Abstract
Background
The prevalence of chronic obstructive pulmonary disease (COPD) in women is fast approaching that in men, and women experience greater symptom burden. Although sex differences in emphysema have been reported, differences in airways have not been systematically characterized.
Purpose
To evaluate whether structural differences in airways may underlie some of the sex differences in COPD prevalence and clinical outcomes.
Materials and Methods
In a secondary analyses of a multicenter study of never-, current-, and former-smokers enrolled from January 2008 to June 2011 and followed up longitudinally until November 2020, airway disease on CT images was quantified using seven metrics: airway wall thickness, wall area percent, and square root of the wall thickness of a hypothetical airway with internal perimeter of 10 mm (referred to as Pi10) for airway wall; and lumen diameter, airway volume, total airway count, and airway fractal dimension for airway lumen. Least-squares mean values for each airway metric were calculated and adjusted for age, height, ethnicity, body mass index, pack-years of smoking, current smoking status, total lung capacity, display field of view, and scanner type. In ever-smokers, associations were tested between each airway metric and postbronchodilator forced expiratory volume in 1 second (FEV1)-to–forced vital capacity (FVC) ratio, modified Medical Research Council dyspnea scale, St George's Respiratory Questionnaire score, and 6-minute walk distance. Multivariable Cox proportional hazards models were created to evaluate the sex-specific association between each airway metric and mortality.
Results
In never-smokers (n = 420), men had thicker airway walls than women as quantified on CT images for segmental airway wall area percentage (least-squares mean, 47.68 ± 0.61 [standard error] vs 45.78 ± 0.55; difference, −1.90; P = .02), whereas airway lumen dimensions were lower in women than men after accounting for height and total lung capacity (segmental lumen diameter, 8.05 mm ± 0.14 vs 9.05 mm ± 0.16; difference, −1.00 mm; P < .001). In ever-smokers (n = 9363), men had greater segmental airway wall area percentage (least-squares mean, 52.19 ± 0.16 vs 48.89 ± 0.18; difference, −3.30; P < .001), whereas women had narrower segmental lumen diameter (7.80 mm ± 0.05 vs 8.69 mm ± 0.04; difference, −0.89; P < .001). A unit change in each of the airway metrics (higher wall or lower lumen measure) resulted in lower FEV1-to-FVC ratio, more dyspnea, poorer respiratory quality of life, lower 6-minute walk distance, and worse survival in women compared with men (all P < .01).
Conclusion
Airway lumen sizes quantified at chest CT were smaller in women than in men after accounting for height and lung size, and these lower baseline values in women conferred lower reserves against respiratory morbidity and mortality for equivalent changes compared with men.
© RSNA, 2022
Summary
In never-smokers, airway lumen at chest CT images was smaller in women than men; in ever-smokers, worsening of lumen size impacted respiratory outcomes more in women than men.
Key Results
■ Airway lumen dimensions were lower in never-smoker women than in men (segmental lumen diameter, 8.1 mm ± 0.2 [SE] vs 9.1 mm ± 0.1; P < .001).
■ Ever-smoker women had narrower segmental lumen diameter (7.8 mm ± 0.05 vs 8.7 mm ± 0.04; P < .001).
■ A unit change in wall thickness or lumen area resulted in more severe airflow obstruction, more dyspnea, worse respiratory quality of life, lower 6-minute walk distance, and worse survival in women compared with men.
Introduction
Chronic obstructive pulmonary disease (COPD) is more frequently diagnosed in men than in women, but with changes in smoking behavior and increasing urbanization, the prevalence of COPD in women is fast approaching that in men (1). Age-adjusted rates for COPD-related deaths have continued to decline in men but not in women (2). Among never-smokers, women account for two-thirds of the prevalence of COPD in population-based studies (3). When smoking burden is accounted for, women have greater airflow obstruction and have a faster rate of lung function decline than men (4–6). Women also report more dyspnea than men with equivalent lung function (7,8). In the United States, hospitalization and death because of COPD are now higher in women than in men (9). Differences in susceptibility to tobacco smoke (4,10), genetic predisposition (11,12), hormonal influences (13), and household biomass smoke exposure (14) have been varyingly implicated in explaining the biologic underpinnings of these sex differences, but they do not explain a significant portion of the variance.
Increasingly, structural differences in the lungs have been reported between men and women with established airflow obstruction. These differences are especially well documented for alveolar disease. Although emphysema occurs in both men and women who smoke, men on average have more emphysema than women after accounting for smoking burden and lung function (15–18). Sex differences in airway remodeling, however, have not been conclusively demonstrated. Histopathologic and quantitative CT analyses of segmental airway wall thickness and lumen have shown conflicting results (15,16,19,20). These analyses were limited to airway measurements on a few sections of medium-sized airways and do not fully inform changes occurring throughout the airway. Studies indicate that airway remodeling extends beyond wall thickness and luminal narrowing; COPD is associated with airway loss and a reduction in the complexity of branching patterns (21–23).
The aim of our study was to evaluate whether structural differences in airways may underlie some of the sex differences in COPD prevalence and clinical outcomes. We sought to explore whether there is a sex predisposition to airway remodeling, and the impact of airway remodeling on patient-reported outcomes and survival. We analyzed quantitative CT measures of airway remodeling in never-smokers, current smokers, and former smokers with and without airflow obstruction to address these questions.
