IgG Fc fragment. Molecular mass markers (kDa) are indicated.
Occurrence of the demA gene in various isolates of S. dysgalactiae.
The presence of demA in 10 different strains, isolated from cases of bovine mastitis in different areas of Sweden, was investigated. A PCR-generated fragment corresponding to the insert of the pGDEMA8 clone encoding the mature DemA protein was radioactively labelled and used as a probe. Chromosomal DNA of the isolates was double digested with the restriction enzymes BamHI and EcoRI, and the fragments were separated by agarose gel electrophoresis. The DNA was transferred to a nylon membrane and incubated with the radioactive probe. The assay revealed that a gene homologous to demA was present in all tested isolates (data not shown).
demA gene is preceded by gene resembling mga gene of S. pyogenes.
Sequencing of the phagemid clone pDEMA1 revealed that ∼170 bp upstream of the demA gene there was an additional ORF present (Fig. 1). Comparison of the sequence to databases revealed homology to the mga genes of Streptococcus pyogenes. However, the mga-like gene, called dmgA (mga-like gene of S. dysgalactiae), of strain 8215 lacked ∼500 bp of the 5′ end of corresponding mga genes in S. pyogenes, but surprisingly, the 276 bp at the 5′ end of the DNA fragment showed high homology to an earlier described insertion sequence (IS) element, IS199, from Streptococcus mutans (25). In order to clone the intact dmgA gene, two parallel PCRs were performed with chromosomal DNA from S. dysgalactiae isolates tested earlier for the presence of the demA gene. One primer set, O4 and O5, was designed to amplify the ΔIS-dmgA junction. The second set of primers, O6 and O7, was used to amplify the 3′ end of the dmgA gene. The results of the two PCRs revealed isolates which contained the dmgA-like gene without the ΔIS-dmgA junction.
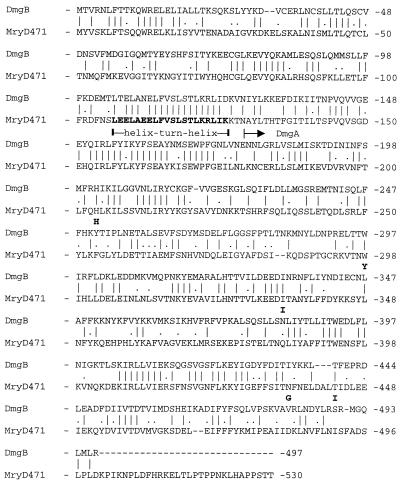
Strain Epi9, lacking the sequence of the ΔIS-dmgA junction, was selected for the cloning of the expected intact mga-like gene. After restriction enzyme cleavage of the chromosomal DNA of Epi9 and identification by Southern blot assay of the fragment harboring this gene, an ∼6-kb EcoRI-BamHI fragment was cloned into plasmid vector pUC19. The nucleotide sequence of the insert corresponding to an mga-like gene, called dmgB, was determined (accession no. AJ243530). The deduced amino acid sequence of the dmgB gene revealed the highest identity (∼45%) to the known mga gene product, Mry, of S. pyogenes D471, according to recent nomenclature equal to Mga (Fig. 4) (32). The N-terminal part of DmgB also contains a region highly homologous to the predicted helix-turn-helix motif of Mry (Fig. 4) (33) while the C-terminal part resembles, like in Mry (amino acids 404 to 528) (28, 32), the receiver domain of response proteins of bacterial two-component regulatory systems. The sequence of the truncated Mga-like protein, DmgA, present in strain 8215 is identical to the corresponding region in the deduced protein, called DmgB, from strain Epi9, with the exception of five amino acid residues. However, in a Northern blot assay, we could detect RNA transcript only from the dmgB gene, not that from the dmgA gene, with a specific probe (826 bp) derived from the dmgB gene (Fig. 5).
FIG. 4.
