Abstract
We have identified the chlamydial heat shock protein Hsp10 as a potential correlate to the immunopathogenic process in women with tubal factor infertility (TFI). The human serologic response to chlamydial Hsp10, Hsp60, and major outer membrane protein (MOMP) was measured by enzyme-linked immunosorbent assay. Three populations of women were studied: uninfected controls (CU), acutely infected (AI) women, and women with TFI. Sera from women in the AI and TFI groups both recognized Hsp10 more frequently and at a higher overall level than sera from healthy uninfected controls. Moreover, the infertile women had significantly greater Hsp10 seroreactivity than acutely infected women, indicating a concomitant increase of Hsp10 recognition in populations with increasing levels of disease severity. Hsp60 reactivity showed a similar correlation in these populations, while MOMP reactivity peaked at the same level in both AI and TFI populations but did not increase with disease severity. Test populations were standardized by level of reactivity to formalin-fixed Chlamydia trachomatis elementary bodies (EBs) to address whether these associations were reflections of increased overall chlamydial exposure rather than a property specific to Hsp10. Associations between Hsp10 seropositivity and TFI were greater in the EB+ subgroup while associations among the EB− subgroup were diminished. When restricted to the EB+ subgroups, Hsp60 and MOMP responses in the TFI population did not increase significantly over the level of AI group responses. Thus, among women with similar exposure to chlamydiae, the serologic response to Hsp10 exhibited a stronger correlation with TFI than did the response to Hsp60 or MOMP. These findings support the hypothesis that the serological response to C. trachomatis heat shock proteins is associated with the severity of disease and identifies Hsp10 as an antigen recognized by a significant proportion of women with TFI.
Chlamydia trachomatis is a prevalent sexually transmitted pathogen that is responsible for over 4 million new cases of urogenital infection in the United States per year (8). Most infections are uncomplicated or asymptomatic and with treatment resolve without serious complications. However, approximately 10% of women who acquire C. trachomatis urogenital infections develop upper genital tract complications, such as salpingitis and pelvic inflammatory disease, chronic inflammation, and subsequent fallopian tube scarring, which greatly increases the risk of ectopic pregnancy and tubal factor infertility (TFI) (22). The chlamydial components responsible for those deleterious responses and how they further the progression of chronic inflammation and tissue damage have not been elucidated. It has been proposed that prolonged exposure to conserved chlamydial antigens is a contributing factor in the pathogenesis of endometrial and tubal damage (4, 6), although the precise mechanism by which that occurs is not fully understood. Repeated or continuous exposure to those antigens, such as through multiple infections or the development of persistent low-level chlamydial growth, may ultimately be the catalyst for immunopathological development. Identification of immunopathogenic chlamydial antigens may lead to new diagnostic approaches for the identification of individuals who have or are likely to develop adverse complications of chlamydial infections.
Chlamydial heat shock proteins are known to be activators of immunopathologic mechanisms which contribute to human disease. Responses to the chlamydial heat shock protein Hsp60, a homologue of Escherichia coli GroEL, have been associated with the sequelae of upper genital tract disease, including ectopic pregnancy (27), pelvic inflammatory disease and chronic pelvic pain (9, 10, 14, 24), perihepatitis (19), and TFI (1, 28, 32, 33). In general, serological reactivity to Hsp60 is low among healthy controls but increases stepwise as disease becomes more severe (6). Since purified Hsp60 elicits mononuclear cell inflammation and tissue damage in animal models of chlamydial infection (20, 23), it has been hypothesized that the increased level of immune reactivity to Hsp60 contributes to the development of immune pathology.
Additional antigens that may participate in the immunopathological response to chlamydiae have not been characterized. A prime candidate, however, is the chlamydial GroES homologue, Hsp10. Reports on the immunogenicity of Hsp10 antigens from other microbial pathogens suggest that the Hsp10 family of proteins are capable of eliciting chronic inflammation and delayed hypersensitivity. In particular, the immune response to the Hsp10 homologues of Mycobacterium leprae and Mycobacterium tuberculosis have been shown to be prominent T-cell antigens and targets of serum antibody responses (3, 12, 13, 17). Both M. leprae and M. tuberculosis Hsp10s elicit strong Th1 phenotype human T-cell responses, with the production of interleukin 2 and gamma interferon, consistent with a delayed-type hypersensitivity (DTH) response (15, 17, 18, 29). Furthermore, sensitized guinea pigs show strong cutaneous DTH responses to purified mycobacterial Hsp10 (17). Human T cells recovered from the site of the Mitsuda reaction, a test that is used as a cutaneous measure of M. leprae DTH, proliferate strongly in response to Hsp10 (29). While such Th1-mediated DTH responses are critical for resolution of disease, they also are associated with much of the immunopathology of leprosy (31, 34).