Materials and Methods
Participants
In a secondary analysis, we included consecutive participants enrolled in the Genetic Epidemiology of COPD (COPDGene) study (ClinicalTrials.gov: NCT00608764). Study details have been previously published (24). Briefly, COPDGene is a prospective multicenter observational cohort study that enrolled current and former smokers, as well as never-smokers, between ages 45 and 80 years at 21 clinical centers across the United States. The eligibility criteria are in Appendix E1 (online). Smokers with at least a 10–pack-year smoking history were included. In this secondary analysis, we included all participants enrolled in round one of COPDGene between October 2006 and January 2011. In addition to the baseline enrollees, which included 107 never-smokers, we also included 347 never-smokers who were enrolled at the second phase of COPDGene after 5 years to increase the number of never-smokers. Self-reported gender and ethnicity were recorded as reported by the participants. Sex was determined using chromosomal information, and XX and XY status aligned with gender was used in all analyses. We excluded 26 participants who had XO, XXX, XXY or XY/XO chromosomal aneuploidies (Fig 1). Never-smokers were defined as those who smoked fewer than 100 cigarettes in their lifetime. Current smokers were defined as having smoked cigarettes within 30 days of study visit.
Figure 1:
Study inclusion flowchart.
All participants underwent prebronchodilator spirometry (Easy-One Spirometer; NDD) to assess lung function. Postbronchodilator spirometry was performed 20–30 minutes after administering 180 μg of albuterol. The presence of airflow obstruction was defined by postbronchodilator forced expiratory volume in 1 second (FEV1)-to–forced vital capacity (FVC) ratio of less than 0.70, and severity of airflow obstruction determined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report (25). GOLD 0 was defined as those at risk for COPD (FEV1-to-FVC ratio, ≥0.70; FEV1 percent predicted, ≥80). Preserved Ratio Impaired Spirometry (known as PRISm) was defined as FEV1-to-FVC ratio of 0.70 or greater but FEV1 percent predicted of less than 80 (26). Respiratory quality of life was estimated by the St George's Respiratory Questionnaire score. The score ranges from 0 to 100, with higher scores indicating worse quality of life (24). Dyspnea was quantified by using the modified Medical Research Council score (24). The score ranges from 0 to 4, with higher scores indicating greater dyspnea. Functional capacity was assessed by distance covered on the 6-minute walk test (24). Vital status was ascertained by follow-up phone calls every 6 months and a search of the Social Security Death Index. Written informed consent was obtained from all participants. Health Insurance Portability and Accountability Act approval was obtained as part of the institutional review board review at the respective hospitals. The COPDGene study protocol was approved by the institutional review board at each of the 21 participating centers.
CT Protocol
High-resolution volumetric noncontrast-enhanced CT images were acquired at full inspiration (total lung capacity) at enrollment (24,27). Image acquisition and reconstruction parameters have been described in detail previously (Appendix E1 [online]).
CT Image Analysis
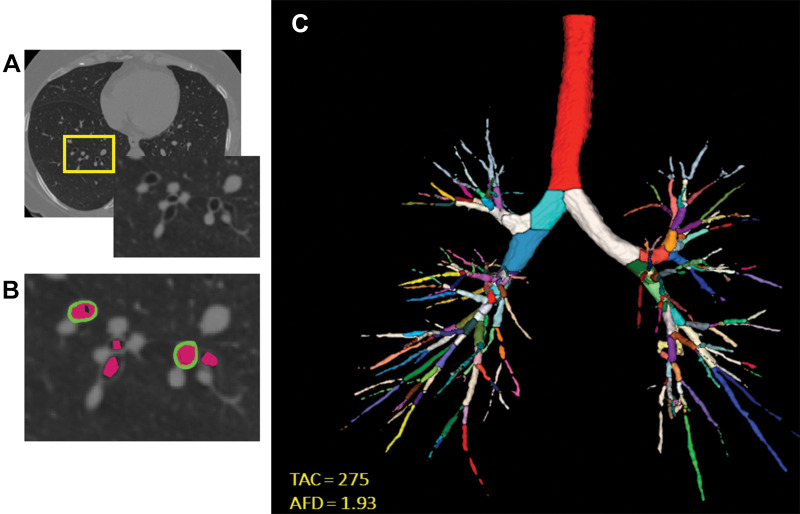
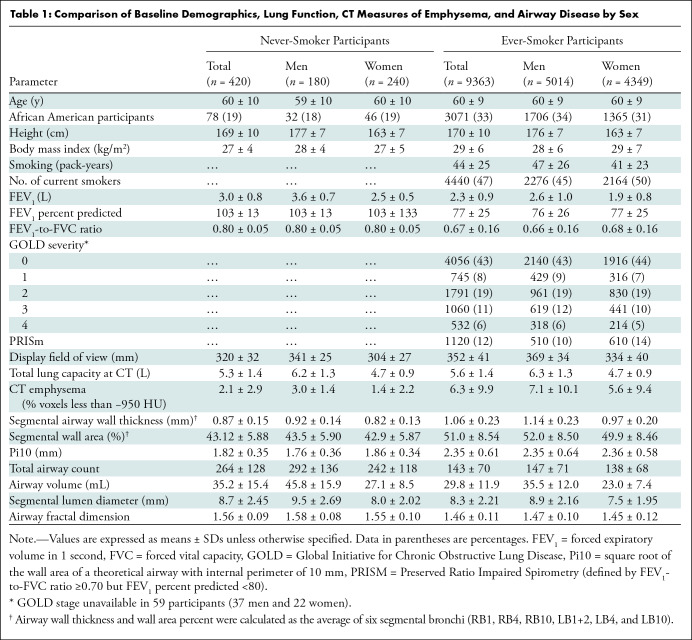
Lung masks were applied, and airway segmentation was performed using lung quantification software (LungQ; Thirona) (28). We quantified emphysema as the percentage of voxels with attenuation less than −950 HU at end inspiration. Although data regarding air trapping and functional small airways disease are available in the COPDGene study, we did not include these as airway measures because they are not direct measures of airway disease and hence not visible. We quantified airway disease with the following metrics (Fig 2):
Airway wall thickness of segmental airways: The mean airway wall thickness was calculated as the average airway wall thickness of six segmental bronchi (RB1, RB4, RB10, LB1+2, LB4, and LB10) in each participant (Thirona) (28).
