Highlights
-
•
Body condition score (BCS) in three German breeds was studied over lifetime.
-
•
BCS differs among breeds, changes non-linearly and breed-specific over lifetime.
-
•
The results suggest an opportunity for improving performance and welfare of dairy cows by adjusting their nutrition specific to breed and age.
Keywords: Body condition, Overconditioning, Underconditioning, Simmental cattle, Brown Swiss cattle
Abstract
Optimal body condition is crucial for the well-being and optimal productivity of dairy cows. However, body condition depends on numerous, often interacting factors, with complex relationships between them. Moreover, most of the studies describe the body condition in Holstein cattle, while condition of some breeds, e.g. Simmental (SIM) and Brown Swiss (BS) cattle, have not been intensively studied yet. Body condition score (BCS) proved to be one of the most effective measures for monitoring body condition in dairy cows. Alterations in BCS were previously mainly studied over a single lactation period, while changes over the lifetime were largely ignored. This study was designed to report BCS of German SIM and BS cows in the light of the broadly accepted BCS in German Holstein (GH) cows and to explore patterns of change in BCS over the productive lifetime of animals. BCS was modeled via linear mixed effects regression, over- and undercondition of animals were studied using mixed effects logistic regressions and condition of animals was explored with the multinomial log-linear model via neural networks. All models included an interaction between breed and age. We found BCS of SIM and BS to be higher than BCS of GH. Our results show that BCS of BS cows did not change over the lifetime. In contrast, the BCS of GH and SIM was found to have a non-linear (quadratic) shape, where BCS increased up to the years of highest productivity and then decreased in aging cows. Patterns of change between SIM and GH, however, differed. GH do not only reach their highest BCS earlier in life compared to SIM, but also start to lose their body condition earlier. Our dataset revealed that 23% of the animals scored were over- and 14% underconditioned. The proportion of cows that were overconditioned was high (>10% of cows) for every breed and every age, while severe underconditioning (>10% of cows) occurred only in middle aged and old GH. Moreover, we found that the probability of underconditioning of animals over lifetime increases, while the overconditioning decreases from the middle to older ages. Our findings highlight the importance of understanding the non-linear nature of BCS, and uncover the potential opportunity for improving the performance and welfare of dairy cows by adjusting their nutrition, not only during lactation, but also highly specific to breed and age.
1. Introduction
Body condition in dairy cows is one of the most important indicators reflecting animal health, energy status, production level, reproductive success and longevity of dairy cows (Metzner et al., 1993; Jones et al., 2016; Roche et al., 2007). To this end, body condition score (BCS) is an important tool in dairy herd management (Metzner et al., 1993; J. R. Roche et al., 2009; John R. Roche et al., 2013). For instance, optimal BCS facilitates the mobilization of body reserves to support milk production (Butler, 2014) and may greatly influence the reproductive success, e.g. increase the success rate for artificial insemination (AI) and conception (Bates & Saldias, 2019). Cows with insufficient BCS and cows with a nadir of BCS in the post partum period below optimum are at risk to have lower conception rates (Domecq et al., 1997; Pryce et al., 2001), to be more prone to metabolic (John R. Roche et al., 2013; Schuster et al., 2020) and infectious diseases (Abunna et al., 2010; Jaja et al., 2017), e.g. endometritis (Heuer et al., 1999; Hoedemaker et al., 2009), to have decreased milk yield (Domecq et al., 1997; Souissi & Bouraoui, 2019) and higher culling rates (Hoedemaker et al., 2009). A steep decrease in BCS was found to increase the odds of lameness in a number of studies (Hoedemaker et al., 2009; Oehm et al., 2020; Randall et al., 2018). Shrinkage of the fat cushion within the horn shoe and subsequent horn disruption lesions were shown to form an important risk factor for claw disorders (Newsome et al., 2017). Some studies, however, reported either no association between BCS and lameness (Daros et al., 2020) or increased lameness prevalence in obese cows (Kellogg, 2010). Moreover, obese cows are also prone to develop health disorders (Locher et al., 2015; Sundrum, 2015) such as dystocia, retained placenta, milk fever, ketosis, downer cow syndrome (Heuer et al., 1999; Kellogg, 2010) and were shown to result in reduced reproductive performance (Correa et al., 1990; Jorritsma et al., 2001). Thus, understanding BCS, as valuable management tool, is crucial for a well-being and optimal productivity of dairy cows (J. R. Roche et al., 2009).
The majority of studies on BCS, however, explored only a small number of cows (Median = 750, IQR [409; 1257], J. R. Roche et al., 2009) in a few, often well-managed commercial farms or herds (e.g. 76 farms, Berry et al., 2007) with animals in a good condition (Waltner et al., 1993) and thus with a narrow BCS range (J. R. Roche et al., 2007) predominantly of the Holstein breed (Waltner et al., 1993; J. R. Roche et al., 2009). Therefore, the knowledge related to body condition of cattle is limited and potentially biased towards healthy Holstein animals. For instance, Hoedemaker et al. (2009) only found a few under- or overconditioned animals among 234 German Holstein cows. In the current study, we try to close this gap in the variability by investigating the BCS of a large sample of 75,641 dairy cows of three main breeds in dairy production in Germany: German Holstein (GH), Brown Swiss (BS) and Simmental cattle (SIM) on 754 farms. The special focus of this study is on the BCS of SIM and BS as these breeds have not been intensively explored yet. Moreover, we explicitly model over- and underconditioning (as separate phenomena) in dairy cows of all three breeds in order to compare them, which, to our knowledge, also has not been done before.
Such large amount of data provides an analytic challenge. Statistical models proved to be one of the best tools for gaining insights from large data sets. In a comprehensive review about BCS, J. R. Roche et al. (2009) described numerous modeling approaches applied to study the body condition of cows. Many of these uncovered non-linear associations between BCS and important variables, such as reproductive success or occurrence of diseases (i.e. incidence of milk fever, Gallo et al., 1996; Koenen et al., 2001; J. R. Roche et al., 2007). Interestingly, most of these non-linear relationships were described for and therefore restricted to the lactation period (Hoedemaker et al., 2009; Rinell & Heringstad, 2018; Wildman et al., 1982). Thus, if non-linearity is yet strongly coupled with BCS during lactation, it is plausible to assume that BCS may non-linearly change over productive lifetime, which is defined as the time from first calving until culling (Compton et al., 2017; Ducrocq et al., 1988; Schuster et al., 2020).
The general aim of this study was to report BCS of Simmental (9140 cows) and Brown Swiss (1184 cows) cattle in comparison to more commonly used and therefore more extensively studied milk-orientated German Holstein breed (65,317 cows). Since SIM and also BS cows to some extent are dual-purpose breeds (milk and meat), predominantly held in mountainous regions of the world, we hypothesize that a difference might exist in BCS across breeds. Specific objectives of our study are, first, to explore changes in BCS over the whole productive life of cows, from an age of 575 up to 6695 days (1.6 – 18.3 years), second, to compare those changes among GH, BS and SIM cattle, and finally, we aim to understand temporal patterns of change in over- and underconditioning and to quantify the associations between body condition score of different breeds with their age.
We hope that our findings can contribute to a better animal welfare of dairy cows and uncover the potential opportunities for improving the performance of dairy cows without compromising health and welfare by adjusting their nutrition, not only during lactation, but also highly specific to breed and age.
2. Materials and methods
2.1. Farm recruitment and data collection
Data were collected in an extensive cross-sectional study across Germany, which had been initiated and funded by the German Federal Ministry of Food and Agriculture through the Federal Office for Agriculture and Food, grant number 2814HS006–8. Dairy farms were located in three geographically different dairying regions in Germany. Particularly, federal states of Lower Saxony and Schleswig-Holstein in the region North, federal states of Thuringia, Saxony-Anhalt, Brandenburg, and Mecklenburg-Western Pomerania in the region East and federal state of Bavaria in the region South were studied. Within the three study regions 754 farms (North: 249; East: 245; South: 260) with a total number of 75,641 dairy cows (North: 22,105 cows (56.6% of observations); East: 42,810 cows (29.2%) South: 10,726 cows (14.2%)) were visited by researchers on a single occasion between December 2016 and August 2019. A total of 250 farms per study region were determined by the power analysis with a power of 80% and a level of significance of 5%. The selection of farms was assigned randomly and based on their administrative district within the federal state and study region. A response rate of 30 – 40% was expected. Within each study region, a total amount of 1250 farms, i.e. 5 times more farms than required for the study, were drawn from the underlying population in order to cover a response rate of at least 20%. Region-specific farm size cut-off values were determined in order to obtain a realistic distribution of farm sizes within the study population and due to structural differences in dairy farming in Germany (Merle et al., 2012). The participation in the study was voluntary with a written consent of interested farm managers for participation and data inspection. All farm-specific information was handled according to the principles of the German and European data protection legislation.
