Abstract
In mammals, energy homeostasis is regulated by the antagonistic action of hormones insulin and glucagon. However, in contrast to the highly conserved insulin, glucagon is absent in most invertebrates. Although there are several endocrine regulators of energy expenditure and catabolism (such as the adipokinetic hormone), no single invertebrate hormone with all of the functions of glucagon has been described so far. Here, we used genetic gain- and loss-of-function experiments to show that the Drosophila gene Ion transport peptide (ITP) codes for a novel catabolic regulator that increases energy expenditure, lowers fat and glycogen reserves, and increases glucose and trehalose. Intriguingly, Ion transport peptide has additional functions reminiscent of glucagon, such as inhibition of feeding and transit of the meal throughout the digestive tract. Furthermore, Ion transport peptide interacts with the well-known signaling via the Adipokinetic hormone; Ion transport peptide promotes the pathway by stimulating Adipokinetic hormone secretion and transcription of the receptor AkhR. The genetic manipulations of Ion transport peptide on standard and Adipokinetic hormone-deficient backgrounds showed that the Adipokinetic hormone peptide mediates the hyperglycemic and hypertrehalosemic effects of Ion transport peptide, while the other metabolic functions of Ion transport peptide seem to be Adipokinetic hormone independent. In addition, Ion transport peptide is necessary for critical processes such as development, starvation-induced foraging, reproduction, and average lifespan. Altogether, our work describes a novel master regulator of fly physiology with functions closely resembling mammalian glucagon.
Keywords: Drosophila, ITP, metabolism, fat, glycogen, trehalose, development, energy homeostasis, insect hormones, crustacean hyperglycemic hormone, Adipokinetic hormone
Introduction
The fine control of energy catabolism and anabolism is crucial for organismal survival and growth. Energy metabolism needs to be adjusted to changing environmental conditions (such as temperature and water and food availability) and intrinsic processes (such as development, reproduction, and other energy-demanding activities) (Gáliková and Klepsatel 2018; Gillette et al. 2021). The key endocrine factors that regulate energy balance are often pleiotropic, i.e. integrate several aspects of animal physiology. Such pleiotropic hormones are also insulin and glucagon. While insulin is a hypoglycemic hormone-promoting anabolism, glucagon is a hyperglycemic peptide that increases catabolism and energy expenditure and inhibits feeding. These 2 antagonistic hormones regulate numerous metabolic processes, including satiety, digestion, energy metabolism of stored reserves, and basal metabolic rate (Guo 2014; Al-Massadi et al. 2019; Kleinert et al. 2019). Thus, both insulin (Das and Arur 2017) and glucagon (Yu et al. 2012; Charron and Vuguin 2015) signaling are essential for processes such as development, reproduction, and average lifespan. While insulin signaling is highly evolutionarily conserved (Das and Arur 2017), insects, including the popular model Drosophila melanogaster, do not seem to have a true homolog of glucagon. Instead, the hyperglycemic functions are governed by the Adipokinetic hormone (AKH) (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015) and, during the immune response, by extracellular adenosine (Zuberova et al. 2010; Bajgar et al. 2015). AKH is often considered a functional analog of glucagon (e.g. Kim and Rulifson 2004; Ahmad et al. 2020; Hughson 2021). The AKH peptide controls a plethora of processes including, for example, fat storage (Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017), levels of circulating sugars (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015), food intake (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017), oxidative stress response (Bednářová et al. 2015; Zemanova et al. 2016), and others. However, apart from the hyperglycemic action, AKH deficiency does not copy phenotypes of mutants in glucagon signaling. For example, glucagon signaling is required for development, reproduction, and lifespan. The mutations in the glucagon receptor (Gcgr) lead to altered placentation, poor fetal growth, and increased fetal–neonatal death (Vuguin et al. 2006; Ouhilal et al. 2012). In adulthood, Gcgr deficiency causes Mahvash disease, which leads to progressive cachexia, severe hypoglycemia, and early death (Yu et al. 2012). In contrast, mutants in the Akh signaling are fully viable (Gáliková et al. 2015; Sajwan et al. 2015). Fecundity analysis of Akh and AkhR mutants carried out during the first 10 days of their lives did not reveal any decrease in reproductive success (Gáliková et al. 2015). Furthermore, when the reproduction of Akh-deficient and w1118 females was compared after a 24-h mating, the Akh mutants even performed better than the controls (Wat et al. 2021). In males, the AKH pathway modulates pheromone production and courtship (Lebreton et al. 2016). The AkhA mutant males have reduced reproductive success when their mating is restricted to a 1-h-long trial (Wat et al. 2021), but the effect disappears when they have access to females over a longer period (Gáliková et al. 2015; Bednářová et al. 2018). In contrast to the detrimental consequences of increased or decreased glucagon (Janah et al. 2019), the AKH manipulations seem to have milder effects on general physiology and lifespan. Some studies showed that males deficient in the peptide have normal lifespan (Bednářová et al. 2018), while other works reported a lifespan-shortening effect of the Akh mutation (Hofbauer et al. 2021) and silencing of the AKH-producing cells (Wat et al. 2021). Interestingly, ubiquitous overexpression of Akh (Katewa et al. 2012) or activation of the AKH-producing cells (Waterson et al. 2014) even increased life expectancy. The differences between AKH and glucagon also extend to their roles in regulating energy intake. Glucagon represses feeding (Schulman et al. 1957; Geary et al. 1993), while AKH has the opposite function (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017). Finally, in contrast to glucagon triggering mobilization of lipid and carbohydrate reserves (Rui 2014; Campbell and Drucker 2015), AKH induces lipolysis but not mobilization of glycogen (Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017; Yamada et al. 2018). Moreover, mice deficient in the Gcgr have reduced adipose tissue and triglycerides (Gelling et al. 2003), while the Akh and AKH receptor (AkhR) mutant flies are obese (Grönke et al. 2007; Gáliková et al. 2015). Altogether, the phenotypes of Akh or AkhR deficiencies do not fully recapitulate the role of glucagon signaling in mammals. Given the conservation of the insulin pathway between flies and mammals, it is natural to expect its antagonistic hormone in both groups. However, the Drosophila genome seems to lack a gene with sequence similarity to glucagon, suggesting that other hormones fulfill its function and regulate fly physiology alongside AKH. Intriguingly, insects, including Drosophila, carry homologs of a gene called Crustacean hyperglycemic hormone (CHH), known for its developmental, reproduction-related, and hyperglycemic functions in crustaceans (Chung et al. 2010; Webster et al. 2012; Chen et al. 2020). The CHH-family peptides have a broad expression pattern that includes tissues such as the eyestalk, pericardial organ, gill, stomach, intestine, and others (Chen et al. 2020). The CHH peptides control numerous processes, including osmoregulation, digestion, and carbohydrate and lipid metabolism (Webster et al. 2012; Chen et al. 2020).
The fly homolog of CHH, Ion transport peptide (ITP), is a relatively unknown hormone expressed mainly in the brain and peripheral nervous system. The brain expression includes ipc-1 and ipc-2 neurosecretory cells and the ipc-3 and ipc-4 interneurons. In the periphery, ITP is expressed in the abdominal ganglion cells and the lateral bipolar dendrite neurons of the abdominal segments A7/A8 (Dircksen et al. 2008; Gáliková et al. 2018). The production of ITP outside of the nervous system has not been fully investigated in the fruit flies, but the FlyAtlas (Chintapalli et al. 2007) and FlyAtlas 2 (Leader et al. 2018; Krause et al. 2022) data suggest expression of the gene in various organs including gut, trachea, heart, and others. The extraneuronal expression of the ITP gene has been confirmed in other insect species. For example, the ITP gene is expressed in the midgut and carcass of the red flower beetle Tribolium castaneum (Begum et al. 2009) and in the male reproductive tract and integument of the brown planthopper, Nilaparvata lugens (Yu, Li, et al. 2016).
The ITP gene codes for 3 peptides—the ITP and the so-called ITP-like peptides 1 and 2. However, only ITP has an amidation signal at the N-terminus, which is crucial for the activity of the hormone. Thus, only ITP is considered an active hormone in Drosophila (Dircksen et al. 2008; Dircksen 2009). Receptor(s) for Drosophila ITP is currently unknown. The existence of multiple receptors for CHH was predicted in crustaceans (Nery et al. 1993). Remarkably, 3 different promiscuous receptors bind ITP in Bombyx mori (Nagai et al. 2014). It is thus possible that more receptors also exist in Drosophila.
The biological functions of ITP are relatively understudied. The first described role of this peptide has been its stimulatory effect on the evening and nocturnal activity (Hermann-Luibl et al. 2014). Later, it was shown that ITP is an antidiuretic hormone that antagonistically regulates water and food intake (Gáliková et al. 2018). ITP also protects from diarrhea by repressing the speed of transit throughout the digestive tract (Gáliková et al. 2018). These functions probably cannot be attributed to specific ITP neurons; it seems that the neurosecretory cells synthesize the hormone redundantly and compensate for a partial ITP knock-down (Gáliková et al. 2018).
In contrast to the relatively well-investigated glucagon-like functions of CHHs, not much is known about the roles of ITP in metabolic homeostasis. However, the roles of ITP in the repression of hunger and the speed of transit throughout the digestive tract (Gáliková et al. 2018) are intriguingly reminiscent of the role of glucagon in the inhibition of feeding (Schulman et al. 1957; Geary et al. 1993) and peristalsis (Paul and Freyschmidt 1976). Therefore, we investigated here the potential roles of ITP in the key glucagon-regulated processes, such as glycemia control, basal metabolic rate, homeostasis of stored energy reserves, development, and reproduction. The functional analyses of ITP are complicated by the complexity (Dircksen et al. 2008; Gáliková et al. 2018) and redundancy (Gáliková et al. 2018) in the ITP expressing neurons, the lethality of the ITP mutants and ubiquitous RNAi treatment (Gáliková et al. 2018), and, most of all, unknown receptors and downstream components of the pathway. Therefore, we focused mainly on ubiquitous adulthood-specific manipulations using the GeneSwitch system, which previously turned out to be useful for discovering the antidiuretic roles of ITP (Gáliková et al. 2018). Intriguingly, our gain- and loss-of-function manipulations of ITP resulted in phenotypes reminiscent of dysregulations of mammalian glucagon signaling. In addition, we found that the well-known AKH signaling is partially under the control of ITP and mediates its hyperglycemic functions.
Materials and methods
Fly lines and husbandry
Flies for the standard UAS–GAL4 and GeneSwitch experiments were reared under a 12 h light–12 h dark cycle at 25°C. The Drosophila medium consisted of 6 g agar, 50 g yeast, 100 g sugar, 5.43 ml propionic acid, and 1.3 g methyl 4-hydroxybenzoate per 1 l of medium. Adult flies were collected within 24 h after eclosion and housed in groups of approximately 30 females and 30 males per vial. Flies for the GeneSwitch experiments were transferred to the medium supplemented with RU-486 (200 µM) or ethanol vehicle control on the third day after eclosion. Flies for the c929-GAL4- and Impl2-GAL4-based TARGET (temporal and regional gene expression targeting) experiments developed at 18°C and on the third day after eclosion were transferred to 29°C for the transgene induction. After induction of the transgene expression, flies were flipped every second day to fresh media. If not stated otherwise, male flies were used for experiments 6–7 days after the induction of the transgene expression. Controls for the non-GeneSwitch experiments were generated by crossing the UAS and GAL4 lines to the w1118 strain. The list of the fly stocks used in the study is available in Supplementary File 1.
Developmental lethality
Approximately 4-day-old parental flies were reared on a standard medium with sprinkled yeast for 3 days. The food was exchanged daily to prevent egg retention. Afterward, flies were transferred on standard medium, and eggs were collected over 4 h. These eggs were used for the analysis of developmental lethality. Embryonic, larval, and pupal lethality was expressed as a proportion of animals that died during the given developmental stage. Viability was examined in at least 3 replicates per genotype. Fisher's exact test was conducted on pooled data. Altogether, the viability of at least 370 eggs was tested per each genotype/treatment.
Developmental timing
Approximately 4-day-old parental flies were reared for 3 days on a standard medium with sprinkled yeast. Food was exchanged daily to prevent egg retention. The flies were then transferred to a new medium, and eggs for the analysis of the developmental timing were collected over 4 h. Developmental timing was measured as the time from the egg deposition until the eclosion of the adult fly (Fig. 1b) and as the time from the egg deposition until the pupation (Supplementary Fig. 1b). All experiments were conducted in 3 replicates.
