Abstract
Small cell lung cancer (SCLC) is a highly aggressive malignancy with exceptionally poor prognosis, comprising approximately 15% of lung cancers. Emerging knowledge of the molecular and genomic landscape of SCLC and recent successful clinical applications of new systemic agents have allowed for precision oncology treatment approaches. Imaging is essential for the diagnosis, staging, and treatment monitoring of patients with SCLC. The role of imaging is increasing with the approval of new treatment agents, including immune checkpoint inhibitors, which lead to novel imaging manifestations of response and toxicities. The purpose of this state-of-the-art review is to provide the reader with the latest information about SCLC, focusing on the subtyping of this malignancy (molecular characterization) and the emerging systemic therapeutic approaches and their implications for imaging. The review will also discuss the future directions of SCLC imaging, radiomics and machine learning.
© RSNA, 2022
Learning Objectives:
After reading the article and taking the test, the reader will be able to:
■ Describe molecular subtypes and genomic alterations of specific transcription factors in small cell lung cancer (SCLC)
■ Identify the current and emerging systemic therapeutic options for SCLC
■ Specify the role of anatomic and molecular imaging in SCLC for diagnosis and therapy response monitoring
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based SA-CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Summary
Radiologists involved in the multidisciplinary management of patients with small cell lung cancer (SCLC) should be aware of how the molecular landscape of SCLC impacts therapy.
Essentials
■ Molecular and genomic characterization of specific transcription factors in small cell lung cancer (SCLC) indicates novel subtypes.
■ Recent developments in systemic therapy for extensive stage SCLC includes immune checkpoint inhibitors as the first-line therapy and a selective inhibitor of oncogenic transcription, lurbinectedin, for those who progressed on platinum-based chemotherapy.
■ Tetraazacyclododecane tetraacetic acid–octreotate, or DOTATATE, PET/CT and machine learning models may help inform treatment planning and outcome prediction for SCLC.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 14% of all lung cancers and is characterized by rapid growth, early metastatic spread, and poor prognosis. It has been estimated that there will be 236 740 new cases of lung cancer in 2022, with most classified as non-SCLC (NSCLC) (82%) or SCLC (14%) (1). Although NSCLC and SCLC are the two major types of lung cancer, these two tumors are distinctly different from each other in terms of clinical, pathologic, and radiologic perspectives. The 5-year survival rate is only 7% for SCLC, in striking comparison with 26% for NSCLC (1). SCLC is categorized as a lung neuroendocrine neoplasm in the World Health Organization Classification of Thoracic Tumors (2), as opposed to NSCLC, which is classified as an epithelial tumor. Most SCLCs are located in the central lung and are typically accompanied by mediastinal or hilar lymphadenopathy (3). SCLC is most commonly staged with use of a two-stage system, classifying it as “limited disease,” in which tumors are confined to one hemithorax with local extensions that can treated in one radiation portal, or “extensive disease,” with disease beyond these boundaries (3,4). This is different from the TNM staging system, which is used for NSCLC. Low-dose CT screening is effective for early detection of NSCLC; however, most cases of SCLC are detected within 1 year of a previous negative screening due to the highly aggressive and rapidly progressing nature of the tumor (5).
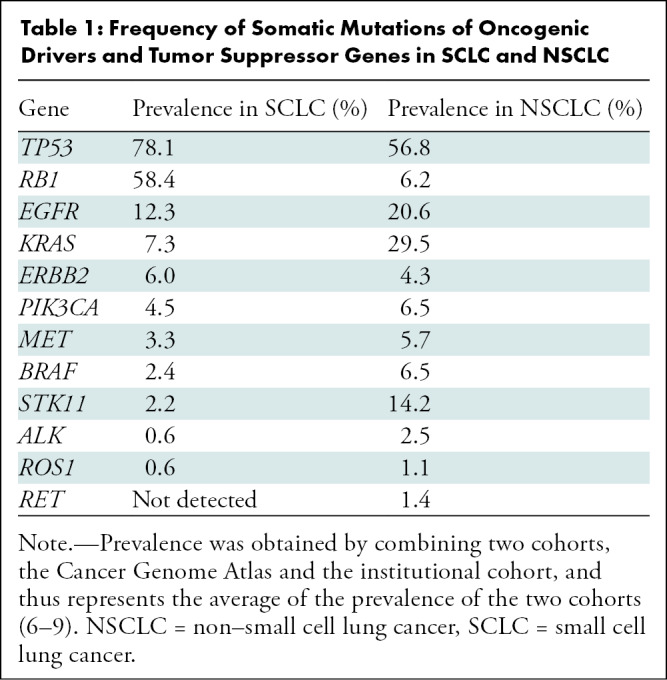
Tumor genomic characterization has further classified SCLC and NSCLC (Table 1) (6–9). The efficacy of immune checkpoint inhibitors (ICIs) has led to regulatory approvals for these agents and has changed the treatment paradigm for patients with SCLC. However, these latest advances in SCLC have not been widely recognized in the radiology community. Imaging has a key role in diagnosis, staging, and monitoring of SCLC. As members of a multidisciplinary team, it is important that radiologists are informed of the rapidly advancing landscape of SCLC and how this impacts imaging and patient care. This review describes the recent advances of molecular characterization and therapeutic approaches for SCLC, discusses their implications for imaging, and is designed to effectively communicate this new information so that radiologists can maximize their contributions to the multidisciplinary management of this important primary lung malignancy.
Table 1:
Frequency of Somatic Mutations of Oncogenic Drivers and Tumor Suppressor Genes in SCLC and NSCLC
Genomic Characterizations and Molecular Subtyping of SCLC
Molecular Subtypes of SCLC Based on Expression of Transcription Factors
In the past few decades, there has been a significant advance in our understanding of the genomic landscape of SCLC with the development of comprehensive genomic studies, including exome, whole-genome, transcriptome, and copy-number alteration analyses. The characteristic genetic alterations in SCLC are inactivation of the tumor-suppressor genes TP53 and RB1, which is a near ubiquitous event in SCLC (10). Loss of TP53 and RB1 results in proliferation of tumor cells and is associated with early metastasis and rapid resistance to chemotherapy (11).
Biologic heterogeneity of gene expression in SCLC has been recently discovered, leading to novel molecular subtypes (Fig 1) (10). Emerging knowledge indicates that subtypes of SCLC are not necessarily defined by their tumor mutational landscape, but instead by the expression of specific transcription factors that can provide a biologic framework for the distinct SCLC entities (10). Three major molecular subtypes have been described, including SCLC-A, SCLC-N, and SCLC-P based on high expression of the transcription factors ASCL1 (achaete-scute homolog 1), NEUROD1 (neurogenic differentiation factor 1), and POU2F3 (POU class 2 homeobox 3), respectively (10,12). SCLC-A is the most common molecular subtype of SCLC, comprising approximately 70% of SCLCs. Further subdivision of SCLC-A into two groups is suggested, including SCLC-A and SCLC-A2, based on difference in expression of other factors including HES1 (hairy and enhancer of split-1) (13,14). These subtypes tend to reflect the differential expression levels of MYC oncogene family members, which are known oncogenic drivers of SCLC and may constitute novel therapeutic targets (15). Increased MYCL expression is associated with SCLC-A, whereas increased MYC expression is noted in the other subtypes (16). MYC also contributes to temporal evolution among subtypes, for example from SCLC-A to SCLC-N, indicating that SCLC molecular subtypes may represent dynamic stages of MYC-driven tumor evolution (17). Another major difference between these subtypes is a distinct degree of neuroendocrine differentiation, where SCLC-P has a less neuroendocrine phenotype compared with SCLC-A and SCLC-N subtypes (10).
Figure 1:
Emerging molecular subtypes of small cell lung cancer (SCLC) (10). Molecular subtypes SCLC-A, SCLC-N, and SCLC-P have been described based on high expression of the transcription factors (achaete-scute homologue 1 [ASCL1], neurogenic differentiation factor 1 [NEUROD1], and POU class 2 homeobox [POU2F3], respectively). For SCLC-A, which is the most common molecular subtype consisting of approximately 70% of SCLCs, further subgroups of SCLC-A and SCLC-A2 are suggested based on difference in expression of other factors. For the remaining SCLCs with low expression of the three transcription factors (indicated in gray in the figure), several subgroups are proposed, including SCLC-Y with high expression of YAP1, a rare subtype with elevated expression of ATOH1, and SCLC-I with “inflamed” gene signatures. Inactivation of the tumor-suppressor genes TP53 and RB1 is noted as a near ubiquitous event in SCLC. Different degree of neuroendocrine differentiation and the differential expression levels of MYC oncogene family members are noted among the subgroups.
In addition, a recent study described a subgroup with low expression of all three transcription factor signatures that has an “inflamed” gene signature (SCLC-I) with uniquely expressed genes including a number of immune checkpoints and human leukocyte antigens. In preclinical studies, SCLC-I showed better response to ICI, emphasizing the clinical implication of understanding molecular subtypes (18). Further investigations are ongoing to further define the subgroups, particularly for those with low expressions of transcription factors, and to determine the therapeutic implications for these new molecular subtypes of SCLC.