Data on breed, days post partum and age collected during the farm visit were recorded via data entry forms and later manually transferred to a central SQL-data base. The individual ear tag number was recorded for each cow. All cows were subjected to scoring for body condition. Body condition score was assessed following the 5-point scale with 0.25-unit increments presented by Edmonson et al. (1989), later modified by Metzner et al. (1993), by 15 trained veterinarians across the three study regions. Inter-observer agreement between all of the involved researches was evaluated three times during the study period. Each of these evaluations was conducted during a two-day workshop which included scoring of cows, general training and discussions. During the first session, 43 dairy cows were scored for BCS, resulting in the Intraclass Correlation Coefficient (ICC) of 0.59. During the second assessment, 59 cows were scored, resulting in the ICC of 0.79. The third assessment date included 60 cows for scoring and yielded an ICC of 0.76. According to guidelines provided by Cicchetti (1994), the agreement in the last two sessions is considered to be excellent (ICC between 0.75 and 1) and suggests successful training. Therefore, we assumed no differences in scoring among professionals and thus neither explicitly explored it here as a fixed effect, nor included individual veterinarians into the model as a random effect.
2.2. Data editing
Since it would hardly be possible to collect BCS data from 75,641 animals over the course of their entire life (which could take up to 20 years), the distribution of BCS collected from differently aged animals in the time of the study (December 2016 – August 2019) were used to define the lifetime. Cows with an age from 575 days (1.6 years) to 6695 days (18.3 years) were considered, yet, the number of animals over nine years old was fairly limited (the histogram for age is provided in the supplementary material, Fig. 6). Due to the violation of the linearity assumption uncovered during the first round of analysis (Fig. 3B & D), the initially numeric variable age, which showed a quadratic (parabolic) non-linear and therefore difficult to interpret trend, was categorized into young (< three years), old (> nine years) and mid-age. The mid-age differed among breeds and was determined by the top of the age-curve (Fig. 3D). Particularly, days 1800 – 2100 showed the highest BCS in GH while days 1900 – 2700 in SIM and BS. This categorization of age naturally resulted into 46,693 Unknown observations of age [category] (Table 2), but did not change the inference of the final model as compared with the final model containing all age values.
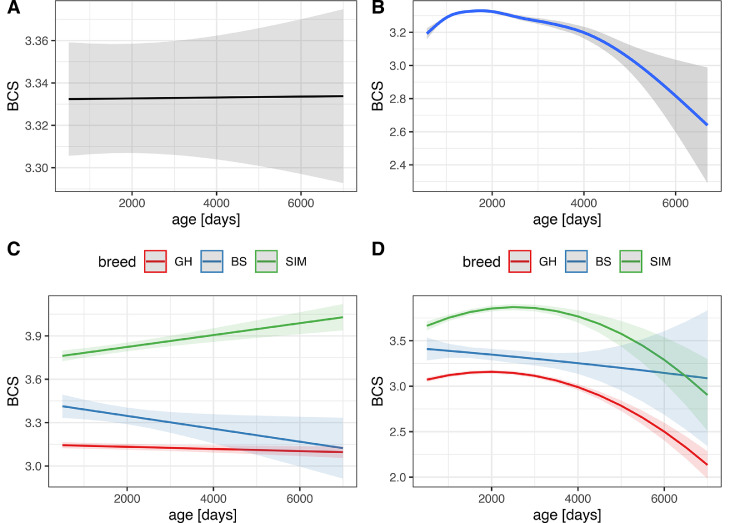
Fig. 3.
Breed specific evolution of variable age in our dataset in regard to BCS (Body condition score), where breeds are: GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle. All subplots show predicted values of BCS. Subplot B shows the results of a non-linear additive model, while subplots A, C and D display results of linear mixed-effects models. A model in subplot D, having a non-linear component in the form of the second polynomial degree for age, is technically still linear, because it allows for a linear combination of predictors.
Table 2.
Basic breed specific descriptive statistics of a dataset, where breeds are: GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle. The lactation stages are determined by the days post-partum in Table 1. Condition categories are based on the optimal BCS range per breed per lactation stage as described in Table 1. Breed specific categorization of age (described in material and methods) into three specific categories resulted in a high amount of missing values (unknown).
| Variable | GH, N = 65,3171 | BS, N = 1,1841 | SIM, N = 9,1401 |
|---|---|---|---|
| region | |||
| East | 42,578 (65.2%) | 63 (5.3%) | 169 (1.8%) |
| North | 21,990 (33.7%) | 27 (2.3%) | 88 (1.0%) |
| South | 749 (1.1%) | 1094 (92.4%) | 8883 (97.2%) |
| age [days] | 1494 (1128, 1997) | 1730 (1322, 2365) | 1603 (1216, 2165) |
| age [category] | |||
| 1. young | 14,767 (60.7%) | 144 (26.6%) | 1504 (36.8%) |
| 2. mid-age | 7904 (32.5%) | 308 (56.8%) | 2229 (54.6%) |
| 3. old | 1652 (6.8%) | 90 (16.6%) | 350 (8.6%) |
| Unknown | 40,994 | 642 | 5057 |
| BCS | 3.25 (2.75, 3.75) | 3.50 (3.00, 3.75) | 3.75 (3.50, 4.00) |
| condition | |||
| normal | 39,201 (61.4%) | 731 (63.4%) | 6366 (71.0%) |
| overconditioned | 15,043 (23.6%) | 329 (28.5%) | 1742 (19.4%) |
| underconditioned | 9565 (15.0%) | 93 (8.1%) | 862 (9.6%) |
| Unknown | 1508 | 31 | 170 |
| lactation stage | |||
| 1.1. third | 4934 (7.7%) | 65 (5.6%) | 803 (9.0%) |
| 1.2. third | 11,993 (18.8%) | 203 (17.6%) | 1719 (19.2%) |
| 2. third | 16,798 (26.3%) | 340 (29.5%) | 2432 (27.1%) |
| 3. third | 15,367 (24.1%) | 284 (24.6%) | 2169 (24.2%) |
| dry | 14,717 (23.1%) | 261 (22.6%) | 1847 (20.6%) |
| Unknown | 1508 | 31 | 170 |
| 1n (%); Median (IQR) | |||
The numeric BCS variable was used to determine the overall condition of individual breeds adjusted for every particular lactation period into three categories: normal condition, overconditioned and underconditioned animals. The limits for this categorization are provided in Table 1 (Kritzinger and Schoder, 2009a, 2009b; Kritzinger et al., 2009), whereas the lowest number was always chosen if a span of a lower limit was provided (e.g. 2.75 for the span from 3.25 to 2.75) and the highest number was always chosen if a span of an upper limit was provided (e.g. 3.75 for the span from 3.75 to 3.40). The lactation stage was determined by the days post partum as displayed in Table 1. A total of 1709 (2.26%) missing days post partum values naturally resulted into 1709 Unknown observations of body condition (Table 2).
Table 1.
Optimal lower (LL) and upper (UL) limits of body conditin score (columns 3–6) for German Holstein (GH), Simmental (SIM) and Brown Swiss (BS) cows adjusted for a lactation stage (Kritzinger et al., 2009; Kritzinger and Schoder 2009a, 2009b). Days post partum (days p.p.) were used to separate lactation stages.
| Lactation stage | Days p.p. | GH & BS LL | GH & BS UL | SIM LL | SIM UL |
|---|---|---|---|---|---|
| 1.1. third | 0 – 29 | 3.25↘2.75 | 3.75↘3.40 | 3.75↘3.30 | 4.25↘4.00 |
| 1.2. third | 30 – 99 | 2.75↘2.50 | 3.40↘3.00 | 3.30↘3.25 | 4.00↘3.75 |
| 2. third | 100 – 199 | 2.50↗2.75 | 3.00↗3.25 | 3.25 | 3.75 |
| 3. third | 200 – 299 | 2.75↗3.25 | 3.25↗3.75 | 3.25↗3.75 | 3.75↗4.25 |
| dry | <0 & >299 | 3.25 | 3.75 | 3.75 | 4.25 |
2.3. Statistical analysis
All analyses were conducted using R Statistical software (R version 4.0.3, 2020; RStudio desktop version 1.4.1103, 2021). All packages used in the current study are listed in the supplementary material (Tab. 5).
First, as recommended by Dohoo et al. (1997), the univariate analyses of predictors age, region and breed (as fixed effects) on BCS (response variable) were performed with linear mixed effects models. Each model included farm as random effect. Any variable having a p-value < 0.2 during the univariate test was selected as a candidate for the multivariate model without interactions. The assumption of multicollinearity among predictors was then checked via the variance-inflation factor (VIF). The importance of variables in the multivariate model was determined via Random Forest algorithm, which allowed to compare variables using the Increased Mean Square-Error (%IncMSE), or mean decrease accuracy. The variables with the highest %IncMSE give the best prediction and thus contribute the most to the model (Breiman, 2001). The numeric variable age was tested for the linearity of association with the outcome BCS using a non-linear generalized additive mixed effects model with different degrees of smoothness (from two to nine) in order to explore potential changes in the shape of the BCS curve throughout the life of animals. Breusch-Pagan test (Breusch & Pagan, 1979) was used to test the assumption of heteroscedasticity of residuals, while the normality of the residuals distribution was assessed visually.