Wall area percent of segmental airways: The luminal area and total airway cross-sectional area were calculated for six segmental bronchi (Thirona), and the airway wall area was estimated by AT – AI, where AT is total airway cross-sectional area and AI is the luminal area. The average airway wall area percent was calculated as [WA%] = [(AT – AI)/AT] × 100 (29), where WA% is the wall area percent.
The square root of the wall area of a hypothetical airway with 10-mm internal perimeter (hereafter, Pi10): To represent airway wall thickness, Pi10 was calculated by plotting the internal perimeters of all segmental and distal airways against the square root of their wall areas (30).
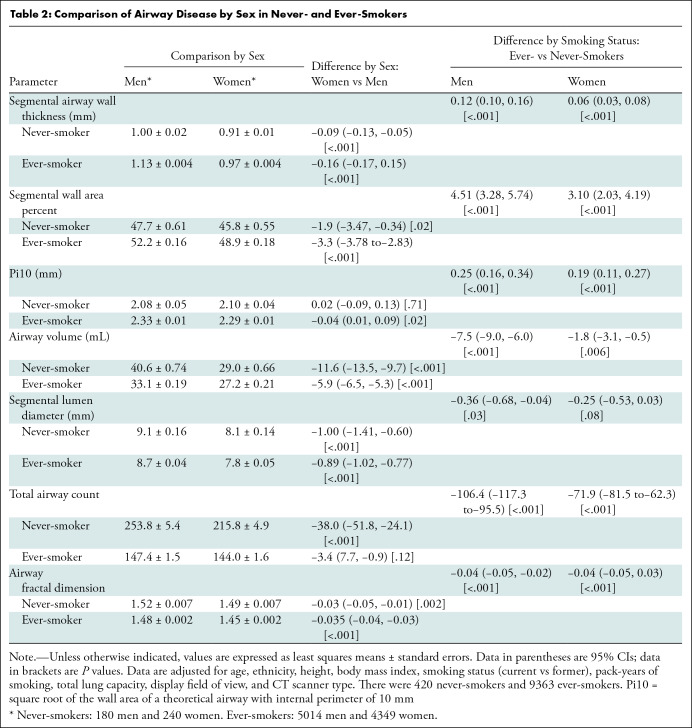
Total airway count: The total airway count was calculated by automated identification of branch points on the airway tree and summing number of branches.
Lumen diameter of segmental airways: The average hydraulic diameter of all segmental airways was calculated as 4A/P, where A is the cross-sectional area and P is the internal perimeter.
Airway volume: Airway volume was estimated from airway trees segmented from inspiratory CT images using regionprops3 function (Matlab R2020a; MathWorks). To adjust to physical units (in cubic millimeters), the total number of airways voxels in the tree was multiplied by the voxel size.
Airway fractal dimension: Airway fractal dimension of the airway lumen was calculated by using the Minkowski-Bougliand box-counting dimension with software (Matlab R2020a; MathWorks) (23). Briefly, cubes of progressively increasing side lengths were iteratively laid over the airway tree. The number of cubes that overlapped with the airway were identified at each iteration. Airway fractal dimension is the slope of the regression line between log (n) and log (1/s), where n indicates number of cubes. Greater AFD indicates greater branching complexity of the airway tree.
Figure 2:
Images show airway metrics. (A) Noncontrast-enhanced axial view chest CT image shows cross sections of segmental airways. (B) Inset shows segmented airway wall (green) and airway lumen (pink). (C) Segmented airway tree with color-coded branches. The resulting total airway count (TAC) and airway fractal dimension (AFD) are shown in C.
Statistical Analysis
Linear regression models were estimated with each airway metric as the dependent variable and sex as an independent variable. These models were adjusted for age, ethnicity, height, body mass index, pack-years of smoking, smoking status (ever vs never), total lung capacity at CT, display field of view, and CT scanner type. Airway models specifically included height, display field of view, and total lung capacity because these can influence airway size but not emphysema. Models were created for percent emphysema with adjustment for age, ethnicity, body mass index, pack-years of smoking, smoking status, and CT scanner type. Least-squares means derived from the linear regression models were compared by sex using the t test. To test whether current smoking affected these CT metrics, we performed additional analyses where smoking status was stratified as never, former, and current smoker.
In ever-smokers, we also estimated separate linear regression models in men and women with clinical outcomes as the dependent variable (postbronchodilator FEV1-to-FVC ratio, St George's Respiratory Questionnaire, and 6-minute walk distance) and airway metric as the independent variable to compare the strength of association between each airway metric and important clinical outcomes in men and women. The same analysis approach was applied to the clinical outcome modified Medical Research Council score by using the ordinal logistic regression model to evaluate the cumulative probability of higher modified Medical Research Council scores. These models were also adjusted for age, ethnicity, height, body mass index, smoking status (never vs ever), pack-years of smoking, FEV1, total lung capacity at CT, emphysema at CT, display field of view, and CT scanner type, and additionally for the interaction between the airway metric and sex. The regression coefficients for the association between each airway metric and the dependent variable were compared by using the t test for difference in slopes. Cox proportional hazards models were similarly estimated to assess sex differences in the association between each airway metric and survival. Two-sided P values less than .05 were considered to indicate statistical significance. All analyses were performed using software (SAS version 9.4; SAS Institute).