The human immune response to chlamydial Hsp10 has not been thoroughly evaluated. In this study, we investigated several fundamental parameters of the human immune response to purified C. trachomatis Hsp10. The immune response to Hsp10 was compared among three groups of women representing differing severities of disease: uninfected, acutely infected, and postimmunopathology. Furthermore, we compared patterns of Hsp10 immunological reactivity with same-patient reactivity to Hsp60 and to major outer membrane protein (MOMP). Our data show that like responses to Hsp60, Hsp10 antibody responses are associated with the immunopathology of severe upper genital tract complications of chlamydial disease in women.
MATERIALS AND METHODS
Patient sera.
Female volunteers were recruited from several institutions throughout the Midwest: Indiana University Hospitals, the University of Wisconsin Student Health Services STD Clinic, the University of Wisconsin Women's Clinic, the University of Iowa Hospitals and Clinics, and the University of Kansas Medical Center. Patients were divided into three unmatched study groups based on the following criteria. Control uninfected women (CU; n = 42) were defined as women with no signs of infection who were seen at the clinic for routine gynecological care. CU women had no stated history of chlamydial infection and were not infected at the time of sample collection. This population served as a C. trachomatis-negative reference by which seropositivity of the test groups could be determined (see below). Acutely infected women (AI; n = 139) had active chlamydial genital tract infection and were found to be positive for the presence of chlamydiae in the genital tract prior to serum sample collection by at least one of the following diagnostic tests: cell culture of cervical swabs, PCR, ligase chain reaction, gene probe, enzyme immunoassay, or direct fluorescent assay. Serotyping of swab cultures, as described elsewhere (30), was performed on 63 of 137 (46%) AI women. The TFI group (n = 33) consisted of women who were seeking infertility treatment and who had laparoscopic or hysterosalpingographic evidence of tubal damage. TFI group candidates were considered infertile if they had had regular unprotected intercourse for at least 1 year without conception.
Antigens.
A panel of antigens was assembled to test relationships between antigen recognition and the antichlamydial immune response. Recombinant chlamydial Hsp10 was purified as previously described (16). To simplify the assay system, a single serovar of chlamydiae, strain E/UW-5, was used in elementary body (EB) and purified MOMP assays. Formalin-fixed density gradient-purified serovar E/UW-5 EBs were prepared as described elsewhere (7). A purified OGP extract of native serovar E/UW-5 MOMP was generously provided by Jim Williams, Indiana University. Of the women in this study who were serotyped, 45 of 63 (71%) had been infected with serovar E. Among these women, the identity of the infecting serovar did not significantly affect the tendency to be seropositive for either formalin-fixed serovar E EBs or purified serovar E MOMP (data not shown).
Production of recombinant chlamydial Hsp60.
Recombinant C. trachomatis serovar A Hsp60 was expressed and purified by using the QIAexpress System (Qiagen Inc., Chatsworth, Calif.). Oligonucleotides (5′-GAGCGCATCCATGGTCGCTAAAAACATTAAA-3′ [primer A] and 5′-CCATTAGAGAGATCTATAGTCCATTCCTGCGCC-3′ [primer B]) that allowed amplification of the hypB sequence coding for the 60-kDa heat shock protein of C. trachomatis serovar A polypeptide from pTA571 (21) were designed. Primer A alters a base 5′ to the ATG start codon to include an NcoI site, which facilitated cloning but did not affect the subsequent coding sequences of the hypB open reading frame. Primer B modified the TAA stop codon to AGA (arginine), which placed the gene in frame with codons encoding six histidine residues and also allowed the introduction of a BglII site to facilitate cloning. A purified NcoI/BglII-digested PCR product was cloned into NcoI/BglII-digested pQE60 vector and transformed into E. coli TG1. Purified plasmid from a positive transformant was used to transform E. coli M15. A positive clone was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting by using the anti-Hsp60 monoclonal antibody A57-B9 (35). The gene encoding the recombinant Hsp60 protein was sequenced to confirm that no errors were introduced during the amplification and cloning procedures. The recombinant Hsp60 polypeptide, expressed as a fusion protein containing eight additional amino acids (arginine and serine followed by six histidine residues) at the carboxyl terminus, was purified by affinity chromatography with Ni-nitrilotriacetic acid resin following the manufacturer's suggested procedures (Qiagen, Inc.). Recombinant protein was eluted from the Ni-nitrilotriacetic acid resin with 250 mM imidazole, dialyzed against 10 mM phosphate-buffered saline (PBS), aliquoted, and stored at −70°C until used. The homogeneity of the recombinant protein as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining was >95%. Protein concentration was determined by measuring absorbance at 280 nm (an optical density of 0.22 = 1.0 mg/ml).
Enzyme-linked immunosorbent assays (ELISAs).
