Abstract
Background
Up to one-third of patients hospitalized for acute severe colitis secondary to inflammatory bowel diseases (IBD) do not adequately respond to intravenous steroids. There is an unmet need to identify a useful predictor for rescue treatment in this cohort of patients.
Aims
The aim of this study was to assess the predictive efficacy of fecal calprotectin in identifying the need for medical or surgical therapy in patients with acute severe colitis.
Methods
We conducted a multicenter retrospective cohort study including patients with ulcerative colitis (UC) who were hospitalized for severe exacerbation of colitis. The primary outcome was the need for in-hospital medical or surgical rescue therapy. Univariate and multivariate logistic regression was performed to identify predictors of rescue therapy.
Results
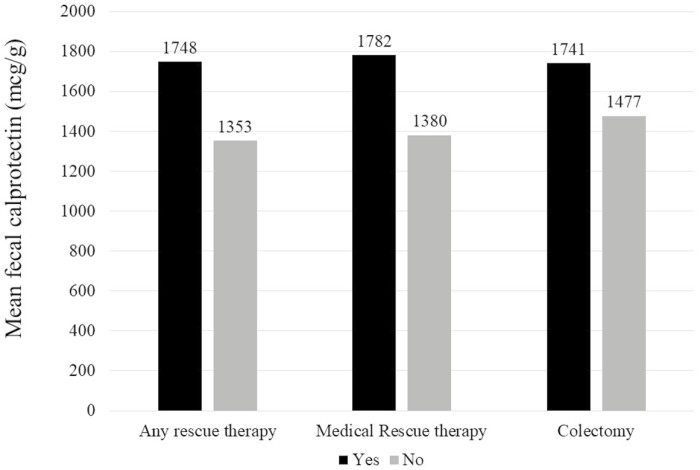
Our study included 147 patients with UC. One-third (33%) required rescue therapy, and 13% underwent colectomy. Patients requiring rescue therapy had significantly higher fecal calprotectin (mean 1748 mcg/g vs 1353 mcg/g, P = .02) compared with those who did not. A fecal calprotectin >800 mcg/g independently predicted the need for inpatient medical rescue therapy (odds ratio, 2.61; 95% CI, 1.12-6.12). An admission calprotectin >800 mcg/g independently predicted surgery within 3 months (odds ratio, 2.88; 95% CI, 1.01-8.17).
Conclusions
Fecal calprotectin levels may serve as a useful noninvasive predictor of medical and surgical risk in individuals with UC presenting with acute severe colitis. This approach can facilitate earlier therapeutic interventions and improve outcomes.
Keywords: ulcerative colitis, biologics, fecal calprotectin, rescue therapy
Introduction
Acute severe colitis, affecting up to 25% of patients with ulcerative colitis, is a severe complication often requiring hospitalization for prompt assessment and intervention.1 Despite treatment with intravenous corticosteroids, up to one-third of patients may have no response or partial response and require medical or surgical rescue therapy2. Early prediction of patients who are likely to require such rescue therapy is essential to reduce treatment delays and morbidity. Existing prediction models incorporate assessment of patient-reported symptoms, serum inflammatory markers such as C-reactive protein (CRP), endoscopic severity, or radiologic features at presentation3,4. However, models with clinical and serum inflammatory markers alone have a suboptimal performance; additionally, models incorporating endoscopic or radiologic scoring lack standardization and generalizability across practice settings that vary in the expertise of the treating physician.
Fecal calprotectin, a cytosolic protein present in neutrophils, is a readily available and commonly used biomarker in the management of IBD.5 Although its use has been extensively studied in differentiating IBD from noninflammatory conditions such as irritable bowel syndrome,6,7 studies have also demonstrated that calprotectin has a strong correlation with endoscopic severity of inflammation in IBD8,9. In longitudinal studies, a single fecal calprotectin measure could predict subsequent relapse in those with quiescent disease and was a responsive marker in therapeutic interventions10. The widespread availability of this objective biomarker, its potential for point-of-care assessment, and its mechanistic relevance makes it an attractive biomarker as a predictor of rescue therapy requirement in acute severe colitis. However, there is a paucity of data regarding its use as a risk stratification tool in this setting.