SCLC Transformation of NSCLC Treated with Molecular Targeted Therapy
In addition to de novo SCLC, histologic transformation to SCLC has been described as one of the mechanisms of acquired resistance to epidermal growth factor receptor (EGFR) inhibitors in EGFR-mutant NSCLC, noted in approximately 3%–10% of acquired resistance cases (19–21), which provides another insight for SCLC biology. A recent study investigated 58 patients with EGFR-mutant NSCLC at diagnosis who subsequently experienced SCLC transformation after receiving EGFR inhibitors. The median time on EGFR inhibitors before transformation was 15.8 months. Most patients (93%) were receiving EGFR inhibitors at the time of SCLC transformation (19). The most common mutations identified in SCLC tumor samples were TP53, RB1, and PIK3CA mutations (19–21). Unlike TP53 and RB1 mutations, which are commonly noted at the initial diagnosis of SCLC, PIK3CA mutations are relatively uncommon in SCLC and NSCLC. PIK3CA-mutated lung cancers are clinically and genetically heterogeneous (22), and the clinical significance remains to be investigated. Patients with SCLC transformations no longer respond to the original targeted therapy to NSCLC, and their tumors often show an aggressive course (Fig 2), with frequent central nervous system metastasis (19). SCLC transformation has also been noted in patients with ALK-positive NSCLC treated with ALK inhibitors (23), indicating it may be one of the general mechanisms of acquired resistance to molecular targeted therapies for NSCLC. Further studies are needed to better understand the biologic characteristics of SCLC due to transformation of NSCLC after molecular targeted therapy as well as to help develop effective therapeutic and preventive strategies for this phenomenon.
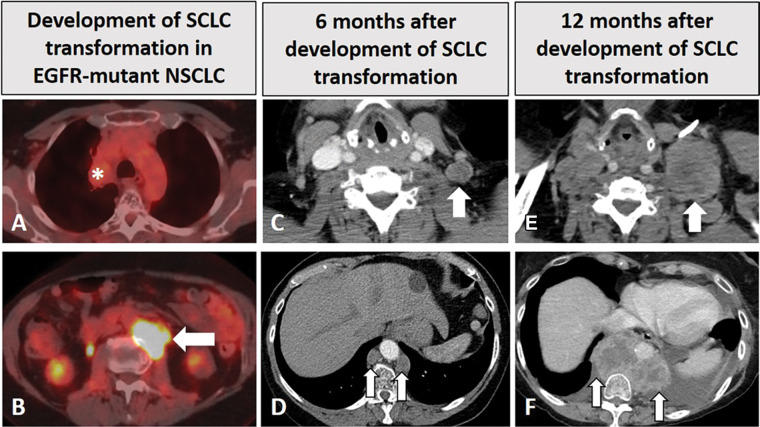
Figure 2:
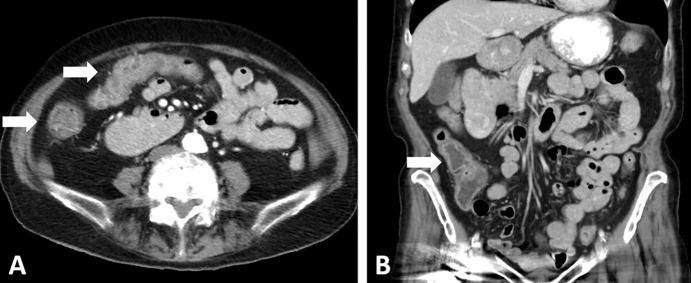
Images in a 68-year-old woman with EGFR-mutant non–small cell lung cancer (NSCLC) with small cell transformation after epidermal growth facto receptor (EGFR) inhibitor therapy. (A, B) The patient was initially treated with EGFR inhibitor, osimertinib, for her NSCLC with sensitizing EGFR mutation (L858R) and responded well, with a treated non-fluorodeoxyglucose (FDG)–avid primary tumor in the right upper lobe (* in A), as shown on FDG PET/CT scan obtained 2 years after the initiation of osimertinib (A). However, on the same FDG PET/CT scan (B), a new FDG-avid paraspinal mass (arrow in B) was noted. The paraspinal mass demonstrated mixed features of EGFR L858R mutant non-SCLC and SCLC at histologic examination, demonstrating SCLC transformation due to acquired resistance to EGFR inhibitor. The patient was treated with carboplatin plus etoposide. (C, D) Contrast-enhanced chest CT scans obtained 6 months later show further progression, with enlarged left supraclavicular node (arrow in C) and paraspinal nodes (arrows in D). The patient was switched to paclitaxel therapy, while continuing osimertinib. (E, F) Contrast-enhanced CT scans obtained at 12 months demonstrate further progression, with significant further enlargement of the left supraclavicular node (arrow in E) and paraspinal nodes (arrows in F) with heterogeneous CT attenuation and the development of left pleural effusion.
Current and Emerging Systemic Therapy Options for SCLC
Although SCLC remains a challenging disease to treat and cure, especially extensive or recurrent disease, there have been several recent advances of systemic therapeutic approaches. Up-to-date knowledge on the current treatment approaches to SCLC is essential for accurate image interpretation, given unique image manifestations of tumor response and toxicities of novel agents.
Summary of Existing Treatment Approaches for SCLC
Surgical resection can be curative in early limited stage SCLC, with an improved overall survival (OS) when compared with chemotherapy, although surgery is performed in only about 3% of patients diagnosed with SCLC (24). Concurrent chemoradiotherapy with etoposide and cisplatin or carboplatin is the standard of care for limited stage SCLC that is not surgically resectable (25). Although patients show good response to initial therapy, the 2-year cumulative risk of developing intracranial metastasis is more than 50% and the median survival time after brain metastasis is only 4–5 months (26). Prophylactic cranial radiation is recommended for patients who achieve good response to initial therapy because it reduces the risk of developing intracranial metastasis and prolongs OS (27).
For extensive stage SCLC, a combination of etoposide and cisplatin or carboplatin has historically been a mainstay of treatment (28). The objective response rates of platinum-based chemotherapy range from 40% to 70%, with up to 10% of patients having complete response. Unfortunately, the responses are often short-lived, with median OS ranging from 7 to 12 months and a 5-year survival rate of less than 2% (28). There have been additional important developments in systemic therapy for extensive stage SCLC, including ICIs and a selective inhibitor of oncogenic transcription, lurbinectedin, which has recently been approved by the U.S. Food and Drug Administration (FDA) (Fig 3) (29). These treatment approaches are discussed further below.
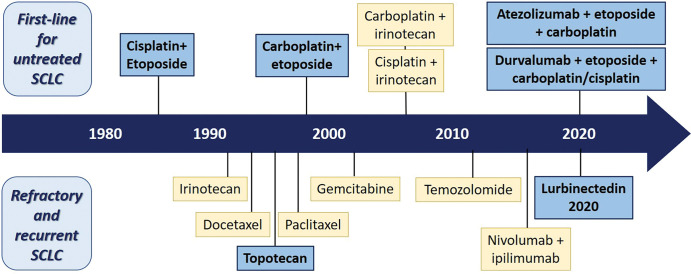
Figure 3:
Diagram shows advances of systemic therapy approaches for small cell lung cancer (SCLC) (12). The advances of systemic treatment options for patients with SCLC during the past decades are shown with the timeline. The blue boxes represent U.S. Food and Drug Administration (FDA)–approved standard-of-care treatment, and the yellow boxes represent treatment options recommended by the National Comprehensive Cancer Network but not currently approved by the FDA. The figure illustrates the paucity of the FDA-approved therapeutic options in the past 3 decades, with new recent additions for the first-line setting and for refractory and/or recurrent disease. Both nivolumab and pembrolizumab were initially approved by the FDA in 2018 and 2019, respectively, for patients with relapsed SCLC based on several trial results showing improved response rate and progression-free survival. However, subsequent trials failed to demonstrate improved survival in the programmed cell death protein 1 (PD-1) inhibitor therapy group and the FDA withdrew the indication of use of these PD-1 inhibitors in relapsed SCLC.
ICIs in SCLC
ICIs have emerged as a promising treatment option for various types of advanced malignancies. The two most commonly used ICI agents are cytotoxic T lymphocyte–associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors, both of which use antibodies to block immune-inhibitory pathways. CTLA-4 inhibitors block an inhibitory pathway that down-regulates the initial stages of T cell activation, ultimately unleashing a pre-existing anticancer T-cell response. PD-1 and PD-L1 inhibitors block the interaction between PD-1 and PD-L1, respectively, enhancing antitumor activity of T cells (30,31). These agents have been tested in trials of SCLC in combination with platinum and etoposide chemotherapy, leading to recent FDA approvals (Table 2).