Wells of Immulon 2 plates (Dynex Technologies) were coated with 0.1 μg of the appropriate antigen in PBS with 0.02% sodium azide for 48 h at 4°C. After coating, plates were washed three times with PBS–0.1% Tween 20 by using a Labsystems Wellwash 4 Mk 2 plate washer and then blocked for 90 min at 37°C with PBS–3% ovalbumin (grade II)–0.1% Tween 20. Plates were then washed three times and incubated with patient sera at a 1:250 dilution in PBS–0.1% ovalbumin (grade V)–0.05% Tween 20 for 1 h at 37°C. Plates were washed three additional times, rinsed once with Tris-buffered saline, and then incubated with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (Jackson Immunoresearch) for 30 min at 37°C. After plates were washed a final three times with PBS-Tween, they were rinsed once with Tris-buffered saline. The substrate, p-nitrophenylphosphate (SigmaFAST tablets; Sigma Chemical Co., St. Louis, Mo.), was added, and color was developed for 30 min at 37°C. Absorbance at 405 nm was read with a Dynatech MR5000 plate spectrophotometer. For each plate, the absorbance value of an appropriately coated well that received no primary serum (dilution buffer-only blank) was subtracted from the values for all test wells for that antigen. Triplicate blanked test absorbance values for each antigen were averaged and reported for each patient. The absorbance values of all populations were log transformed to approximate a normal distribution before analysis. Data analysis was performed with the InStat (GraphPad Software, San Diego, Calif.) software package for the Macintosh.
Data analyses.
Serological responses were analyzed to determine the magnitude of seroreactivity and the frequency of a positive response within each of the test groups. The magnitude, or amount of antigen-specific antibody present in the serum, was indicated by each population's net ELISA absorbance values. Statistical differences in the level of antibody reactivity between groups and subgroups were determined by analysis of variance with Student-Newman-Keuls post tests. In addition, patterns of antigen reactivity of individuals within each group were examined for trends. Best-fit regression lines and corresponding coefficients of correlation (r values) were calculated with Cricket Graph III software (Computer Associates, Islandia, N.Y.). The frequency of a positive serological response was also determined. Seropositivity was defined as having a response to an antigen that was significantly greater than the response made by the control uninfected population. To be deemed seropositive, a patient's antigen-specific ELISA absorbance had to be greater than or equal to the mean plus 2 standard deviations of the CU population's same-antigen reactivity. Significant differences in frequency between test groups were determined with Fisher's exact test.
EB+ and EB− subgroups.
In this study, women with tubal damage were compared with women who had either acute infections or no history of prior chlamydial infection. This allowed us to note differences in antigenic reactivity that may correlate with the severity of disease. To avoid potential bias that could be explained by different rates of Chlamydia seropositivity among the patient populations, any associations of increased reactivity to a specific test antigen with a disease state were viewed relative to the rate of overall immunoreactivity to chlamydiae in that population. We accomplished this by standardizing those responses by the patient's overall seroreactivity to whole Chlamydia organisms. EB seropositivity, as determined by ELISA reactivity to whole formalin-fixed serovar E EBs, served as a common denominator by which specific antigenic reactivity was compared between patient populations.
RESULTS
EB seropositivity.
Patients in each of the test groups were divided into EB-seropositive (EB+) and EB-seronegative (EB−) subpopulations based on their seroreactivity to whole formalin-fixed serovar E EBs relative to the CU population. Division into subgroups resulted in 1 of 42 (2%) of CU women, 48 of 137 (35%) of AI women, and 23 of 39 (59%) women with TFI being deemed EB+. Patients that did not have an antibody level significantly greater than that of the control population were placed in the EB− subgroup.
Magnitude of response.
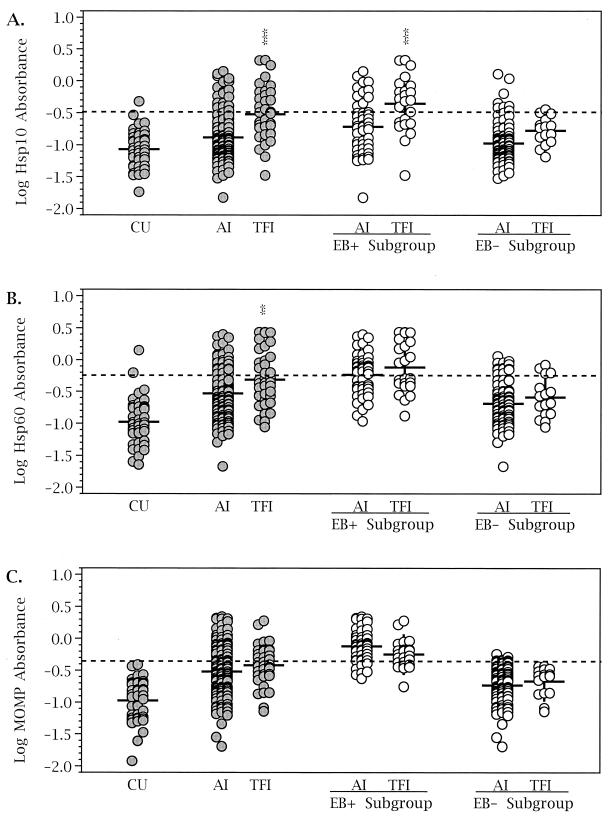
Antigen-specific immunoglobulin G reactivity to Hsp10, Hsp60, and MOMP was measured by ELISA for the three test groups (Fig. 1). The levels of reactivity to Hsp10, Hsp60, and MOMP observed in the CU population were low, not significantly different from each other, and not significantly different from CU reactivity to the irrelevant protein ovalbumin (data not shown). Women with AI had a higher level of reactivity to Hsp60 (P < 0.001) and to MOMP (P < 0.001) than the uninfected controls. Sera from the AI population, however, did not significantly react with Hsp10 despite showing an upward trend (Fig. 1A). Sera from TFI patients reacted strongly to all three antigens (Fig. 1). Serological responses to the panel of antigens were then compared between the TFI and the AI populations. A significantly higher level of reactivity to Hsp10 (P < 0.001) and Hsp60 (P < 0.01) was observed in the TFI group (Fig. 1A and B). In contrast, anti-MOMP responses were not different between the AI and TFI groups (Fig. 1C).