Results
Baseline Characteristics of Participants
After exclusions (26 participants with mosaic chromosomes and 843 participants with inadequate airway quantification at CT), 420 lifetime nonsmokers (mean age, 60 years ± 10 [SD]; 240 women [57%]; 342 non-Hispanic White participants [81%]) and 9363 ever-smokers (mean age, 60 ± 9 years, 5014 [54%] men, and 6292 [67%] non-Hispanic White participants) were included in the final analyses (Consolidated Standards of Reporting Trials, known as CONSORT, diagram; Fig 1). Table 1 shows univariable comparisons of demographics, lung function, and CT measures of emphysema and airway disease by sex. In both never- and ever-smokers, women had lower FEV1 and FVC, but the FEV1-to-FVC ratio was similar. Women were shorter and had lower lung size at CT in both never- and ever-smokers, and accordingly a smaller display field of view was used. Among ever-smokers, women had lower pack-years of smoking, and fewer women were active smokers at enrollment compared with men.
Table 1:
Comparison of Baseline Demographics, Lung Function, CT Measures of Emphysema, and Airway Disease by Sex
Airway Metrics in Never-Smokers
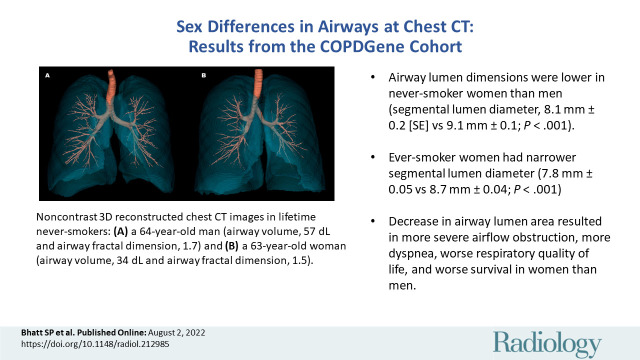
There was a positive correlation between airway volume and height (r = 0.61; P < .001) and between airway volume and total lung capacity (r = 0.71; P < .001). In analyses adjusted for age, ethnicity, height, body mass index, total lung capacity, display field-of-view, and CT scanner type, men had thicker airway walls as estimated by segmental airway wall thickness (1.00 mm ± 0.02 vs 0.91 mm ± 0.01; P < .001) and segmental wall area percent (47.68 ± 0.61 vs 45.78 ± 0.55; P = .02), but we found no evidence of a difference in the Pi10, a wall thickness measure that accounts for the size of the airways (2.08 mm ± 0.05 vs 2.10 mm ± 0.04; P = .71) (Table 2). However, the following measures were lower in women than in men: the lumen parameters of airway volume (29.03 mL ± 0.66 vs 40.61 mL ± 0.74; P < .001), diameter of segmental airways (8.05 mm ± 0.14 vs 9.05 mm ± 0.16; P < .001), total airway count (215.8 ± 4.9 vs 253.8 ± 5.4; P < .001), and airway fractal dimension (1.49 ± 0.007 vs 1.52 ± 0.007; P < .001) (Table 2). Figure 3 shows illustrative examples of airway disease in representative individuals who are lifetime never-smokers.
Table 2:
Comparison of Airway Disease by Sex in Never- and Ever-Smokers
Figure 3:
Representative examples of airways in lifetime never-smoker men and women. (A) Noncontrast-enhanced three-dimensional reconstructed chest CT image in a lifetime never-smoker 64-year-old man (height, 1.74 m; total lung capacity, 8.1 L; forced expiratory volume in 1 second [FEV1]-to–forced vital capacity [FVC] ratio, 0.80; and FEV1, 127% predicted) shows airway volume of 57 dL, total airway count of 526, and airway fractal dimension of 1.695. (B) Noncontrast-enhanced three-dimensional reconstructed chest CT image in a lifetime never-smoker 63-year-old woman (height, 1.73 m; total lung capacity, 6.1 L; FEV1-to-FVC ratio, 0.76; and FEV1, 100% predicted) shows airway volume of 34 dL, 299 viewable airway branches, and airway fractal dimension of 1.513.
Airway Metrics in Ever-Smokers
In analyses adjusted for age, ethnicity, height, body mass index, current smoking status, pack-years of smoking, total lung capacity, display field of view, and CT scanner type, men had thicker airway walls as estimated by segmental airway wall thickness (1.13 mm ± 0.004 vs 0.97 mm ± 0.004; P < .001) and segmental wall area percent (52.19 ± 0.16 vs 48.89 ± 0.18; P < .001), and Pi10 (2.33 mm ± 0.01 vs 2.29 mm ± 0.01 ; P = .02; Table 2). Similar to the findings in never-smokers, the lumen parameters of airway volume (27.20 dL ± 0.21 vs 33.13 dL ± 0.19; P < .001), diameter of segmental airways (7.80 mm ± 0.05 vs 8.69 mm ± 0.04; P < .001), and airway fractal dimension (1.45 ± 0.002 vs 1.48 ± 0.002; P < .001) were lower in ever-smoker women than in men (Table 2). We found no evidence of a difference in total airway count between women and men (144.0 ± 1.6 vs 147.4 ± 1.5; P = .12). In comparing ever-smokers with never-smokers according to sex, the sex difference in airway wall metrics was accentuated with greater remodeling in men than in women but was attenuated for airway lumen metrics (Table 2). Similar differences were noted when smokers were further classified as current and former (Table E1 [online]). These differences persisted across GOLD stages of disease severity (Table E2 [online]).