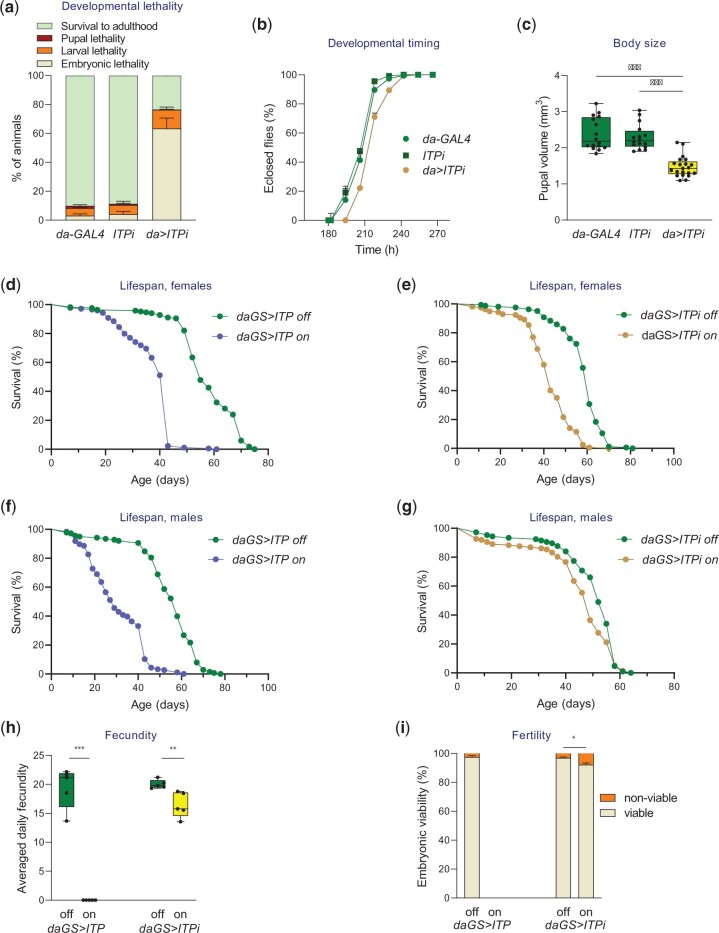
Fig. 1.
ITP is required for development, reproduction, and lifespan. a) ITP is required for the development. Daughterless-GAL4-driven ITP RNAi (da>ITPi) results in increased embryonic and larval lethality when compared to controls (UAS and GAL4 lines crossed to w1118 background). Fischer’s exact test: P < 0.001 for comparison with each control for both embryonic and larval survival. Animals were analyzed in 3 replicates; Fischer’s exact test was performed on pooled data. Sample size: da-GAL4, n = 382; ITPi, n = 381; da>ITPi, n = 370. Error bars represent SEM. b) A decrease in ITP via da>ITPi results in a prolonged development. Gehan–Breslow–Wilcoxon test: P < 0.001 for comparison with each control. Sample size: da-GAL4, n = 167; ITPi, n = 140; da>ITPi, n = 110. c) A decrease in ITP via da>ITPi results in a smaller body size (estimated as pupal volume). Two-tailed Student’s t-test: P < 0.001 for comparison with each control. Sample size: da-GAL4, n = 16; ITPi, n = 16; da>ITPi, n = 21. d–g) ITP is required for a normal lifespan. Adulthood-specific upregulation and downregulation of ITP via daughterless-GeneSwitch (daGS) decrease lifespan in males and females. d) Effect of ITP overexpression in females: log-rank test P < 0.001. Sample size daGS>ITP off n = 164, daGS>ITP on n = 174. e) Effect of ITPi in females: log-rank test P < 0.001. Sample size daGS>ITPi off n = 163, daGS>ITPi on n = 157. f) Effect of ITP overexpression in males: log-rank test P < 0.001. Sample size daGS>ITP off n = 138, daGS>ITP on n = 184. g) Effect of ITPi in males: log-rank test P < 0.01. Sample size daGS>ITPi off n = 106, daGS>ITPi on n = 137. h) An increase in ITP signaling via daGS>ITP inhibits egg production. Two-tailed Student’s t-test: P < 0.001. A decrease in ITP signaling via daGS>ITPi reduces egg production. Two-tailed Student’s t-test: P < 0.01. i) A decrease in ITP signaling via daGS>ITPi reduces embryonic viability (estimated as the percentage of eggs that hatch into the 1st larval instar). Fischer’s exact test: P < 0.05. Animals were analyzed in 5 replicates; Fischer’s exact test was performed on pooled data. Sample size: daGS>ITP off n = 306; daGS>ITP on females were completely sterile, no embryos were produced; daGS>ITPi off n = 291; daGS>ITPi on n = 223. Error bars represent SEM.
Body size measurement
Body size was estimated as the pupal volume, using a previously described method (Delanoue et al. 2010), based on the formula 4/3π (L/2)(l/2)2 (L = length; l = diameter of the pupae). Photographs were taken by Leica WILD M32 stereomicroscope and analyzed by ImageJ.
Lifespan determination
Freshly eclosed flies (24-h cohorts) were collected, transferred to standard food, and allowed to mate for 3 days. Afterward, male and female flies were separated and randomly distributed on the GeneSwitch food (200 µM RU-486 or ethanol as vehicle control). Flies were flipped on fresh media every other day. At least 5 replicates (altogether, at least 106 animals) were tested per sex/genotype/treatment.
Fecundity assays
Fecundity was measured in 5 replicates, each consisting of 3 females and 3 males. The number of eggs laid was determined on days 7 and 8 after the induction of the transgene expression (overexpression experiments) and on days 7, 8, and 10 after the induction of the transgene expression (RNAi experiments).
Fertility assay
Fertility was estimated as embryonic viability, i.e. as the proportion of eggs that hatched into the 1st larval instar. The experiment was conducted on the eggs from the fecundity assay. At least 291 eggs were tested per genotype/treatment.
Determination of dry weight
Flies were desiccated for 2 days at 65°C and weighed on a microbalance. At least 5 replicates (each consisting of 5 flies) were tested per genotype/treatment.
Determination of the whole body fat, glycogen, protein, and trehalose content
Lipids, proteins, glycogen, trehalose, and glucose were quantified by methods adapted from Tennessen et al. (2014) with modifications described previously (Gáliková et al. 2015; Gáliková, Klepsatel, Münch, et al. 2017). Briefly, 5 flies per replicate were homogenized in 600 ml of 0.05% Tween-20 in PBS and heat-inactivated at 70°C. After centrifugation, the supernatant was transferred into deep-well plates and further used for the colorimetric assays. Lipids were measured by the Triglycerides (liquid) assay (Randox, TR1697), proteins were measured by the Pierce BCA Protein assay kit (Thermo Fisher Scientific), and glycogen was determined by the Glucose assay kit (GO, Sigma) supplemented with 3 U of amyloglucosidase (Sigma) per 1 ml of the reagent. Glycogen content was calculated as the difference between the total glucose after the incubation with amyloglucosidase and the free glucose before the incubation with the enzyme. Trehalose was measured by the GO kit supplemented with 3 µl of porcine trehalase (Sigma, T8778‐1U) per 1 ml of the reagent. Trehalose content was calculated as the difference between the total glucose after the incubation with trehalase and the free glucose before the incubation with the enzyme. The homogenates for the trehalose, glucose, and glycogen measurements were diluted 1:3 in PBS prior to the experiments.
Determination of circulating sugars
Circulating trehalose was measured by a method adapted from Tennessen et al. (2014) with modifications described before (Gáliková et al. 2015). Briefly, the hemolymph was collected by centrifugation (6 min, 9,000 rcf at 4°C) of decapitated flies. These flies were placed in a holder tube with bottom holes, which was inserted in a 0.5-ml collection tube. Each sample contained hemolymph collected from 50 flies. A total of 1 μl of the collected hemolymph was diluted with 99 μl of 0.05% Tween-20 in PBS, heat-inactivated, further diluted 1:6 in PBS, and used for the measurements using the same method as in the case of the whole body homogenates.
Quantitative PCR
RNA was extracted using the Zymo Research QuickRNA MicroPrep kit. cDNA was synthesized by the QuantiTect Reverse Transcription Kit (Qiagen) using 1 μg of the total RNA. Quantitative PCR was performed in the StepOne Real-Time PCR System (Applied Biosystems) in the SensiFAST SYBR Hi-ROX Kit (Bioline) reaction mix. Expression levels were normalized to the Actin 5C (Act5C) and Ribosomal protein L32 (Rpl32, data in the main text) or to β-Tubulin at 60D (βTub60D, confirmatory data in the Supplementary material). Primer sequences are found in Supplementary File 1.
Metabolic rate measurements
The basal metabolic rate was estimated by manometric respirometry as the volume of consumed O2 according to the protocol described by Yatsenko et al. (2014). Six replicates (each consisting of 5 male flies) were tested per each genotype/treatment. The metabolic rate was measured over 4 h.
Determination of the spontaneous locomotor activity
Spontaneous activity of individual males was tested using the Drosophila Activity Monitor 2 system (TriKinetics). Monitoring tubes contained the standard medium supplemented with RU-486 or the vehicle control (ethanol). Spontaneous locomotion was determined as the total number of midline crossings during the tested period of time (3 days). At least 22 flies were tested per genotype/treatment.
Determination of starvation resistance
Starvation resistance was measured as the time survived on a medium containing 0.6% agarose cooked in distilled water. At least 3 replicates (each with a minimum of 22 flies) were tested per sex/genotype/treatment.
Analysis of the starvation-induced hyperactivity
The starvation-induced hyperactivity was determined as the total activity during the last 12 h of life under starvation as described previously (Gáliková et al. 2015). After 6 days of the transgene induction in standard vials, males were loaded individually into the monitor tubes for the Drosophila Activity Monitor 2 system. The tubes contained 0.3% agarose as a water source. The starvation-induced hyperactivity was analyzed by visual inspection of individual locomotion patterns (Fig. 3g) and by statistical analysis of midline crossings during the last 12 h premortem (Fig. 3h). At least 23 flies were tested per genotype/treatment.
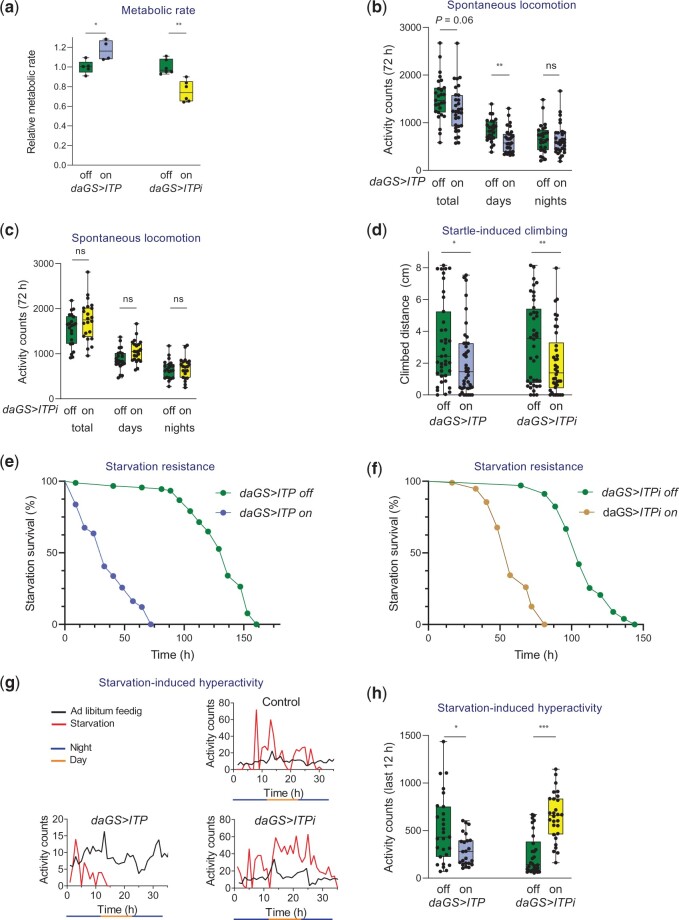
Fig. 3.
ITP regulates metabolic rate, startle-induced climbing, starvation resistance, and starvation-induced hyperactivity. a) ITP regulates metabolic rate. Short-term overexpression of ITP results in increased metabolic rate. Two-tailed Student’s t-test: P < 0.05. ITPi decreases metabolic rate. Two-tailed Student’s t-test: P < 0.001. Data are normalized to their controls. For raw data, see Supplementary Fig. 7a. b) Overexpression of ITP affects spontaneous locomotor activity. ITP marginally nonsignificantly reduces total activity tested over 3 days (2-tailed Student’s t-test: P = 0.06), significantly decreases diurnal activity (2-tailed Student’s t-test: P < 0.01), but does not change nocturnal activity. c) ITPi does not affect spontaneous locomotion during the day or during the night. d) Both ITP overexpression and ITPi reduce the startle-induced climbing (negative geotaxis assay). Two-tailed Student’s t-test for the effect of overexpression: P < 0.05, for the effect of RNAi: P < 0.01. e) Overexpression of ITP reduces starvation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITP off n = 91; daGS>ITP on n = 74. f) Reduction of ITP via RNAi reduces starvation resistance. Log-rank test: P < 0.001. Sample size: daGS>ITPi off n = 102; daGS>ITPi on n = 96. g) Representative patterns of locomotor activity of individual flies upon starvation vs ad libitum feeding. Depicted are control flies, flies overexpressing ITP (daGS>ITP), and flies with ITP RNAi (daGS>ITPi). Note that starvation increases activity, which is particularly pronounced shortly before death. Overexpression of ITP abolishes this increase in activity, while ITPi enhances this behavior. h) Quantification and statistical analysis of starvation-induced hyperactivity during the last 12 h. Overexpression of ITP reduces (2-tailed Student’s t-test: P < 0.05) while ITPi increased this activity (2-tailed Student’s t-test: P < 0.001).