FIG. 1.
Comparison of the magnitude of the serological response to C. trachomatis antigens among test groups and subgroups. The amount of antigen-specific antibody present in sera from each population was determined by ELISA. For further analysis, test groups were divided into EB+ and EB− subgroups (see Materials and Methods). Significant differences between AI and TFI groups and subgroups were determined by analysis of variance with Student-Newman-Keuls post tests (∗∗, P < 0.01; ∗∗∗, P < 0.001). Dashed lines indicate cutoff values for determination of a positive response (see Materials and Methods). The mean for each population is indicated by a short horizontal line.
The AI and TFI groups were each divided into EB+ and EB− subgroups to standardize the populations based on their level of reactivity to chlamydiae. Anti-MOMP activity was not different between TFI and AI populations regardless of subgrouping (Fig. 1C). Patients in the EB+ subgroup, however, had a higher range of anti-MOMP reactivity, while the reactivity of patients in the EB− subgroup approached the level of the CU population. The levels of anti-Hsp60 reactivity (Fig. 1B), though different in the TFI and AI groups, were not significantly different when the data were restricted by EB reactivity. Thus, the anti-Hsp60 responses in the TFI population correlated with a greater overall serological response to chlamydiae rather than disease severity. In contrast, the elevated response to Hsp10 (Fig. 1A) in the EB+ TFI subgroup compared to that in the EB+ AI subgroup (P < 0.001) correlated with an increase in disease severity, since EB seropositivity was not different between those groups.
Frequency of response.
The number of patients seropositive for Hsp10, Hsp60, and MOMP was determined for each of the test groups and the EB+ and EB− subgroups (Table 1). The AI and TFI groups each had more patients with seropositive antibody levels than the CU women. Separating the patients into EB+ and EB− subgroups revealed a dramatic difference in antigenic reactivity between the two subgroups. In all cases, the majority of patients seropositive for Hsp10, Hsp60, or MOMP tended to be in the EB+ subgroup rather than the EB− subgroup. The frequency of a positive anti-MOMP response in the TFI population was no different from that of the AI population (Table 1). Hsp60 seropositivity showed a modest increase from the AI group to the TFI group (P = 0.05); however, there was no significant difference in the rates of Hsp60 seropositivity between TFI and AI groups within either the EB+ or EB− subgroup. Hsp10 seropositivity had a different pattern among these groups. Hsp10 seropositivity was significantly greater in the TFI group than in the AI group (P < 0.001). Moreover, this trend was maintained within the EB+ subgroups, which represent women with otherwise similar chlamydial seroreactivity. Thus, when overall chlamydial seropositivity was used as a common denominator, only anti-Hsp10 seropositivity was significantly associated with infertility.
TABLE 1.
Number of seropositive women in each group and subgroupa
| Group | n | No. (%) positive for:
|
||
|---|---|---|---|---|
| Hsp10 | Hsp60 | MOMP | ||
| Control | 42 | 1 (0.24) | 2 (4.76) | 0 |
| AI | 137 | 21 (15.44) | 39 (28.47) | 38 (27.73) |
| TFI | 39 | 17 (44.74) | 18 (46.15)∗ | 14 (35.90) |
| EB+ subgroups | ||||
| AI | 48 | 12 (25.00) | 26 (54.17) | 36 (75.00) |
| TFI | 23 | 15 (65.22)∗∗ | 14 (60.87) | 14 (60.87) |
| EB− subgroups | ||||
| AI | 89 | 9 (10.22) | 13 (14.61) | 2 (2.25) |
| TFI | 16 | 2 (13.33) | 4 (25.00) | 0 |
A positive result was defined as reactivity greater than or equal to the mean for the CU population plus 2 standard deviations. Fisher's exact test was used to compare AI and TFI values within a subgroup: ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Patterns of antigenic responses.