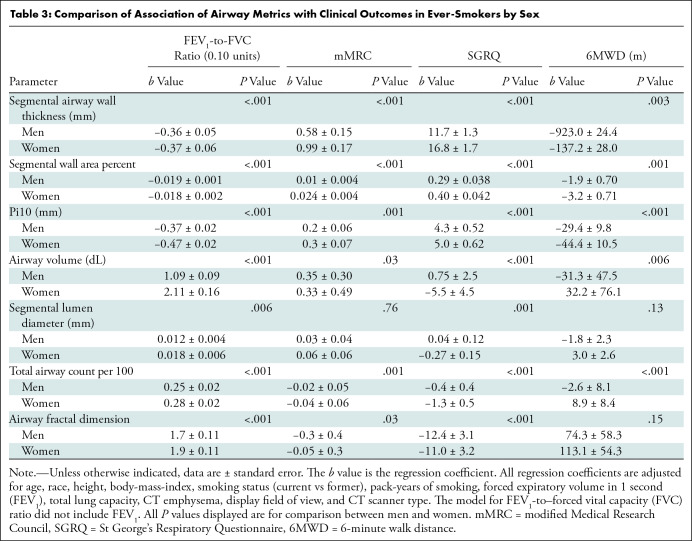
Association with Clinical Outcomes
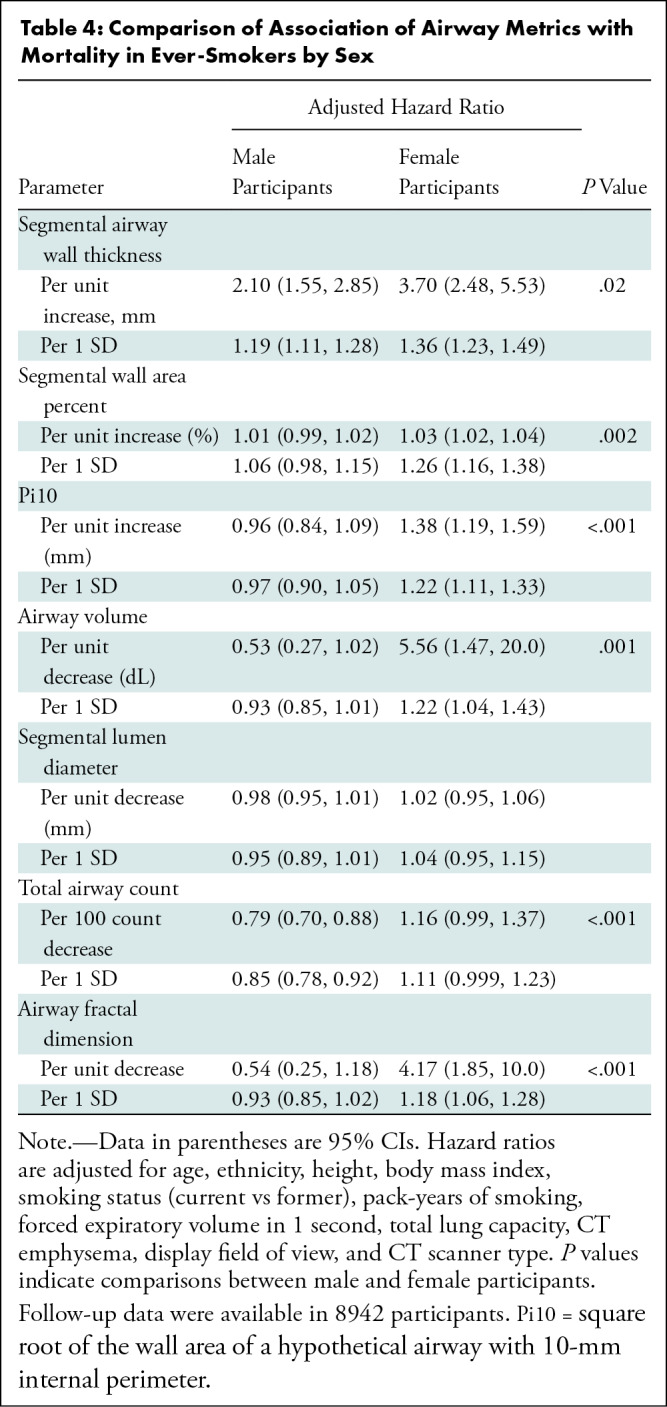
Both higher wall thickness parameters and lower lumen metrics were associated with worse clinical outcomes in women than in men. The association between a unit change (greater wall or lower lumen) in each airway metric and FEV1-to-FVC ratio was greater in women than in men. Similarly, derangements in the majority of the airway metrics were more strongly associated with greater dyspnea, worse respiratory quality of life, and lower 6-minute walk distance in women than in men, even after adjustment for lung function (Table 3). For the ever-smokers, follow-up data were available in 8942 of 9363 (96%) individuals, with a median follow-up time of 7.6 years (25th–75th percentile, 6.2–8.6 years). At follow-up, 1567 of 8942 (18%) participants died (19% of the male participants and 14% of the female participants). After adjustment for age, ethnicity, height, body mass index, smoking status, pack-years of smoking, FEV1, total lung capacity, CT emphysema, display field of view, and CT scanner type, each airway metric was more strongly associated with all-cause mortality in women than in men. Table 4 shows hazard ratios for all-cause mortality per unit change and per 1 SD change in each airway metric.