We performed this study to examine the predictive utility of a single fecal calprotectin measurement obtained during hospitalization in patients with acute severe colitis in determining the need for medical or surgical rescue therapy.
Methods
Study Population
This study included all UC-related hospitalizations within the MassGeneral Brigham health care system, comprising 2 tertiary referral centers (Massachusetts General Hospital, Brigham and Women’s Hospital) and affiliated hospitals in the Greater Boston metropolitan region from 2018-2020. Potential cases were identified using the Research Practice Data Registry, a data warehouse of all inpatient and outpatient visits within the MassGeneral Brigham network through use of the diagnostic codes for ulcerative colitis (ICD-9 556.x, ICD-10 K51.x). Each potential case was reviewed to confirm a diagnosis of ulcerative colitis, hospitalization related to a severe exacerbation, treatment with intravenous corticosteroid therapy, and an available calprotectin level obtained either during the hospitalization or within 2 weeks preceding admission. All patients met the definition for acute severe colitis as defined by Truelove and Witts.11 Hospital stays not related to IBD, patients with Crohn’s disease, autoimmune enteropathy or common variable immunodeficiency (CVID)-related disease, admissions for elective surgery, and admissions for pouch-related disorders were excluded.
Covariates
Information was extracted regarding age, sex, and smoking status. Disease extent was classified according to the Montreal classification. Severity at the time of hospitalization was quantified using admission hemoglobin, albumin, peak erythrocyte sedimentation rate (ESR), CRP, and endoscopic severity where available. Endoscopic severity was classified using the Mayo endoscopic score (MES) and stratified as severe (presence of ulcerations and spontaneous bleeding) or nonsevere. Our main predictor of interest was fecal calprotectin measured on a single stool sample and quantified as mcg/g stool. Fecal calprotectin levels were measured using an enzyme-linked immunosorbent assay (ELISA) platform at the Mayo Clinic laboratories (Rochester, MN).
Outcomes
Our primary outcome was need for in-hospital medical or surgical rescue therapy. Medical rescue treatment included initiation of infliximab, cyclosporine, or tofacitinib. However, in a sensitivity analysis accounting for the patients who may have already been infliximab-experienced, we defined medical rescue therapy to also include a new in-hospital start of adalimumab, vedolizumab, or ustekinumab in patients who were naïve to these agents. Surgical rescue therapy referred to in-hospital colectomy during the index hospitalization.
Postdischarge outcomes were assessed at 3 months and 1 year after index hospitalization. Clinical remission at these time points was ascertained through retrospective review of the medical records, defining remission using a composite of UC-related symptoms, inflammatory markers, endoscopy, or radiologic examination. We also examined if an interval UC-related surgery occurred by these time points.
Statistical Analysis
Statistical analysis was performed using Stata 15.2 (StataCorp, College Station, TX). Continuous variables were summarized using means and standard deviations and compared using the t test. Variables that were not normally distributed were summarized using medians and interquartile ranges and compared using nonparametric tests. Categorical variables were presented as proportions and compared using the χ2 test; Fisher exact test was used when necessary. Univariate logistic regression was performed to identify predictors of requiring medical or surgical rescue therapy. Variables significant on univariate analysis at P < .2 were included in a multivariable model to identify independent predictors.
In the primary analysis, fecal calprotectin was modeled as a continuous variable. We then used the Youden index, a summary measure for the performance of the receiver operator characteristics (ROC) curve, to identify the optimal fecal calprotectin threshold to differentiate responders from nonresponders. In subsequent analyses, we modeled fecal calprotectin as a dichotomous measure above and below this cutoff to define its predictive value. We repeated the analysis separately for need of medical rescue therapy and in-hospital surgery. We performed a sensitivity analysis that defined rescue therapy as use of only infliximab or cyclosporine. Two-sided P-value < .05 indicated independent statistical significance in the multivariable model. The study was approved by the institutional review board of MassGeneral Brigham.