Measurement of the startle-induced climbing
The startle-induced climbing (negative geotaxis) was analyzed using the Rapid iterative negative geotaxis assay (Gargano et al. 2005) modified after Liao et al. (2021). Briefly, male flies were housed in empty transparent vials (25 mm × 95 mm) placed in a holder with an attached ruler. The holder accommodated 3 replicates for each treatment (GeneSwitch on vs off). After a brief recovery (around 15 min), the flies were tapped to the bottom of the tube. Four seconds later, photographs were taken to record the position of individual flies. Subsequently, we measured the distance that flies managed to climb using ImageJ software. At least 22 flies were tested per genotype/treatment.
Immunohistochemistry
Male flies were dissected in ice-cold Drosophila Ca2+-free saline. The brain-thoracic/abdominal ganglia complexes were fixed overnight in Zamboni's fixative at room temperature, washed, and treated as described before (Dircksen et al. 2008). Primary antibodies were a polyclonal rabbit anti-ITP diluted 1:10,000 (Hermann-Luibl et al. 2014), polyclonal rabbit anti-AKH diluted 1:1,000 (Kerafast EGA261, Mark R. Brown, University of Georgia), and monoclonal mouse anti-GFP (Invitrogen) diluted 1:1,000. Secondary antibodies were goat antirabbit Alexa 546 and goat antimouse Alexa 488 (Invitrogen), both diluted 1:1,000. Samples were mounted in Mowiol supplemented with 2.5% 1,4-diazabicyclo-[2.2.2]-octane (DABCO, Sigma, D2522) and imaged under a Zeiss LSM 780 confocal microscope. Images were processed using the Zeiss ZEN software for maximum intensity projections of z-stacks. Cell fluorescence was measured using standard procedures. Briefly, the area of the CC cells was selected and their area, integrated density, and mean gray values were measured. For each sample, the background values for 5 adjacent regions were measured and averaged. The corrected total cell fluorescence (CTCF) was calculated using the standard equation: CTCF = integrated density − (the area of selected cell × the mean fluorescence of the background readings).
Food intake measurement by the Capillary Feeder assay
Food intake was measured by a modification of the Capillary Feeder assay (Ja et al. 2007) in a device constructed out of 24-well plates as described before (Gáliková, Klepsatel, Xu, et al. 2017). Capillaries with food (Hirschmann minicaps, 5µ) were exchanged daily. The volume of ingested food was measured over 5 days in at least 18 males per genotype/treatment and corrected for the evaporation rate. The liquid food contained the same concentration of RU-486 (200 µM) or vehicle control (ethanol) as during the prefeeding period.
Measurement of the speed of the food transit throughout the digestive tract
The speed of food transit was measured as the time from the intake of the blue-dyed food until the excretion of the dye in feces, as described in detail previously (Gáliková et al. 2018). Flies were housed in vials with a drop (approximately 0.2 ml) of food medium containing 0.5% Brilliant Blue (Sigma) and allowed to feed continuously. The cumulative numbers of feces that contained the blue dye were counted every hour until 5 h after the switch to the blue-dyed medium. Four replicates were tested per genotype/treatment, each consisting of 22 male flies.
Statistical analyses
We used a 2-tailed Student's t-test to analyze the pupal volume, dry weight, fecundity, lipid, glycogen, protein, trehalose, and glucose contents, metabolic rate, locomotor activity, starvation-induced activity, food intake, differences in the AKH fluorescence, and expression differences. For experiments conducted in both standard and AkhA genetic backgrounds, we performed, in addition, a 2-way ANOVA with Akh background and ITP manipulation as fixed effects. The details of these analyses are included in Supplementary File 3. Differences in developmental timing were evaluated by the Gehan–Breslow–Wilcoxon test. Data on developmental lethality and fertility were analyzed using Fisher's exact test. Differences in lifespan and starvation survival were assessed using the log-rank test. To analyze the defecation rate, we performed a 2-way ANOVA with 2 fixed factors, genotype and time, and their interaction. P values are indicated by asterisk symbols (*P < 0.05, **P < 0.01, ***P < 0.001). Error bars represent SEM. Data were analyzed using Excel (Microsoft) and GraphPad Prism 8 (GraphPad Software Inc.). Raw data are included in Supplementary File 2.
Results
ITP is essential for development, reproduction, and lifespan
Previous works have shown that the ITP mutation is embryonically lethal (Park 2004), and its partial knock-down reduces survival to adulthood (Gáliková et al. 2018). Here, we further examined the roles of ITP in preadult development, testing the developmental consequences of its genetic downregulation. The expression pattern of ITP involves multiple central and peripheral neurons, which probably synthesize the hormone redundantly (Gáliková et al. 2018). Therefore, we knocked down the hormone production ubiquitously, using the daughterless-GAL4 driver and 2 RNAi lines that have been previously shown to efficiently reduce ITP levels (Gáliková et al. 2018). We found that ITP is required for embryonic and larval development, as RNAi increased lethality during these stages but not during the pupal phase (Fig. 1a). Escapers that survived to adulthood had delayed development (Fig. 1b) and reduced body size (Fig. 1c). These developmental roles of ITP were also confirmed using an alternative RNAi line and ubiquitous daughterless-GeneSwitch (daGS) system (Supplementary Fig. 1), which allowed us to compare genetically identical animals (Tricoire et al. 2009).
Subsequently, we analyzed the effects of ITP gain of function and loss of function during the adult stage.
We used the same manipulations that led to the discovery of the roles of ITP in water balance (Gáliková et al. 2018)—the daGS-driven RNAi to reduce the ITP signaling and the overexpression of the ITP transcript to increase the pathway. To avoid the confounding effects of the developmental roles of ITP, we induced the GeneSwitch 3 days after eclosion. Both up- and downregulation of ITP shortened the lifespan of females (Fig. 1, d and e and Supplementary Fig. 2a) and males (Fig. 1, f and g and Supplementary Fig. 2b). These manipulations also reduced fecundity; overexpression of ITP blocked egg production completely, whereas ITPi reduced the egg-laying to a considerable extent (Fig. 1h). The fertility of these flies (estimated as the viability of embryos produced by parents with reduced ITP signaling) was mildly decreased as well (Fig. 1i).
Altogether, our data show that similar to mammalian glucagon signaling (Vuguin et al. 2006; Ouhilal et al. 2012; Yu et al. 2012), ITP is necessary for development, normal body size, reproduction, and lifespan.
ITP is a hyperglycemic hormone necessary for the maintenance of glycogen and lipid reserves
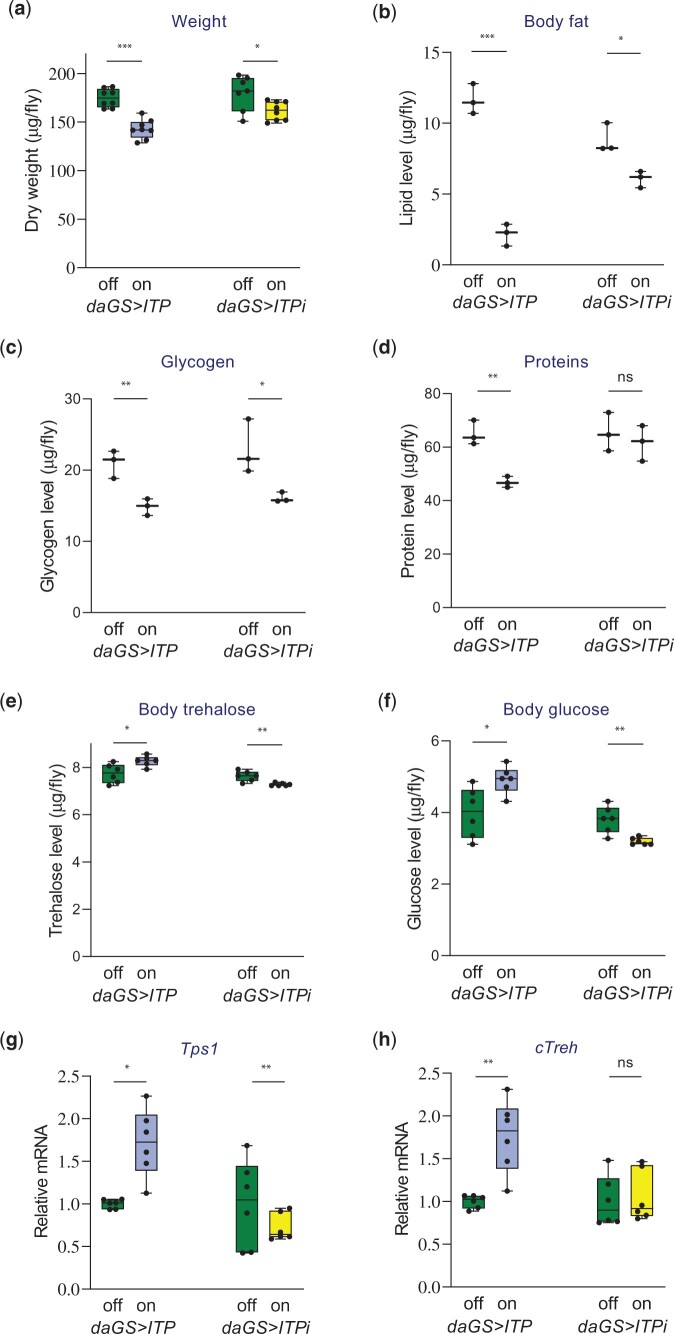
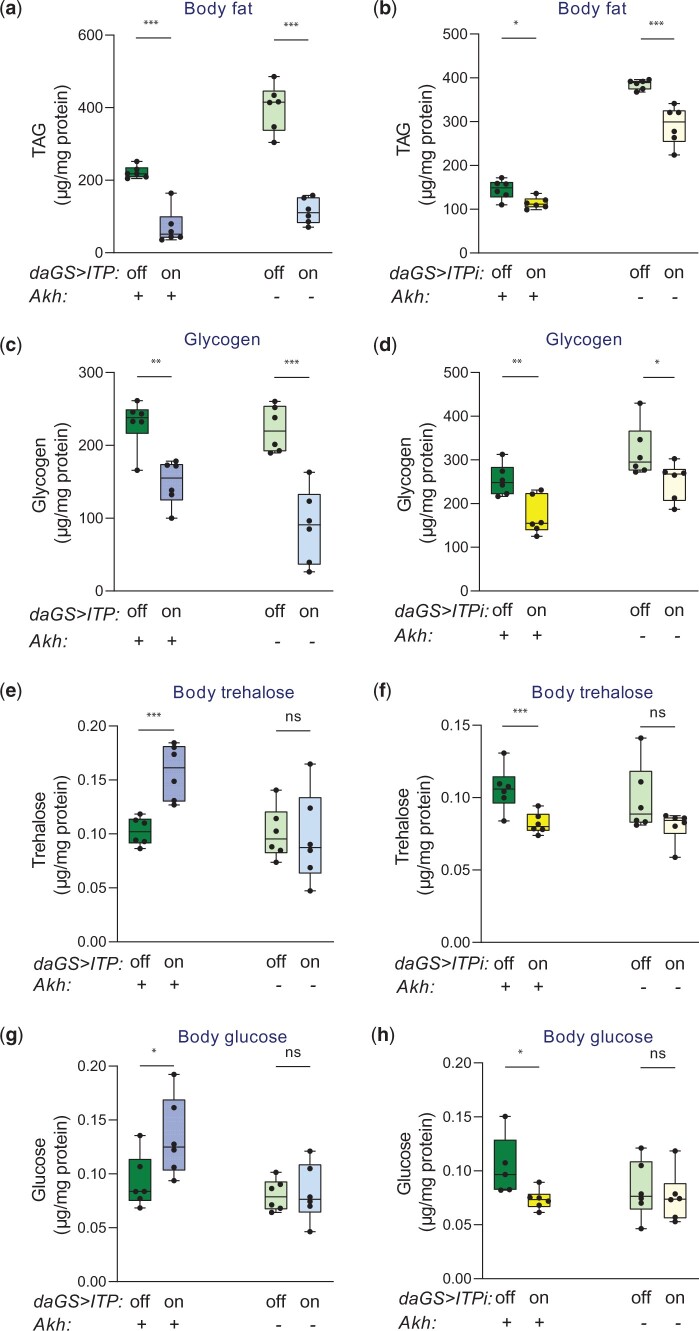
It has been shown that ITP inhibits both feeding and the speed of transit throughout the digestive tract (Gáliková et al. 2018). Thus, individuals with ITP overexpression gain fewer nutrients due to reduced feeding, while flies with downregulated ITP suffer from a diarrhea-like phenotype (Gáliková et al. 2018). Consistent with these results, we observed that the dry weight of flies significantly decreased upon both ITP manipulations in males (Fig. 2a) and females (Supplementary Fig. 3). Both sex and ITP manipulation had a significant effect on the dry weight. The effect of ITP seems to be more pronounced in females (2-way ANOVA for both ITP treatments, sex and genetic manipulations as fixed factors; the effect of ITP: P < 0.001, the effect of sex: P < 0.001). The weight-decreasing effect of ITPi was confirmed by an independent RNAi line (Supplementary Fig. 4a). Both RNAi lines have shown that the reduction in the dry weight was caused by the depletion of lipids (Fig. 2b and Supplementary Fig. 4b) and glycogen (Fig. 2c and Supplementary Fig. 4c). Moreover, ITP overexpression increased the catabolism of proteins (Fig. 2d). Our work also showed that the fly ITP is a hypertrehalosemic hormone; overexpression of ITP elevated the whole body trehalose, while ITPi caused its reduction (Fig. 2e and Supplementary Fig. 4d). Like CHH (Webster, Keller, and Dircksen 2012), also ITP is a hyperglycemic hormone; overexpression of ITP increased, while ITPi reduced the body glucose (Fig. 2f). The hyper- and hypotrehalosemic phenotypes driven by the genetic manipulations of ITP were accompanied by corresponding changes in the expression of Trehalose-6-phosphate synthase (Tps1) (Fig. 2g), which codes for the key enzyme in the synthesis of trehalose (Yoshida et al. 2016). ITP overexpression increased, while ITPi decreased expression of this gene (Fig. 2g). The ITP overexpressing flies had likely increased the turnover of trehalose suggested by the elevated expression of cytoplasmic trehalase (cTreh, Fig. 2h), which codes for a trehalose-degrading enzyme. These expression changes were confirmed by normalization to different housekeeping genes (Act5C and Rpl32 in Fig. 2, g and h and βTub60D in Supplementary Fig. 5).