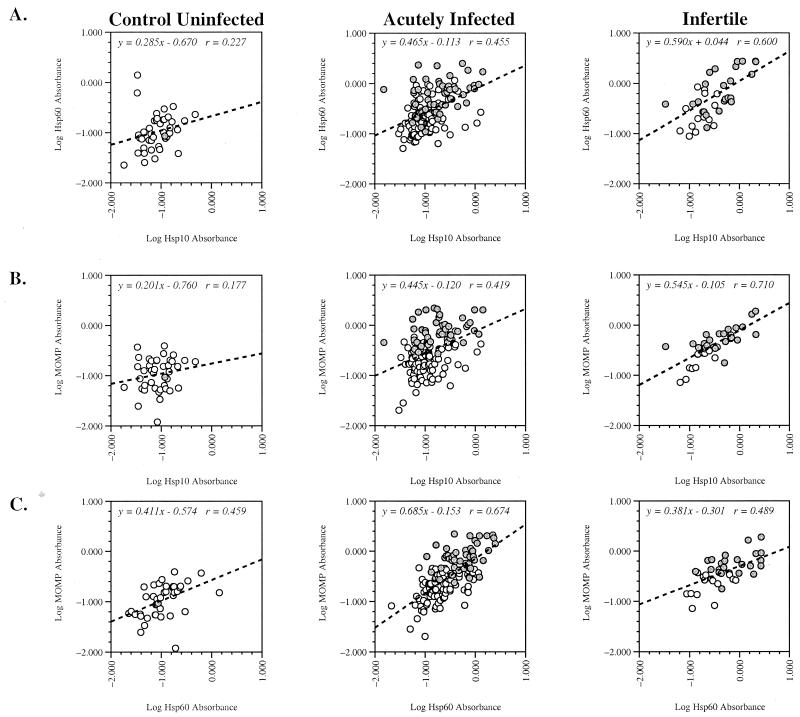
Relationships between the immune responses to Hsp10, Hsp60, and MOMP were explored among the three test groups (Fig. 2). To determine how patient reactivity to one chlamydial antigen correlated with reactivity to other antigens, levels of antigenic reactivity to sets of antigens were compared among the CU, AI, and TFI groups. Absorbance values for pairs of test antigens were plotted, and a regression line and corresponding coefficient of correlation were determined. The following sets of antigens were compared: Hsp10 and Hsp60 (Fig. 2A), Hsp10 and MOMP (Fig. 2B), and Hsp60 and MOMP (Fig. 2C).
FIG. 2.
Patterns of patient-specific serological reactivity to pairs of chlamydial antigens for the three test groups. A computer-interpolated regression line and corresponding line equation with coefficient of correlation are provided in each panel. Shaded circles, patients in the EB+ subgroup; open circles, patients in the EB− subgroup.
A notable direct relationship was observed in the TFI population, where the level of reactivity to Hsp10 appeared to be reflective of the level of Hsp60 reactivity (Fig. 2A). The AI population showed an intermediate relationship in which the degree of Hsp10 reactivity was predictive of the level of Hsp60 reactivity but the converse did not hold. For example, patients with high levels of seroreactivity to Hsp10 tended to have high levels of seroreactivity to Hsp60, while patients with high levels of seroreactivity to Hsp60 did not necessarily have high levels of Hsp10 seroreactivity. Those patterns were especially apparent in the EB+ subpopulations (Fig. 2A).
Both anti-Hsp10 and anti-Hsp60 responses were found to be predictive of the level of anti-MOMP response (Fig. 2B and C) in the TFI population. Since the anti-MOMP response was very closely related to the anti-EB response (r = 0.91 for either AI or TFI; data not shown), this pattern likely reflects a greater overall serological response to chlamydiae. The AI population, however, reacted differently to Hsp10 than to Hsp60. The relationship of anti-Hsp10 responses to anti-MOMP responses was considerably different from the relationship between anti-Hsp60 responses and anti-MOMP responses. Higher anti-Hsp10 levels were generally predictive of higher anti-MOMP levels, but high anti-MOMP levels did not necessarily predict anti-Hsp10 levels (Fig. 2B). Thus, the seroreactivity of Hsp10 appeared to be independent of the seroreactivity of MOMP and EBs. In contrast, the seroreactivities of Hsp60 and MOMP were predictive of each other in both the AI and TFI populations. Those patterns suggest that the level of Hsp60 seroreactivity was influenced by the overall level of response to chlamydial antigens, but the level of Hsp10 seroreactivity was independent of MOMP and Hsp60 responses and correlated with disease severity.
Frequency of seropositivity to multiple chlamydial antigens.
The interrelationship of the immune response to Hsp10, Hsp60, and MOMP was also examined by determining the number of patients in each group or subgroup that were seropositive for two or more of those chlamydial antigens (Table 2). The TFI population was more likely to be positive for both Hsp10 and Hsp60 than the AI population. This relationship also held when the analysis was restricted to the EB+ subgroup to control for overall chlamydial exposure. Similarly, a greater number of TFI patients than AI patients were positive for both Hsp10 and MOMP. However, the frequency of seropositivity to both Hsp60 and MOMP was not significantly different between the TFI and AI populations. An increased overall serological response to chlamydiae, as indicated by placement into the EB+ and EB− subgroups, had the greatest influence on the likelihood that a patient's serum would recognize multiple chlamydial antigens. Sera from patients in the EB− subgroup recognized more than one chlamydial antigen only rarely (Table 2). Thus, TFI patients were more likely than AI patients to be seropositive for more than one test antigen even when both the AI and TFI groups were serologically EB+. Those observations provided further evidence that greater overall antigenic exposure to chlamydiae is associated with infertility and is responsible for the increased immunoreactivity seen to most antigens in TFI patients. Only the serological response to Hsp10 increased in relation to severity of disease rather than as a function of increased overall chlamydial seroreactivity.