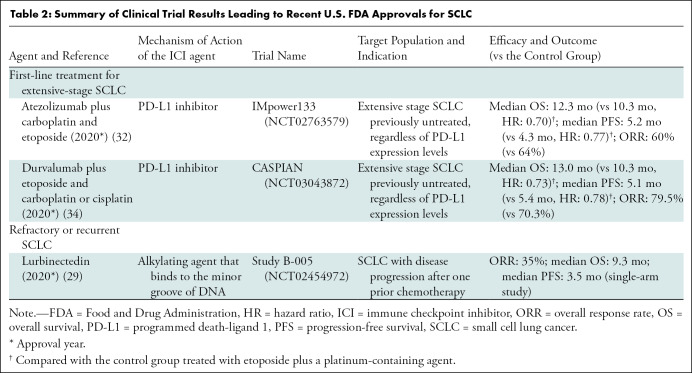
Table 2:
Summary of Clinical Trial Results Leading to Recent U.S. FDA Approvals for SCLC
PD-L1 inhibitor in combination with chemotherapy has become the new frontline standard of care for patients with extensive stage SCLC. The IMpower133 trial showed that a combination of carboplatin and etoposide with atezolizumab (PD-L1 inhibitor) improved OS compared with chemotherapy alone (12.3 months vs 10.3 months, respectively; P = .007) in extensive stage SCLC (32). Similar results were demonstrated in the phase III CASPIAN trial, where the addition of durvalumab (PD-L1 inhibitor) to chemotherapy significantly improved OS compared with chemotherapy alone (OS at 3 years: 17.6% vs 5.8%, respectively) (33–35). Based on these clinical trial results, atezolizumab and durvalumab were approved by the FDA in 2019 and 2020, respectively, for use in combination with platinum-based chemotherapy for first-line treatment of patients with extensive stage SCLC. These two treatment regimens are the only two new approvals for first-line therapy for SCLC since 1999 (Fig 3), representing a new milestone for SCLC treatment. However, even with these therapies, tumor progression is inevitable for most patients, and the prolongation of OS by approximately 2 months with the addition of immunotherapy to chemotherapy highlights its limited efficacy (Fig 4). Further treatment strategies are sorely needed to further delay or, ideally, prevent disease progression and to better manage refractory and relapsed SCLC.
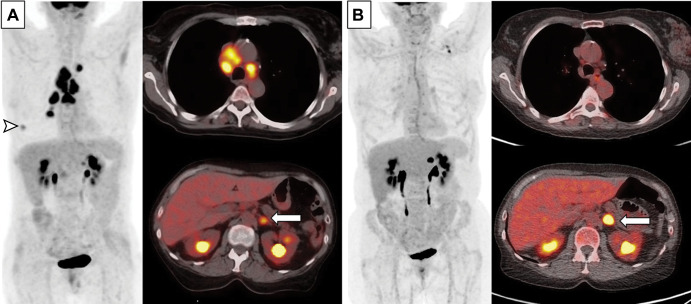
Figure 4:
Images in a 63-year-old woman with extensive small cell lung cancer detected at screening CT. (A) Baseline whole-body PET scan (left) and fused axial PET/CT images (right) demonstrate fluorodeoxyglucose-avid right lower lobe nodule (arrowhead, whole-body PET scan) and mediastinal and hilar lymphadenopathy (fused axial image on top right) as well as left adrenal lesion (arrow, fused axial image on bottom right). First-line therapy with the programmed death-ligand 1 inhibitor atezolizimab was initiated. (B) Follow-up whole-body PET scan (left) and fused axial PET/CT images (right) obtained after four cycles of atezolizimab, carboplatin, and etoposide show significantly improved thoracic lymphadenopathy and resolution of right lower lobe nodule. However, the left adrenal metastasis (arrow) has progressed and was treated with radiation therapy.
Controversy remains in the role of ICI therapy for cases of relapsed SCLC. The PD-1 inhibitors nivolumab and pembrolizumab were both granted accelerated FDA approval in 2018 and 2019, respectively, for patients with SCLC whose disease has progressed after platinum-based chemotherapy and at least one other line of therapy (36–38). However, subsequent trials failed to demonstrate improved survival in the PD-1 inhibitor group (39,40). Therefore, these indications for nivolumab and pembrolizumab in relapsed SCLC were subsequently withdrawn. There are ongoing clinical trials assessing the clinical efficacy and safety of ICI therapy in patients with limited stage SCLC in addition to chemoradiation. In addition, trials are ongoing to evaluate the addition of novel ICI agents targeting, for example, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3), lymphocyte activating 3 (LAG3), or T-cell immunoreceptor with immunoglobulin and ITIM domain (TIGIT), to PD-1 or PD-L1 inhibitors in advanced solid tumors including relapsed SCLC to further expand the application of ICIs in patients with SCLC.
Another unmet need in ICI therapy in SCLC is a lack of predictive biomarkers. PD-L1 expression at immunohistochemistry is one of the most commonly used biomarkers to predict response to ICI in NSCLC and other solid malignancies. However, most SCLC tumors do not express PD-L1. Tumor mutation burden is another biomarker known to correlate with treatment response in other malignancies, and SCLC has a relatively high tumor mutation burden. Tumor mutation burden as a predictor of benefit from ICIs was noted to be promising in patients with relapsed SCLC treated in a trial of combinations of nivolumab and ipilimumab (41) and in a few retrospective clinical studies (42). However, in the IMpower133 trial, blood-based tumor mutation burden demonstrated no clear predictive value in the first-line setting. A paucity of tumor tissue specimens is another challenge in SCLC, for which the only diagnostic specimens obtained tend to be small and necrotic (32,43). Emerging molecular subtypes according to expression levels of transcription factors may also have a role in predicting response to ICI therapy, as suggested in a recent preclinical study of SCLC-I subtype (18).
Lurbinectedin for SCLC
Another newly approved therapeutic option for SCLC is lurbinectedin, which is an alkylating agent that binds to the minor groove of DNA and affects transcription. Acting as a selective inhibitor of oncogenic transcription, lurbinectedin promotes tumor cell death. In the recent single-arm, open-label phase II basket trial, lurbinectedin showed overall response of 35.2%, with median progression-free survival (PFS) of 3.5 months in 105 patients with SCLC (44). Lurbinectedin was approved by the FDA in June of 2020 for patients with SCLC who have disease progression on or after platinum-based chemotherapy (29,44). Before this approval of lurbinectedin, topotecan was the only FDA-approved agent for the treatment of patients with recurrent or progressive SCLC with platinum-sensitive disease (29).
Emerging Molecular Targets for Precision Therapy
Increasing understanding of the genomic landscape of SCLC also indicates potential therapeutic targets in the DNA damage repair pathway and cell cycle checkpoints. In addition, certain genomic alterations can be potential biomarkers for predicting treatment response to advance precision therapy for SCLC (Table 3). Poly (adenosine diphosphate-ribose) polymerase 1 (PARP1) is upregulated in SCLC and is involved in various tumorigenic processes, including cell differentiation, proliferation, and transformation. Patients with SCLC included in a phase I study treated with the poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitor talazoparib showed an overall response rate of 9%, and the clinical benefit for 16 weeks or longer was noted in 26% (45). In a phase II clinical trial, high schlafen family member 11 (SLFN11) expression (H-score ≥1) was associated with favorable clinical outcome in patients with SCLC treated with temozolomide plus the PARP inhibitor veliparib (46). Novel agents for other molecular targets, including Aurora kinase and hepatocyte growth factor/ mesenchymal epithelial transition (HGF/MET) pathway have also been tested in trials of SCLC (Table 3).
Table 3:
Emerging Molecular Targets for Precision Therapy Applications in SCLC
Role of Imaging in the Current Era of SCLC Diagnosis and Treatment
Challenges in Early Detection of SCLC in CT Lung Cancer Screening
Lung cancer screening using low-dose CT is recommended in a high-risk population and has demonstrated cancer-specific mortality reduction (47). However, unlike NSCLC, there was no significant outcome benefits of early detection of SCLC with screening CT (5,48), presumably due to local-regional aggressive behavior and frequent widespread metastasis at the time of diagnosis (Fig 4). According to the analysis of characteristics and clinical outcome of SCLC detected by using low-dose CT screening in the National Lung Screening Trial (5), most SCLC cases were detected within 1 year after a negative screening examination, indicating the rapid growth and aggressive biology of the tumor. Most SCLC cases were late stage, and there was no difference in stage or survival among SCLCs detected in screening, interval, postscreening, or unscreened groups. Only 14% of screen-detected SCLCs were stage I when the average nodule size was 3 mm to less than 7 mm, and there was no stage I SCLC when the average nodule size was 7 mm or larger (5). There is clearly an unmet need for a better strategy for screening and early detection of SCLC.
TNM and Veterans’ Administration Lung Study Group Staging Systems and the Role of Different Imaging Modalities
The Veterans’ Administration Lung Study Group (VALSG) staging system was introduced in 1957 and has been widely used for clinical staging of SCLC (4). This system divides SCLC into “limited” and “extensive” disease depending on whether all known tumors could be treated within a single radiation therapy portal. Limited disease is defined as tumors confined to one hemithorax without extrathoracic metastases, although local extension and ipsilateral supraclavicular nodes could be present if they can be included in the same radiation portal as the primary tumor. All other diseases are classified as extensive disease (eg, malignant pleural and pericardial effusions, contralateral hilar or supraclavicular lymph nodes, and metastatic disease beyond a single radiation port). In 1989, the International Association for the Study of Lung Cancer proposed modification of the VALSG staging system, which includes contralateral mediastinal or supraclavicular lymph nodes and ipsilateral pleural effusions (whether benign or malignant) in the limited stage (4). VALSG and modified VALSG staging systems are most widely accepted and used in clinical management planning of SCLC. Approximately 70% of patients with SCLC are diagnosed at the extensive stage (24).
The American Joint Committee on Cancer TNM staging system defines stages I–IV based on primary tumor size (T), nodal spread (N), and metastasis (M), which is less frequently used for SCLC staging. The utility of TNM staging in SCLC was described in 349 cases of resected SCLC, which showed that survival after resection correlates with both T and N category, with nodal status having a stronger influence on survival (49). TNM staging can provide more refined assessment of prognosis and optimal treatment and identify SCLC patient subgroups with different prognoses, especially in patients with limited stage disease, which includes heterogeneous diseases from early to locally advanced tumors (50).