Results
Study Population
Our study included 147 patients admitted with acute severe colitis. The mean age was 39 years (range 7-87 years). Just over half (51%) of the cohort were female (n = 75). Of the entire cohort, 33% (n = 48) required rescue therapy after failure to respond to intravenous steroids (2 tofacitinib; the rest received infliximab or cyclosporine). An additional 10 patients were initiated on adalimumab, vedolizumab, or ustekinumab during the hospitalization. A total of 19 patients (13%) underwent rescue surgery during the index hospitalization. Eight patients who underwent colectomy had previously received medical rescue therapy during the same hospitalization.
Table 1 compares the characteristics of patients who failed intravenous steroids and required rescue therapy to steroid responders. There was no significant difference in age, age at disease diagnosis, smoking status, and prior disease severity including immunomodulator or biologic use at time of admission. However, patients requiring rescue with either medical or surgical therapy had significantly higher fecal calprotectin (median 1588 mcg/g vs 1000 mcg/g; P = .02; Figure 1), lower hemoglobin (mean 9.2 g/dL vs 10.5 g/dL), and lower albumin (mean 3.4 g/dL vs 3.7 g/dL; P = .002). In addition, patients who required rescue therapy were more likely to have severe disease on endoscopic evaluation (80%) compared with those who were steroid responsive (59%, P = .013). Using the Youden method, the optimal cutoff for fecal calprotectin to separate those who required medical or surgical rescue therapy from steroid responders was ~800 mcg/g with an AUC of 0.61. At this cutoff, the sensitivity and specificity for medical or surgical rescue therapy was 0.80 and 0.42, respectively.
Table 1.
Baseline characteristics of hospitalized patients with ulcerative colitis who received rescue therapy (n = 59) and those who did not (n = 88).
| Characteristic | Rescue Therapy (Medical or Surgical) | No Rescue | P |
|---|---|---|---|
| n = 59 | n = 88 | ||
| Age(median) (IQR) | 32.0 (27-51) | 34.5 (22-55) | 0.61 |
| Age at diagnosis | 0.45 | ||
| < or =16 | 12% | 16% | |
| 17–40 | 61% | 51% | |
| >40 | 27% | 34% | |
| Male n (%) | 51% | 48% | 0.71 |
| Female n (%) | 49% | 52% | |
| Smoking status | 0.47 | ||
| Never | 63% | 72% | |
| Current | 2% | 2% | |
| Former | 35% | 26% | |
| Disease location/pancolitis | 68% | 71% | 0.71 |
| Medication use at time of admission | |||
| Immunomodulators | 15% | 13% | 0.63 |
| Anti-TNF agents | 39% | 44% | 0.52 |
| Labs during admission–Median (IQR) | |||
| Calprotectin | 1588 (913–2900) | 1000 (474–2274) | 0.02 |
| ESR peak (pretreatment) | 37 (22–53) | 33 (16–45) | 0.56 |
| CRP peak (pretreatment) | 54 (20–121) | 28 (7–77) | 0.08 |
| Hgb nadir | 9.2 (7.4–10.9) | 10.6 (9.2–11.9) | 0.002 |
| Albumin | 3.5 (2.9–3.8) | 3.6 (3.3–4.1) | 0.07 |
| Endoscopic findings | |||
| Mild | 20% | 41% | 0.013 |
| Severe | 80% | 59% | |
Figure 1.
Fecal calprotectin levels and the need for inpatient rescue therapy in acute severe colitis.
On multivariate analysis, a higher serum hemoglobin (odds ratio [OR], 0.76 for each 1 g/dL increase; 95% CI, 0.65-0.90) was associated with lower likelihood of rescue therapy. A fecal calprotectin >800 mcg/g was associated with a 2-fold increase in risk for rescue therapy (OR, 2.53; 95% CI, 1.15–5.59; Table 2). A fecal calprotectin >800 mcg/g independently predicted the need for inpatient medical rescue therapy (OR, 2.61; 95% CI, 1.12-6.12) and demonstrated a trend towards predicting in-hospital surgery (OR, 2.03; 95% CI 0.64-6.49). The median interval between date of hospitalization and fecal calprotectin testing was 0 days (interquartile range, 0-1 day). Of the 147 patients in the cohort, 130 (88%) had calprotectin levels checked within 48 hours after initiation of intravenous steroids. Restricting the analysis to this group alone did not alter our findings (OR, 2.77; 95% CI, 1.27-6.03).