TABLE 2.
Percentage of patients in each group and subgroup that were seropositive for two or more chlamydial antigensa
| Group | % Positive for:
|
|||
|---|---|---|---|---|
| Hsp10 + Hsp60 | Hsp10 + MOMP | Hsp60 + MOMP | Hsp10 + Hsp60 + MOMP | |
| Control | 0 | 0 | 0 | 0 |
| AI | 9.5 | 8.8 | 16.1 | 5.8 |
| TFI | 25.6∗∗ | 30.8∗∗∗ | 23.1 | 20.5∗∗ |
| EB+ subgroups | ||||
| AI | 18.8 | 22.9 | 45.8 | 16.7 |
| TFI | 39.1 | 52.2∗ | 39.1 | 34.8 |
| EB− subgroups | ||||
| AI | 4.5 | 1.1 | 0 | 0 |
| TFI | 6.3 | 0 | 0 | 0 |
Combinations are not mutually exclusive. Fisher's exact test was used to compare AI and TFI values within a subgroup: ∗, P < 0.05; ∗∗, P < 0.05; ∗∗∗, P < 0.001.
DISCUSSION
The immune response to C. trachomatis not only is critical to the resolution of infection but also may be responsible for disease progression. Because of this dichotomy, it is imperative to understand the nature and substance of the anti-Chlamydia immune response and how it differs during a mild infection and during chronic inflammation with severe consequences. The precise mechanisms that lead to immunopathology and consequent organ dysfunction in humans remains largely unknown; however, identification of the antigens associated with immunopathology is a crucial step in understanding these processes. Ultimately the development of a safe and effective vaccine or a serological test to determine probable risk of developing disease sequelae will depend on understanding the interplay between chlamydial antigens and the host immune responses that evoke protection or pathology.
We have evaluated the human immune response to chlamydial Hsp10 and assessed the potential of that immune response to be a marker of advanced chlamydial disease. Hsp10 was identified to be a target of the human serological response after infection and after the development of immunopathology. Women with either active infections or tubal factor infertility recognized Hsp10 more frequently and to a greater degree than healthy uninfected women. Moreover, sera from women with TFI recognized Hsp10 more frequently and exhibited higher antibody titers than sera from AI women. Thus, a stepwise increase in seroreactivity was observed: a very low level of reactivity in the CU women, an intermediate level of seroreactivity in the AI population, and a high level of seroreactivity in the population of women with TFI. Therefore, the levels of Hsp10 seroreactivity related directly to the level of disease severity. Serological reactivity to Hsp10 in our patient populations was similar to the pattern of responses reported by several groups of investigators for Hsp60 (1, 10, 14, 19, 24, 28). They find that seropositivity is greater in patient populations with severe disease and increased risk of immunopathology than in healthy populations or populations with uncomplicated acute infection. The association of anti-Hsp60 responses with disease sequelae is supported by the findings that chlamydial Hsp60 induces immunopathology in certain experimental models (20, 23); however, high serological titers of antibody to Hsp60 do not always indicate the presence of increased pathology (25, 26). Although our observations of patient seroreactivity to Hsp10 also fit this pattern, a direct role for Hsp10 in the induction of immunopathology is currently undefined. The association of the serological response to chlamydial Hsp10 with tubal infertility may not necessarily implicate Hsp10 in immunopathogenesis but is indicative of advanced disease.
The increased levels or incidence of antigen-specific responses that are associated with immunopathogenesis may occur as a result of either multiple infections or persistent exposure to chlamydial antigens (4–6, 11). To avoid misconstruing anti-Hsp responses as mere reflections of exposure to chlamydiae, we used seropositivity to whole EBs as a common denominator for several of our analyses, and thereby each subgroup was standardized to have like levels of overall antichlamydial activity. Although the antichlamydial serological response may often include serovar-specific epitopes on MOMP (and therefore EBs), it was not evident that the use of only serovar E EBs and MOMP compromised the outcome of our analyses (see Materials and Methods). The serological response to MOMP and Hsp60 in our patient populations was measured to provide a framework from which to interpret Hsp10 results. Anti-MOMP antibody titers and seropositivity rates did not associate with tubal infertility. Although MOMP seropositivity was dependent on EB seropositivity, there was no change in outcome when data were compared within subgroups. In contrast, assigning patients to EB+ or EB− subgroups had a marked effect on Hsp60 seroreactivity patterns, such that differences between AI and infertile populations became insignificant when the analysis was restricted to respective EB subgroups. Conversely, the association of Hsp10 seroreactivity with TFI remained significant when the analysis was restricted to the EB+ subgroup. The relationship of the serological responses to Hsp60 and MOMP also reflect these findings. Hsp60 seroresponses correlated with MOMP seroresponses more closely than did Hsp10 seroresponses in the AI population. In addition, the relationship between Hsp60 and Hsp10 seroreactivity differed between the AI and TFI populations. In both the AI and TFI populations, women with high Hsp10 seroreactivity tended to also have high Hsp60 seroreactivity. The converse, however, was true only in the TFI population, suggesting that Hsp10 is less immunogenic than Hsp60 or MOMP; thus, repeated or persistent exposure to Hsp10 may be required to induce a significant serological response. This profile may therefore be useful in the development of a diagnostic reagent that distinguishes between acute chlamydial infection and severe disease such as tubal infertility.