Alignment of the amino acid sequences of proteins DmgB and Mry. The amino acid sequence of protein DmgB from S. dysgalactiae Epi9 was aligned with the Mry (later designated Mga) of S. pyogenes D471 (32) with the PALIGNE program of PCGENE. A vertical bar indicates identical residues while a dot indicates similar residues. Gaps introduced to improve the alignment are indicated by a dashed line. The horizontal arrow above the sequence indicates the beginning of the N-terminally truncated DmgA protein of S. dysgalactiae 8215. The positions of the five changed amino acids in DmgA in comparison to those in DmgB are indicated by bold letters above the sequence. The predicted helix-turn-helix motif in Mry (33) is indicated by bold letters within the sequence. The region in Mry containing amino acids 404 to 528 exhibits homology to the receiver domain of response regulators (32). The alignment reveals an overall 61% similarity (45% identity) between the DmgB and Mry proteins.
FIG. 5.
Northern blot analysis of the RNA transcripts of the dmg and the dem genes. Twenty micrograms of total RNA isolated from cultures of S. dysgalactiae strains 8215 and Epi9 grown to mid-log phase in normal (lanes 1) or 10% CO2-enriched (lanes 2) atmosphere were separated in an 1% agarose gel, vacuum blotted to a nylon membrane, and hybridized with specific DNA probes derived from dmgB and demB (as presented in Materials and Methods). The positions of the 23S and 16S rRNA bands are indicated.
Analysis of the demB gene in strain Epi9.
Chromosomal DNA of S. dysgalactiae strain Epi9 was PCR amplified with forward primer O8, which hybridized to the sequence corresponding to the 3′ end of the dmgA gene, while the reverse primer O9 annealed to the nucleotides coding for the cell wall anchoring sequence QLPSTG in protein DemA. After sequencing the PCR fragment, we found an ∼1.5-kb ORF coding for a C-terminally truncated protein. By additional cloning, the missing 3′ end of the gene, called demB, was obtained (accession no. AJ243530). Analysis showed that the demB gene consists of 1,608 nucleotides encoding a protein, called DemB, of 536 amino acid residues with a molecular mass of ∼57.5 kDa. The alignment of the amino acid sequence of this protein to DemA revealed high similarity. The predicted signal sequences of the two proteins are 100% homologous, but the region preceding the A repeats of both proteins (between residues 42 and 152 of DemB) showed only 44% similarity (36% identity). Comparisons of the remaining parts of the proteins revealed 97% similarity (93% identity). Furthermore, the DemB protein contains only 2.5 A repeats instead of the 3.5 repeats present in the DemA protein of strain 8215. Further analysis showed that 11 residues from the C-terminal part of A1 and 10 residues from the N-terminal part of the A2 repeat from the DemA protein in strain 8215 are deleted in DemB of strain Epi9.
Expression of Fg binding in S. dysgalactiae strains 8215 and Epi9.
Cells of the two isolates used for cloning of the dem and dmg genes were also tested for Fg binding. Cultured in ambient atmosphere, strain 8215 showed low (7,000 cpm) binding while Epi9 had moderate (13,000 cpm) binding to Fg in comparison to the S. zooepidemicus strain ZV (65,000 cpm), which served as a positive control in the radioimmunoassays. Cells of E. coli TG1 were used as a negative control (400 cpm). However, when the two isolates were grown in an atmosphere enriched with 10% CO2, Epi9 showed a four- to sixfold increased capacity to bind Fg while the Fg binding of strain 8215 was unaffected (data not shown). Northern blot analysis also confirmed an increased transcription level of the demB gene in strain Epi9 grown in an atmosphere with elevated CO2 (Fig. 5).
Mutanolysine extracts of both strains were subjected to SDS-PAGE, transferred to an NC membrane, and probed with 125I-Fg. For both strains, positive signals were obtained in the range of 75 to 95 kDa (data not shown).
Competitive binding assays with S. dysgalactiae 8215 and Epi9.
The capacity of the GDEMA8 recombinant protein to inhibit the binding of 125I-Fg to cells was also tested in radioimmunoassays. This molecule corresponds to the mature DemA protein without the membrane spanning domain. The data in Fig. 6 show that the fusion protein was able to completely inhibit the binding of the labelled Fg to the cells of both strains while the GST protein alone had no inhibitory activity. Complete inhibition was also obtained in experiments with cells of Epi9 grown in the presence of CO2 (data not shown).
FIG. 6.