Models (e.g. with and without interaction) were manually compared using four main performance quality indicators: Akaike's Information Criterion (AIC), Bayesian Information Criterion (BIC), Conditional coefficient of determination and Marginal coefficient of determination . The final model was supposed to keep only non-multicollinear and significant (p < 0.01) variables and interactions and to have the best combination of predictive (AIC and BIC) and fitting () power.
A final multiple linear mixed effects model (estimated using REML and nloptwrap optimizer) was defined to study the association between the outcome variable BCS with predictors categorical age and breed, with the interaction between them. Variable region was removed due to the multicollinearity with breed and due to the lower importance measured by random forest's %IncMSE as compared to breed. The final model included farm as random effect. The 95% Confidence Intervals (CIs) and p-values were computed using Wald approximation.
| (1) |
where:
-
•
BCS is body condition score from one to five.
-
•
Age is categorized into “1. young,” “2. mid-age” and “3. old” animals.
-
•
Breed represents German Holstein (GH), Simmental (SIM) and Brown Swiss (BS) cows.
-
•
With a random farm effect fitted on the intercept.
Contrasts (differences) between particular categories of breed and age were assessed after the model fit by the estimated least-squares marginal means (emmeans), with the Benjamini & Hochberg p-value correction for multiple comparisons (Benjamini et al., 1995). An error level of 0.01 was used to declare statistical significance, while < 0.05 was used for associations trending for statistical significance. A stricter level of significance was used in order to reduce the probability of a Type I error due to the high amount of data.
Percentages of animals with different body conditions (normal, over- and underconditioned) were first determined separately for breed and age categories and the association between them was assessed by a Pearson's Chi-Square test. Secondly, both over- and underconditioning were separately modeled with a logistic mixed effects regression (estimated using Maximum Likelihood and Nelder-Mead optimizer) in order to predict the over- or undercondition with an interaction between categorical age and breed. Categorical age instead of the numeric age was chosen due to the non-linear effect of age on animal's condition. Each of these models included farm as random effect. Finally, in order to double-check the results of two logistic regressions, we modeled the probabilities of all three conditions (normal, over- and underconditioned) together in a single non-parametric multinomial log-linear classification model via (single-hidden-layer) neural network.
Since cows have several lactations during their life, BCS changes during lactation (days −60 – 500) would create a “time-conflict” as compared to BCS changes over the whole life of animals (days 575 – 6695, being a focus in this study). Therefore, days post partum was not considered as a potential predictor.
3. Results
Some descriptive statistics of the whole dataset are displayed in Table 2. BCS scores of a total of 75,641 cows were included in the present study.
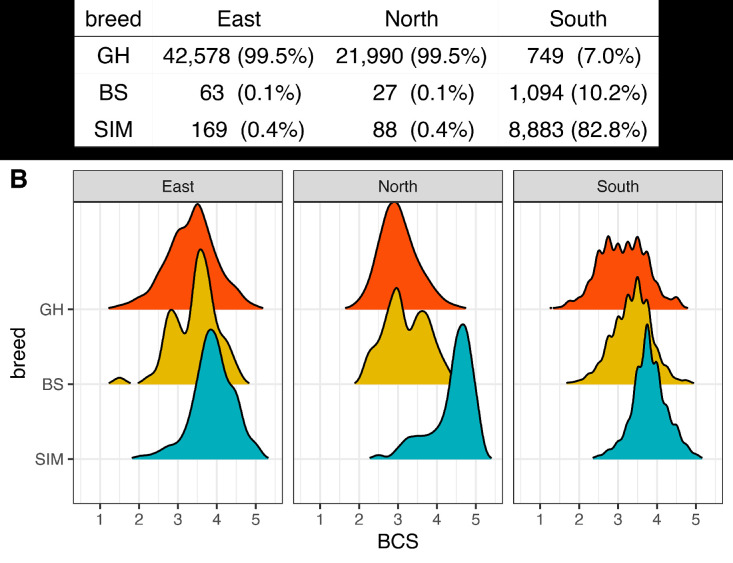
Most of the animals, 99.5% in regions East and North were GH, whereas region South had 82.8% of SIM, 10.2% of BS and only 7.0% of GH (Fig. 1A). The frequency distribution of the BCS in each region indicated SIM to have the highest BCS among all three breeds, while BS seemed to have higher BCS than GH in regions North and South (Fig. 1B). The univariate analyses showed that both breed and region strongly affected BCS (both with p < 0.001), while age did not (p = 0.95).
Fig. 1.
A - Distribution of animals (% (n)) over three breeds and three regions (East, North and South). B - Density of BCS (Body condition score) per breed per region. Breeds are: GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle.
3.1. Breed
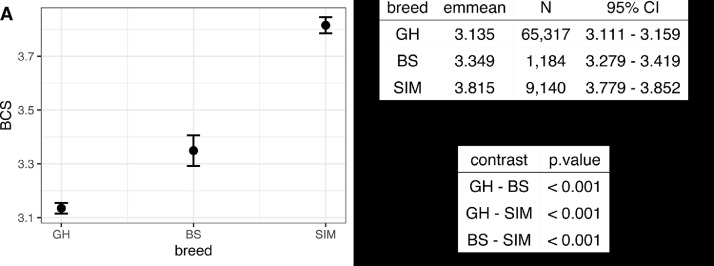
Brown Swiss proved to have higher ( = 0.21, 95% CI [0.16, 0.27], p < 0.001) BCS as compared with GH, while Simmental cattle showed the highest BCS which was also higher than BCS of GH ( = 0.68, 95% CI [0.65, 0.71], p < 0.001). The differences between breeds are significant and are displayed in the form of predicted BCS-values in Fig. 2A and as estimated contrasts in Fig. 2C. The non-overlapping confidence intervals on Fig. 2A corroborate the contrasts shown in Fig. 2C, which suggests the SIM breed to have the highest BCS among all studied breeds, while GH cows have the lowest. BCS of BS cows ranged between both other breeds being higher than BCS of GH while lower than BCS of SIM.
Fig. 2.
Results of the univariate model of the association between breed (GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle) and BCS (Body condition score). A - predicted values of BCS. B - estimated marginal means (least-squares means) of BCS per breed with 95% confidence intervals. C - pairwise comparisons of breeds among each other.
3.2. Age
Age did not appear to have any effect on BCS (p = 0.95) in the univariate analyses. The visualization of a linear model for the association between BCS and age (Fig. 3A) showed a linear trend. However, after checking the linearity assumption between the numeric outcome (BCS) and a numeric predictor (age), we discovered a non-linear pattern (Fig. 3B).
The results of the non-linear generalized additive mixed-effects model showed a non-linear development of BCS throughout the life of SIM and HF and a slight linear decrease in BCS from young to mid-aged to old BS animals (Fig. 3D). Different degrees of smoothness (from two to nine) in additive models were applied to study the change in shape of the curves. The models for both SIM and GH cattle remained quadratic (shaped as a parabola) for every degree of smoothness tested, while for BS the trend also remained linear. Generally, the curves showed lower BCS of animals in their early lactations (< 1000 days or ca. three years) and towards the end of their productive life (> 4000 days or ca. 11 years) of SIM and GH cattle, while higher BCS in the middle (ca. 2000 – 3000 days, or simply the top of the curve) stage of life. Interestingly, the “mid-age” period between SIM and GH differed. Namely, for SIM cows the BCS peak (vertex) of the curve was around 1900 – 2700 days of age (ca. 5.2 – 7.4 years), while for the GH animals around 1800 – 2100 days (ca. 4.9 – 5.8 years). These findings prompted us to transform age to the second polynomial degree in order to mimic and display the quadratic (parabolic) curve progression (Fig. 3D).
However, the coefficients of polynomial models in veterinary science are difficult to interpret (J. R. Roche et al., 2009). Thus, in order to be sure about our findings and to allow for a better interpretability of the results, we have split the continuous variable age into three categories, young animals (< three years), mid-age (5.2 – 7.4 years for SIM and BS and 4.9 - 5.8 years for GH) and old animals (> nine years) for a final model. The relationship between BCS and both quadratic age and categorized age proved to be highly relevant (p < 0.001, exploratory modeling, therefore not shown). Thus, age which did not have any effect on BCS in the linear model, showed a strong effect in the non-linear model.