Table 3:
Comparison of Association of Airway Metrics with Clinical Outcomes in Ever-Smokers by Sex
Table 4:
Comparison of Association of Airway Metrics with Mortality in Ever-Smokers by Sex
Emphysema
After adjustment for age, ethnicity, body mass index, pack-years of smoking, and CT scanner type, men had greater percent emphysema than women, regardless of smoking status in never-smokers (4.21% ± 0.67 vs 2.02% ± 0.59; difference, 2.07% [95% CI: 0.44, 3.70]; P = .01), former smokers (10.60% ± 0.21 vs 8.76% ± 0.21; difference, 1.85% [95% CI: 1.34, 2.35]; P < .001), and current smokers (4.69% ± 0.19 vs 4.15% ± 0.20; difference, 0.53%; [95% CI: 0.06, 1.01]; P = .03). These differences were not noted in GOLD disease stages 3 and 4 (Table E2 [online]).
Discussion
By using CT imaging data from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease study (known as the COPDGene study), we examined differences according to sex in airway wall and lumen structure and the impact of changes in airway structure on lung function, quality of life, dyspnea, functional capacity, and survival. We found that in never-smokers (n = 420), men had thicker airway walls than did women at CT for segmental airway wall area percentage, whereas airway lumen dimensions were lower in women than men after accounting for height and total lung capacity. In ever-smokers (n = 9363), men had greater segmental airway wall area percentage, whereas women had narrower segmental lumen diameter. A unit change in each of the airway metrics (higher wall or lower lumen measure) resulted in lower forced expiratory volume in 1 second–to–forced vital capacity ratio, more dyspnea, poorer respiratory-quality of life, lower 6-minute walk distance, and worse survival in women compared with men (all P < .01).
The assumption that women have smaller airways is long standing and is on the basis of comparisons of the trachea and main stem bronchi, but few studies have compared distal airways in healthy individuals or accounted for differences in lung size. Two small studies that included nonsmokers found that men have greater segmental wall area percentage than women (31,32), but these comparisons were not adjusted for potential confounders including height and lung size. Furthermore, thickness of the segmental airway wall is affected by the size of the airway measured and hence precludes direct comparisons by sex. Wall area percent adjusts for airway size but is more affected by wall thickness than changes in the luminal diameter (33). Although a proportion of wall thickness may be caused by adventitial thickening, thicker airway walls likely result in a narrower lumen. We extend the literature by confirming that the wall thickness of the segmental airways is greater in men than in women even in lifetime never-smokers. It is noteworthy that the Pi10, a summary measure of wall thickness of airways of various sizes that is less likely to be affected by the differences in lung sizes, was not different by sex. We also found that women have narrower medium-size conducting airways and lower total airway volume, both indicating smaller airway lumen. Correspondingly, the airway fractal dimension was lower in women than in men, reflecting a lower complexity of branching pattern. The total number of airways viewed was lower in women than in men, likely reflecting that airways are uniformly smaller and hence a larger number of airways are below the resolution of current CT scanner protocols. With the additional adjustment for factors that may confound airway dimensions in women, including height, lung volume, display field-of-view, and body mass index, these data support the existence of a true sex difference in airway dimensions in never-smokers.
In ever-smokers, airway wall thickness was greater in men than in women. These findings are consistent with previous studies of wall changes, which demonstrated thicker walls in men (15,16,20,34–36). Although histopathologic examination of resected lung tissue in a subset of participants in the National Emphysema Treatment Trial, or NETT, showed greater wall thickness in the peripheral airways in women than in men, CT analyses of the entire cohort demonstrated thicker airway walls in men (15). NETT was also limited by only including patients with severe emphysema. The thinner airway walls in women are also compatible with findings that women appear to have more compliant airways with greater collapsibility than men (37,38). Greater collapsibility is also noted in more central airways in women (37). The impact of wall thickness on luminal narrowing depends on whether the thickening accrues inward or outward because of adventitial thickening. It is plausible that the increased airway wall thickness associated with cigarette smoking disproportionately affects women who have smaller airways.
The size of the airway lumen, with direct implications for higher airway resistance, was lower in women with no smoking history compared with men and was lower in female smokers compared with male smokers, even with adjustments for smoking burden and lung size. Previous CT studies have documented smaller luminal diameter in the central airways in women (20,36). We additionally found that total airway count and airway fractal dimension were lower in women than in men. The reasons for the luminal loss and remodeling are unclear but the likely mechanisms include mucus plugs, mucosal thickening, adventitial fibrosis, and loss of axial airway fibers resulting in obliteration of the airways. We found that the sex difference in airway wall remodeling noted in never-smokers was accentuated in ever-smokers whereas sex difference in airway lumen was attenuated, suggesting that the airways in men may be more susceptible to cigarette smoke–associated remodeling. This finding needs confirmation because we did not account for possible sex differences in smoking topography. However, even after decades of smoking, the lumen size remains lower in women, suggesting that the size limitation in women persists with chronic smoking exposure.