Inhibition of binding of 125I-Fg to S. dysgalactiae cells by recombinant DemA protein. Overnight cultures of S. dysgalactiae strains 8215 and Epi9 grown in ambient atmosphere were centrifuged and washed in PBS. The pellets were resuspended in PBS–0.1% ovalbumin–0.05% Tween 20, and the OD600s were adjusted to 1.4 and 0.75, respectively. Three hundred microliters of the respective cell suspensions were mixed with 100-μl serial dilutions of the GST-DemA fusion protein GDEMA8 and 100 μl of the iodine-labelled Fg. The mixtures were incubated with end-over-end rotation for 4 h at RT. The cells were pelletized and washed twice in PBS–0.1% ovalbumin–0.05% Tween 20, and the radioactivity associated with the cells was measured in a γ-counter. A similar assay was performed with the GST protein as the inhibitor of the binding of 125I-Fg to S. dysgalactiae Epi9 cells to exclude a possible effect of the GST part of the fusion protein on the binding.
DISCUSSION
During an infection, pathogenic bacteria like streptococci interact with their hosts by different mechanisms. Much interest has been focused on the interplay between the bacterial cell surface and the host. The M proteins have for a long time been considered one of the major virulence factors of S. pyogenes by mediating resistance to phagocytosis (11, 20, 36). Although not a prerequisite for inhibition of phagocytosis, Fg and IgG binding is a common feature among these proteins (9, 12). Concerning the virulence factors in S. dysgalactiae, our knowledge is limited (6). Using the phage display technique, we have continued to study the cell surface proteins expressed by this species. Construction of a phage display library and affinity selection of phagemid particles against Fg resulted in an enrichment of clones expressing Fg-binding activity (Fig. 1). By aligning the sequences of the inserts of these clones, it was possible to identify an ORF, which when deduced was found to encode a C-terminally truncated cell wall associated protein, called DemA, with a typical N-terminal signal sequence and a cell wall anchoring sequence (Fig. 1 and 2). By additional cloning, the complete demA gene was identified. The deduced amino acid sequence revealed characteristic features of a cell surface protein of a gram-positive bacterium. Further computer analysis predicted a protein with a typical coil-coiled secondary structure. Using different phagemid clones (Fig. 1) and the two GST fusion constructs (Fig. 1 and 3a, A), the Fg-binding domain was mapped to a 238-amino-acid region in the N-terminal part of the DemA protein (Fig. 1 and 3a, B). Another Western blot assay revealed that protein GDEMA8 bound predominantly to the β-chain of the Fg (Fig. 3b, B). Furthermore, the affinity-purified GST fusion protein GDEMA8 was found to completely inhibit the binding of labelled Fg to cells of strain 8215 of S. dysgalactiae, which suggests that the cell-surface-displayed Fg-binding activity is encoded by the cloned gene (Fig. 6). In the Northern blot assay, there was no RNA transcript of demA detected from strain 8215 despite the observed Fg-binding property of the cells. This is most likely due to a low level of transcription of the demA gene under the experimental conditions used.
The finding that the sequence of DemA has the same overall organization and partially displays a high degree of similarity to FgBP and SeM (Fig. 2), M proteins from S. equi subsp. equi, is important, since these proteins are claimed to be major virulence factors in this species (29, 40), similar to M proteins in S. pyogenes (11, 36).
Since several M or M-like proteins from S. pyogenes are also able to bind other plasma proteins, e.g., IgG, the IgG-binding properties of the DemA protein were tested with phage stocks from various phagemid clones and the recombinant proteins GDEMA7 and GDEMA8 (Fig. 1, 3a [A, C to E], and 3b [A and B]). The protein was found to have a higher affinity to polyclonal human IgG compared to cow IgG, while no interaction was found with pig IgG (data not shown). In Western blots, the GDEMA8 protein reacted with human, rabbit, and horse IgGs (Fig. 3a, C to E) and bound only the Fc fragment of human IgG (Fig. 3b, B), thus having IgG-binding properties similar to type IIa IgG-binding proteins of group A streptococci (2). It is therefore possible that strain 8215 expresses two unrelated IgG Fc-binding molecules, proteins MAG (16) (a type III IgG-binding protein) and DemA, on the cell surface. Interestingly, despite the high sequence homology between the DemA protein of S. dysgalactiae and the FgBP protein of S. equi subsp. equi, the FgBP did not bind horse IgG (29).