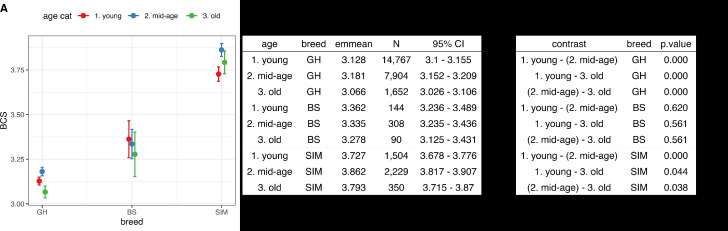
3.3. The final model for BCS
The final multiple linear mixed effects model showed the BCS of SIM to be higher than BCS of BS and GH independent of age (see non-overlapping confidence intervals between breeds in Fig. 4A). The BCS of young GH was lower than BCS of GH cows in their mid-age, while higher than in older animals (Fig. 4B-C). The BCS of GH in their mid-age was higher than the BCS of both young and old GH cattle (both p < 0.01). SIM showed similar results, however, the difference between mid-aged and old and the difference between young and old animals was only suggestive (p = 0.038 and p = 0.044 accordingly, Fig. 4B-C). Despite the steady, though not significant decline in BCS of BS cattle, there was not enough evidence (all p-values > 0.05) for BCS change over the course of their lives.
Fig. 4.
A - Predicted values of BCS (Body condition score) per breed (GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle) per age category (described in material and methods). B - estimated marginal means of BCS. C - post-hoc tests of the final model representing contrasts (differences) in BCS between age categories for a particular breed.
3.4. Models for different body conditions
A substantial proportion (63%, p < 0.001, Table 3) of animals in our dataset was in normal condition, 23% were overconditioned and 14% were underconditioned. Brown Swiss cows showed the highest percentage of overconditioned animals and the lowest percentage of underconditioned animals as compared to SIM and GH. SIM showed the lowest percentage of overconditioned animals, while GH cows showed the highest percentage of underconditioned animals.
Table 3.
Percentage of normal, over- and underconditioned animals among different breeds (GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle). Condition categories are based on the optimal BCS (Body condition score) range per breed per lactation stage as described in Table 1.
| Breed | |||||
|---|---|---|---|---|---|
| GH | BS | SIM | Total | p-value1 | |
| condition | <0.001 | ||||
| normal | 39,201 (61.4%%) | 731 (63.4%%) | 6366 (71.0%%) | 46,298 (62.6%%) | |
| overconditioned | 15,043 (23.6%%) | 329 (28.5%%) | 1742 (19.4%%) | 17,114 (23.1%%) | |
| underconditioned | 9565 (15.0%%) | 93 (8.1%%) | 862 (9.6%%) | 10,520 (14.2%%) | |
| Total | 63,809 (100.0%%) | 1153 (100.0%%) | 8970 (100.0%%) | 73,932 (100.0%%) | |
Pearson's Chi-squared test.
Table 4 indicates that general (across breeds) overconditioning declines from the mid-age of animals towards the end of their life, while the percentage of underconditioned animals increases throughout the whole life. The association between the categorical age and body condition is significant (p < 0.001).
Table 4.
Percentage of normal, over- and underconditioned animals in different age group. Breed specific categorization of age is described in material and methods. Condition categories are based on the optimal BCS (Body condition score) range per breed per lactation stage as described in Table 1.
| Age_cat | |||||
|---|---|---|---|---|---|
| 1. Young | 2. Mid-age | 3. Old | Total | p-value1 | |
| condition | <0.001 | ||||
| normal | 11,266 (68.6%%) | 6018 (59.4%%) | 1051 (53.8%%) | 18,335 (64.3%%) | |
| overconditioned | 3960 (24.1%%) | 2534 (25.0%%) | 367 (18.8%%) | 6861 (24.1%%) | |
| underconditioned | 1186 (7.2%%) | 1585 (15.6%%) | 535 (27.4%%) | 3306 (11.6%%) | |
| Total | 16,412 (100.0%%) | 10,137 (100.0%%) | 1953 (100.0%%) | 28,502 (100.0%%) | |
Pearson's Chi-squared test.
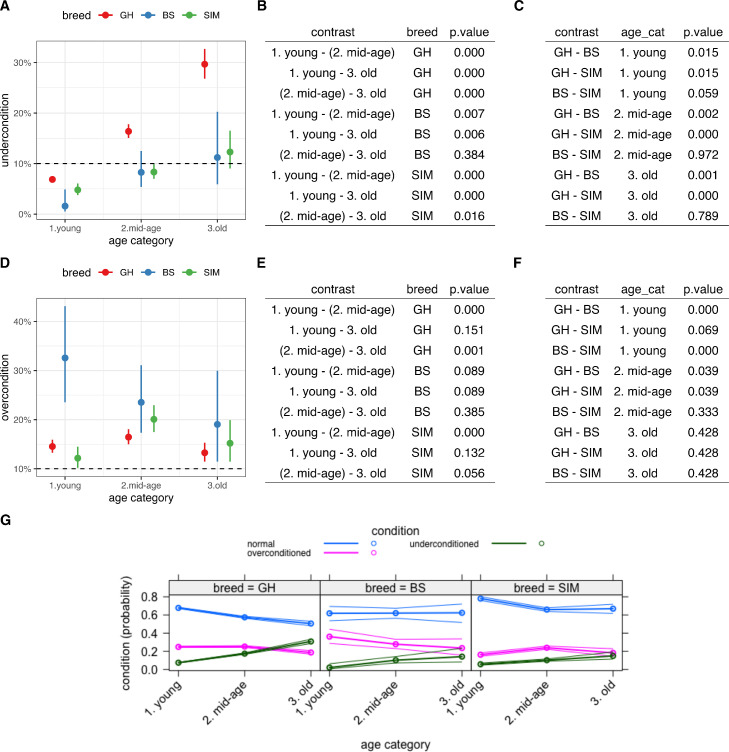
Underconditioning in every particular breed, studied separately from other conditions by the logistic mixed effects model, showed similar results to the results of the not breed specific Chi-Square test (Table 4). Particularly, only the difference between middle aged and older BS cows was not significant (p = 0.384, Fig. 5A & B) and the difference between middle aged and old SIM cows was suggestive to grow (p = 0.016, Fig. 5A & B), while all other contrasts between age categories indicated strong increase of underconditioning over the course of animal's life (p < 0.01, Fig. 5A & B). SIM and BS did not show differences among each other independently of the age, while both were less frequently (though only slightly (p = 0.015) in young age) underconditioned as compared with GH (Fig. 5C). The amount of underconditioned animals of mid and old aged GH cows was higher than 10%, while the amount of underconditioned animals in SIM and BS did not cross the 10% (dashed lines on Fig. 5A, D).
Fig. 5.
Dynamics of under- (A, B, C) and overconditioning (D, E, F) of breeds (GH - German Holstein, SIM - Simmental and BS - Brown Swiss cattle) in different age groups (see material and methods). A, D - predicted values of BCS (Body condition score) per age category. Condition categories are based on the optimal BCS range per breed per lactation stage as described in Table 1. B, E - pairwise comparisons of age categories among each other. C, F - pairwise comparisons of breeds among each other. G - dynamics of all conditions of breeds in different age groups by the means of non-parametric multinomial neural network based model. Dashed lines indicate the threshold of a percentage of animals in a herd, below which the herd is considered well managed (Kelogg, 2010).
Overconditioning of all breeds, also studied by the logistic mixed effects model, showed different dynamics as compared with underconditioning. The percentage of overconditioned GH animals first increased from their young (14.5%) to their middle (16.4%) ages (p < 0.01, Fig. 5D & E) and then decreased from their middle to older (13.3%) ages (p < 0.01). Interestingly, the percentage of overconditioned young (14.5%) and old (13.3%) GH animals did not differ (p = 0.151). Despite the decline of the percentage of overconditioned animals over the course of their productive life in BS cows, from 32.6% in young to 23.5% in mid to 19% in old ages, this change did not appear to be relevant (p > 0.05, Fig. 5D & E). The overconditioning of SIM cows increased (p < 0.01, Fig. 5D & E) from the young (12.2%) to the middle age (20%), then slightly dropped (15.2%) in older age to the point where it did not differ anymore from the young (p = 0.132) or mid (p = 0.056) ages.
The non-parametric multinomial neural network-based model, which aimed to provide an additional validation of the result, confirmed the trends uncovered by both mixed effects models and also showed the increase in underconditioning of animals over lifetime, while decrease in overconditioning from the middle to older ages (Fig. 5, G). Explicitly modeled normal condition here showed declining probability to be in normal condition from young to the middle-aged SIM cows, while steadily decreasing probability to be in the normal condition throughout the lifetime of GH cows.