Our current study identifies Hsp10 as an antigen recognized by a significant proportion of women with TFI. The association of the immune response to Hsp10 with severity of genital tract disease, and the recent report that the chlamydial Omp2 may elicit immunopathology (2), suggests that proteins other than Hsp60 may have a role in the development of chlamydial disease or may prove useful as immunologic markers of advanced disease. Continued investigation to better define how the immune system recognizes and responds to Hsp10 in protection and/or immunopathology will be needed to determine whether Hsp10 participates directly in the development of disease and whether anti-Hsp10 serology can be used for the diagnosis of advanced chlamydial disease.
ACKNOWLEDGMENTS
This work was supported by grants AI 19782 (G.I.B.) and AI 38991 (R.P.M.) and the ACOG/Curatek Research Award in Lower Genital Tract Infections (K.A.A.).
We thank the nurses and staff at the Indianapolis, Madison, and Iowa City hospitals and clinics for collecting and processing the patient sera used in this study.
REFERENCES
- 1.Ault K, Statland B, Smith King M, Dozier D, Joachims M, Gunter J. Antibodies to the chlamydial 60 kilodalton heat shock protein in women with tubal factor infertility. Infect Dis Obstet Gynecol. 1998;6:163–167. doi: 10.1002/(SICI)1098-0997(1998)6:4<163::AID-IDOG5>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmaier K, Neu N, de la Maza L M, Pal S, Hessel A, Penninger J M. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283:1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P F, Mehra V, Rivoire B, Fong S-J, Brennan P J, Voegtline M S, Minden P, Houghten R A, Bloom B, Modlin R L. Immunoreactivity of a 10 kDa antigen of Mycobacterium tuberculosis. J Immunol. 1992;148:1835–1840. [PubMed] [Google Scholar]
- 4.Beatty W L, Byrne G I, Morrison R P. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 5.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Recommendations for the prevention and management of Chlamydia trachomatis infections, 1993. Morbid Mortal Weekly Rep. 1993;42:1–39. [PubMed] [Google Scholar]
- 9.Domeika M, Domeika K, Paavonen J, Mårdh P-A, Witkin S S. Humoral immune response to conserved epitopes of Chlamydia trachomatis and human 60-kDa heat-shock protein in women with pelvic inflammatory disease. J Infect Dis. 1998;177:714–719. doi: 10.1086/514218. [DOI] [PubMed] [Google Scholar]
- 10.Eckert L, Hawes S, Wölner-Hanssen P, Money D, Peeling R, Brunham R, Stevens C, Eschenbach D, Stamm W. Prevalence and correlates of antibody to chlamydial heat shock protein in women attending sexually transmitted disease clinics and women with confirmed pelvic inflammatory disease. J Infect Dis. 1997;175:1453–1458. doi: 10.1086/516479. [DOI] [PubMed] [Google Scholar]
- 11.Grayston J T, Wang S P, Yeh L J, Kuo C-C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 12.Iangumaran S, Ramanathan S, Shankernarayan N, Ramu B, Muthukkarauppan V. Immunological profiles of leprosy patients and healthy family contacts toward M. leprae antigens. Int J Lepr Other Mycobact Dis. 1996;64:6–14. [PubMed] [Google Scholar]
- 13.Kim J, Sette A, Rodda S, Southwood S, Sieling P A, Mehra V, Ohmen J D, Oliveros J, Apella E, Higashimoto Y, Rea T H, Bloom B, Modlin R L. Determinants of T cell reactivity to the Mycobacterium leprae GroES homologue. J Immunol. 1997;159:335–343. [PubMed] [Google Scholar]
- 14.Kimani J, Maclean I W, Bwayo J J, MacDonald K, Oyugi J, Maitha G M, Peeling R W, Cheang M, Nagelkerke N J, Plummer F A, Brunham R C. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis. 1996;173:1437–1444. doi: 10.1093/infdis/173.6.1437. [DOI] [PubMed] [Google Scholar]
- 15.Launois P, Niang N'Diaye M, Cartel J L, Mane I, Drowart A, van Vooren J P, Sarthou J L, Huygen K. Fibronectin-binding antigen 85 and the 10 kilodalton GroES-related heat shock protein are the predominant TH-1 response inducers in leprosy contacts. Infect Immun. 1995;63:88–93. doi: 10.1128/iai.63.1.88-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaVerda D, Byrne G I. Use of monoclonal antibodies to facilitate identification, cloning, and purification of Chlamydia trachomatis Hsp10. J Clin Microbiol. 