From strain Epi9 of S. dysgalactiae, a gene homologous to demA was identified and sequenced. This gene, called demB, encodes a protein which, except for 110 amino acids in the N-terminal part of the mature protein, shows very high identity (93%) and similarity (97%) to the DemA protein. This is in agreement with earlier findings that different M and M-like proteins from group A streptococci also have diverging amino acid sequences in their N-terminal regions, while the C-terminal regions are more conserved (11). Studies on the binding of 125I-Fg to cells of Epi9 and a Northern blot analysis of total RNA confirmed the expression of DemB in Epi9 as well as the upregulation of the gene expression as a response to an elevated level of CO2 in the atmosphere (Fig. 5). The binding of labelled Fg to cells of strain Epi9 was also completely inhibited by the recombinant Fg-binding protein derived from DemA (Fig. 6), even for cells grown in the presence of 10% CO2 (data not shown). In addition, mutanolysine treatment of cells from both strains (8215 and Epi9) released Fg-binding proteins detected as bands in the range of 75 to 95 kDa, compared to the calculated molecular masses of the mature DemA (54 kDa) and DemB (53 kDa) proteins. The discrepancy between the calculated and observed values may be explained by the presence of other cell wall components associated with the mutanolysine-released proteins.
In S. pyogenes, the expression of genes encoding M or M-like proteins is regulated by the mga gene, which codes for a trans-acting positive regulator (7, 32, 33). During the identification of the demA gene, which encodes the Fg-binding activity in strain 8215, an ORF directly upstream of the demA gene was recognized, which when deduced, showed high sequence similarity to Mga proteins (Fig. 4). However, the 5′ end of the presumed gene, called dmgA, was missing and instead we found part of an IS element (Fig. 1). A complete mga-like gene, called dmgB, was cloned from strain Epi9. Alignment of the region of the deduced amino acid sequence of the DmgB protein corresponding to the truncated DmgA protein showed differences in only five amino acid residues, indicating that these proteins are highly conserved (Fig. 4).
It is known that the Mga protein in S. pyogenes is part of a two-component regulatory system (32) involved in the regulation of genes in the mga regulon. The N-terminal part of the Mga protein has been reported to contain DNA-binding region(s), while at the C-terminal end (amino acids 404 to 528) it exhibits homology to the phosphorylation receiver domain of response proteins (28, 32, 33). The alignment of DemB to MryD721 shows that highly similar regions are also present in DemB (Fig. 4). Thus, if the dmg and dem genes in strains 8215 and Epi9 are part of a regulon similar to the mga regulon found in S. pyogenes, the finding that the dmgA gene of strain 8215 contains an inserted part of an IS element in the 5′ end after the potential helix-turn-helix motif (Fig. 4) suggests that a functional dmgA product is lacking in this strain. The observed low Fg binding by cells of strain 8215 is most likely the result of a basal transcription of the demA gene, although the presence of another gene, one that encodes an alternative Fg-binding protein with identical or overlapping binding epitope(s) with DemA on the Fg molecule, can not be fully excluded. However, all analyzed Fg affinity-selected phagemid clones contained inserts derived only from the demA gene. Northern blot analysis confirmed the expression of demB and dmgB genes in Epi9. Moreover, it showed that CO2 has an enhancing effect on the transcription of demB, as it does on the expression of genes belonging to the mga regulon in S. pyogenes. These data indicate that dmgB and demB form a functional mga-like regulon, while the similar regulon in 8215 is inactivated by the inserted IS element (8, 30).
In conclusion, the finding of the overall similarity in both the deduced primary and the predicted secondary structures of DemA and DemB proteins to M proteins, the recognized divergence in amino acid sequences in the N-terminal regions of the two proteins, and the detected binding properties of DemA are all characteristics which strongly suggest that DemA and DemB are M-like proteins. Furthermore, the data from the Northern blot analyses strongly support the idea that the dmg and dem genes are members of an mga-like regulon in S. dysgalactiae. Further studies will focus on the coupling between the expression of the dmg and dem genes concerning the response to other environmental factors earlier reported to influence the expression of the genes in the mga regulon in S. pyogenes (27). It will also be interesting to find out whether Dmg controls the expression of other genes within or outside the putative regulon. The observation that a demA-derived probe hybridized to chromosomal DNA from all 10 tested mastitis isolates of S. dysgalactiae and preliminary results from PCR amplification studies with dmg derived primers revealing the presence of a dmg-like gene in 9 out of the 10 tested strains indicates that the encoded proteins are of importance for the virulence of this species.