4. Discussion
4.1. Body condition
The comparison of BCS of German Simmental and German Brown Swiss cows with German Holstein breed revealed higher average BCS in SIM and BS compared with GH, which might be interpreted in two ways. On one side, higher BCS provides more energy and therefore a higher potential for adipose tissue mobilization (Bauman & Bruce Currie, 1980), which might result in a higher milk yield. Thus, increase in milk yield with increasing BCS seems reasonable. However, such energy mobilization is strongly coupled with the genetic predisposition of a specific breed (J. R. Roche et al., 2009). It has been calculated that up to 60% of variations in BCS are due to differences in genetic makeup (J. R. Roche et al., 2009). Particularly, GH was intensively selected for higher milk performance and is, as a purely milk oriented breed, not predisposed to gain muscle mass, but predisposed to a higher conversion rate from body resources (in form of fat) into a final product - milk. Thus, GH may assure higher milk yields having lower body condition than dual-purpose breeds such as SIM and BS. This suggestion is in line with other studies which showed that genetically superior milk producers tend to have generally lower BCS (Buckley et al., 2000; Veerkamp & Brotherstone, 1997). SIM and (original) BS breeds, in contrast, have been selected for the dual-purpose final products, milk and beef. Walsh et al. (2008) found two other dual-purpose breeds, namely Montbéliarde and Normande, to also have significantly higher BCS as compared with GH. Thus, dual-purposefulness of SIM and BS intuitively explains their significantly higher BCS in our study, while suggesting nothing about higher or lower milk yield. The relationship between BCS of SIM and BS and their milk yield still needs to be explored. Similar differences among breeds in their ability to partition energy towards milk production or body reserves have often been reported before (Mao et al., 2004; Sinclair et al., 1998; Yan et al., 2006), however the Simmental and Brown Swiss cattle have been rarely studied.
One notable exception is the study of Arango et al. (2002) who tracked BCS changes of different breeds, including Simmental crosses with Hereford and Angus, over the productive lifetime (two - seven years) of animals. They found a slight increase in BCS of SIM crosses from the second (5.75–7.90) to the seventh (6.11–6.39) year on an one to ten points BCS scale. Pure Simmental breeds in our study also achieved their maximum BCS around the seventh year of life. We can only speculate that if Arango et al. (2002) extended their BCS recordings to older animals, they would have probably seen a similar decline in BCS as we found here. Arango et al. (2002) also noticed that smaller and lighter breeds, Jersey cows in their study, tend to mature and to begin accumulating fat at earlier ages than taller and heavier breeds, e.g. Charolais. This is in line with our results, where GH, being a lighter and milk-oriented breed, reached their highest BCS point of the parabola (Fig. 3D) earlier (4.9 – 5.7 years) than SIM (5.2 – 7.4 years).
A slower increase of BCS in SIM cattle over the course of life compared with GH assumes an opportunity for earlier achievement of the optimal productivity period (assuming the top of the BCS curve on Fig. 3D, leads to the highest milk yield) by changes in feeding strategy. Moreover, breed- and time- specific nutrition management might also reduce losses in body condition towards the end of life. Such measures could maximize the sustainable yield of milk for both SIM and GH, while at the same time greatly extend not only the most productive period of milk production at the mid-age, but also the span of the productive life of cows. Otherwise, the drop from the highest BCS at the mid-age (vertex of the curve) relatively early in life, especially in GH breed, may lead to a faster and potentially preliminary culling.
Unfortunately, despite such potential benefits of longer productive life, the average time spent by dairy cattle in the herd declines globally since the start of the 21st century (Rushen & Passillé, 2013; Schuster et al., 2020). A dairy cow, which is biologically capable of a life span of up to 20 years (Nowak & Walker., 1999), currently spends between 4.5 and 5.5 years in the herd on average (Knaus, 2009; Wathes et al., 2008). The top of the BCS parabola or, as we defined it, the optimal productive time of GH cows, uncovered in our study by the non-linear model (Fig. 3D) is surprisingly consistent with these averages, namely 4.9 – 5.7 years. The increase in the probability of underconditioning with increasing age found in this study might decrease the welfare of animals and therefore justifies such earlier culling by the modern dairying practices. Besides, since welfare of animals is not a single factor determining modern dairying practices in respect to age of animals, such management practices are unlikely to change any time soon. However, culling of animals after 5.5 years not only misses their biological potential, but also suggests general management deficiencies, especially in regard to older animals. Since less than 10% of the herd should be over- or underconditioned (Kellogg, 2010), higher percentages of over- and underconditioned animals observed in this study corroborate with the latter suggestion. Particularly, the predictions of a higher than 10% percentage of overconditioned animals for each breed and at different ages is quite concerning (Fig. 5D). While higher BCS can potentially lead to a higher milk production via increased adipose tissue mobilization (Bauman & Bruce Currie, 1980), some studies showed that normally conditioned cows of the same breed produced greater milk yields than overconditioned cows (Garnsworthy, 1988; Garnsworthy & Topps, 1982; Treacher et al., 1986). Excessive BCS in Czech Simmental cows was found to be highly related to ovarian cyst cases (Stadnik et al., 2017; Zulu et al., 2002). Overconditioning may be especially related to reduced fertility on dairy farms. That is why preventing cows from overconditioning should principally be considered to obtain fertile cows. A high BCS during the dry period was shown to increase the incidence of periparturient health disorders (Drackley, 1999; Rukkwamsuk et al., 1999). In contrast, the probability of underconditioning (as a modeled phenomenon) in our study is higher than 10% only in GH in their mid and old ages (Fig. 5A) as compared with SIM and BS herds. While underconditioning of cows in young age (not an issue in our dataset) could have been explained by an additional energy demand for continued growth (Dechow et al., 2002; Koenen et al., 1999), so that they invest less in body reserves, the drop in body condition in older animals is most likely due to the environmental and external factors, such as herd management, feeding strategies or diseases (e.g. Johne's disease). The probability of 30% with 95% CI [27 – 33%] of older GH cows in our dataset to be underconditioned is particularly alarming. Since underconditioning is strongly coupled with lowered conception rates (Domecq et al., 1997; Pryce et al., 2001), increased occurrence of lameness (Green et al., 2014; Lim et al., 2015; Oehm et al., 2019; Randall et al., 2015) and increased probability of metabolic (John R. Roche et al., 2013; Schuster et al., 2020) and infectious diseases (Abunna et al., 2010; Jaja et al., 2017), earlier culling of animals directly or indirectly due to underconditioning is almost naturally guaranteed. Thus, our results suggest that GH herds have the highest volatility in BCS as compared with BS and SIM, which seem to be more robust. Such volatility might be due to the natural ability of GH to a higher and faster conversion from fat into milk, while dual-purposefulness (milk and meat) and therefore the genetic predisposition for muscle growth in SIM and BS might make their BCS more muscular and therefore less volatile, as compared to GH. This suggests that GH herds have the highest potential for yield optimization and improvement of animal welfare independent of reasons for misconditioning. Exploring factors (be it overstocking, lameness, diseases, milk yield, parity etc.) which have the biggest influence on the over- and underconditioning themselves (as separate phenomena) would be an interesting branch of future research which might deliver useful insights, especially if such factors would turn out to be different across breeds.
4.2. BCS modeling framework
Using non-linear models to uncover temporal patterns is corroborated by a study on two Dutch (Koenen et al., 2001) and three Danish (Friggens & Badsberg, 2007) breeds during lactation, which implemented polynomial and exponential functions accordingly in order to allow for non-linearity. However, the use of solely polynomial degrees (from two to nine) in our study was not optimal. Particularly, polynomial degrees higher than two violently forced the BCS curve to bend resulting in several different patterns. For instance, polynomial degrees from fine to nine all resulted in a s-curve, with BCS growing towards the end of life (ca. 4000 days) after a convex valley in the curve, which for degree nine made BCS of 11 years old animals even higher than the peak (vertex) of the curve at their mid-age (ca. seven years), which does not appear plausible from a biological point of view.
Using exponential functions, Friggens and Badsberg (2007) modeled BCS during lactation in three different ways: one model for lactation only until the point of nadir, one for pregnancy starting from the nadir, and then a combined model for both periods. This seems perfectly reasonable and very accurately describes the BCS during lactation as an overturned parabola, however, assuming the exponential course of the BCS curve. In contrast, our data did not show a strongly exponential change of BCS during lactation, but rather looked like two wobbly, but still clearly linear curves, one as a drop before nadir, and another as a steady increase after nadir (not shown). Furthermore, Friggens and Badsberg (2007) reported numerous attempts to identify starting (primer) values for the models before they could converge, which does not assure that these models are applicable to a new data sets.
Hence, despite the advantages which polynomial and exponential models bring to the table, we here used generalized additive models with integrated smoothness estimation, which were specifically designed to describe non-linear relationships regardless of the course of the curve (Hastie & Tibshirani, 1986). In contrast to polynomial models, which forced the BCS curve to bend, additive models with different degrees of smoothness (also from two to nine) all finished up with a quadratic (parabolic) shape, where higher degrees of smoothness only made the curve more refined. Such quadratic nature of BCS over the lifetime has, to our knowledge, not been discussed yet and hence, this is the first study to unravel a potential quadratic nature of BCS over lifetime. In contrast to the mathematically sound, though primer (starting values) sensitive exponential model, which would also force the curve to bend in a particular manner, our purely statistical approach can be applied to any kind of data without the need of starting values, because the additive model will always fit existing data and thus will always uncover existing (linear or non-linear) trends.