Fig. 2.
ITP is a hyperglycemic hormone required for the homeostasis of energy reserves. a) Both upregulation (daGS>ITP) and downregulation of ITP (daGS>ITPi) results in reduced dry weight. Two-tailed Student’s t-test: P < 0.001 for the ITP overexpression; P < 0.05 for the ITPi. b) Both upregulation (daGS>ITP) and downregulation of ITP (daGS>ITPi) result in reduced lipid reserves. Two-tailed Student’s t-test: P < 0.001 for the ITP overexpression; P < 0.05 for the ITPi. c) Both upregulation (daGS>ITP) and downregulation of ITP (daGS>ITPi) results in reduced glycogen reserves. Two-tailed Student’s t-test: P < 0.01 for the ITP overexpression; P < 0.05 for the ITPi. d) Overexpression of ITP results in the catabolism of proteins. Two-tailed Student’s t-test: P < 0.01. e) ITP increases trehalose levels. Overexpression of ITP increases, while ITPi reduces trehalose content. Two-tailed Student’s t-test for the effect of overexpression: P < 0.05, Student’s t-test for the effect of RNAi: P < 0.01. f) ITP increases glucose levels. Overexpression of ITP increases, while ITPi reduces glucose content. Two-tailed Student’s t-test for the effect of overexpression: P < 0.05, Student’s t-test for the effect of RNAi: P < 0.01. g) ITP regulates the expression of Trehalose-6-phosphate synthase (Tps1). Overexpression of ITP increases, while ITPi reduces Tps1 mRNA. Two-tailed Student’s t-test for the effect of overexpression: P < 0.05, Student’s t-test for the effect of RNAi: P < 0.01. h) Overexpression of ITP increases mRNA of the trehalose-degrading enzyme, cytoplasmic Trehalase (cTreh). Two-tailed Student’s t-test P < 0.01.
Since trehalose and glucose are often determined in the hemolymph samples, their concentration depends on the amount of body water. Previous work has shown that ITP is an antidiuretic hormone; its overexpression increases while its downregulation decreases water content (Gáliková et al. 2018). Consistently with the dilution of sugars by increased body fluids, the hemolymph of the ITP overexpressing flies does not contain a higher concentration of sugars (Supplementary Fig. 6), despite the increased trehalose and glucose in the whole body samples (Fig. 2, e and f).
It is important to note that to exclude potential confounding developmental effects on body size, the above-mentioned GeneSwitch manipulations were initiated in adult flies. Thus, the differences in body weight, protein, and energy reserves can be directly attributed to the metabolic functions of ITP. However, we cannot differentiate whether the changes in energy reserves are caused solely by the roles of ITP in feeding and digestion or whether they are also contributed by some direct effects of ITP on the fat body tissue.
Altogether, our results show that similar to glucagon (Bürger and Brandt 1935) and AKH (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015; Sajwan et al. 2015), ITP regulates trehalose and glucose levels. Unlike AKH (Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017; Yamada et al. 2018), but like glucagon (Rui 2014; Campbell and Drucker 2015), ITP reduces both lipid and glycogen stores. Moreover, in contrast to the obese Akh mutants (Gáliková et al. 2015), flies with reduced ITP suffer from reduced lipid reserves, resembling the cachectic mutants in glucagon signaling (Gelling et al. 2003; Yu et al. 2012).
ITP increases basal metabolic rate
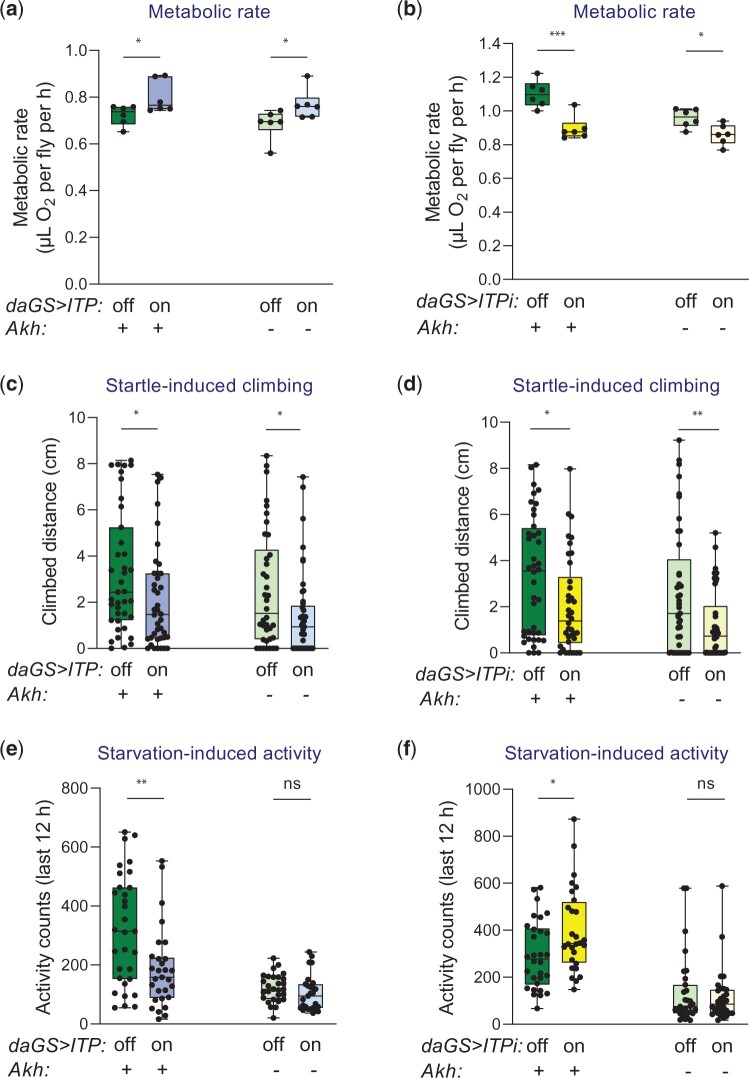
Like glucagon, ITP diminishes energy intake (Gáliková et al. 2018). Therefore, we tested whether the analogy between glucagon and ITP extends to the regulation of energy expenditure. By using manometric respirometry to measure O2 consumption, we found that ITP increases basal metabolic rate. Overexpression of ITP elevated O2 consumption already after 24 h of the GeneSwitch induction (Fig. 3a). Consistently, ITPi via both tested RNAi lines reduced O2 consumption (Fig. 3a and Supplementary Fig. 7). However, long-term overexpression of ITP decreased the metabolic rate (Supplementary Fig. 8), probably due to the depletion of energy reserves (Fig. 2, a–c).
Altogether, our data suggest that, similar to glucagon (Breton et al. 1983), ITP controls energy expenditure by increasing metabolic rate.
ITP does not seem to be required for spontaneous walking but is necessary for the startle-induced locomotion
In the next step, we analyzed whether ITP contributes to energy balance by regulating spontaneous locomotion. We measured the spontaneous walking activity of individual flies using the Drosophila activity monitoring system. ITP manipulations did not affect the total activity (Fig. 3b), although the analyses of locomotor behavior showed that overexpression of ITP significantly reduced spontaneous diurnal activity (Fig. 3b). ITP RNAi did not have any statistically significant effect on locomotor activity (Fig. 3c). Thus, these results suggest that the manipulations of ITP do not alter energy balance by changing spontaneous locomotor activity.
Subsequently, we examined the potential roles of ITP in startle-induced locomotion, which can be considered an escape reaction. We measured the vertical distance flies walked upon a startle impulse (tapping to the bottom of a vial). Both ITP overexpression and RNAi reduced the climbing distance (Fig. 3d). Thus, although ITP is not required for spontaneous locomotion under normal conditions, this peptide is essential for startle-induced vertical climbing (negative geotaxis).
ITP inhibits the starvation-induced hyperactivity
The locomotion of flies depends on their nutritional balance. Under nutritional shortage, flies increase their foraging activity (Lee and Park 2004). This process is independent of circadian rhythms and becomes particularly pronounced during the terminal phase of starvation (Lee and Park 2004). The starvation-induced hyperactivity depends on the previous diet (Huang et al. 2020) and is regulated by the main nutritional pathways such as insulin (Yu, Huang, et al. 2016) and AKH signaling (Lee and Park 2004; Isabel et al. 2005; Braco et al. 2012; Gáliková et al. 2015; Huang et al. 2020). Therefore, we tested wherever ITP regulates this behavior as well. Consistently with the reduced energy reserves (Fig. 2, a–c), both ITP overexpression and downregulation dramatically decreased starvation resistance (Fig. 3, e and f). Visual inspection of the locomotor activity patterns of individual flies under starvation revealed that the ITP overexpressing flies often failed to increase their activity, while downregulation of ITP enhanced the starvation-induced locomotion (Fig. 3g). Consistently, a quantitative analysis of the locomotor activity toward the end of starvation proved that ITP indeed inhibits this process—overexpression of ITP diminished while ITPi RNAi increased the total activity during the last 12 h of life (Fig. 3h).
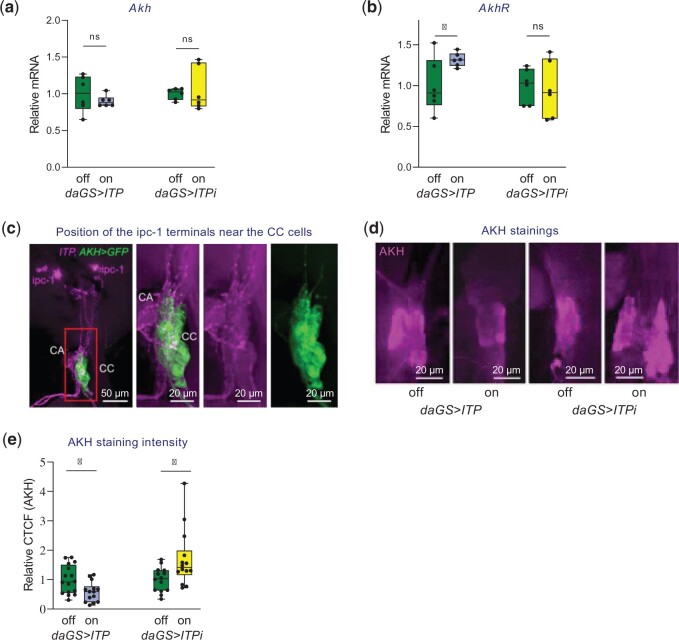
ITP activates the AKH pathway by stimulating the secretion of AKH
Several of the biological functions affected by ITP manipulations are also controlled by AKH (reviewed in e.g. Koyama et al. 2020). Therefore, we aimed to test whether ITP regulates these processes via AKH or independently of this pathway. First, we examined whether ITP manipulations alter AKH signaling. We did not find a significant effect of ITP manipulations on the expression of Akh (Fig. 4a) when the expression data were normalized to Act5C and Rpl32. However, when we used βTub60D as a reference gene, the ITP overexpression increased while ITP RNAi reduced Akh mRNA (Supplementary Fig. 9a). In addition, both normalization approaches confirmed that ITP increases the mRNA of the AKH receptor AkhR (Fig. 4b and Supplementary Fig. 9b). These data thus suggest that ITP may regulate the AKH pathway at the transcriptional level.
Fig. 4.
ITP regulates AKH signaling. a) Manipulations of ITP do not change the expression of Akh when expression data are normalized to Act5C and Rpl32. Two-tailed Student’s t-test: P > 0.05 for both overexpression of ITP and ITPi. b) ITP regulates the expression of the AKH receptor AkhR. Overexpression of ITP increases levels of AkhR (2-tailed Student’s t-test: P < 0.05). c) Anatomy of the ITP-producing ipc neurons. The ITP-producing ipc neurons send their axons toward the neurohemal release sites, which are located nearby the corpora allata (CA) and corpora cardiaca (CC). The ipc neurons are stained with the ITP antibody (in magenta), and the CCs are visualized via AKH-GAL4-driven GFP (in green). d) Representative staining of CC (the AKH-producing organs) with the AKH antibody. Overexpression of ITP reduces the AKH levels in the CCs, which is indicative of increased secretion of AKH. ITPi increases the AKH in CCs, suggesting a reduced release of AKH. e) Quantification of AKH staining in CCs of flies with genetically manipulated ITP signaling. Overexpression of ITP increases AKH secretion (2-tailed Student’s t-test: P < 0.05), while ITP RNAi reduces it (2-tailed Student’s t-test: P < 0.05). Plotted is the CTCF normalized to the values of the genetically-matching controls.