Imaging plays a central role in the diagnosis of SCLC and in the evaluation of the extent of disease involvement. Centrally located tumor with mediastinal or hilar lymphadenopathy is the typical finding in SCLC at chest CT. Due to the central location of disease, narrowing of the airway(s), atelectasis, and major vessel involvement are commonly seen (3). Intratumoral calcifications may be present. Only 5%–10% of SCLCs manifest as a peripheral nodule without associated lymphadenopathy (3). SCLC demonstrates intense fluorodeoxyglucose (FDG) uptake at PET/CT (3), which contributes to systemic evaluation of the extent of disease involvement and spread.
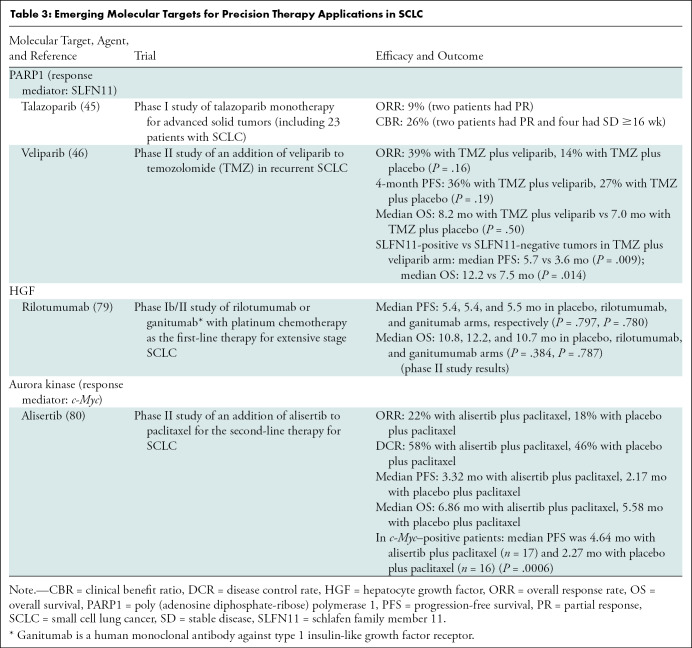
A few studies examined the value of different imaging modalities for SCLC staging. In a systematic review of the imaging literature (years 2000–2015) for the pretreatment staging of SCLC, the following conclusions were found in 408 patients with SCLC: (a) FDG PET/CT is more sensitive than multi–detector row CT for detecting osseous metastases (Fig 5), (b) FDG PET/CT is more sensitive than bone scintigraphy for detecting osseous metastases, and (c) standard staging plus FDG PET/CT is more sensitive than standard staging alone for detecting any distant metastases (51). The data are particularly sparse for other imaging modalities such as MRI and PET/MRI. Subsequent studies have also shown that FDG PET/CT can help detect occult distant metastasis, which would upstage the tumor stage. Stages have changed from limited disease to extensive disease in 10%–15% of patients with SCLC with FDG PET/CT compared with anatomic imaging alone, mostly due to medullary bone metastasis, leading to change in management to systemic therapy (52,53).
Figure 5:
Images in an 81-year-old man who presented with cough and back pain. The patient was a former smoker. (A, B) Fluorodeoxyglucose (FDG) PET/CT scans demonstrate a left hilar mass (arrow in A) with mediastinal and left hilar lymphadenopathy (arrow in B) and bone metastasis, representing extensive stage small cell lung cancer. (C, D) Bone metastasis in the left iliac bone is better seen on PET/CT scan (C) as intense focal FDG uptake (circle in C) without definitive correlate on CT scan (D) (circle in D). (E) Chest CT scan at diagnosis also demonstrates severe centrilobular emphysema with mild peribronchial thickening in the underlying lungs in this former smoker. The patient received several lines of systemic therapy, including carboplatin and etoposide, irinotecan, and nivolumab; however, disease progressed and the patient died 10 months after the diagnosis.
Therapy Response Imaging in SCLC
Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 was published in 2009 and has been widely used for assessment of treatment response of solid tumors including lung cancer (54,55). RECIST version 1.1 is based on the sum of unidimensional measurement of target lesions and assigns response categories on follow-up scans during therapy. Most clinical trials for SCLC use RECIST for response assessment, as in trials of NSCLC and other solid tumors (32,34,44).
The value of RECIST evaluations to predict clinical outcome of patients with SCLC has not been extensively studied. In a study of 134 patients with limited stage SCLC treated in a trial of concurrent radiation therapy with cisplatin plus etoposide, partial response and stable disease were not associated with PFS or OS. The percentage change in primary mediastinal tumor diameter was associated with longer PFS (hazard ratio [HR], 0.98; P = .004) and longer OS (HR, 0.98; P = .001) (56). Although the data are limited in extensive stage SCLC, given the aggressive nature of the disease and frequent systemic spread with multiple sites, the assessment of metabolic activity using FDG PET/CT may provide additional information (Fig 6).
Figure 6:
Images in a 69-year-old woman who presented with chronic cough. (A) Baseline whole-body fluorodeoxyglucose (FDG) PET scan (left) and fused axial FDG PET/CT image (right) show FDG-avid left perihilar lesion with extensive pleural metastasis (*) and osseous metastases (arrows) in the left pelvic bone, consistent with extensive small cell lung cancer. (B) Follow-up whole-body FDG PET scan (left) and fused axial FDG PET/CT scan (right) obtained after four cycles of therapy with carboplatin, etoposide, and atezolizumab demonstrate excellent response, with decreased tumor burden and residual FDG-avid disease in the left hilar region and pleura (arrowheads).
With the introduction of ICI therapy, atypical patterns of response are observed. These changes have been given the term “pseudoprogression.” Pseudoprogression is defined as the imaging appearance of an initial increase in tumor size or the appearance of new lesion during ICI therapy that is followed by a subsequent reduction of tumor burden (30,31). The initial increase in tumor burden is thought to be due to immune cell infiltrate instead of tumor cell proliferation. To capture this atypical response pattern, new immune-related response criteria were proposed to better characterize the response to ICI therapy (30,31). These criteria require confirmation of progressive disease on the subsequent scan to differentiate pseudoprogression from true progression. However, studies have shown that pseudoprogression is a rare event, with an overall incidence of 6% in a meta-analysis of 3402 patients from ICI trials of solid tumors (57). The incidence of pseudoprogression was even lower and was 1% or less in patients with advanced NSCLC treated with PD-1 inhibitors (58,59). Immune-related response patterns in patients with SCLC are understudied and not systematically described, indicating the need for further studies as the use of ICI increases for patients with SCLC.
Imaging of Underlying Lung Diseases in SCLC: Emphysema and Interstitial Lung Disease
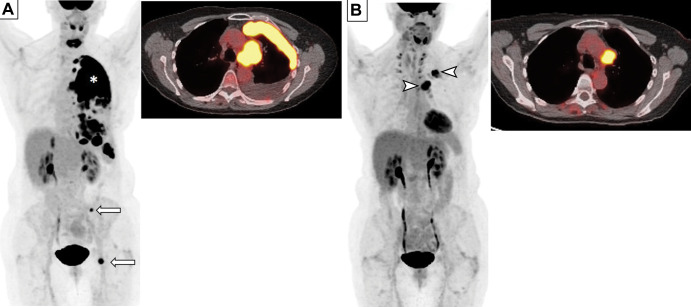
Patients with SCLC have high rates of a positive smoking history; thus, most patients with SCLC also have emphysema and interstitial lung disease (ILD) as comorbidities. Attention to these additional findings is important to provide prognostic markers in patients with SCLC (Figs 5, 7). In a study of 149 patients with SCLC, where 87% of the cohort had a positive smoking history, emphysema was present in 111 patients (74.5%) on chest CT scans. Coexisting centrilobular and paraseptal emphysema was the most common type (72.9%). Emphysema was associated with older age, male sex, poorer performance status, smoking history, and a significantly higher pack-year history of smoking. Higher severity of emphysema at CT was associated with shorter OS at multivariable analyses after adjustment for age and other significant variables, including extensive stage, elevated lactate dehydrogenase level, and supportive care only (60). Another study evaluated 122 patients with SCLC who were receiving platinum-based combination chemotherapy for the presence of preexisting ILD, which was present in 28 patients (23%) at diagnosis (60). Drug-related pneumonitis was significantly more common in patients with preexisting ILD (60). The median OS was significantly longer in patients without preexisting ILD than in those with preexisting ILD (17.8 months vs 10.7 months, respectively). Absence of preexisting ILD remained as a significant predictor of longer OS after adjusting for other significant variables including performance status and limited stage (60). These observations highlight the role of imaging in patients with SCLC for evaluation and monitoring of underlying lungs in addition to the evaluation of their tumors.
Figure 7:
Images in a 79-year-old man with extensive small cell lung cancer (SCLC) and underling fibrotic interstitial lung disease. The patient was a former smoker and presented with worsening cough and dyspnea on exertion. (A–C) Chest CT scans demonstrate a left upper lobe lesion (* in A) and peritoneal and retroperitoneal deposits (arrows in C) that were biopsied and showed metastatic SCLC. Notably, the clinical record indicated that the annual low-dose CT screening 6 months earlier was negative for cancer. Underlying lungs show fibrotic interstitial lung disease in peripheral and somewhat basilar distribution (arrows in A and B), accompanied by interlobular septal thickening, ground-glass and reticular opacities, traction bronchiectasis, and a few areas suggestive of early honeycombing. The patient was treated with several lines of systemic therapy, including carboplatin plus etoposide and nivolumab; however, he died 6 months after the diagnosis.