Fig. 3C reveals that the linear model for SIM suggests a steady increase in body condition towards higher age, implying severe overconditioning of older cows, whereas the opposite is actually the case: their BCS declines. Presenting a linear trend on Fig. 3C highlights the danger to believe linear models, especially if only coefficients (i.e. ordinary least squares means or odds ratios) and (often) significant p-values are presented, while the assumption of linearity is not checked, because it may lead to a biologically misleading inference. Interestingly, the linear trend of BS remained perfectly linear even when modeled via additive regression with high degrees of smoothness. Thus, a non-linear model would always catch a linear trend, if one exists, while, in contrast, a linear model will always miss any dynamic relationship, which is the case with the age of SIM and GH cattle in our study. Thus, we here strongly advocate the use of additive models as an important exploratory tool. However, we used a linear mixed model as our final model for several reasons.
First of all, additive non-linear models are by no means perfect. Their ability to uncover patterns (flexibility) is traded off by a challenging interpretability (James et al., 2013). Secondly, the intentional categorization of age produced the same results as the additive model, i.e. a significant difference between young and middle-aged and between middle-aged and older animals and a less pronounced difference between young and old cows (Fig. 4). While categorization of a numeric variable usually reduces the amount of information a variable contains (Altman & Royston, 2006; Royston et al., 2006), the categorization after applying additive models is yet justified and allows for a straightforward interpretation via p-values, estimated means (i.e. of BCS) and probabilities (i.e. of over- and underconditioning). Using linear mixed effects models also simplified the process of checking model assumptions. Consequently, the categorization of age after additive modeling here provided rather an advantage without a loss of information.
A correlation (multicollinearity) between region and breed in our data (model) was not surprising, since 99.5% of animals in regions North and East were GH, while approximately 83% in region South were SIM cattle. Such low amount of data for BS and SIM (only 0.5% of observations combined) breeds in regions North and East could not produce meaningful insights for these breeds in case the interaction term between region and breed would be considered. Moreover, the interaction between region and breed also turned out to be highly multicollinear (VIF = 504). Thus, studying regions and breeds in our dataset is mutually exclusive since they provide very similar and therefore redundant (or even overlapping) information.
This implies that in order to assess the regional effect the regions should be analyzed separately (i.e. one model per particular region), or, if only regions North and East are considered, the variable region could be analyzed as a fixed effect. For the purpose of this study these analyses would not have been helpful as these models would have prevented useful outcome for the more important variable breed. Nevertheless, a possible collinear effect of region has to be considered when interpreting the results for breed, here.
Further predictors and interactions might have improved the explanatory power of our final model. We are aware that e.g. pasture access, feeding strategies or disease prevalence have been associated with BCS (Ruegg & Milton, 1995; Walsh et al., 2008; Washburn et al., 2002). However, since the main objective of this study was to report the previously underexplored BCS of Simmental and Brown-Swiss cattle, adding more factors would have diluted the focus. Moreover, in order to keep the study focused on BCS changes throughout the whole productive life of animals, we intentionally did not thoroughly explore changes in BCS during lactation period and between lactation stages, that would be a topic for a separate study.
4.3. Limitations of the study
Due to a lower number of Brown Swiss cows in our study, their estimates are less precise than those of Simmental and Holstein-Friesien cows. This caused wider and often overlapping confidence intervals in these estimates, which might have caused missing some potential insights. The probability of Type II Error is therefore potentially higher for BS as compared with GH and SIM. Since body condition highly depends on diet (Berry et al., 2006; Coffey et al., 2004; McCarthy et al., 2007), one of the possible biases could be present in the study due to the absence of the information about nutrition. However, including nutrition variables would shift the focus from reporting BCS of underexplored SIM and BS over lifetime to a different study. Such studies are important and are to be explored. Furthermore, it is important to acknowledge that enrollment was based on voluntary participation of interested farmers. This may be a source of bias as those farmers with particularly well managed farms, probably being of a more proactive personality, may be over-represented which may have yielded results that show improved conditions compared with the underlying dairy cow population. On the other hand, specifically those farmers with poor management and impaired animal health may have been inclined to participate which would result in outcomes reflecting a situation worse than in the underlying population. Furthermore, the outcomes are in alignment with previous work and biological reasoning which indicates that selection bias may well not have been a major concern.
5. Conclusions
Failing to hold linearity and multicollinearity model assumptions, became the most revealing part of this study. Significant differences between the GH and dual-purpose breeds demand a breed-specific management during the respective life and production phases of dairy cattle, which might help to fully exploit genetic potential of every breed without compromising welfare of animals, could help to prevent the losses of body reserves during the later stages of life, and therefore reduce the probability of preliminary culling.
Author contributions
AOE and YZ initiated and conceptualized the study and interpreted the results. MH developed the concept of the PraeRi study, applied for funding and provided the overall management of the study. AOE participated in data acquisition and data pre-processing and supervised the study. YZ conducted statistical analyses. GKS was involved in planning of the study and interpretation of the results. AC conceptualized and supervized the epidemiological part of the PraeRi study and commented on the statistical analyses within this manuscript. All authors were involved in creating and revising the final version of the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Martina Hoedemaker reports financial support was provided by German Federal Ministry of Food and Agriculture through the Federal Office for Agriculture and Food.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2022.100275.
Appendix. Supplementary materials
References
- Abunna Fufa, Asfaw Loma, Megersa Bekele, Regassa Alemayehu. Bovine fasciolosis: Coprological, abattoir survey and its economic impact due to liver condemnation at Soddo municipal abattoir, Southern Ethiopia. Tropical Animal Health and Production. 2010 doi: 10.1007/s11250-009-9419-3. [DOI] [PubMed] [Google Scholar]
- Altman Douglas G., Royston Patrick. The cost of dichotomising continuous variables. BMJ (Clinical Research ed.) 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango J.A., Cundiff L.V., Van Vleck L.D. Breed comparisons of Angus, Charolais, Hereford, Jersey, Limousin, Simmental, and South Devon for weight, weight adjusted for body condition score, height, and body condition score of cows. Journal of Animal Science. 2002 doi: 10.2527/2002.80123123x. [DOI] [PubMed] [Google Scholar]
- Bates A.J., Saldias B. A comparison of machine learning and logistic regression in modelling the association of body condition score and submission rate. Preventive Veterinary Medicine. 2019 doi: 10.1016/j.prevetmed.2019.104765. [DOI] [PubMed] [Google Scholar]
- Bauman Dale E., Bruce Currie W. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. Journal of Dairy Science. 1980;63(9):1514–1529. doi: 10.3168/jds.S0022-0302(80)83111-0. [DOI] [PubMed] [Google Scholar]
- Benjamini Yoav, Hochberg Yosef, Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B. 1995:289–300. http://arxiv.org/abs/95/57289 [Google Scholar]