Table 2.
Multivariate analysis of risk factors predicting need for inpatient rescue therapy (medical or surgical) in hospitalized patients with acute severe colitis.
| Characteristic | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Lowest hemoglobin (for each 1 g/dL increase) | 0.76 | 0.65–0.90 | 0.002 |
| Fecal calprotectin > 800 mcg/g | 2.53 | 115–5.89 | 0.021 |
With respect to long-term outcomes, patients requiring colectomy by 3 months after the index hospitalization had a median calprotectin level of 1951 mcg/g compared with those who did not (1000 mcg/g, P = .006). A similar difference was also noted in colectomy by 12 months (mean 1843 mcg/g vs 1021 mcg/g; P = .018; Table 3). Calprotectin >800 mcg/g independently predicted surgery within 3 months of the index hospitalization (OR, 2.88; 95% CI, 1.01-8.17); this association did not meet statistical significance at 12 months.
Table 3.
Fecal calprotectin during hospitalization and posthospitalization outcomes at 3 and 12 months among patients hospitalized with severe colitis.
| Time After Index Hospitalization | Outcome | Median Calprotectin Among Those With Outcome (mcg/g) (SD) | Median Calprotectin Among Those Without Outcome (mcg/g) (SD) | P |
|---|---|---|---|---|
| 3 months | ||||
| Colectomy | 1951 | 1000 | 0.006 | |
| Remission | 1000 | 1015 | 0.74 | |
| Steroid use | 1382 | 1000 | 0.34 | |
| 12 months | ||||
| Colectomy | 1843 | 1021 | 0.018 | |
| Remission | 1000 | 1598 | 0.31 | |
| Steroid use | 1000 | 1382 | 0.42 | |
Discussion
Acute severe colitis is a morbid complication of inflammatory bowel diseases. In the one-third of patients who do not respond satisfactorily to intravenous steroids, early initiation of medical or surgical rescue therapy is required.2 Thus, it is an important clinical and research goal to identify biomarkers that can be quantified at presentation and that can accurately identify the subgroup of steroid nonresponders. Existing models utilizing clinical parameters are insufficiently accurate, and those relying on endoscopic or radiologic assessment are challenged by standardization across different practice settings. In this retrospective cohort study, we demonstrate that a single admission fecal calprotectin level can accurately determine patients who are likely to require medical and surgical rescue therapy and suggest the need for more routine use of calprotectin in this setting.
Several predictive models have been proposed to predict responsiveness to therapy in acute severe colitis; but recently, the applicability and relevance of some of these models have been challenged by the increasing number of patients who have already previously failed multiple rescue therapies including infliximab prior to hospitalization. The Ho and Oxford scoring systems were developed to predict likelihood of nonresponse to intravenous steroids and risk of colectomy using a combination of reported clinical symptoms, serum biomarkers, and radiologic imaging.12 Initially these indices demonstrated high accuracy in predicting response. Although their performance has been more mixed due to the changing population and evolving treatment patterns in acute severe colitis, including a much lower rate of rescue in-hospital colectomy.4 A recent study assessing the use of Ho and Oxford scoring systems highlighted that surgical risk was decreased from previous studies (33.1% vs 11.8% and 34.0% vs 9.7%, respectively)3. The Ulcerative Colitis Endoscopic Index of Severity (UCEIS) has gained recognition as a tool to quantify endoscopic activity and as a predictor of response in acute severe colitis.13–15 Although data have been promising, it is invasive and resource limiting. Further, it relies on gastroenterologist familiarity with scoring index, limiting its potential to be generalizable and standardized in diverse clinical practices.