1997;35:1209–1215. doi: 10.1128/jcm.35.5.1209-1215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra V, Bloom B, Bajardi A C, Grisso C L, Sieling P A, Alland D, Convit J, Fan X, Hunter S W, Brennan P J, Rea T H, Modlin R L. A major T cell antigen of Mycobacterium leprae is a 10 kDa heat shock cognate protein. J Exp Med. 1992;175:275–284. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minden P, Houghton R A, Spear J R, Shinnick T M. A chemically synthesized peptide which elicits humoral and cellular immune responses to mycobacterial antigens. Infect Immun. 1986;53:560–564. doi: 10.1128/iai.53.3.560-564.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Money D, Hawes S, Eschenbach D, Peeling R, Brunham R, Wölner-Hanssen P, Stamm W. Antibodies to the chlamydial 60 kd heat-shock protein are associated with laparoscopically confirmed perihepatitis. Am J Obstet Gynecol. 1997;176:870–877. doi: 10.1016/s0002-9378(97)70613-6. [DOI] [PubMed] [Google Scholar]
- 20.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kd protein. J Exp Med. 1989;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison R P, Su H, Lyng K, Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groE stress response operon of Escherichia coli. Infect Immun. 1990;58:2701–2705. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease. Hum Reprod Update. 1996;2:519–529. doi: 10.1093/humupd/2.6.519. [DOI] [PubMed] [Google Scholar]
- 23.Patton D L, Cosgrove-Sweeney Y T, Kuo C-C. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 24.Peeling R W, Kimani J, Plummer F, Maclean I, Cheang M, Bwayo J, Brunham R C. Antibody to chlamydial hsp60 predicts an increased risk for chlamydial pelvic inflammatory disease. J Infect Dis. 1997;175:1153–1158. doi: 10.1086/516454. [DOI] [PubMed] [Google Scholar]
- 25.Rank R G, Dascher C, Bowlin A K, Bavoil P M. Systemic immunization with Hsp60 alters the development of chlamydial ocular disease. Invest Ophthalmol Vis Sci. 1995;36:1344–1351. [PubMed] [Google Scholar]
- 26.Rank R G, Sanders M M, Patton D L. Increased incidence of oviduct pathology in the guinea pig after repeat vaginal inoculation with the chlamydial agent of guinea pig inclusion conjunctivitis. Sex Transm Dis. 1995;22:48–54. doi: 10.1097/00007435-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Sziller I, Witkin S S, Ziegert M, Csapó Z, Ujházy A, Papp Z. Serological responses of patients with ectopic pregnancy to epitopes of the Chlamydia trachomatis 60 kDa heat shock protein. Hum Reprod. 1998;13:1088–1093. doi: 10.1093/humrep/13.4.1088. [DOI] [PubMed] [Google Scholar]
- 28.Toye B, Laferriere C, Claman P, Jessamine P, Peeling R. Association between antibody to the chlamydial heat-shock protein and tubal infertility. J Infect Dis. 1993;168:1236–1240. doi: 10.1093/infdis/168.5.1236. [DOI] [PubMed] [Google Scholar]
- 29.Uyemura K, Ohmen J D, Grisso C L, Sieling P A, Wyzykowski R, Reisinger D M, Rea T H, Modlin R L. Limited T cell receptor β-chain diversity of a T helper cell type 1-like response to Mycobacterium leprae. Infect Immun. 1992;60:4542–4548. doi: 10.1128/iai.60.11.4542-4548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van der Pol B, Williams J A, Jones R B. Rapid antigen detection assay for identification of Chlamydia trachomatis infection. J Clin Microbiol. 1995;33:1920–1921. doi: 10.1128/jcm.33.7.1920-1921.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker K B, Butler R, Colston M J. Role of Th1 lymphocytes in the development of protective immunity against Mycobacterium leprae. J Immunol. 1992;148:1885–1889. [PubMed] [Google Scholar]
- 32.Witkin S S, Jeremias J, Toth M, Ledger W J. Cell-mediated immune response to the recombinant 57-kDa heat-shock protein of Chlamydia trachomatis in women with salpingitis. J Infect Dis. 1993;167:1379–1383. doi: 10.1093/infdis/167.6.1379. [DOI] [PubMed] [Google Scholar]
- 33.Witkin S S, Askienazy-Elbhar M, Henry-Suchet J, Belaisch-Allart J, Tort-Grumbach J, Sarjdine K. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60 kDa heat shock protein (hsp60) in infertile couples and its relationship to antibodies to C. trachomatis surface antigens and the Escherichia coli and human HSP60. Hum Reprod. 1998;13:1175–1179. doi: 10.1093/humrep/13.5.1175. [DOI] [PubMed] [Google Scholar]
- 34.Yamamura M, Wang X-H, Ohmen J D, Uyemura K, Rea T H, Bloom B, Modlin R L. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]
- 35.Yuan Y, Lyng K, Zhang Y X, Rockey D D, Morrison R P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]