Imaging of Treatment-related Toxicities in SCLC
Because chemoradiotherapy is the standard of care in limited stage SCLC, radiation pneumonitis is one of the most common adverse events after definitive therapy. Most radiation pneumonitis occurs within 4–12 weeks after completion of radiation therapy. The initial manifestation of the radiation pneumonitis on an imaging study can be a ground glass or consolidative opacity along the radiation field (61). The lung becomes fibrotic when the inflammation is healed, accompanied by traction bronchiectasis, architectural distortion, and lung volume loss (61).
With the advent of upfront use of immune-checkpoint inhibition, a unique set of immune-related adverse events has been observed in patients with SCLC (Figs 8, 9). In a retrospective study investigating the incidence of immune-related adverse events among patients with advanced SCLC, colitis and pneumonitis were the most common immune-related adverse events, noted in 17% of patients (nine of 53) (62). Pneumonitis and thyroiditis were more frequent in patients with a history of thoracic radiation therapy (62), justifying an increasing concern for pneumonitis in the setting of previous or concurrent chest radiation therapy. Given the recent approval of ICI therapy as the first-line treatment for SCLC, the awareness of the unique set of immune-related adverse events and their imaging manifestations is essential for treatment monitoring of patients with SCLC. Further details of the imaging manifestations of immune-related adverse events in various organs have been previously described (30).
Figure 8:
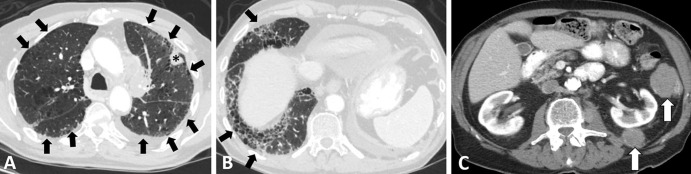
Images in a 68-year-old woman with relapsed small cell lung cancer (SCLC) after chemoradiotherapy for limited stage SCLC. The patient was treated with nivolumab and ipilimumab. (A, B) Axial (A) and coronal (B) CT images obtained 7 weeks after immune checkpoint inhibitor (ICI) therapy. Abdominal CT scans demonstrate diffuse wall thickening and increased mucosal enhancement of the ascending and transverse colon (arrows in A) and a fluid-filled lumen in the ascending colon (arrow in B), representing a characteristic appearance of ICI-related pancolitis.
Figure 9:
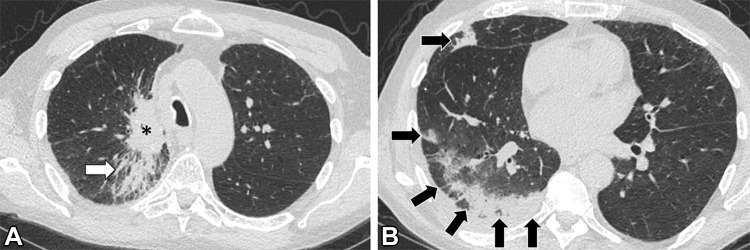
Images in a 69-year-old man with extensive stage small cell lung cancer (SCLC) treated with first-line atezolizumab plus carboplatin and etoposide. The patient also has a remote history of tracheal squamous cell carcinoma, which had been treated with chemoradiotherapy. (A, B) Chest CT scans obtained 6 weeks after initiation of atezolizumab therapy demonstrate development of peripheral areas of consolidation in the right lung (arrows in B), indicative of immune checkpoint inhibitor–related pneumonitis with an organizing pneumonia pattern. A dominant lung mass from SCLC is noted in the right upper lobe (* in A), with underlying postradiation changes from prior radiation therapy to the tracheal tumor (arrow in A). The patient had increasing shortness of breath but no fever, and results of infectious work-up, including COVID-19, were negative. Atezolizumab was held and the patient was treated with oral corticosteroids.
Future Directions of SCLC Imaging
FDG PET/CT for Treatment Planning and Prognostication of SCLC
SCLC typically shows intense uptake on FDG PET/CT scans, reflecting its high metabolic activity. FDG PET/CT can play a role in identifying the extent of active disease more accurately compared with CT, which is important to determine the extent of the radiation field in limited stage SCLC (Fig 10). In a study of 33 patients with limited stage SCLC who underwent baseline FDG PET/CT before radiation therapy, gross tumor volume for radiation therapy was defined using FDG uptake in primary disease and nodal metastasis at PET/CT (53). The outcome of these patients was better than that reported in the literature, indicating that precise delineation of gross tumor volume by means of FDG uptake at PET/CT may lead to better local-regional disease control after radiation therapy (63). A prospective study using pretreatment FDG PET/CT for defining a target volume of primary tumor and mediastinal nodal disease for radiation therapy showed lower rates of nodal recurrence and radiation esophagitis compared with CT-based treatment planning (64).
Figure 10:
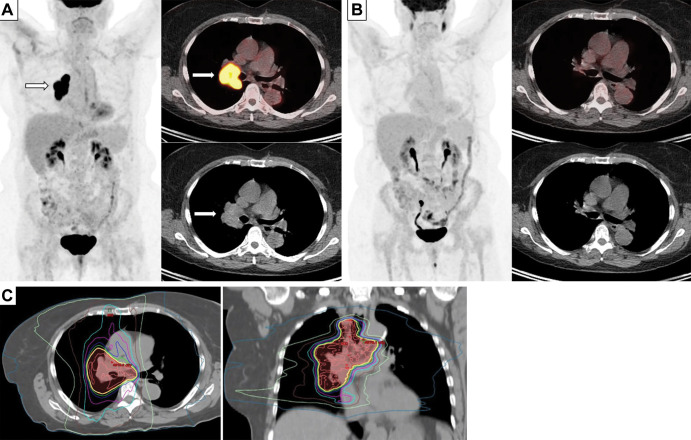
Images in a 70-year-old woman who presented with dyspnea and chest pain. (A) Baseline whole-body fluorodeoxyglucose (FDG) PET image (left), fused axial FDG PET/CT scan (top right), and CT scan (bottom right) demonstrate intense FDG uptake in the enlarged right hilar mass and nodal conglomerate (arrows) with luminal narrowing of right bronchus, consistent with limited stage small cell lung cancer. (B) Whole-body FDG PET image (left), fused axial FDG PET/CT scan (top right), and CT image (bottom right) obtained after four cycles of cisplatin and etoposide and radiation therapy show resolution of FDG-avid right hilar lesions, indicating complete response to therapy. (C) CT images used for treatment planning for radiation therapy show delineation of the gross tumor volume corresponding to the FDG-avid tumor burden on the initial PET/CT scans, for delivery of 45 Gy radiation in 30 fractions (twice daily) to the tumor.
Metabolic parameters on pretreatment and posttreatment PET/CT scans can be used as a predictive marker of clinical outcome of SCLC. Maximum standardized uptake value (SUVmax), metabolic tumor volume, and total lesion glycolysis are commonly used metabolic parameters in FDG PET/CT. Higher SUVmax values of the primary tumor at pretherapy FDG PET/CT were observed in extensive stage compared with limited stage SCLC (65), and higher metabolic tumor burden on pretreatment scans is associated with poor clinical outcome. In limited stage SCLC, the high SUVmax group (>5.9) showed lower 2-year OS rates compared with the low SUVmax group after definitive chemoradiotherapy (15% vs 56%, respectively) (53). In addition to standardized uptake value, total lesion glycolysis and metabolic tumor volume at pretreatment FDG PET/CT have prognostic implication in SCLC. Patients with SCLC who had high total lesion glycolysis (>443.8) at pretreatment FDG PET/CT showed significantly shorter OS compared with patients with low total lesion glycolysis (median OS: 13.4 vs 25.7 months, respectively; P = .018) (52). Patients with high total metabolic tumor volume (>72.4) at pretreatment FDG PET/CT also showed significantly shorter PFS than those with low total metabolic tumor volume (median PFS of 12.1 and 26.2 months, respectively; P = .005) (52).
Metabolic activity at posttreatment FDG PET/CT can also help predict clinical outcome. In a retrospective study of 29 patients with SCLC (16 with extensive disease and 13 with limited disease) treated with chemotherapy, complete metabolic response at posttreatment FDG PET/CT, defined as visual disappearance of all metabolically active tumor, was associated with longer OS (66). In another study of 59 patients with SCLC (32 with extensive disease and 27 with limited disease) who were treated with chemotherapy (n = 37) or concurrent chemoradiotherapy with or without additional chemotherapy (n = 22), a peak standardized uptake value change of –46.8% or less on posttreatment scans compared with pretreatment scans was an independent prognostic factor for OS (HR, 2.6; P = .002). High metabolic tumor volume (>9.8) on posttreatment scans (HR, 2.8; P = .001), extensive disease stage (HR, 2.7; P = .003), and a lack of RECIST response (HR, 2.0; P = .023) were independent prognostic factors for PFS (67). FDG uptake may also reflect tumor biology in the tumor microenvironment, which is an important predictive factor for ICI response. In a recent study of 98 patients with SCLC, high SUVmax was associated with low CD8+ and CD4+ tumor-infiltrating T lymphocytes in the tumor microenvironment and was an independent predictor of shorter OS in limited stage SCLC (68).