Fecal calprotectin is a noninvasive and sensitive biomarker. It correlates well with endoscopic disease activity. It is also superior to serum markers such as C-reactive protein.8, 9, 16–18 A prospective study comparing calprotectin levels with MES found that median calprotectin levels of 35.2 mcg/g, 103.3 mcg/g, 295.0 mcg/g, and 751.9 mcg/g correlated with an MES score of 0, 1, 2, and 3, respectively (P < .01)17. Although, there is only sparse literature examining its role in acute severe colitis. Ho et al examined the discriminant ability of fecal calprotectin in 90 hospitalized patients with ASUC to predict inpatient outcomes including steroid responsiveness, need for second-line agents such as infliximab (at 5 mg/kg dosing), and eventual need for colectomy.19 In their study cohort, 23% required infliximab therapy, and 34% required colectomy. Fecal calprotectin levels were higher in patients requiring colectomy (1200 mcg/g vs 887 mcg/g; P = .04), with a cutoff of 1922.5 mcg/g having optimal performance. Similarly, Beswick et al conducted a prospective study involving 24 patients with steroid-refractory ASUC initiated on infliximab20. Twelve (50%) patients achieved remission at 3 months, and 6 (25%) underwent colectomy. Fecal calprotectin level predicted clinical remission with 100% sensitivity and future colectomy with 89% sensitivity. Jain et al examined the value of both UCEIS and fecal calprotectin in 49 patients with acute severe colitis. They found a fecal calprotectin level >1000 mcg/g on day 3 of starting steroid therapy to be predictive of in-hospital outcomes. However, these studies were limited by sample sizes, reliance on infliximab as the sole rescue therapy (which is less reflective of the current practice of multiple biologic failures), and short duration of follow-up.
There are some important implications for our findings. First, our findings demonstrate that a single fecal calprotectin measurement can be an important risk assessment tool in patients with acute severe colitis. If robustly confirmed in other larger cohorts, it can be used for inexpensive and noninvasive risk stratification among ASUC patients by identifying those who are candidates for early initiation of rescue therapy due to high likelihood of steroid failure. It can also allow identification of an enriched patient population for trials of treatment strategies in acute severe colitis, where such patients with a high baseline fecal calprotectin can be preferentially treated with rescue agents at hospitalization rather than after a trial of intravenous steroids. There is also the need to examine repeated measurements of fecal calprotectin during hospitalization to assess its utility as a dynamic early marker of response. In addition, the availability of fecal calprotectin quantification through point-of-care testing makes it a particularly attractive tool for risk stratification in the setting of this rapidly evolving and morbid complication of IBD.21–24
We acknowledge several limitations to our study. Owing to the retrospective study design, there is the potential for missing information. Although calprotectin measurements were frequently performed in our hospital, they were not consistently performed at time of admission in all patients. There may also be a bias in the selection of the patients. Moreover, serial monitoring of calprotectin levels in response to treatment could also not be assessed because most patients had only a single assessment. It will be important to define both the timing of calprotectin measurement most relevant to predicting the outcome of the hospitalization and the utility of serial measurements of calprotectin. In terms of longitudinal analysis, there were some patients who were lost to follow-up by 1 year.
In conclusion, we demonstrate that fecal calprotectin levels can serve as a useful noninvasive predictor of disease severity and surgical risk. Larger prospective studies to validate the use of calprotectin as a predictor of longer-term outcome merits further investigation. This added information can serve as an essential prognostic tool to facilitate earlier interventions surrounding medical and surgical management and improve clinical outcomes.
Contributor Information
Saranya Sasidharan, Division of Gastroenterology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, United States.
Alexa N Sasson, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, United States.
Kevin M Shannon, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, United States.
Ashwin N Ananthakrishnan, Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts, United States.
Author Contributions
A.A. contributed to the study concept, design data analysis, and study supervision. S.S. contributed to the data extraction, analysis, and drafting of the manuscript. A.S. contributed to the data analysis and manuscript revision. K.S. contributed to the data extraction. All authors have approved the final version of the manuscript.
Funding
None
Conflicts of Interest
A.A. has served on the scientific advisory boards of Gilead, Ikena therapeutics, and Sun Pharma. A.A. is supported by grants from the National Institutes of Health, Crohn’s and Colitis Foundation, and Chleck Family Foundation. The other authors have no conflicts of interest to disclose.
Data Availability
Due to institutional research committee guidelines, the data are not publicly available.