ACKNOWLEDGMENTS
This investigation was supported by grants from the Swedish Council for Forestry and Agricultural Research (32.0646/97).
REFERENCES
- 1.Almeida R A, Oliver S P. Invasion of bovine mammary epithelial cells by Streptococcus dysgalactiae. J Dairy Sci. 1995;78:1310–1317. doi: 10.3168/jds.S0022-0302(95)76752-2. [DOI] [PubMed] [Google Scholar]
- 2.Boyle M D. Variation of multifunctional surface binding proteins—a virulence strategy for group A streptococci? J Theor Biol. 1995;173:415–426. doi: 10.1006/jtbi.1995.0073. [DOI] [PubMed] [Google Scholar]
- 3.Boyle M D, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. doi: 10.1086/515241. [DOI] [PubMed] [Google Scholar]
- 4.Calvinho L F, Almeida R A. Influence of Streptococcus dysgalactiae surface hydrophobicity on adherence to mammary epithelial cells and phagocytosis by mammary macrophages. Zentbl Vetmed Reihe B. 1996;43:257–266. doi: 10.1111/j.1439-0450.1996.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 5.Calvinho L F, Oliver S P. Invasion and persistence of Streptococcus dysgalactiae within bovine mammary epithelial cells. J Dairy Sci. 1998;81:678–686. doi: 10.3168/jds.S0022-0302(98)75623-1. [DOI] [PubMed] [Google Scholar]
- 6.Calvinho L F, Almeida R A, Oliver S P. Potential virulence factors of Streptococcus dysgalactiae associated with bovine mastitis. Vet Microbiol. 1998;61:93–110. doi: 10.1016/s0378-1135(98)00172-2. [DOI] [PubMed] [Google Scholar]
- 7.Caparon M G, Scott J R. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci USA. 1987;84:8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caparon M G, Geist R T, Perez-Casal J, Scott J R. Environmental regulation of virulence in group A streptococci: transcription of the gene encoding M protein is stimulated by carbon dioxide. J Bacteriol. 1992;174:5693–5701. doi: 10.1128/jb.174.17.5693-5701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleary P, Retnoningrum D. Group A streptococcal immunoglobulin-binding proteins: adhesins, molecular mimicry or sensory proteins? Trends Microbiol. 1994;2:131–136. doi: 10.1016/0966-842x(94)90600-9. [DOI] [PubMed] [Google Scholar]
- 10.Cue D, Dombek P E, Lam H, Cleary P P. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horstmann R D, Sievertsen H J, Leippe M, Fischetti V A. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect Immun. 1992;60:5036–5041. doi: 10.1128/iai.60.12.5036-5041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsson K, Frykberg L. Cloning of ligand-binding domains of bacterial receptors by phage display. BioTechniques. 1995;18:878–885. [PubMed] [Google Scholar]
- 14.Jacobsson K, Frykberg L. Phage display shot-gun cloning of ligand-binding domains of prokaryotic receptors approaches 100% correct clones. BioTechniques. 1996;20:1070–1081. doi: 10.2144/96206rr04. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson H, Müller H-P. The type-III Fc receptor from Streptococcus dysgalactiae is also an α2-macroglobulin receptor. Eur J Biochem. 1994;220:819–826. doi: 10.1111/j.1432-1033.1994.tb18684.x. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson H, Frykberg L, Rantamäki L, Guss B. MAG, a novel plasma protein receptor from Streptococcus dysgalactiae. Gene. 1994;143:85–89. doi: 10.1016/0378-1119(94)90609-2. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson H, Burtsoff-Asp C, Guss B. Streptococcal protein MAG—a protein with broad albumin binding specificity. Biochim Biophys Acta. 1995;1249:65–71. doi: 10.1016/0167-4838(95)00065-3. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson H, Lindmark H, Guss B. A protein G-related cell surface protein in Streptococcus zooepidemicus. Infect Immun. 1995;63:2968–2975. doi: 10.1128/iai.63.8.2968-2975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kihlberg B M, Cooney J, Caparon M G, Olsën A, Björck L. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb Pathog. 1995;19:299–315. doi: 10.1016/s0882-4010(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 20.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 21.Leigh J A, Hodgkindson S M, Lincoln R A. The interaction of Streptococcus dysgalactiae with plasmin and plasminogen. Vet Microbiol. 1998;61:121–135. doi: 10.1016/s0378-1135(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 22.Lindgren P E, Speziale P, McGavin M, Monstein H J, Höök M, Visai L, Kostiainen T, Bozzini S, Lindberg M. Cloning and expression of two different genes from Streptococcus dysgalactiae encoding fibronectin receptors. J Biol Chem. 1992;267:1924–1931. [PubMed] [Google Scholar]