Molecular Imaging of SCLC Using Novel Tracers
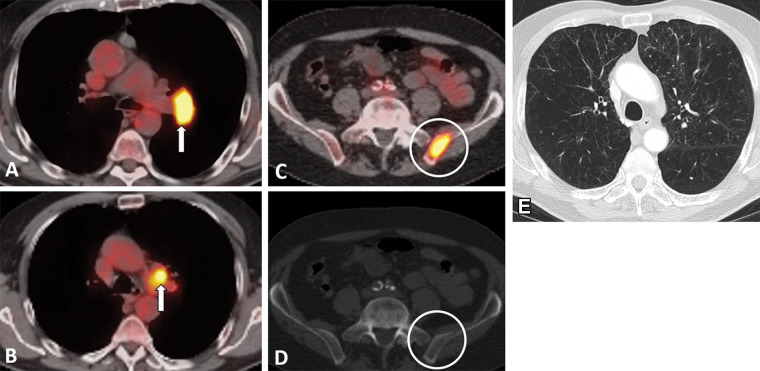
Tetraazacyclododecane tetraacetic acid–octreotate (DOTATATE) selectively binds to somatostatin receptor 2a, which is abundant in tumors with neuroendocrine features (69). Gallium 68–labeled DOTATATE is widely used in imaging of neuroendocrine tumor, and lutetium 177 (177Lu)– or yttrium 90–labeled compound can be used for therapy (69,70). As most SCLCs shows neuroendocrine features, ongoing efforts attempt to evaluate the expression of somatostatin receptors on DOTATATE PET/CT scans for potential use of the receptor as a treatment target (71,72) (Fig 11). In a retrospective study of 21 patients with SCLC, 10 patients demonstrated somatostatin receptor expression on DOTATATE PET/CT scans (four showed high uptake and six showed intermediate uptake). Among four patients treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE and/or DOTA Phe1-Tyr3-octreotide, or DOTATOC, three had clinical benefit (one had partial response, one had stable disease for more than 1 year, and one had improved performance status) (71). Peptide receptor radionuclide therapy might be considered as an alternative treatment option in the subset of patients with SCLC exhibiting sufficient somatostatin receptor expression for therapy (72).
Figure 11:
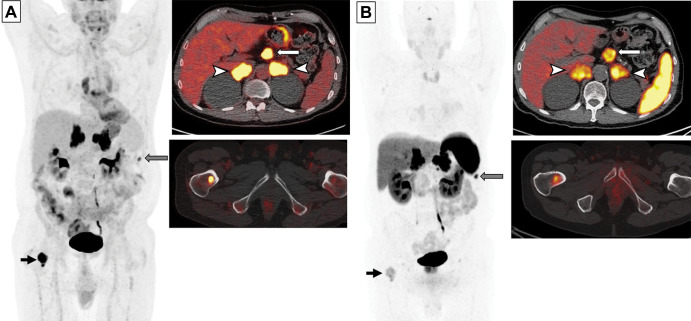
Images in a 65-year-old man with small cell lung cancer who was previously treated with carboplatin and etoposide and radiation therapy to the brain and thoracic lymph nodes. The patient presented with progression of disease. (A) Whole-body fluorodeoxyglucose (FDG) PET image (left) and fused axial FDG PET/CT scans (right) show intense FDG uptake in the bilateral adrenal lesions (arrowheads) and upper abdominal lymphadenopathy (white arrow), a peritoneal nodule (gray arrow), and a right femoral bone lesion (black arrow). FDG uptake in the bilateral paramediastinal region corresponds to the postradiation change. (B) Gallium 68 tetraazacyclododecane tetraacetic acid–octreotate (DOTATATE) whole-body PET scan (left) and fused axial PET/CT scans (right) obtained 17 days after FDG PET/CT show intense radiotracer uptake (ie, greater than the uptake in the liver) in the adrenal lesions (arrowheads) and upper abdominal lymph nodes (white arrow), a peritoneal nodule (gray arrow), and moderate radiotracer uptake (ie, similar to the uptake in the liver) in the bone (black arrow). Note a lack of DOTATATE uptake in the mediastinum in the areas of postradiation inflammatory changes, indicating high specificity of DOTATATE PET/CT for tumors compared with FDG PET/CT.
Novel radiolabeled imaging tracers for therapeutic target molecules of SCLC are also under investigation. For example, a recent preclinical study demonstrated that fluorine 18–labeled PARP inhibitor can quantify target engagement of chemically diverse small molecule inhibitors in mice at PET/CT (73). Further investigations are ongoing to translate these approaches into human imaging for therapeutic guidance and monitoring.
Radiomics and Machine Learning Approaches for SCLC
Radiomics refers to a quantitative feature extraction and analysis from high-throughput image data to support clinical decision making. Radiomics has been applied to diagnosis, prognosis, and assessment of treatment response in various cancers. Radiomics features commonly used in oncology are first-order statistics (ie, mean, median, SD, skewness, and kurtosis of imaging intensity values), heterogeneity and texture features (eg, gray-level co-occurrence matrix, gray-level run-length matrix), shape and volume of tumors, tumor microenvironment, and vascularity radiomics (74). In addition, machine learning is increasingly applied to efficiently process large radiomics data sets in oncologic imaging.
Unlike NSCLC, the knowledge of radiomics in SCLC has been limited and accumulating gradually. Radiomics features were shown to help differentiate SCLC and lung adenocarcinoma (75,76). Chen et al (76) studied CT images of peripheral lung cancer and extracted histogram-based features (max, min mean, range, entropy, variance, skewness and kurtosis) as well as texture features including gray-level co-occurrence matrix, gray-level run-length matrix, gray-level size-zone matrix, and neighborhood gray-tone difference matrix. A CT radiomic model with a neural network classifier was able to differentiate SCLC (n = 35) and NSCLC (n = 34), with an area under the curve of 0.93. In a prior study of CT volumetric features as a predictive marker of survival in 105 patients diagnosed with limited stage SCLC treated with chemoradiotherapy, three-dimensional maximal diameter of the tumor was significantly associated with local-regional recurrence, distant metastasis, and OS (77).
Application of machine learning allows for the building of diagnostic models combining multiple radiomics features and assessment of their diagnostic performance. In a study of 92 patients with SCLC treated with first-line etoposide and cisplatin, a diagnostic model combining texture analysis parameters of gray-level histogram analysis, spatial gray-level dependence matrices, and neighborhood gray-tone difference matrix of the primary tumor with clinicopathologic factors can better predict the response to chemotherapy compared with clinicopathologic factors alone (area under the curve: 0.797 vs 0.670, respectively) (78). The study provided preliminary observations to develop prognostic imaging markers to guide treatment decisions in patients with SCLC.
Conclusion
Emerging knowledge of small cell lung cancer (SCLC) biology, including genomic characterizations and molecular subtyping, and new effective treatment approaches have the potential to lead to advances of patient care for this highly aggressive and lethal disease, toward the goal of improving clinical outcome. Awareness of these new discoveries regarding SCLC is essential for radiologists, to provide accurate image interpretations for diagnosis and monitoring of this malignancy. Ongoing imaging research indicates the promising future of radiologic contributions to the clinical applications of molecular imaging and machine learning techniques for the development of objective imaging markers for SCLC.
Current address: Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital at Linkou and Chang Gung University, Taoyuan, Taiwan.
M.N. and H.H. supported by the National Cancer Institute (grants R01CA203636 and U01CA209414).
Disclosures of conflicts of interest: H.P. No relevant relationships. S.C.T. No relevant relationships. L.M.S. Grants to institution from Genentech and Lilly; consulting fees to institution from Genentech. H.H. Research grants to hospital from Canon Medical Systems and Konica-Minolta; consulting fees from Mitsubishi Chemical and Canon Medical Systems. M.M.A. Grants to institution from Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, and Lilly; consulting fees from Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, Maverick, Blueprint Medicine, Syndax, Ariad/Takeda, Nektar, Gritsone, ArcherDXm Mirati, NextCure, Novartix, EMD Serono, and Panvaxal/NovaRx; data safety monitoring board for Bristol-Myers Squibb. M.N. Grants to institution from Canon Medical Systems, AstraZeneca, and Daiichi Sankyo; consulting fees unrelated to submitted work from AstraZeneca and Daiichi Sankyo; serves on Radiology editorial board as Diagnosis Please editor.