- Berry D.P., Buckley F., Dillon P. Body condition score and live-weight effects on milk production in Irish Holstein-Friesian dairy cows. Animal : an International Journal of Animal Bioscience. 2007 doi: 10.1017/S1751731107000419. [DOI] [PubMed] [Google Scholar]
- Berry D.P., Veerkamp R.F., Dillon P. Phenotypic profiles for body weight, body condition score, energy intake, and energy balance across different parities and concentrate feeding levels. Livestock Science. 2006 doi: 10.1016/j.livsci.2006.02.012. [DOI] [Google Scholar]
- Breiman Leo. Random forests. Machine Learning. 2001 doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- Breusch T.S., Pagan A.R. A Simple test for heteroscedasticity and random coefficient variation. Econometrica: Journal of the Econometric Society. 1979;47(5) doi: 10.2307/1911963. [DOI] [Google Scholar]
- Buckley F., Dillon P., Rath M., Veerkamp R.F. The relationship between genetic merit for yield and live weight, condition score, and energy balance of spring calving Holstein Friesian dairy cows on grass based systems of milk production. Journal of Dairy Science. 2000 doi: 10.3168/jds.S0022-0302(00)75060-0. [DOI] [PubMed] [Google Scholar]
- Butler S.T. Nutritional management to optimize fertility of dairy cows in pasture-based systems. Animal : An International Journal of Animal Bioscience. 2014 doi: 10.1017/S1751731114000834. [DOI] [PubMed] [Google Scholar]
- Cicchetti Domenic V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994 doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- Coffey M.P., Simm G., Oldham J.D., Hill W.G., Brotherstone S. Genotype and diet effects on energy balance in the first three lactations of dairy cows. Journal of Dairy Science. 2004 doi: 10.3168/jds.S0022-0302(04)73577-8. [DOI] [PubMed] [Google Scholar]
- Compton C.W.R., Heuer C., Thomsen P.T., Carpenter T.E., Phyn C.V.C., McDougall S. Invited review: A systematic literature review and meta-analysis of mortality and culling in dairy cattle. Journal of Dairy Science. 2017 doi: 10.3168/jds.2016-11302. [DOI] [PubMed] [Google Scholar]
- Correa Maria T., Curtis Charles R., Erb Hollis N., Scarlett Janet M., David Smith R. An ecological analysis of risk factors for postpartum disorders of Holstein-Friesian Cows from thirty-two New York farms. Journal of Dairy Science. 1990 doi: 10.3168/jds.S0022-0302(90)78819-4. [DOI] [PubMed] [Google Scholar]
- Daros Ruan R., Eriksson Hanna K., Weary Daniel M., Keyserlingk Marina A.G.Von. The relationship between transition period diseases and lameness, feeding time, and body condition during the dry period. Journal of Dairy Science. 2020 doi: 10.3168/jds.2019-16975. [DOI] [PubMed] [Google Scholar]
- Dechow C.D., Rogers G.W., Clay J.S. Heritability and correlations among body condition score loss, body condition score, production and reproductive performance. Journal of Dairy Science. 2002 doi: 10.3168/jds.S0022-0302(02)74393-2. [DOI] [PubMed] [Google Scholar]
- Dohoo I.R., Ducrot C., Fourichon C., Donald A., Hurnik D. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Preventive Veterinary Medicine. 1997 doi: 10.1016/S0167-5877(96)01074-4. [DOI] [PubMed] [Google Scholar]
- Domecq J.J., Skidmore A.L., Lloyd J.W., Kaneene J.B. Relationship between body condition scores and milk yield in a large dairy herd of high yielding Holstein cows. Journal of Dairy Science. 1997 doi: 10.3168/jds.S0022-0302(97)75917-4. [DOI] [PubMed] [Google Scholar]
- Drackley James K. Biology of dairy cows during the transition period: The final Frontier? Journal of Dairy Science. 1999 doi: 10.3168/jds.s0022-0302(99)75474-3. [DOI] [PubMed] [Google Scholar]
- Ducrocq V., Quaas R.L., Pollak E.J., Casella G. Length of productive life of dairy cows. 1. Justification of a Weibull model. Journal of Dairy Science. 1988 doi: 10.3168/jds.S0022-0302(88)79906-3. [DOI] [Google Scholar]
- Edmonson A.J., Lean I.J., Weaver L.D., Farver T., Webster G. A body condition scoring chart for Holstein dairy cows. Journal of Dairy Science. 1989 doi: 10.3168/jds.S0022-0302(89)79081-0. [DOI] [Google Scholar]
- Friggens N.C., Badsberg J.H. The effect of breed and parity on curves of body condition during lactation estimated using a non-linear function. Animal: An International Journal of Animal Bioscience. 2007 doi: 10.1017/S1751731107691861. [DOI] [PubMed] [Google Scholar]
- Gallo L., Garnier P., Cassandro M., Mantovani R., Bailoni L., Contiero B., Bittante G. Change in body condition score of Holstein cows as affected by parity and mature equivalent milk yield. Journal of Dairy Science. 1996 doi: 10.3168/jds.S0022-0302(96)76452-4. [DOI] [PubMed] [Google Scholar]
- Garnsworthy, P.C. (1988). “The effect of energy reserves at calving on performance of dairy cows.” In Nutrition and lactation in the dairy cow, 157–170. https://doi.org/10.1016/b978-0-408-00717-7.50014-9.
- Garnsworthy P.C., Topps J.H. The effect of body condition of dairy cows at calving on their food intake and performance when given complete diets. Animal Production. 1982 doi: 10.1017/S0003356100000878. [DOI] [Google Scholar]
- Green L.E., Huxley J.N., Banks C., Green M.J. Temporal associations between low body condition, lameness and milk yield in a UK dairy herd. Preventive Veterinary Medicine. 2014 doi: 10.1016/j.prevetmed.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Hastie Trevor, Tibshirani Robert. Generalized additive models. Statistical Science. 1986 doi: 10.1214/ss/1177013604. [DOI] [PubMed] [Google Scholar]
- Heuer C., Schukken Y.H., Dobbelaar P. Postpartum body condition score and results from the first test day milk as predictors of disease, fertility, yield, and culling in commercial dairy herds. Journal of Dairy Science. 1999 doi: 10.3168/jds.S0022-0302(99)75236-7. [DOI] [PubMed] [Google Scholar]
- Hoedemaker M., Prange D., Gundelach Y. Body condition change ante- and postpartum, health and reproductive performance in German Holstein Cows. Reproduction in Domestic Animals. 2009 doi: 10.1111/j.1439-0531.2007.00992.x. [DOI] [PubMed] [Google Scholar]
- Jaja Ishmael Festus, Mushonga Borden, Green Ezekiel, Muchenje Voster. Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Veterinary and Animal Science. 2017 doi: 10.1016/j.vas.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Gareth, Witten Daniela, Hastie Trevor, Tibishirani Robert. Springer; New York: 2013. An introduction to statistical learning with applications in R.http://books.google.com/books?id=9tv0taI8l6YC [Google Scholar]
- Jones C.M., Heinrichs J., Ishler V.A. Body condition scoring as a tool for dairy herd management. Penn State Extension. 2016 [Google Scholar]
- Jorritsma R., Jorritsma H., Schukken Y.H., Bartlett P.C., Wensing T., Wentink G.H. Prevalence and indicators of post partum fatty infiltration of the liver in nine commercial dairy herds in The Netherlands. Livestock Production Science. 2001 doi: 10.1016/S0301-6226(00)00208-6. [DOI] [Google Scholar]
- Kellogg Wayne. Body condition scoring with dairy cattle. Agriculture and Natural Resources. 2010 [Google Scholar]
- Knaus Wilhelm. Dairy cows trapped between performance demands and adaptability†. Journal of the Science of Food and Agriculture. 2009 doi: 10.1002/jsfa.3575. [DOI] [Google Scholar]
- Koenen E.P.C., Groen A.F., Gengler N. Phenotypic variation in live weight and live-weight changes of lactating Holstein-Friesian cows. Animal Science. 1999 doi: 10.1017/s135772980005013x. [DOI] [Google Scholar]
- Koenen E.P.C., Veerkamp R.F., Dobbelaar P., De Jong G. Genetic analysis of body condition score of lactating Dutch Holstein and red-and-white heifers. Journal of Dairy Science. 2001 doi: 10.3168/jds.S0022-0302(01)74588-2. [DOI] [PubMed] [Google Scholar]
- Kritzinger F., Schoder G. Gesund und fit bringt optimale Leistung, BCS - body condition scoring für Fleckvieh. Oberösterreichischer Tiergesundheitsdienst, Linz. 2009:1–2. no. a. [Google Scholar]
- Kritzinger F., Schoder G., Mader C., Winkler R. Gesund und fit bringt optimale Leistung, BCS - body condition scoring für Braunvieh. Braunvieh Tirol u. Tiroler Tiergesundheitsdienst, Innsbruck. 2009 [Google Scholar]
- Kritzinger, Schoder Gesund und fit bringt optimale Leistung, BCS - body condition scoring für Holstein. Oberösterreichischer Tiergesundheitsdienst, Linz. 2009:1–2. no. b. [Google Scholar]
- Lim P.Y., Huxley J.N., Willshire J.A., Green M.J., Othman A.R., Kaler J. Unravelling the temporal association between lameness and body condition score in dairy cattle using a multistate modelling approach. Preventive Veterinary Medicine. 2015 doi: 10.1016/j.prevetmed.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Locher L., Häussler S., Laubenthal L., Singh S.P., Winkler J., Kinoshita A., Kenez, et al. Effect of increasing body condition on key regulators of fat metabolism in subcutaneous adipose tissue depot and circulation of nonlactating dairy cows. Journal of Dairy Science. 2015 doi: 10.3168/jds.2014-8710. [DOI] [PubMed] [Google Scholar]