Our findings have implications for airflow limitation and the consequent clinical outcomes (22,23). The rising prevalence of COPD in women and the greater predisposition to airflow obstruction than in men when exposed to similar smoking burden conflicts with the multitude of studies that have documented a greater degree of emphysema in men (4,15–18,34,39). These sex differences are despite the more synaptic growth of airways and alveoli in women, such that the FEV1-to-FVC ratio is greater in women than in men as reflected by population-based normative data (40,41). We confirmed that men have more emphysema than women with equivalent smoking burden, and our results suggest that the lower reserve conferred by smaller airways predisposes women to develop airflow limitation predominantly through the airway phenotype. All airway remodeling changes were associated with more dyspnea, worse respiratory-quality of life, and lower functional capacity in women than in men. The smaller airways in women can result in higher airway resistance and more turbulent airflow, and thus place a higher ventilatory constraint during exertion. Indeed, endurance‐trained women experience expiratory flow limitation earlier during exercise compared with endurance‐trained men (42). Our findings explain in part why women experience worse symptoms for a given degree of airflow obstruction (7,8). Alteration in each airway measure was also associated with worse survival in women than in men, partially explaining the comparable mortality between the sexes for COPD despite the differing degrees of emphysema.
Our study has several strengths. First, we included a large number of never-smokers as well as participants with COPD of varying severity. Second, we performed a comprehensive evaluation of airway remodeling that included measures of both airway wall and airway lumen. Third, we confirmed sex by chromosomal analyses in all participants. Fourth, unlike previous studies, we adjusted for the display field of view to reduce variability in the measurement of airways; this is especially important in women in whom the display field of view tends to be smaller and airway wall thickness may appear artificially low, whereas lumen-based parameters such as total airway count and airway fractal dimension can appear artificially higher.
Our study had limitations. First, a higher proportion of men were active smokers compared with women and, although we adjusted for smoking status, some of the airway wall differences may be from the impact of active cigarette smoking on airway wall thickness. Second, the cross-sectional comparisons between never- and ever-smokers precludes ascribing causality to the smoking-associated changes. Third, we analyzed larger airways and did not examine differences in smaller airways because these are below current CT resolution. Fourth, although parametric response mapping data are available in COPDGene, this technique results in an indirect assessment of functional small airways disease. Fifth, we did not adjust for medication use when analyzing outcomes; however, current medications are not known to affect airway remodeling. Finally, cause-specific mortality adjudication was not completed, and we limited the survival analyses to all-cause mortality.
In conclusion, there are differences in airway structure and size between men and women as quantified at CT, after accounting for height and lung size. Although these differences by sex are attenuated in ever-smokers, the airway lumen size difference in women persists even with chronic smoking exposure and is associated with worse outcomes in women than in men. These structural differences may underlie some of the differences in predisposition to chronic obstructive pulmonary disease in men and women. These findings have implications for studies targeting disease progression.
S.P.B. and A.N. supported by the National Heart, Lung, and Blood Institute (S.P.B. and A.N., R01 HL151421; S.P.B., K23HL133438); S.P.B. supported by the National Institute of Biomedical Imaging and Bioengineering (R21EB027891); study supported by the National Heart, Lung, and Blood Institute (U01 HL089897, U01 HL089856); J.M.R. supported by the National Institutes of Health Roy J. Carver Charitable Trust (R01HL142625); E.A.H. supported by a grant from the National Institutes of Health; D.L.D. supported by a grant from the National Institutes of Health (R01 HG 011393). COPDGene is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion.
Disclosures of conflicts of interest: S.P.B. Grants/contracts from Sanofi/Regeneron Nuvaira to institute; consulting fees to author from Sanofi/Regeneron; payment for lectures from Integrity, Boehringer Ingelheim. S.B. Disclosed no relevant relationships. A.N. Disclosed no relevant relationships. Y.I.K. Disclosed no relevant relationships. J.M.R. Royalties from Vida Diagnostics; payment for lectures from Boehringer Ingelheim; payment for expert testimony from Desmarais; stock or stock options from Vida Diagnostics. E.A.H. Royalties from Vida Diagnostics; stock options from Vida Diagnostics, where he is a founder and shareholder. A.M. Patent pending for Regional Pulmonary V/Q via image registration and Multi-Energy CT. C.G.W. Disclosed no relevant relationships. S.M.H. Disclosed no relevant relationships. E.A.R. Disclosed no relevant relationships. D.L.D. Grant support from Bayer; honoraria from Novartis.