Abbreviations:
- FDA
- Food and Drug Administration
- FDG
- fluorodeoxyglucose
- HR
- hazard ratio
- ICI
- immune checkpoint inhibitor
- ILD
- interstitial lung disease
- NSCLC
- non-SCLC
- OS
- overall survival
- PARP
- poly (adenosine diphosphate-ribose) polymerase
- PD-1
- programmed cell death protein 1
- PD-L1
- programmed death-ligand 1
- PFS
- progression-free survival
- RECIST
- Response Evaluation Criteria in Solid Tumors
- SCLC
- small cell lung cancer
- SUVmax
- maximum standardized uptake value
References
- 1. Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer statistics, 2022 . CA Cancer J Clin 2022. ; 72 ( 1 ): 7 – 33 . [DOI] [PubMed] [Google Scholar]
- 2. Nicholson AG , Tsao MS , Beasley MB , et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015 . J Thorac Oncol 2022. ; 17 ( 3 ): 362 – 387 . [DOI] [PubMed] [Google Scholar]
- 3. Chong S , Lee KS . Spectrum of findings and usefulness of integrated PET/CT in patients with known or suspected neuroendocrine tumors of the lung . Cancer Imaging 2007. ; 7 ( 1 ): 195 – 201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stahel RA , Ginsberg R , Havemann K , et al. Staging and prognostic factors in small cell lung cancer: a consensus report . Lung Cancer 1989. ; 5 ( 4-6 ): 119 – 126 . [Google Scholar]
- 5. Thomas A , Pattanayak P , Szabo E , Pinsky P . Characteristics and Outcomes of Small Cell Lung Cancer Detected by CT Screening . Chest 2018. ; 154 ( 6 ): 1284 – 1290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell JD , Alexandrov A , Kim J , et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas . Nat Genet 2016. ; 48 ( 6 ): 607 – 616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao J , Aksoy BA , Dogrusoz U , et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal . Sci Signal 2013. ; 6 ( 269 ): pl1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cerami E , Gao J , Dogrusoz U , et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data . Cancer Discov 2012. ; 2 ( 5 ): 401 – 404 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. AACR Project GENIE: Data. American Association for Cancer Research . https://www.aacr.org/professionals/research/aacr-project-genie/aacr-project-genie-data/. Updated March 4, 2022. Accessed June 9, 2022 .
- 10. Rudin CM , Brambilla E , Faivre-Finn C , Sage J . Small-cell lung cancer . Nat Rev Dis Primers 2021. ; 7 ( 1 ): 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. George J , Lim JS , Jang SJ , et al. Comprehensive genomic profiles of small cell lung cancer . Nature 2015. ; 524 ( 7563 ): 47 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabari JK , Lok BH , Laird JH , Poirier JT , Rudin CM . Unravelling the biology of SCLC: implications for therapy . Nat Rev Clin Oncol 2017. ; 14 ( 9 ): 549 – 561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wooten DJ , Groves SM , Tyson DR , et al. Systems-level network modeling of Small Cell Lung Cancer subtypes identifies master regulators and destabilizers . PLOS Comput Biol 2019. ; 15 ( 10 ): e1007343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson KL , Stoney R , Frese KK , et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity . Nat Can 2020. ; 1 ( 4 ): 437 – 451 . [DOI] [PubMed] [Google Scholar]
- 15. Brägelmann J , Böhm S , Guthrie MR , Mollaoglu G , Oliver TG , Sos ML . Family matters: How MYC family oncogenes impact small cell lung cancer . Cell Cycle 2017. ; 16 ( 16 ): 1489 – 1498 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mollaoglu G , Guthrie MR , Böhm S , et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition . Cancer Cell 2017. ; 31 ( 2 ): 270 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ireland AS , Micinski AM , Kastner DW , et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate . Cancer Cell 2020. ; 38 ( 1 ): 60 – 78.e12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gay CM , Stewart CA , Park EM , et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities . Cancer Cell 2021. ; 39 ( 3 ): 346 – 360.e7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcoux N , Gettinger SN , O’Kane G , et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes . J Clin Oncol 2019. ; 37 ( 4 ): 278 – 285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JK , Lee J , Kim S , et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas . J Clin Oncol 2017. ; 35 ( 26 ): 3065 – 3074 . [DOI] [PubMed] [Google Scholar]
- 21. Niederst MJ , Sequist LV , Poirier JT , et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer . Nat Commun 2015. ; 6 ( 1 ): 6377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarris EG , Saif MW , Syrigos KN . The Biological Role of PI3K Pathway in Lung Cancer . Pharmaceuticals (Basel) 2012. ; 5 ( 11 ): 1236 – 1264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hofman P . Detecting Resistance to Therapeutic ALK Inhibitors in Tumor Tissue and Liquid Biopsy Markers: An Update to a Clinical Routine Practice . Cells 2021. ; 10 ( 1 ): 168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kauffmann-Guerrero D , Walter J , Kovács J , et al. The Role of Thoracic Surgery in Small Cell Lung Cancer - A Large Longitudinal Analysis (2002-2015) Based on Real-World Data . Clin Lung Cancer 2022. ; 23 ( 3 ): 244 – 252 . [DOI] [PubMed] [Google Scholar]
- 25. Pignon JP , Arriagada R , Ihde DC , et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer . N Engl J Med 1992. ; 327 ( 23 ): 1618 – 1624 . [DOI] [PubMed] [Google Scholar]
- 26. Quan AL , Videtic GM , Suh JH . Brain metastases in small cell lung cancer . Oncology (Williston Park) 2004. ; 18 ( 8 ): 961 – 972 ; discussion 974,979–980, 987 . [PubMed] [Google Scholar]
- 27. Arriagada R , Le Chevalier T , Borie F , et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission . J Natl Cancer Inst 1995. ; 87 ( 3 ): 183 – 190 . [DOI] [PubMed] [Google Scholar]
- 28. Jackman DM , Johnson BE . Small-cell lung cancer . Lancet 2005. ; 366 ( 9494 ): 1385 – 1396 . [DOI] [PubMed] [Google Scholar]
- 29. Singh S , Jaigirdar AA , Mulkey F , et al. FDA Approval Summary: Lurbinectedin for the Treatment of Metastatic Small Cell Lung Cancer . Clin Cancer Res 2021. ; 27 ( 9 ): 2378 – 2382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishino M , Hatabu H , Hodi FS . Imaging of Cancer Immunotherapy: Current Approaches and Future Directions . Radiology 2019. ; 290 ( 1 ): 9 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishino M , Ramaiya NH , Hatabu H , Hodi FS . Monitoring immune-checkpoint blockade: response evaluation and biomarker development . Nat Rev Clin Oncol 2017. ; 14 ( 11 ): 655 – 668 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horn L , Mansfield AS , Szczęsna A , et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer . N Engl J Med 2018. ; 379 ( 23 ): 2220 – 2229 . [DOI] [PubMed] [Google Scholar]
- 33. Goldman JW , Dvorkin M , Chen Y , et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial . Lancet Oncol 2021. ; 22 ( 1 ): 51 – 65 . [DOI] [PubMed] [Google Scholar]
- 34. Paz-Ares L , Dvorkin M , Chen Y , et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial . Lancet 2019. ; 394 ( 10212 ): 1929 – 1939 . [DOI] [PubMed] [Google Scholar]
- 35. Mathieu L , Shah S , Pai-Scherf L , et al. FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer . Oncologist 2021. ; 26 ( 5 ): 433 – 438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung HC , Lopez-Martin JA , Kao SC-H , et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158 . J Clin Oncol 2018. ; 36 ( 15_suppl ): 8506 . [Google Scholar]
- 37. Chung HC , Piha-Paul SA , Lopez-Martin J , et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies . J Thorac Oncol 2020. ; 15 ( 4 ): 618 – 627 . [DOI] [PubMed] [Google Scholar]
- 38. Ready NE , Ott PA , Hellmann MD , et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort . J Thorac Oncol 2020. ; 15 ( 3 ): 426 – 435 . [DOI] [PubMed] [Google Scholar]
- 39. Spigel DR , Vicente D , Ciuleanu TE , et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331} . Ann Oncol 2021. ; 32 ( 5 ): 631 – 641 . [DOI] [PubMed] [Google Scholar]
- 40. Owonikoko TK , Park K , Govindan R , et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451 . J Clin Oncol 2021. ; 39 ( 12 ): 1349 – 1359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hellmann MD , Callahan MK , Awad MM , et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer . Cancer Cell 2018. ; 33 ( 5 ): 853 – 861.e4 [Published correction appears in Cancer Cell 2019;35(2):329.] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ricciuti B , Kravets S , Dahlberg SE , et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer . J Immunother Cancer 2019. ; 7 ( 1 ): 87 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iams WT , Porter J , Horn L . Immunotherapeutic approaches for small-cell lung cancer . Nat Rev Clin Oncol 2020. ; 17 ( 5 ): 300 – 312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trigo J , Subbiah V , Besse B , et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial . Lancet Oncol 2020. ; 21 ( 5 ): 645 – 654 . [DOI] [PubMed] [Google Scholar]