References
- 1. Whaley KG, Rosen MJ.. Contemporary medical management of acute severe ulcerative colitis. Inflamm Bowel Dis. 2019;25:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Faubion WA, Jr, LoftusEV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. [DOI] [PubMed] [Google Scholar]
- 3. Lynch RW, Churchhouse AM, Protheroe A, et al. Predicting outcome in acute severe ulcerative colitis: comparison of the Travis and Ho scores using UK IBD audit data. Aliment Pharmacol Ther. 2016;43:1132–1141. [DOI] [PubMed] [Google Scholar]
- 4. Moore AC, Bressler B.. Acute severe ulcerative colitis: the Oxford criteria no longer predict in-hospital colectomy rates. Dig Dis Sci. 2020;65:576–580. [DOI] [PubMed] [Google Scholar]
- 5. Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. [DOI] [PubMed] [Google Scholar]
- 6. Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. [DOI] [PubMed] [Google Scholar]
- 7. Bertani L, Mumolo MG, Tapete G, et al. Fecal calprotectin: current and future perspectives for inflammatory bowel disease treatment. Eur J Gastroenterol Hepatol. 2020;32:1091–1098. [DOI] [PubMed] [Google Scholar]
- 8. Xiang JY, Ouyang Q, Li GD, et al. Clinical value of fecal calprotectin in determining disease activity of ulcerative colitis. World J Gastroenterol. 2008;14:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YW, Lee KM, Lee JM, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019;34:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. [DOI] [PubMed] [Google Scholar]
- 11. Truelove SC, Witts LJ.. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holvoet T, Lobaton T, Hindryckx P.. Optimal management of acute severe ulcerative colitis (ASUC): challenges and solutions. Clin Exp Gastroenterol. 2021;14:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jong D, C, Lowenberg M, Koumoutsos I, et al. Validation and investigation of the operating characteristics of the ulcerative colitis endoscopic index of severity. Inflamm Bowel Dis. 2019;25:937–944. [DOI] [PubMed] [Google Scholar]
- 14. Di Ruscio M, Variola A, Vernia F, et al. Role of Ulcerative Colitis Endoscopic Index of Severity (UCEIS) versus Mayo Endoscopic Subscore (MES) in predicting patients’ response to biological therapy and the need for colectomy. Digestion. 2021;102:534–545. [DOI] [PubMed] [Google Scholar]
- 15. Jain S, Kedia S, Bopanna S, et al. Faecal calprotectin and UCEIS predict short-term outcomes in acute severe colitis: prospective cohort study. J Crohns Colitis. 2017;11:1309–1316. [DOI] [PubMed] [Google Scholar]
- 16. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819; quiz 820; quiz 820. [DOI] [PubMed] [Google Scholar]
- 17. Kawashima K, Ishihara S, Yuki T, et al. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kristensen V, Klepp P, Cvancarova M, et al. Prediction of endoscopic disease activity in ulcerative colitis by two different assays for fecal calprotectin. J Crohns Colitis. 2015;9:164–169. [DOI] [PubMed] [Google Scholar]
- 19. Ho GT, Lee HM, Brydon G, et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol. 2009;104:673–678. [DOI] [PubMed] [Google Scholar]
- 20. Beswick L, Rosella O, Rosella G, et al. Exploration of predictive biomarkers of early infliximab response in acute severe colitis: a prospective pilot study. J Crohns Colitis. 2018;12:289–297. [DOI] [PubMed] [Google Scholar]
- 21. Rogler G, Aldeguer X, Kruis W, et al. Concept for a rapid point-of-care calprotectin diagnostic test for diagnosis and disease activity monitoring in patients with inflammatory bowel disease: expert clinical opinion. J Crohns Colitis. 2013;7:670–677. [DOI] [PubMed] [Google Scholar]
- 22. Vinding KK, Elsberg H, Thorkilgaard T, et al. Fecal calprotectin measured by patients at home using smartphones—a new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:336–344. [DOI] [PubMed] [Google Scholar]
- 23. Bello C, Roseth A, Guardiola J, et al. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017;49:991–996. [DOI] [PubMed] [Google Scholar]
- 24. Hejl J, Theede K, Mollgren B, et al. Point of care testing of fecal calprotectin as a substitute for routine laboratory analysis. Pract Lab Med. 2018;10:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to institutional research committee guidelines, the data are not publicly available.