Abbreviations:
- COPD
- chronic obstructive pulmonary disease
- COPDGene
- Genetic Epidemiology of COPD
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
References
- 1.Ntritsos G, Franek J, Belbasis L, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2018;13:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni HXJ. COPD-related mortality by sex and race among adults aged 25 and over: United States, 2000–2014. NCHS data brief, no 256. Hyattsville, Md: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 3.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011;139(4):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med 2006;100(6):1110–1116. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Varela MV, Montes de Oca M, Halbert RJ, et al. Sex-related differences in COPD in five Latin American cities: the PLATINO study. Eur Respir J 2010;36(5):1034–1041. [DOI] [PubMed] [Google Scholar]
- 6.Amaral AFS, Strachan DP, Burney PGJ, Jarvis DL. Female Smokers Are at Greater Risk of Airflow Obstruction Than Male Smokers. UK Biobank. Am J Respir Crit Care Med 2017;195(9):1226–1235. [DOI] [PubMed] [Google Scholar]
- 7.DeMeo DL, Ramagopalan S, Kavati A, et al. Women manifest more severe COPD symptoms across the life course. Int J Chron Obstruct Pulmon Dis 2018;13:3021–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celli B, Vestbo J, Jenkins CR, et al. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease. The TORCH experience. Am J Respir Crit Care Med 2011;183(3):317–322. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins CR, Chapman KR, Donohue JF, Roche N, Tsiligianni I, Han MK. Improving the Management of COPD in Women. Chest 2017;151(3):686–696. [DOI] [PubMed] [Google Scholar]
- 10.Sørheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010;65(6):480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman EK, Chapman HA, Drazen JM, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 1998;157(6 Pt 1):1770–1778. [DOI] [PubMed] [Google Scholar]
- 12.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162(6):2152–2158. [DOI] [PubMed] [Google Scholar]
- 13.Moll M, Regan EA, Hokanson JE, et al. The Association of Multiparity with Lung Function and Chronic Obstructive Pulmonary Disease-Related Phenotypes. Chronic Obstr Pulm Dis (Miami) 2020;7(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sana A, Somda SMA, Meda N, Bouland C. Chronic obstructive pulmonary disease associated with biomass fuel use in women: a systematic review and meta-analysis. BMJ Open Respir Res 2018;5(1):e000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 2007;176(3):243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest 2009;136(6):1480–1488. [DOI] [PubMed] [Google Scholar]
- 17.Sverzellati N, Calabrò E, Randi G, et al. Sex differences in emphysema phenotype in smokers without airflow obstruction. Eur Respir J 2009;33(6):1320–1328. [DOI] [PubMed] [Google Scholar]
- 18.Hardin M, Foreman M, Dransfield MT, et al. Sex-specific features of emphysema among current and former smokers with COPD. Eur Respir J 2016;47(1):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu S, Deng X, Li Q, Sun X, Xu J, Li H. Gender differences of chronic obstructive pulmonary disease associated with manifestations on HRCT. Clin Respir J 2017;11(1):28–35. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Dai YL, Yu N, Guo YM. Sex-related differences in bronchial parameters and pulmonary function test results in patients with chronic obstructive pulmonary disease based on three-dimensional quantitative computed tomography. J Int Med Res 2018;46(1):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365(17):1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby M, Tanabe N, Tan WC, et al. Total Airway Count on Computed Tomography and the Risk of Chronic Obstructive Pulmonary Disease Progression. Findings from a Population-based Study. Am J Respir Crit Care Med 2018;197(1):56–65. [DOI] [PubMed] [Google Scholar]
- 23.Bodduluri S, Puliyakote ASK, Gerard SE, et al. Airway fractal dimension predicts respiratory morbidity and mortality in COPD. J Clin Invest 2018;128(12):5374–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 26.Wan ES, Balte P, Schwartz JE, et al. Association Between Preserved Ratio Impaired Spirometry and Clinical Outcomes in US Adults. JAMA 2021;326(22):2287–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatt SP, Washko GR, Hoffman EA, et al. Imaging Advances in Chronic Obstructive Pulmonary Disease. Insights from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) Study. Am J Respir Crit Care Med 2019;199(3):286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charbonnier JP, Pompe E, Moore C, et al. Airway wall thickening on CT: Relation to smoking status and severity of COPD. Respir Med 2019;146:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000;162(3 Pt 1):1102–1108. [DOI] [PubMed] [Google Scholar]
- 30.Patel BD, Coxson HO, Pillai SG, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;178(5):500–505. [DOI] [PubMed] [Google Scholar]
- 31.Zach JA, Newell JD Jr, Schroeder J, et al. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol 2012;47(10):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Y, Ji W, An S, Han SS, Lee SJ, Kim WJ. Sex differences of COPD phenotypes in nonsmoking patients. Int J Chron Obstruct Pulmon Dis 2016;11:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Washko GR, Diaz AA, Kim V, et al. Computed tomographic measures of airway morphology in smokers and never-smoking normals. J Appl Physiol (1985) 2014;116(6):668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography: emphysema and airway wall thickness by sex, age and smoking. Eur Respir J 2009;34(4):858–865. [DOI] [PubMed] [Google Scholar]
- 35.Kim WJ, Silverman EK, Hoffman E, et al. CT metrics of airway disease and emphysema in severe COPD. Chest 2009;136(2):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YI, Schroeder J, Lynch D, et al. Gender differences of airway dimensions in anatomically matched sites on CT in smokers. COPD 2011;8(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt SP, Terry NL, Nath H, et al. Association Between Expiratory Central Airway Collapse and Respiratory Outcomes Among Smokers. JAMA 2016;315(5):498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camiciottoli G, Diciotti S, Bigazzi F, et al. Is intrathoracic tracheal collapsibility correlated to clinical phenotypes and sex in patients with COPD? Int J Chron Obstruct Pulmon Dis 2015;10:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest 2007;132(2):464–470. [DOI] [PubMed] [Google Scholar]
- 40.Hibbert M, Lannigan A, Raven J, Landau L, Phelan P. Gender differences in lung growth. Pediatr Pulmonol 1995;19(2):129–134. [DOI] [PubMed] [Google Scholar]
- 41.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 2007;581(Pt 3):1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]



![Representative examples of airways in lifetime never-smoker men and women. (A) Noncontrast-enhanced three-dimensional reconstructed chest CT image in a lifetime never-smoker 64-year-old man (height, 1.74 m; total lung capacity, 8.1 L; forced expiratory volume in 1 second [FEV1]-to–forced vital capacity [FVC] ratio, 0.80; and FEV1, 127% predicted) shows airway volume of 57 dL, total airway count of 526, and airway fractal dimension of 1.695. (B) Noncontrast-enhanced three-dimensional reconstructed chest CT image in a lifetime never-smoker 63-year-old woman (height, 1.73 m; total lung capacity, 6.1 L; FEV1-to-FVC ratio, 0.76; and FEV1, 100% predicted) shows airway volume of 34 dL, 299 viewable airway branches, and airway fractal dimension of 1.513.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4b62/9713451/68548c07f672/radiol.212985.fig3.jpg)