However, the AKH signaling is regulated primarily at the level of hormone secretion (Van der Horst et al. 2001), and we have thus investigated whether ITP manipulations also affect this process. AKH is produced by a neuroendocrine organ called corpora cardiaca (CC) (Kim and Rulifson 2004; Lee and Park 2004), and the secretion of the peptide is inversely correlated with the intensity of its immunolabeling in these cells. Therefore, immunostainings are frequently used to study the release of this hormone (e.g. Braco et al. 2012; Kim and Neufeld 2015). We used this approach to investigate the potential effects of ITP on the secretion of AKH. Overexpression of ITP increased secretion of AKH, while ITPi inhibited the release of the peptide (Fig. 4, d and e). This supports the conclusion that ITP regulates the AKH pathway at the level of AKH secretion.
Interestingly, a small subset of ITP-producing cells called ipc-1 and ipc-2a send their axons toward the AKH-producing cells of CCs (Gáliková et al. 2018; Fig. 4c). This suggests that they may directly regulate AKH release. Intriguingly, these cells have already been shown to regulate metabolism (Kahsai et al. 2010). Thus, we have tested their potential roles in sugar homeostasis. However, the ITPi in the pattern of these neurosecretory cells [covered by the previously tested Impl2 (Gáliková et al. 2018) and c929 (Kahsai et al. 2010) GAL4 drivers] did not recapitulate the effects obtained by the ubiquitous RNAi, such as reduced trehalose levels (Supplementary Fig. 10). Accordingly, this observation suggests that the AKH release from CCs is likely regulated by ITP-producing cells other than ipc-1 and ipc-2a or that the ITP-producing cells act redundantly [as shown previously for their role in the water balance (Gáliková et al. 2018)].
Altogether, our results indicate that the ITP pathway interacts with the AKH signaling; ITP increases the expression of the AKH receptor and triggers the secretion of AKH from the CC cells.
The ITP-dependent regulation of energy intake and expenditure does not require AKH
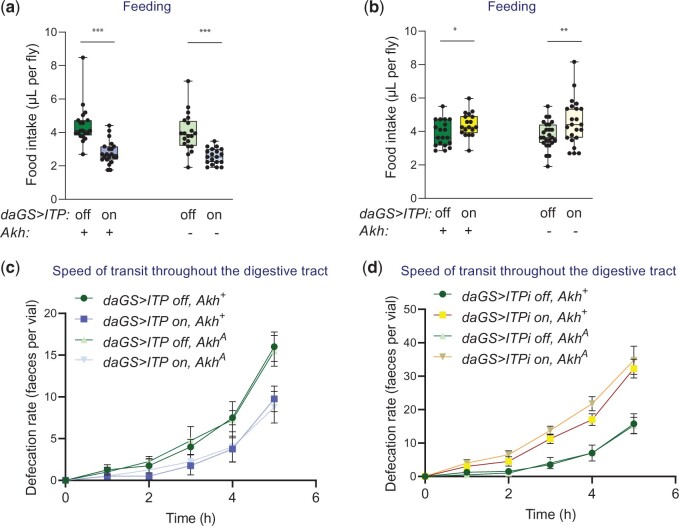
Because the ITP manipulations affect the AKH release, we next explored whether the functions of ITP are mediated via the AKH peptide or whether ITP controls Drosophila physiology independently of this hormone. We reasoned that if ITP modulates the tested processes via AKH, the effects of ITP manipulations will disappear in the absence of the AKH peptide. Therefore, we analyzed the consequences of ITP up- and downregulations in flies with the previously described functional null mutation of Akh, AkhA (Gáliková et al. 2015). We focused on the biological processes that we discovered as ITP regulated and that were previously shown to be under AKH control. First, we examined the role of ITP in energy intake and expenditure. Overexpression of ITP reduced food intake in both standard and AKH-deficient backgrounds (Fig. 5a), and RNAi increased feeding independently of the presence of AKH (Fig. 5b). Thus, the anorexigenic effect of ITP seems to be AKH independent. Similarly, the regulation of the speed of transit throughout the digestive tract via ITP does not require AKH. The experimental increase in ITP decreased the defecation rate (Fig. 5c), while the loss of ITP led to a diarrhea-like phenotype in standard and in the AkhA background (Fig. 5d). The hypermetabolic effect of ITP appears to be AKH independent as well; overexpression of ITP increased (Fig. 6a), while ITPi decreased (Fig. 6b) the O2 consumption in the absence of AKH.
Fig. 5.
ITP regulates energy intake independently of AKH signaling. a, b) ITP represses feeding independently of AKH. a) Overexpression of ITP reduces food intake in the standard genetic background (2-tailed Student’s t-test: P < 0.001), as well as in the AKH-deficient background (2-tailed Student’s t-test: P < 0.001). The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITP overexpression as fixed effects; the effect of ITP: P < 0.001, the effect of interaction: P > 0.05). b) Reduction of ITP via RNAi increases food intake in a standard (2-tailed Student’s t-test: P < 0.05), as well as in the AKH-deficient background (2-tailed Student’s t-test: P < 0.01). The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITP RNAi as fixed effects; the effect of ITPi: P < 0.01, the effect of interaction: P > 0.05). c, d) The antidiarrheal effect of ITP does not require AKH. c) Overexpression of ITP reduces the pace of transit through the digestive tract irrespective of AKH. Two-way ANOVA for the standard as well as in the AkhA background: ITP and time as fixed factors; the effect of ITP: P < 0.05, the effect of time: P < 0.001, the effect of the interaction: P > 0.05. Error bars represent SEM. d) Reduction of ITP via RNAi increases the pace of transit through the digestive tract in the presence as well as in the absence of AKH. Two-way ANOVA for the standard genetic background: ITP and time as fixed factors; the effect of ITPi: P < 0.01, the effect of time: P < 0.001, the effect of the interaction: P > 0.05. Two-way ANOVA for the AkhA genetic background: ITPi and time as fixed factors; the effect of ITPi: P < 0.05, the effect of time: P < 0.001, the effect of the interaction: P > 0.05. Error bars represent SEM.
Fig. 6.
ITP regulates the metabolic rate and startle-induced climbing independently of AKH, but its effects on starvation-induced hyperactivity disappear in the absence of AKH. a, b) ITP increases metabolic rate independently of AKH. a) Overexpression of ITP increases basal metabolic rate in the standard genetic background (2-tailed Student’s t-test: P < 0.05) as well as in the AkhA background (2-tailed Student’s t-test: P < 0.05). The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITP overexpression as fixed effects; the effect of ITP: P < 0.01, the effect of interaction: P > 0.05). b) ITPi decreases basal metabolic rate in the standard genetic background (2-tailed Student’s t-test: P < 0.001) as well as in the AkhA background (2-tailed Student’s t-test: P < 0.05). The background modulates the effect of ITP (2-way ANOVA with the Akh background and ITP RNAi as fixed effects; the effect of ITPi: P < 0.001, the effect of interaction: P < 0.001). c, d) Proper ITP levels are required for the startle-induced vertical climbing independently of AKH. c) Overexpression of ITP impairs vertical climbing in both standard and AkhA backgrounds (2-tailed Student’s t-test: P < 0.05. The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITP overexpression as fixed effects; the effect of ITP: P < 0.05, the effect of interaction: P > 0.05). d) ITPi impairs vertical climbing in both standard genetic background (2-tailed Student’s t-test: P < 0.05) as well as in the AkhA background (2-tailed Student’s t-test: P < 0.01). The background does not alter the effect of ITPi (2-way ANOVA with the Akh background and ITPi as fixed effects; the effect of ITPi: P < 0.001, the effect of interaction: P > 0.05). e, f) The ITP-dependent starvation-induced hyperactivity requires AKH. e) Overexpression of ITP reduces the starvation-induced foraging in the standard genetic background (2-tailed Student’s t-test: P < 0.01), but not in the already hypoactive AkhA background (2-tailed Student’s t-test: P > 0.05). f) Reduction of ITP via RNAi enhances the starvation-induced foraging in the standard genetic background (2-tailed Student’s t-test: P < 0.05), but not in the AkhA background (2-tailed Student’s t-test: P > 0.05).
Taken together, under standard feeding conditions, ITP regulates energy intake and energy expenditure independently of the AKH pathway.
ITP inhibits starvation-induced locomotion only in the presence of a functional AKH peptide
Both startle-induced vertical climbing (Bednářová et al. 2018) and starvation-induced locomotion (Gáliková et al. 2015) are dependent on functional AKH peptide. Our results presented in Fig. 3 show that these processes also require ITP. We have therefore tested whether these ITP phenotypes might be mediated by AKH, i.e. whether the effect of ITP persists in the AKH-deficient background. Overexpression of ITP reduced the distance that flies climbed upon the startle in the standard as well as in the AKH-deficient background (Fig. 6c). Similarly, ITPi impaired the startle-induced climbing irrespective of AKH presence (Fig. 6d). Consistently, overexpression of Akh did not revert the impaired climbing of the ITPi-treated flies (Supplementary Fig. 11). Thus, ITP is necessary alongside AKH for the standard startle-induced locomotion.
In contrast to vertical climbing, the role of ITP in inhibiting hunger-driven locomotion seems to depend on AKH. ITP overexpression reduces (Fig. 6e) and ITPi elevates (Fig. 6f) this phenotype only in the presence of the functional AKH peptide.
ITP regulates glycemia via AKH, but the requirement of ITP for homeostasis of lipids and glycogen is AKH independent
Consistently with the AKH-independent roles in energy intake and expenditure, ITP is required for the maintenance of proper energy reserves irrespective of the AKH presence. Manipulations of ITP reduce lipid (Fig. 7, a and b) and glycogen (Fig. 7, c and d) reserves in both standard and AKH-deficient backgrounds. In contrast to stored energy reserves, circulating energy sources seem to be ITP-regulated via AKH. ITP upregulation increased trehalose levels in control but not in the AkhA background (Fig. 7e). Consistently, ITP RNAi reduced trehalose levels in a standard genetic background but not in the absence of AKH (Fig. 7f). Similar to the regulation of trehalose, glucose levels are also controlled via the ITP–AKH axis. Overexpression of ITP increased glucose content in a standard but not in the AKH-deficient background (Fig. 7g), and ITPi reduced levels of this saccharide in a standard, but not in the AkhA background (Fig. 7h).
Fig. 7.
ITP regulates energy reserves independently of AKH signaling, while the hypertrehalosemic and hyperglycemic effects of ITP are AKH dependent. a, b) ITP is required for proper fat storage independently of AKH. Overexpression of ITP reduces fat content in the standard genetic background, as well as in the AkhA background (2-tailed Student’s t-test: P < 0.001 for both comparisons). The background modulates the effect of ITP (2-way ANOVA with the Akh background and ITP overexpression as fixed effects; the effect of ITP: P < 0.001, the effect of interaction: P < 0.01). ITPi reduces fat content in the standard genetic background, as well as in the AkhA background (2-tailed Student’s t-test: P < 0.05 for the standard and P < 0.001 for the AkhA background). The background modulates the effect of ITP (2-way ANOVA with the Akh background and ITPi as fixed effects; the effect of ITPi: P < 0.001, the effect of interaction: P < 0.01). c, d) ITP is required for proper glycogen storage independently of AKH. Overexpression of ITP reduces glycogen content in the standard genetic background, as well as in the AkhA background (2-tailed Student’s t-test: P < 0.01 for the standard and P < 0.001 for the AkhA background). The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITP overexpression as fixed effects; the effect of ITP: P < 0.001, the effect of interaction: P > 0.05). ITPi reduces glycogen content in the standard genetic background (2-tailed Student’s t-test: P < 0.01), as well as in the AkhA background (2-tailed Student’s t-test: P < 0.05). The background does not alter the effect of ITP (2-way ANOVA with the Akh background and ITPi as fixed effects; the effect of ITPi: P < 0.001, the effect of interaction: P > 0.05). e, f) The hypertrehalosemic function of ITP requires functional AKH. Overexpression of ITP increases trehalose content in the standard genetic background (2-tailed Student’s t-test: P < 0.001) but not in the absence of AKH (2-tailed Student’s t-test: P > 0.05). ITPi decreases trehalose content in the standard genetic background (2-tailed Student’s t-test: P < 0.001) but not in the absence of AKH (2-tailed Student’s t-test: P > 0.05). g, h) The hyperglycemic function of ITP requires functional AKH. Overexpression of ITP increases body glucose in the standard genetic background (2-tailed Student’s t-test: P < 0.05) but not in the absence of AKH (2-tailed Student’s t-test: P > 0.05). ITPi decreases glucose in the standard genetic background (2-tailed Student’s t-test: P < 0.05) but not in the absence of AKH (2-tailed Student’s t-test: P > 0.05).