- 23.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 24.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 25.Macrina F L, Jones K R, Laloi P. Characterization of IS199 from Streptococcus mutans V403. Plasmid. 1996;36:9–18. doi: 10.1006/plas.1996.0026. [DOI] [PubMed] [Google Scholar]
- 26.Mamo W, Fröman G, Sundås A, Wadström T. Binding of fibronectin, fibrinogen and type II collagen to streptococci isolated from bovine mastitis. Microb Pathog. 1987;2:417–424. doi: 10.1016/0882-4010(87)90048-9. [DOI] [PubMed] [Google Scholar]
- 27.McIver K S, Heath A S, Scott J R. Regulation of virulence by environmental signals in group A streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect Immun. 1995;63:4540–4542. doi: 10.1128/iai.63.11.4540-4542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIver K S, Heath A S, Green B D, Scott J R. Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meehan M, Nowlan P, Owen P. Affinity purification and characterization of a fibrinogen-binding protein complex which protects mice against lethal challenge with Streptococcus equi subsp. equi. Microbiology. 1998;144:993–1003. doi: 10.1099/00221287-144-4-993. [DOI] [PubMed] [Google Scholar]
- 30.Okada N, Geist R T, Caparon M G. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol. 1993;7:893–903. doi: 10.1111/j.1365-2958.1993.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 31.Ozeri V, Rosenshine I, Mosher D F, Fässler R, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podbielski A, Flosdorff A, Weber-Heynemann J. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raeder R, Boyle M D. Association of type II immunoglobulin-G-binding protein expression and survival of group A streptococci in human blood. Infect Immun. 1993;61:3696–3702. doi: 10.1128/iai.61.9.3696-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rantamäki L K, Müller H-P. Phenotypic characterization of Streptococcus dysgalactiae isolates from bovine mastitis by their binding to host derived proteins. Vet Microbiol. 1995;46:415–426. doi: 10.1016/0378-1135(95)00046-d. [DOI] [PubMed] [Google Scholar]
- 36.Robinson J H, Kehoe M A. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol Today. 1992;13:362–367. doi: 10.1016/0167-5699(92)90173-5. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Schneewind O, Fowler A, Faull K F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 39.Sting R, Schauffus P, Blobel H. Isolation and characterisation of hyaluronidase from Streptococcus dysgalactiae, S. zooepidemicus and S. equi. Zentbl Bakteriol. 1990;272:276–282. doi: 10.1016/s0934-8840(11)80028-9. [DOI] [PubMed] [Google Scholar]
- 40.Timoney J F, Artiushin S C, Boschwitz J S. Comparison of the sequences and functions of Streptococcus equi M-like proteins SeM and SzPSe. Infect Immun. 1997;65:3600–3605. doi: 10.1128/iai.65.9.3600-3605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todhunter D A, Smith K L, Hogan J S. Environmental streptococcal intramammary infections of the bovine mammary gland. J Dairy Sci. 1995;78:2366–2374. doi: 10.3168/jds.S0022-0302(95)76864-3. [DOI] [PubMed] [Google Scholar]
- 42.Valentin-Weigand P, Traore M Y, Blobel H, Chhatwal G S. Role of α2-macroglobulin in phagocytosis of group A and C streptococci. FEMS Microbiol Lett. 1990;70:321–324. doi: 10.1111/j.1574-6968.1990.tb13997.x. [DOI] [PubMed] [Google Scholar]
- 43.Vasi J, Svensson J, Frick I M, Müller H-P. Five homologous repeats of the protein G-related protein MIG cooperate in binding to goat immunoglobulin G. Infect Immun. 1999;67:413–416. doi: 10.1128/iai.67.1.413-416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]