- Mao I.L., Sloniewski K., Madsen P., Jensen J. Changes in body condition score and in its genetic variation during lactation. Livestock Production Science. 2004 doi: 10.1016/j.livprodsci.2003.12.005. [DOI] [Google Scholar]
- McCarthy S., Berry D.P., Dillon P., Rath M., Horan B. Influence of Holstein-Friesian strain and feed system on body weight and body condition score lactation profiles. Journal of Dairy Science. 2007 doi: 10.3168/jds.2006-501. [DOI] [PubMed] [Google Scholar]
- Merle Roswitha, Busse Marc, Rechter Galina, Meer Uwe. Regionalisierung Deutschlands anhand landwirtschaftlicher Strukturdaten. Berliner Und Munchener Tierarztliche Wochenschrift. 2012;125(1–2) doi: 10.2376/0005-9366-125-52. [DOI] [PubMed] [Google Scholar]
- Metzner M., Heuwieser W., Klee W. Die beurteilung der körperkondition (body conditions coring) im herdenmanagement. Der Praktische Tierarzt. 1993;11 [Google Scholar]
- Newsome R.F., Green M.J., Bell N.J., Bollard N.J., Mason C.S., Whay H.R., Huxley J.N. A prospective cohort study of digital cushion and corium thickness. Part 1: Associations with body condition, lesion incidence, and proximity to calving. Journal of Dairy Science. 2017 doi: 10.3168/jds.2016-12012. [DOI] [PubMed] [Google Scholar]
- Nowak R.M., Walker E.P. Walker's mammals of the world. Choice Reviews Online. 1999 doi: 10.5860/choice.37-2177. [DOI] [Google Scholar]
- Oehm Andreas W., Jensen Katharina Charlotte, Tautenhahn Annegret, Mueller Kerstin Elisabeth, Feist Melanie, Merle Roswitha. Factors associated with lameness in tie stall housed dairy cows in South Germany. Frontiers in Veterinary Science. 2020 doi: 10.3389/fvets.2020.601640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehm, Andreas W., Knubben-Schweizer, Gabriela, Rieger, Anna, Stoll, Alexander, & Hartnack, Sonja (2019). “A systematic review and meta-analyses of risk factors associated with lameness in dairy cows.” https://doi.org/10.1186/s12917-019-2095-2. [DOI] [PMC free article] [PubMed]
- Pryce J.E., Coffey M.P., Simm G. The relationship between body condition score and reproductive performance. Journal of Dairy Science. 2001 doi: 10.3168/jds.S0022-0302(01)70184-1. [DOI] [PubMed] [Google Scholar]
- Randall L.V., Green M.J., Chagunda M.G.G., Mason C., Archer S.C., Green L.E., Huxley J.N. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. Journal of Dairy Science. 2015 doi: 10.3168/jds.2014-8863. [DOI] [PubMed] [Google Scholar]
- Randall L.V., Green M.J., Green L.E., Chagunda M.G.G., Mason C., Archer S.C., Huxley J.N. The contribution of previous lameness events and body condition score to the occurrence of lameness in dairy herds: A study of 2 herds. Journal of Dairy Science. 2018 doi: 10.3168/jds.2017-13439. [DOI] [PubMed] [Google Scholar]
- Rinell E., Heringstad B. The effects of crossbreeding with Norwegian Red dairy cattle on common postpartum diseases, fertility and body condition score. Animal: An International Journal of Animal Bioscience. 2018 doi: 10.1017/S175173111800037X. [DOI] [PubMed] [Google Scholar]
- Roche J.R., Berry D.P., Lee J.M., Macdonald K.A., Boston R.C. Describing the body condition score change between successive calvings: A novel strategy generalizable to diverse cohorts. Journal of Dairy Science. 2007 doi: 10.3168/jds.2006-729. [DOI] [PubMed] [Google Scholar]
- Roche J.R., Friggens N.C., Kay J.K., Fisher M.W., Stafford K.J., Berry D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. Journal of Dairy Science. 2009 doi: 10.3168/jds.2009-2431. [DOI] [PubMed] [Google Scholar]
- Roche J.R., Macdonald K.A., Burke C.R., Lee J.M., Berry D.P. Associations among body condition score, body weight, and reproductive performance in seasonal-calving dairy cattle. Journal of Dairy Science. 2007 doi: 10.3168/jds.S0022-0302(07)72639-5. [DOI] [PubMed] [Google Scholar]
- Roche, John R., Kay, Jane K., Friggens, Nic C., Loor, Juan J., & Berry, Donagh P. (2013). “Assessing and managing body condition score for the prevention of metabolic disease in dairy cows.” https://doi.org/10.1016/j.cvfa.2013.03.003. [DOI] [PubMed]
- Royston Patrick, Altman Douglas G., Sauerbrei Willi. Dichotomizing continuous predictors in multiple regression: A bad idea. Statistics in Medicine. 2006 doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Ruegg P.L., Milton R.L. Body condition scores of Holstein cows on prince Edward Island, Canada: Relationships with yield, reproductive performance, and disease. Journal of Dairy Science. 1995 doi: 10.3168/jds.S0022-0302(95)76666-8. [DOI] [PubMed] [Google Scholar]
- Rukkwamsuk Theera, Wensing Theo, Geelen Math J.H. Effect of overfeeding during the dry period on the rate of esterification in adipose tissue of dairy cows during the periparturient period. Journal of Dairy Science. 1999 doi: 10.3168/jds.S0022-0302(99)75339-7. [DOI] [PubMed] [Google Scholar]
- Rushen J., Passillé A.M. Cow longevity conference. 2013. The importance of improving cow longevity. [Google Scholar]
- Schuster, Jesse C., Barkema, Herman W., Vries, Albert De, Kelton, David F., & Orsel, Karin (2020). “Invited review: Academic and applied approach to evaluating longevity in dairy cows.” https://doi.org/10.3168/jds.2020-19043. [DOI] [PubMed]
- Sinclair K.D., Yildiz S., Quintans G., Broadbent P.J. Annual energy intake and the performance of beef cows differing in body size and milk potential. Animal Science. 1998 doi: 10.1017/S1357729800009218. [DOI] [Google Scholar]
- Souissi Wissal, Bouraoui Rachid. Book: Lactation in farm animals. 2019. Relationship between body condition score, milk yield, reproduction, and biochemical parameters in dairy cows. [DOI] [Google Scholar]
- Stadnik Ludek, Atasever Savas, Ducháček Jaromir. Effects of body condition score and daily milk yield on reproduction traits of Czech Fleckvieh cows. Animal Reproduction. 2017 doi: 10.21451/1984-3143-AR944. [DOI] [Google Scholar]
- Sundrum, Albert. (2015). “Metabolic disorders in the transition period indicate that the dairy cows’ ability to adapt is overstressed.” https://doi.org/10.3390/ani5040395. [DOI] [PMC free article] [PubMed]
- Treacher R.J., Reid I.M., Roberts C.J. Effect of body condition at calving on the health and performance of dairy cows. Animal Production. 1986 doi: 10.1017/S0003356100018286. [DOI] [Google Scholar]
- Veerkamp R.F., Brotherstone S. Genetic correlations between linear type traits, food intake, live weight and condition score in Holstein Friesian dairy cattle. Animal Science. 1997 doi: 10.1017/S1357729800015976. [DOI] [Google Scholar]
- Walsh S., Buckley F., Pierce K., Byrne N., Patton J., Dillon P. Effects of breed and feeding system on milk production, body weight, body condition score, reproductive performance, and postpartum ovarian function. Journal of Dairy Science. 2008 doi: 10.3168/jds.2007-0818. [DOI] [PubMed] [Google Scholar]
- Waltner S.S., McNamara J.P., Hillers J.K. Relationships of body condition score to production variables in high producing Holstein dairy cattle. Journal of Dairy Science. 1993 doi: 10.3168/jds.S0022-0302(93)77679-1. [DOI] [PubMed] [Google Scholar]
- Washburn S.P., White S.L., Green J.T., Benson G.A. Reproduction, mastitis, and body condition of seasonally calved Holstein and jersey cows in confinement or pasture systems. Journal of Dairy Science. 2002 doi: 10.3168/jds.S0022-0302(02)74058-7. [DOI] [PubMed] [Google Scholar]
- Wathes D.C., Brickell J.S., Bourne N.E., Swali A., Cheng Z. Factors influencing heifer survival and fertility on commercial dairy farms. Animal : An International Journal of Animal Bioscience. 2008 doi: 10.1017/S1751731108002322. [DOI] [PubMed] [Google Scholar]
- Wildman E.E., Jones G.M., Wagner P.E., Boman R.L., Troutt H.F., Lesch T.N. A dairy cow body condition scoring system and its relationship to selected production characteristics. Journal of Dairy Science. 1982 doi: 10.3168/jds.S0022-0302(82)82223-6. [DOI] [Google Scholar]
- Yan T., Mayne C.S., Keady T.W.J., Agnew R.E. Effects of dairy cow genotype with two planes of nutrition on energy partitioning between milk and body tissue. Journal of Dairy Science. 2006 doi: 10.3168/jds.S0022-0302(06)72170-1. [DOI] [PubMed] [Google Scholar]
- Zulu Victor Chisha, Sawamukai Yutaka, Nakada Ken, Kida Katsuya, Moriyoshi Masaharu. Relationship among insulin-like growth factor-I, blood metabolites and postpartum ovarian function in dairy cows. Journal of Veterinary Medical Science. 2002 doi: 10.1292/jvms.64.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.