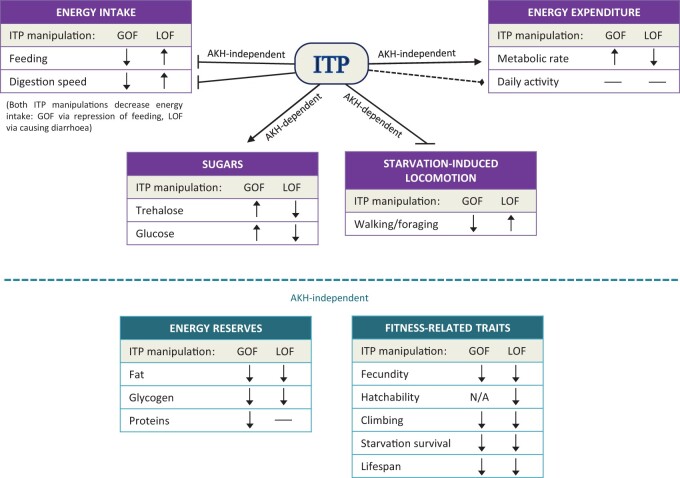
In summary, ITP has metabolic functions intriguingly similar to mammalian glucagon—it inhibits energy intake while increasing metabolic rate. The peptide also increases trehalose and glucose and reduces stored energy reserves. Our work describes a novel hormonal axis, where ITP acts upstream of the well-known AKH peptide. ITP activates the AKH pathway by triggering the secretion of AKH and by inducing the transcription of its receptor AkhR. The hyperglycemic function of ITP is mediated by the AKH pathway, while its roles in energy intake, expenditure, and homeostasis of stored energy reserves are AKH independent (Fig. 8).
Fig. 8.
Summary of ITP functions identified by the daughterless-GeneSwitch driven ITP overexpression (gain of function, GOF) and RNAi (loss of function, LOF). ITP overexpression reduces feeding, the speed of transit throughout the digestive tract (i.e. protects from diarrhea), and increases metabolic rate. The ITPi results in the opposite phenotype. Although ITP overexpression and ITPi have antagonistic effects on the tested traits, both manipulations reduce energy intake: ITP overexpression via feeding inhibition, while ITPi via diarrhea. Accordingly, we have observed a decline in energy reserves (fat and glycogen) and energy-demanding and fitness-related traits upon both manipulations. Testing the direct vs indirect effects of ITP on these traits requires further studies, including the identification of the peptide receptor(s). We have observed that ITP stimulates the secretion of the catabolic hormone AKH. Subsequently, we have tested whether the metabolic roles of ITP depend on this peptide. Our data showed that the effects of ITP on starvation-induced locomotion and trehalose and glucose levels depend on the presence of the functional AKH. However, the rest of the ITP functions in energy intake, expenditure, and energy reserves are independent of this peptide. Altogether, our study shows that ITP is a novel metabolic regulator that controls energy homeostasis alongside AKH.
Discussion
Hormonal regulation of energy catabolism
Despite the growing popularity of Drosophila as an invertebrate model for metabolism, the regulation of energy catabolism is a relatively understudied topic. In mammals, the hormone glucagon induces catabolic processes at several levels—it reduces energy intake, increases energy expenditure via enhanced metabolic rate, and induces catabolism of lipids, glycogen, and proteins. The situation in invertebrates seems to be more complicated. To our best knowledge, no single hormone with all these functions has been described so far. The AKH hormone mobilizes fat (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017), but not glycogen (Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017; Yamada et al. 2018) or proteins (Mochanová et al. 2018). In contrast to glucagon, AKH does not reduce food intake but, on the contrary, enhances appetite (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017). Its role in energy expenditure is unclear. While some studies found increased metabolic rate upon application of AKH in insects such as the firebug Pyrrhocoris apterus (Gautam et al. 2020), others described normal oxygen consumption in flies overexpressing this peptide (Gáliková, Klepsatel, Xu, et al. 2017) and slight reduction of metabolic rate in the Akh mutants (Sajwan et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017). Another peptide with possible catabolic functions is the neuropeptide corazonin. Corazonin depletes glycogen but does not alter fat, trehalose, or glucose levels (Kubrak et al. 2016). Similar to AKH (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017), corazonin increases food intake (Kubrak et al. 2016). Metabolic rate and fat accumulation are also regulated by the fly counterpart of norepinephrine—octopamine (Li et al. 2016). Like AKH, octopamine induces lipolysis (Socha et al. 2008) and increases food intake in insects (Youn et al. 2018). Our work shows that ITP is another peptide that regulates fly metabolism alongside AKH and the above-mentioned catabolic hormones.
The CHH/ITP family and its functional similarity to mammalian glucagon
The CHH/ITP hormone family is evolutionarily conserved in ecdysozoan invertebrates. These peptides were described in arthropods (crustaceans, insects, myriapods, and arachnids) and nematode worms (Dircksen 2009; Webster et al. 2012). Physiological functions of CHH have been first characterized in crustaceans. The name originates from its hyperglycemic effect studied in blue crabs Callinectes sapidus and fiddler crabs Uca pugilator (Abramowitz et al. 1944). Interestingly, CHH regulates lipid metabolism in several crustacean species (Santos et al. 1997). In addition, several studies described the osmoregulatory, developmental, and reproduction-related functions of CHH, suggesting a general requirement of the hormone for almost all aspects of physiology (Chung et al. 2010; Webster et al. 2012). Our study shows that the developmental, reproduction- and metabolism-related roles of the CHH family are conserved in Drosophila. The fly CHH, encoded by the ITP gene, controls energy homeostasis at several key levels, including energy intake, expenditure, and accumulation of energy reserves. ITP is necessary for traits such as development, reproduction, and lifespan. Since ITP is an essential regulator of water and energy balance (Gáliková et al. 2018), it remains to be investigated whether the ITP dysregulations decrease energy balance and fitness-related traits indirectly (due to the impairment of energy and water balance) or whether ITP has additional signaling functions.
The ITP gain- and loss-of-function phenotypes are intriguingly similar to the effects observed in mammalian models upon glucagon administration and in the Gcgr mutants, respectively (Vuguin et al. 2006; Ouhilal et al. 2012; Yu et al. 2012; Campbell and Drucker 2015; Müller et al. 2017). For example, the Gcgr deficiency lowers body size at birth, impairs reproduction (Vuguin et al. 2006; Ouhilal et al. 2012), leads to cachexia, and considerably decreases lifespan (Yu et al. 2012). These developmental phenotypes are similar to the effects of ITP manipulations; deficiency for ITP is embryonically lethal (Park 2004), while partial knock-down results in increased developmental lethality, viability, smaller body size, and shortened lifespan. The hormones have a similar impact on energy homeostasis; glucagon regulates energy balance by repressing energy intake (feeding, peristalsis, and gastric emptying) and increasing energy expenditure [basal metabolism (Campbell and Drucker 2015; Müller et al. 2017)]. ITP seems to have the same functions in Drosophila. Previous work showed that ITP decreases appetite and prolongs the transit time throughout the digestive tract (Gáliková et al. 2018), and our study reveals that this hormone increases energy expenditure by enhancing basal metabolic rate. Glucagon is known for inducing lipolysis, glycogenolysis, and gluconeogenesis. Consistently, overexpression of ITP resulted in the depletion of fat, glycogen reserves, and proteins, suggesting catabolic effects of ITP. Nevertheless, it is unknown whether ITP acts on the fat body directly or if the low energy reserves result only from the reduced energy intake of the flies.
Balanced glucagon signaling is critical for the maintenance of energy reserves. Defects in this pathway (as described in the Gcgr deficient mice) decrease the amount of energy reserves, leading to cachexia (Yu et al. 2012). Similarly, we have observed that the reduction of ITP depletes glycogen and fat stores. Thus, both up- and downregulation of ITP result in low energy reserves, although the underlying mechanism differs. The gain of function is connected with reduced feeding (Gáliková et al. 2018) and increased metabolic rate, while the loss of function is coupled with diarrhea (Gáliková et al. 2018). In addition, it is possible that ITP directly induces catabolism of energy stores and gluconeogenesis. Like glucagon, ITP acts as a hyperglycemic hormone. ITP increases trehalose and glucose levels and stimulates the expression of the key enzyme for the synthesis of trehalose, Tps1 (Yoshida et al. 2016). The exact mechanism behind the hyperglycemic and hypertrehalosemic actions of ITP is unclear, but it seems to rely on the AKH signaling because the effects of ITP overexpression and RNAi disappear in an Akh-deficient background.
The ITP–AKH axis
AKH is an important hyperglycemic and lipolytic hormone in Drosophila (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015; Gáliková, Klepsatel, Xu, et al. 2017), with pleiotropic functions in the defense against oxidative stress (Bednářová et al. 2015; Gáliková et al. 2015; Kodrik et al. 2015; Zemanova et al. 2016), immunity (Ibrahim et al. 2017, 2018), promotion of appetite (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017), starvation-induced foraging (Lee and Park 2004; Isabel et al. 2005; Gáliková et al. 2015), daily locomotor activity (Pauls et al. 2020), courtship (Lebreton et al. 2016), mating (Wat et al. 2021), and alteration of metabolism in response to nutrition deficiency experienced during larval development (Hughson et al. 2021). Thus, the AKH signaling appears to be an important mechanism contributing to the fine-tuning of energy catabolism to various needs. The AKH-producing CC cells constitute a hub regulated by diverse inputs, including circulating sugars (Kim and Neufeld 2015), neuropeptides Unpaired 2 (Zhao and Karpac 2017), Allatostatin A (Hentze et al. 2015), octopaminergic/tyraminergic neurons (Pauls et al. 2018), glucose sensing CN neurons (Oh et al. 2019), and Bursicon-responsive DLgr2 neurons (Scopelliti et al. 2018). Our work shows that ITP is another regulator of the CC cells. ITP seems to increase AKH signaling at the level of the hormone secretion and by increasing transcription of the AKH receptor AkhR. Interestingly, the ITP-producing ipc-1 and ipc-2a secretory neurons have their branches and release sites nearby the AKH-producing cells of CC. However, knock-down of ITP in the ipcs did not cause changes in the trehalose or glucose levels. This suggests that either ITP-producing cells other than the ipc-1 and ipc-2a regulate the secretion of AKH from the CCs or that the ITP cells act redundantly. In addition, we cannot exclude that the ipc-1 and ipc-2a (or other ITP cells) regulate the release of other CC-produced peptides, such as the decretin Limostatin (Alfa et al. 2015).
In addition to triggering the secretion of AKH, ITP seems to control AKH signaling via transcriptional control of AkhR. However, it remains to be investigated whether ITP acts on the CCs and fat body (tissue-expressing AkhR) directly or indirectly via ITP-regulated metabolites or other factors. Altogether, the hyperglycemic action of ITP depends on the AKH peptide. AKH is one of the most studied insect hormones, and its hyperglycemic and hypertrehalosemic functions are well known (Kim and Rulifson 2004; Lee and Park 2004; Gáliková et al. 2015). However, the mechanism behind these roles is not clear. As glycogen is considered to be the major source of trehalose in insects (Becker et al. 1996; Van der Horst 2003), AKH has been expected to exert its hyperglycemic functions by inducing glycogenolysis (Van der Horst 2003). Nevertheless, at least in the fly model, this seems not to be the case (Gáliková, Klepsatel, Xu, et al. 2017; Yamada et al. 2018). Moreover, insects cannot carry out gluconeogenesis from lipid substrates (due to the absence of the glyoxylate cycle). Thus, the hyperglycemic effects of AKH likely do not result from its lipolytic functions. Altogether, the mechanism behind the hyperglycemic and hypertrehalosemic roles of the ITP–AKH axis remains enigmatic.
It also remains to be investigated whether the functions of the ITP–AKH axis are conserved in other ecdysozoan invertebrates. Crustaceans have a structural homolog of AKH, called Red pigment concentrating hormone (RPCH). Interestingly, a recent study showed that it has a hyperglycemic effect (Alexander et al. 2018). It has been suggested that RPCH regulates glycemia via an eyestalk-produced factor (Alexander et al. 2018), and it is thus tempting to hypothesize that RPCH is under the control of CHH.
The AKH-independent roles of ITP
Our results show that except for the hyperglycemic and hypertrehalosemic effects of ITP, the other metabolic functions of the peptide (such as the control of feeding, metabolic rate, and energy reserves) do not require AKH. Interestingly, hunger-related phenotypes are regulated by AKH and ITP in an antagonistic manner. AKH induces (Jourjine et al. 2016; Gáliková, Klepsatel, Xu, et al. 2017) while ITP represses feeding (Gáliková et al. 2018), and AKH is necessary for starvation-induced foraging (Lee and Park 2004; Isabel et al. 2005; Gáliková et al. 2015), while ITP inhibits this process.
Altogether, AKH seems to be part of a broader axis that is functionally similar to the glucagon pathway in mammals. Nevertheless, it is unclear how the AKH-independent effects of ITP are mediated. For example, the essential role of ITP in the control of energy intake and expenditure may be responsible for the metabolic and fitness-related functions of the peptide. Alternatively, ITP may also have direct signaling effects. Understanding the molecular mechanism behind these ITP functions will require, first of all, the identification of ITP receptor(s) and downstream targets.
The open questions in ITP expression, splice variants, receptor(s), and downstream targets
Our study was based primarily on ubiquitous manipulations of ITP. Thus, we cannot differentiate whether specific subpopulations of ITP-producing cells are responsible for the tested phenotypes or whether the ITP neurons produce the peptide redundantly. Although the antibody stainings detected the fly ITP only in the nervous system (Dircksen et al. 2008; Gáliková et al. 2018), we cannot exclude the existence of additional ITP-producing cells. The extraneuronal expression is suggested by the microarray (Chintapalli et al. 2007) and RNA seq (Leader et al. 2018; Krause et al. 2022) data, and the expression of ITP homologs in other insects (Begum et al. 2009; Yu, Li, et al. 2016) and crustaceans (Chen et al. 2020).