- 45. de Bono J , Ramanathan RK , Mina L , et al. Phase I, Dose-Escalation, Two-Part Trial of the PARP Inhibitor Talazoparib in Patients with Advanced Germline BRCA1/2 Mutations and Selected Sporadic Cancers . Cancer Discov 2017. ; 7 ( 6 ): 620 – 629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pietanza MC , Waqar SN , Krug LM , et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer . J Clin Oncol 2018. ; 36 ( 23 ): 2386 – 2394 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. National Lung Screening Trial Research Team ; Aberle DR , Adams AM , et al. Reduced lung-cancer mortality with low-dose computed tomographic screening . N Engl J Med 2011. ; 365 ( 5 ): 395 – 409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silva M , Galeone C , Sverzellati N , et al. Screening with Low-Dose Computed Tomography Does Not Improve Survival of Small Cell Lung Cancer . J Thorac Oncol 2016. ; 11 ( 2 ): 187 – 193 . [DOI] [PubMed] [Google Scholar]
- 49. Vallières E , Shepherd FA , Crowley J , et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer . J Thorac Oncol 2009. ; 4 ( 9 ): 1049 – 1059 . [DOI] [PubMed] [Google Scholar]
- 50. Salem A , Mistry H , Hatton M , et al. Association of Chemoradiotherapy With Outcomes Among Patients With Stage I to II vs Stage III Small Cell Lung Cancer: Secondary Analysis of a Randomized Clinical Trial . JAMA Oncol 2019. ; 5 ( 3 ): e185335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Treadwell JR , Mitchell MD , Tsou A , Torigian D , Aggarwal C , Schoelles KM . AHRQ Comparative Effectiveness Reviews . Imaging for the Pretreatment Staging of Small Cell Lung Cancer . Rockville, Md: : Agency for Healthcare Research and Quality; , 2016. . [PubMed] [Google Scholar]
- 52. Zer A , Domachevsky L , Rapson Y , et al. The Role of 18F-FDG PET/CT on Staging and Prognosis in Patients with Small Cell Lung Cancer . Eur Radiol 2016. ; 26 ( 9 ): 3155 – 3161 . [DOI] [PubMed] [Google Scholar]
- 53. Martucci F , Pascale M , Valli MC , et al. Impact of 18F-FDG PET/CT in Staging Patients With Small Cell Lung Cancer: A Systematic Review and Meta-Analysis . Front Med (Lausanne) 2020. ; 6 : 336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eisenhauer EA , Therasse P , Bogaerts J , et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) . Eur J Cancer 2009. ; 45 ( 2 ): 228 – 247 . [DOI] [PubMed] [Google Scholar]
- 55. Nishino M , Jagannathan JP , Ramaiya NH , Van den Abbeele AD . Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know . AJR Am J Roentgenol 2010. ; 195 ( 2 ): 281 – 289 . [DOI] [PubMed] [Google Scholar]
- 56. Halvorsen TO , Herje M , Levin N , et al. Tumour size reduction after the first chemotherapy-course and outcomes of chemoradiotherapy in limited disease small-cell lung cancer . Lung Cancer 2016. ; 102 : 9 – 14 . [DOI] [PubMed] [Google Scholar]
- 57. Park HJ , Kim KW , Pyo J , et al. Incidence of Pseudoprogression during Immune Checkpoint Inhibitor Therapy for Solid Tumors: A Systematic Review and Meta-Analysis . Radiology 2020. ; 297 ( 1 ): 87 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nishino M , Dahlberg SE , Adeni AE , et al. Tumor Response Dynamics of Advanced Non-small Cell Lung Cancer Patients Treated with PD-1 Inhibitors: Imaging Markers for Treatment Outcome . Clin Cancer Res 2017. ; 23 ( 19 ): 5737 – 5744 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nishino M , Hong F , Ricciuti B , Hatabu H , Awad MM . Tumor Response Dynamics During First-Line Pembrolizumab Therapy in Patients With Advanced Non-Small-Cell Lung Cancer . JCO Precis Oncol 2021. ; 5 ( 5 ): 501 – 509 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee HY , Kim EY , Kim YS , Ahn HK , Kim YK . Prognostic significance of CT-determined emphysema in patients with small cell lung cancer . J Thorac Dis 2018. ; 10 ( 2 ): 874 – 881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi YW , Munden RF , Erasmus JJ , et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis . RadioGraphics 2004. ; 24 ( 4 ): 985 – 997 ; discussion 998 . [DOI] [PubMed] [Google Scholar]
- 62. Park H , Hatabu H , Ricciuti B , Aijazi SJ , Awad MM , Nishino M . Immune-related adverse events on body CT in patients with small-cell lung cancer treated with immune-checkpoint inhibitors . Eur J Radiol 2020. ; 132 : 109275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ulger S , Demirci NY , Aydinkarahaliloglu E , et al. PET-CT guided curative conformal radiation therapy in limited stage small cell lung cancer . J Thorac Dis 2015. ; 7 ( 3 ): 295 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bradley J , Thorstad WL , Mutic S , et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer . Int J Radiat Oncol Biol Phys 2004. ; 59 ( 1 ): 78 – 86 . [DOI] [PubMed] [Google Scholar]
- 65. van der Leest C , Smit EF , Baas J , et al. SUVmax during 18FDG-PET scanning in small cell lung cancer: similar information as in non-small cell lung cancer? Lung Cancer 2012. ; 76 ( 1 ): 67 – 71 . [DOI] [PubMed] [Google Scholar]
- 66. Ziai D , Wagner T , El Badaoui A , et al. Therapy response evaluation with FDG-PET/CT in small cell lung cancer: a prognostic and comparison study of the PERCIST and EORTC criteria . Cancer Imaging 2013. ; 13 ( 1 ): 73 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim H , Yoo IR , Boo SH , Park HL , O JH , Kim SH . Prognostic Value of Pre- and Post-Treatment FDG PET/CT Parameters in Small Cell Lung Cancer Patients . Nucl Med Mol Imaging 2018. ; 52 ( 1 ): 31 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kasahara N , Kaira K , Yamaguchi K , et al. Fluorodeoxyglucose uptake is associated with low tumor-infiltrating lymphocyte levels in patients with small cell lung cancer . Lung Cancer 2019. ; 134 : 180 – 186 . [DOI] [PubMed] [Google Scholar]
- 69. Haug AR , Cindea-Drimus R , Auernhammer CJ , et al. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors . J Nucl Med 2012. ; 53 ( 11 ): 1686 – 1692 . [DOI] [PubMed] [Google Scholar]
- 70. Strosberg J , El-Haddad G , Wolin E , et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors . N Engl J Med 2017. ; 376 ( 2 ): 125 – 135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lapa C , Hänscheid H , Wild V , et al. Somatostatin receptor expression in small cell lung cancer as a prognostic marker and a target for peptide receptor radionuclide therapy . Oncotarget 2016. ; 7 ( 15 ): 20033 – 20040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lehman JM , aMassion PP . Somatostatin receptor 2 targeting in small cell lung carcinoma: perspectives . Oncotarget 2019. ; 10 ( 46 ): 4727 – 4730 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carney B , Kossatz S , Lok BH , et al. Target engagement imaging of PARP inhibitors in small-cell lung cancer . Nat Commun 2018. ; 9 ( 1 ): 176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bera K , Braman N , Gupta A , Velcheti V , Madabhushi A . Predicting cancer outcomes with radiomics and artificial intelligence in radiology . Nat Rev Clin Oncol 2022. ; 19 ( 2 ): 132 – 146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. E L , Lu L , Li L , Yang H , Schwartz LH , Zhao B . Radiomics for Classification of Lung Cancer Histological Subtypes Based on Nonenhanced Computed Tomography . Acad Radiol 2019. ; 26 ( 9 ): 1245 – 1252 . [DOI] [PubMed] [Google Scholar]
- 76. Chen BT , Chen Z , Ye N , et al. Differentiating Peripherally-Located Small Cell Lung Cancer From Non-small Cell Lung Cancer Using a CT Radiomic Approach . Front Oncol 2020. ; 10 : 593 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kamran SC , Coroller T , Milani N , et al. The impact of quantitative CT-based tumor volumetric features on the outcomes of patients with limited stage small cell lung cancer . Radiat Oncol 2020. ; 15 ( 1 ): 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wei H , Yang F , Liu Z , et al. Application of computed tomography-based radiomics signature analysis in the prediction of the response of small cell lung cancer patients to first-line chemotherapy . Exp Ther Med 2019. ; 17 ( 5 ): 3621 – 3629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Glisson B , Besse B , Dols MC , et al. A Randomized, Placebo-Controlled, Phase 1b/2 Study of Rilotumumab or Ganitumab in Combination With Platinum-Based Chemotherapy as First-Line Treatment for Extensive-Stage Small-Cell Lung Cancer . Clin Lung Cancer 2017. ; 18 ( 6 ): 615 – 625.e8 . [DOI] [PubMed] [Google Scholar]
- 80. Owonikoko TK , Niu H , Nackaerts K , et al. Randomized Phase II Study of Paclitaxel plus Alisertib versus Paclitaxel plus Placebo as Second-Line Therapy for SCLC: Primary and Correlative Biomarker Analyses . J Thorac Oncol 2020. ; 15 ( 2 ): 274 – 287 . [DOI] [PubMed] [Google Scholar]



![Emerging molecular subtypes of small cell lung cancer (SCLC) (10). Molecular subtypes SCLC-A, SCLC-N, and SCLC-P have been described based on high expression of the transcription factors (achaete-scute homologue 1 [ASCL1], neurogenic differentiation factor 1 [NEUROD1], and POU class 2 homeobox [POU2F3], respectively). For SCLC-A, which is the most common molecular subtype consisting of approximately 70% of SCLCs, further subgroups of SCLC-A and SCLC-A2 are suggested based on difference in expression of other factors. For the remaining SCLCs with low expression of the three transcription factors (indicated in gray in the figure), several subgroups are proposed, including SCLC-Y with high expression of YAP1, a rare subtype with elevated expression of ATOH1, and SCLC-I with “inflamed” gene signatures. Inactivation of the tumor-suppressor genes TP53 and RB1 is noted as a near ubiquitous event in SCLC. Different degree of neuroendocrine differentiation and the differential expression levels of MYC oncogene family members are noted among the subgroups.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/996c/9713457/7b1b892ce754/radiol.220585.fig1.jpg)