ITP is considered the only functional hormone produced by the ITP gene, because only this peptide bears the amidation signal crucial for its activity (Dircksen et al. 2008; Dircksen 2009). Consistently, mutational analysis of the gene showed that the N-terminus is necessary for the ion transport in the hindgut of Schistocerca gregaria (Zhao et al. 2005), and only ITP (but not ITPLs) is needed for the development in the brown planthopper N. lugens (Yu, Li, et al. 2016). Nevertheless, the ITP of the red flower beetle T. castaneum lacks the canonical amidation signal, and the ITPL forms are required for development and reproduction (Begum et al. 2009). Thus, we cannot exclude the possibility that the 2 long forms of ITP, ITPLs, may also have certain biological functions in the fly. Moreover, our RNAi approach reduced mRNA for all 3 peptides, although the overexpression experiments were ITP specific.
Receptor(s) for Drosophila ITP is currently unknown, but flies carry homologs of the ITP receptors identified in silkworm B. mori (Nagai et al. 2014). The most related fly genes are the Tachykinin-like receptor at 99D (TkR 99D), Tachykinin-like receptor at 86C (TkR 86C), Pyrokinin 2 receptor 2 (PK2-R2), and Pyrokinin 2 receptor 1 (PK2-R1) (Nagai et al. 2014). However, it is unclear whether any of these GPCRs interact with the ITP peptide in Drosophila.
Altogether, although our study brought the first characterization of metabolic roles of ITP in the fly model, it still left many questions open. Further understanding of the exact metabolic actions of ITP requires the identification of the target tissues, receptor(s), and other members of the ITP signaling pathway.
The role of ITP in the integration of water and energy balance
As an antidiuretic hormone (Gáliková et al. 2018), ITP seems to fulfill the functions of mammalian renin–angiotensin and vasopressin systems. Interestingly, mammalian glucagon is also involved in water homeostasis; it cooperates with vasopressin to increase glomerular filtration after a protein-rich meal (Ahloulay et al. 1992; Bankir et al. 2015, 2018). ITP integrates water metabolism with energy homeostasis as well. Briefly, ITP increases body water by promoting water intake while inhibiting its excretion. At the same time, the peptide decreases energy balance by inhibiting energy intake and increasing metabolic rate. This may actually be the primary, overarching function of ITP. In contrast to the protected laboratory conditions with a balanced diet providing sufficient water and energy, animals are often exposed to a shortage of food or water in nature. Thus, animals need to adjust water and food intake to maintain the osmolarity of body fluids. When the food is too dry, ITP increases the ingestion of free water while reducing feeding. At the same time, the hormone promotes water conservation by inhibiting excretion (Gáliková et al. 2018). To cope with the reduced energy intake, ITP likely stimulates the catabolism of stored energy reserves, which also releases bound water. Given the natural transcriptional responses of ITP to desiccation and osmotic stress, ITP signaling may be one of the critical factors integrating water and energy homeostasis.
Supplementary Material
Acknowledgments
We are thankful to Diana Knoblochová and Alexander Baranovič for technical assistance. We are very grateful to prof. Heinrich Dircksen (Stockholm University) for his generous help and support throughout the project. We acknowledge the support of prof. Dick Nässel (Stockholm University) when the project was in its infancy. We acknowledge the support of Dr. Dušan Žitňan (Institute of Zoology, Slovak Academy of Sciences). We thank Véronique Monnier, Hervé Tricoire, Charlotte Helfrich-Förster, Hugo Stocker, the Vienna Drosophila Resource Center, and the Bloomington Drosophila Stock Center (NIH P40OD018537) for fly stocks. We acknowledge the critical resources and information provided by FlyBase (Thurmond et al. 2019). FlyBase is supported by a grant from the National Human Genome Research Institute at the U.S. National Institutes of Health (U41 HG000739) and by the British Medical Research Council (MR/N030117/1).
Funding
This study was supported by grants from the Slovak Academy of Sciences (MoRePro, Seal of Excellence) and the Slovak Research and Development Agency (APVV-19-0196) to MG and by the Carl Trygger’s Foundation grant to Dick R. Nässel. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Conflicts of interest
None declared.
Contributor Information
Martina Gáliková, Institute of Zoology, Slovak Academy of Sciences, 845 06 Bratislava, Slovakia; Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden.
Peter Klepsatel, Institute of Zoology, Slovak Academy of Sciences, 845 06 Bratislava, Slovakia; Institute of Molecular Physiology and Genetics, Centre of Biosciences, Slovak Academy of Sciences, 840 05 Bratislava, Slovakia.
Data Availability
All data necessary for confirming the conclusions presented in this article are available in the article and its online supplementary material. Fly stocks and reagents are available upon request.
Supplemental material is available at GENETICS online.
Literature cited
- Abramowitz AA, Hisaw FL, Papandrea DN.. The occurrence of a diabetogenic factor in the eyestalks of crustaceans. Biol Bull. 1944;86(1):1–5. [Google Scholar]
- Ahloulay M, Bouby N, Machet F, Kubrusly M, Coutaud C, Bankir L.. Effects of glucagon on glomerular filtration rate and urea and water excretion. Am J Physiol. 1992;263(1 Pt 2):F24–F36. doi: 10.1152/ajprenal.1992.263.1.F24. [DOI] [PubMed] [Google Scholar]
- Ahmad M, He L, Perrimon N.. Regulation of insulin and adipokinetic hormone/glucagon production in flies. Wiley Interdiscip Rev Dev Biol. 2020;9(2):e360. doi: 10.1002/wdev.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Massadi O, Ferno J, Dieguez C, Nogueiras R, Quinones M.. Glucagon control on food intake and energy balance. Int J Mol Sci. 2019;20(16). doi: 10.3390/ijms20163905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JL, Oliphant A, Wilcockson DC, Audsley N, Down RE, Lafont R, Webster SG.. Functional characterization and signaling systems of corazonin and red pigment concentrating hormone in the green shore crab, Carcinus maenas. Front Neurosci .2018;11:752. doi: 10.3389/fnins.2017.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa RW, Park S, Skelly KR, Poffenberger G, Jain N, Gu XY, Kockel L, Wang J, Liu Y, Powers AC, Kim SK. Suppression of insulin production and secretion by a decretin hormone. Cell Metab .2015;21(2):323–333. doi: 10.1016/j.cmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar A, Kucerova K, Jonatova L, Tomcala A, Schneedorferova I, Okrouhlik J, Dolezal T.. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol. 2015;13(4):e1002135. doi: 10.1371/journal.pbio.1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankir L, Bouby N, Speth RC, Velho G, Crambert G.. Glucagon revisited: coordinated actions on the liver and kidney. Diabetes Res Clin Pract. 2018;146:119–129. doi: 10.1016/j.diabres.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Bankir L, Roussel R, Bouby N.. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. Am J Physiol Renal Physiol. 2015;309(1):F2–F23. doi: 10.1152/ajprenal.00614.2014. [DOI] [PubMed] [Google Scholar]
- Becker A, Schloder P, Steele JE, Wegener G.. The regulation of trehalose metabolism in insects. Experientia .1996;52(5):433–439. [DOI] [PubMed] [Google Scholar]
- Bednářová A, Kodrík D, Krishnan N.. Knockdown of adipokinetic hormone synthesis increases susceptibility to oxidative stress in Drosophila—a role for dFoxO? Comp Biochem Physiol C: Toxicol Pharmacol. 2015;171:8–14. doi: 10.1016/j.cbpc.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Bednářová A, Tomčala A, Mochanová M, Kodrík D, Krishnan N.. Disruption of adipokinetic hormone mediated energy homeostasis has subtle effects on physiology, behavior and lipid status during aging in Drosophila. Front Physiol. 2018;9. doi: 10.3389/fphys.2018.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum K, Li B, Beeman RW, Park Y.. Functions of ion transport peptide and ion transport peptide-like in the red flour beetle Tribolium castaneum. Insect Biochem Mol Biol. 2009;39(10):717–725. doi: 10.1016/j.ibmb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Braco JT, Gillespie EL, Alberto GE, Brenman JE, Johnson EC.. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics. 2012;192(2):457–466. doi: 10.1534/genetics.112.143610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton L, Clot J.P., Baudry M.. Effects of glucagon on basal metabolic rate and oxidative phosphorylation of rat liver mitochondria. Horm Metab Res. 1983;15(9):429–432. doi: 10.1055/s-2007-1018747. [DOI] [PubMed] [Google Scholar]
- Bürger M, Brandt W.. Über das Glukagon (die hyperglykämisierende Substanz der Pankreas). Z Ges Exp Med. 1935;96:375. [Google Scholar]
- Campbell JE, Drucker DJ.. Islet alpha cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329–338. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- Charron MJ, Vuguin PM.. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. J Endocrinol. 2015;224(3):R123–R130. doi: 10.1530/JOE-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Toullec JY, Lee CY.. The crustacean hyperglycemic hormone superfamily: progress made in the past decade. Front Endocrinol. 2020;11. doi: 10.3389/fendo.2020.578958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA.. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39(6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Chung JS, Zmora N, Katayama H, Tsutsui N.. Crustacean hyperglycemic hormone (CHH) neuropeptides family: functions, titer, and binding to target tissues. Gen Comp Endocrinol. 2010;166(3):447–454. doi: 10.1016/j.ygcen.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Das D, Arur S.. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol Reprod Dev. 2017;84(6):444–459. doi: 10.1002/mrd.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoue R, Slaidina M, Leopold P.. The steroid hormone ecdysone controls systemic growth by repressing dMyc function in Drosophila fat cells. Dev Cell .2010;18(6):1012–1021. doi: 10.1016/j.devcel.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Dircksen H. Insect ion transport peptides are derived from alternatively spliced genes and differentially expressed in the central and peripheral nervous system. J Exp Biol. 2009;212(Pt 3):401–412. doi: 10.1242/jeb.026112. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Tesfai LK, Albus C, Nässel DR.. Ion transport peptide splice forms in central and peripheral neurons throughout postembryogenesis of Drosophila melanogaster. J Comp Neurol .2008;509(1):23–41. doi: 10.1002/cne.21715. [DOI] [PubMed] [Google Scholar]
- Gáliková M, Diesner M, Klepsatel P, Hehlert P, Xu YJ, Bickmeyer I, Predel R, Kühnlein RP.. Energy homeostasis control in Drosophila Adipokinetic hormone mutants. Genetics. 2015;201(2):665–683. doi: 10.1534/genetics.115.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gáliková M, Dircksen H, Nässel DR.. The thirsty fly: Ion transport peptide (ITP) is a novel endocrine regulator of water homeostasis in Drosophila. PLoS Genet. 2018;14(8):e1007618. doi: 10.1371/journal.pgen.1007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gáliková M, Klepsatel P.. Obesity and aging in the Drosophila model. Int J Mol Sci. 2018;19(7). doi: 10.3390/ijms19071896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gáliková M, Klepsatel P, Münch J, Kühnlein RP.. Spastic paraplegia-linked phospholipase PAPLA1 is necessary for development, reproduction, and energy metabolism in Drosophila. Sci Rep. 2017;7. doi: 10.1038/srep46516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gáliková M, Klepsatel P, Xu YJ, Kühnlein RP.. The obesity-related adipokinetic hormone controls feeding and expression of neuropeptide regulators of Drosophila metabolism. Eur J Lipid Sci Tech. 2017;119(3). doi: 10.1002/ejlt.201600138. [DOI] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS.. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40(5):386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Gautam UK, Hlavkova D, Shaik HA, Karaca I, Karaca G, Sezen K, Kodrík D.. Adipokinetic hormones enhance the efficacy of the entomopathogenic fungus Isaria fumosorosea in model and pest insects. Pathogens. 2020;9(10). doi: 10.3390/pathogens9100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Le Sauter J, Noh U.. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264(1 Pt 2):R116–R122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100(3):1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette CM, Tennessen JM, Reis T.. Balancing energy expenditure and storage with growth and biosynthesis during Drosophila development. Dev Biol. 2021;475:234–244. doi: 10.1016/j.ydbio.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Muller G, Hirsch J, Fellert S., Andreou A, Haase T, Jäckle H, Kühnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5(6):e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220(2):T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze JL, Carlsson MA, Kondo S, Nässel DR, Rewitz KF.. The neuropeptide allatostatin A regulates metabolism and feeding decisions in Drosophila. Sci Rep. 2015;5:11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Luibl C, Yoshii T, Senthilan PR, Dircksen H, Helfrich-Forster C.. The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. J Neurosci. 2014;34(29):9522–9536. doi: 10.1523/Jneurosci.0111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer HF, Heier C, Sen Saji AK, Kühnlein RP.. Lipidome remodeling in aging normal and genetically obese Drosophila males. Insect Biochem Mol Biol. 2021;133:103498. doi: 10.1016/j.ibmb.2020.103498. [DOI] [PubMed] [Google Scholar]
- Huang R, Song T, Su H, Lai Z, Qin W, Tian Y, Dong X, Wang L. High-fat diet enhances starvation-induced hyperactivity via sensitizing hunger-sensing neurons in Drosophila .eLife. 2020;9. doi: 10.7554/eLife.53103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson BN. The glucagon-like adipokinetic hormone in Drosophila melanogaster—biosynthesis and secretion. Front Physiol. 2021;12:710652. doi: 10.3389/fphys.2021.710652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson BN, Shimell M, O'Connor MB.. AKH signaling in D. melanogaster alters larval development in a nutrient-dependent manner that influences adult metabolism. Front Physiol. 2021;12. doi: 10.3389/fphys.2021.619219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim E, Dobeš P, Kunc M, Hyršl P, Kodrík D.. Adipokinetic hormone and adenosine interfere with nematobacterial infection and locomotion in Drosophila melanogaster. J Insect Physiol. 2018;107:167–174. doi: 10.1016/j.jinsphys.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Ibrahim E, Hejníková M, Shaik HA, Doležel D, Kodrík D.. Adipokinetic hormone activities in insect body infected by entomopathogenic nematode. J Insect Physiol. 2017;98:347–355. doi: 10.1016/j.jinsphys.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Isabel G, Martin JR, Chidami S, Veenstra JA, Rosay P.. AKH-producing neuroendocrine cell ablation decreases trehalose and induces behavioral changes in Drosophila. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R531–R538. doi: 10.1152/ajpregu.00158.2004. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104(20):8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janah L, Kjeldsen S, Galsgaard KD, Winther-Sorensen M, Stojanovska E, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and glucagon resistance. Int J Mol Sci .2019;20(13). doi: 10.3390/ijms20133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourjine N, Mullaney BC, Mann K, Scott K.. Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell. 2016;166(4):855–866. doi: 10.1016/j.cell.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L, Kapan N, Dircksen H, Winther AM, Nässel DR.. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS One. 2010;5(7):e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, Perrimon N, Melov S, Kapahi P. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012;16(1):97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Neufeld TP.. Dietary sugar promotes systemic TOR activation in Drosophila through AKH-dependent selective secretion of Dilp3. Nat Commun. 2015;6:6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Rulifson EJ.. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature .2004;431(7006):316–320. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- Kleinert M, Sachs S, Habegger KM, Hofmann SM, Müller TD.. Glucagon regulation of energy expenditure. Int J Mol Sci. 2019;20(21). doi: 10.3390/ijms20215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodrík D, Bednářová A, Zemanová M, Krishnan N.. Hormonal regulation of response to oxidative stress in insects—an update. Int J Mol Sci. 2015;16(10):25788–25816. doi: 10.3390/ijms161025788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Texada MJ, Halberg KA, Rewitz K.. Metabolism and growth adaptation to environmental conditions in Drosophila. Cell Mol Life Sci. 2020;77(22):4523–4551. doi: 10.1007/s00018-020-03547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause SA, Overend G, Dow JAT, Leader DP.. FlyAtlas 2 in 2022: enhancements to the Drosophila melanogaster expression atlas. Nucleic Acids Res. 2022;50(D1):D1010–D1015. doi: 10.1093/nar/gkab971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubrak OI, Lushchak OV, Zandawala M, Nässel DR.. Systemic corazonin signalling modulates stress responses and metabolism in Drosophila. Open Biol. 2016;6(11). doi: 10.1098/rsob.160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT.. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 2018;46(D1):D809–D815. doi: 10.1093/nar/gkx976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton S, Mansourian S, Bigarreau J, Dekker T.. The adipokinetic hormone receptor modulates sexual behavior, pheromone perception and pheromone production in a sex-specific and starvation-dependent manner in Drosophila melanogaster. Front Ecol Evol. 2016;3. doi: 10.3389/fevo.2015.00151. [DOI] [Google Scholar]
- Lee G, Park JH.. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics .2004;167(1):311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hoffmann J, Li Y, Stephano F, Bruchhaus I, Fink C, Roeder T. Octopamine controls starvation resistance, life span and metabolic traits in Drosophila. Sci Rep. 2016;6. doi: 10.1038/srep35359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Amcoff M, Nässel DR.. Impact of high-fat diet on lifespan, metabolism, fecundity and behavioral senescence in Drosophila. Insect Biochem Mol Biol. 2021;133:103495. doi: 10.1016/j.ibmb.2020.103495. [DOI] [PubMed] [Google Scholar]
- Mochanová M, Tomčala A, Svobodová Z, Kodrík D.. Role of adipokinetic hormone during starvation in Drosophila. Comp Biochem Phys B. 2018;226:26–35. doi: 10.1016/j.cbpb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH.. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–766. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- Nagai C, Mabashi-Asazuma H, Nagasawa H, Nagata S.. Identification and characterization of receptors for ion transport peptide (ITP) and ITP-like (ITPL) in the silkworm Bombyx mori. J Biol Chem. 2014;289(46):32166–32177. doi: 10.1074/jbc.M114.590646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery LEM, Santos EA, Bianchini A, Goncalves AA.. Effects of crustacean hyperglycemic hormones from carcinus-maenas and orconectes-limosus on blood and muscle glucose and glycogen concentration of chasmagnathus-granulata. Braz J Med Biol Res. 1993;26(12):1291–1296. [PubMed] [Google Scholar]
- Oh Y, Lai JS, Mills HJ, Erdjument-Bromage H, Giammarinaro B, Saadipour K, Wang JG, Abu F, Neubert TA, Suh GSB. A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila. Nature. 2019;574(7779):559–564. doi: 10.1038/s41586-019-1675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhilal S, Vuguin P, Cui L, Du XQ, Gelling RW, Reznik SE, Russell R, Parlow AF, Karpovsky C, Santoro N, et al. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: a role for glucagon action in pregnancy maintenance. Am J Physiol Endocrinol Metab. 2012;302(5):E522–E531. doi: 10.1152/ajpendo.00420.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim H, Li D, Adams M. A novel function of ion transport peptide in Drosophila ecdysis. (Program and Abstracts. 45th Annual Drosophila Research Conference, Washington, DC); 2004. Abstract 774C (Flybase ID: FBrf0174159).
- Paul F, Freyschmidt J.. Anwendung von Glukagon bei endoskopischen und rontgenologischen Untersuchungen des Gastrointestinaltrakts [The use glucagon for endoscopic and radiological examination of the gastrointestinal tract]. Rofo. 1976;125(1):31–37. [DOI] [PubMed] [Google Scholar]
- Pauls D, Blechschmidt C, Frantzmann F, El Jundi B, Selcho M.. A comprehensive anatomical map of the peripheral octopaminergic/tyraminergic system of Drosophila melanogaster. Sci Rep. 2018;8(1):15314. doi: 10.1038/s41598-018-33686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D, Selcho M, Räderscheidt J, Amatobi K, Fekete A, Krischke M, Hermann-Luibl CH, Ozbek-Unal AG, Ehmann N, Itskov PM, et al. Endocrine signals fine-tune daily activity patterns in Drosophila Curr Biol. 2021;31(18):4076–4087. doi: 10.1016/j.cub.2021.07.002. [DOI] [PubMed] [Google Scholar]
- Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajwan S, Sidorov R, Stašková T, Žaloudíková A, Takasu Y, Kodrík D, Zurovec M.. Targeted mutagenesis and functional analysis of adipokinetic hormone-encoding gene in Drosophila. Insect Biochem Mol Biol 2015;61:79–86. doi: 10.1016/j.ibmb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Santos EA, Nery LEM, Keller R, Goncalves AA.. Evidence for the involvement of the crustacean hyperglycemic hormone in the regulation of lipid metabolism. Physiol Zool. 1997;70(4):415–420. doi: 10.1086/515846. [DOI] [PubMed] [Google Scholar]
- Schulman JL, Carleton JL, Whitney G, Whitehorn JC.. Effect of glucagon on food intake and body weight in man. J Appl Physiol. 1957;11(3):419–421. doi: 10.1152/jappl.1957.11.3.419. [DOI] [PubMed] [Google Scholar]
- Scopelliti A, Bauer C, Yu Y, Zhang T, Kruspig B, Murphy DJ, Vidal M, Maddocks ODK, Cordero JB. A neuronal relay mediates a nutrient responsive gut/fat body axis regulating energy homeostasis in adult Drosophila .Cell Metab. 2018;29(2):269–284. doi: 10.1016/j.cmet.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha R, Kodrík D, Zemek R.. Stimulatory effects of bioamines norepinephrine and dopamine on locomotion of Pyrrhocoris apterus (L.): is the adipokinetic hormone involved? Comp Biochem Physiol B: Biochem Mol Biol. 2008;151(3):305–310. doi: 10.1016/j.cbpb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Tennessen JM, Barry WE, Cox J, Thummel CS.. Methods for studying metabolism in Drosophila. Methods. 2014;68(1):105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmond J, Goodman JL, Strelets VB, Attrill H, Gramates LS, Marygold SJ, Matthews BB, Millburn G, Antonazzo G, Trovisco V, et al. FlyBase 2.0: the next generation. Nucleic Acids Res. 2019;47(D1):D759–D765. doi: 10.1093/nar/gky1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire H, Battisti V, Trannoy S, Lasbleiz C, Pret AM, Monnier V.. The steroid hormone receptor EcR finely modulates Drosophila lifespan during adulthood in a sex-specific manner. Mech Ageing Dev. 2009;130(8):547–552. doi: 10.1016/j.mad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ. Insect adipokinetic hormones: release and integration of flight energy metabolism. Comp Biochem Physiol B: Biochem Mol Biol. 2003;136(2):217–226. [DOI] [PubMed] [Google Scholar]
- Van der Horst DJ, Van Marrewijk WJ, Diederen JH.. Adipokinetic hormones of insect: release, signal transduction, and responses. Int Rev Cytol. 2001;211:179–240. [DOI] [PubMed] [Google Scholar]
- Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M., et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147(9):3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat LW, Chowdhury ZS, Millington JW, Biswas P, Rideout EJ.. Sex determination gene transformer regulates the male-female difference in Drosophila fat storage via the adipokinetic hormone pathway. eLife. 2021;10. doi: 10.7554/eLife.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson MJ, Chung BY, Harvanek ZM, Ostojic I, Alcedo J, Pletcher SD.. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc Natl Acad Sci USA .2014;111(22):8137–8142. doi: 10.1073/pnas.1315461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SG, Keller R, Dircksen H.. The CHH-superfamily of multifunctional peptide hormones controlling crustacean metabolism, osmoregulation, moulting, and reproduction. Gen Comp Endocrinol. 2012;175(2):217–233. doi: 10.1016/j.ygcen.2011.11.035. [DOI] [PubMed] [Google Scholar]
- Yamada T, Habara O, Kubo H, Nishimura T.. Fat body glycogen serves as a metabolic safeguard for the maintenance of sugar levels in Drosophila . Development. 2018;145(6). [DOI] [PubMed] [Google Scholar]
- Yatsenko AS, Marrone AK, Kucherenko MM, Shcherbata HR.. Measurement of metabolic rate in Drosophila using respirometry. J Vis Exp .2014;(88):e51681. doi: 10.3791/51681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Matsuda H, Kubo H, Nishimura T.. Molecular characterization of Tps1 and Treh genes in Drosophila and their role in body water homeostasis. Sci Rep. 2016;6. doi: 10.1038/srep30582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H, Kirkhart C, Chia J, Scott K.. A subset of octopaminergic neurons that promotes feeding initiation in Drosophila melanogaster. PLoS One. 2018;13(6). doi: 10.1371/journal.pone.0198362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Li DT, Wang SL, Xu HJ, Bao YY, Zhang CX.. Ion transport peptide (ITP) regulates wing expansion and cuticle melanism in the brown planthopper, Nilaparvata lugens. Insect Mol Biol. 2016;25(6):778–787. doi: 10.1111/imb.12262. [DOI] [PubMed] [Google Scholar]
- Yu R, Ren SG, Mirocha J.. Glucagon receptor is required for long-term survival: a natural history study of the Mahvash disease in a murine model. Endocrinol Nutr. 2012;59(9):523–530. [DOI] [PubMed] [Google Scholar]
- Yu Y, Huang R, Ye J, Zhang V, Wu C, Cheng G, Jia J, Wang L.. Regulation of starvation-induced hyperactivity by insulin and glucagon signaling in adult Drosophila . eLife . 2016;5. doi: 10.7554/eLife.15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanová M, Stašková T, Kodrík D.. Role of adipokinetic hormone and adenosine in the anti-stress response in Drosophila melanogaster. J Insect Physiol . 2016;91–92:39–47. doi: 10.1016/j.jinsphys.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Zhao X, Karpac J.. Muscle directs diurnal energy homeostasis through a myokine-dependent hormone module in Drosophila. Curr Biol. 2017;27(13):1941–1955.e6. doi: 10.1016/j.cub.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Meredith J, Brock HW, Phillips JE.. Mutational analysis of the N-terminus in Schistocerca gregaria ion-transport peptide expressed in Drosophila Kc1 cells. Arch Insect Biochem Physiol. 2005;58(1):27–38. doi: 10.1002/arch.20028. [DOI] [PubMed] [Google Scholar]
- Zuberova M, Fenckova M, Simek P, Janeckova L, Dolezal T.. Increased extracellular adenosine in Drosophila that are deficient in adenosine deaminase activates a release of energy stores leading to wasting and death. Dis Model Mech. 2010;3(11–12):773–784. doi: 10.1242/dmm.005389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary for confirming the conclusions presented in this article are available in the article and its online supplementary material. Fly stocks and reagents are available upon request.
Supplemental material is available at GENETICS online.