Abstract
Background
To estimate the incidence of endocrinopathy in children and adolescents with craniopharyngioma after treatment with photon-based conformal and intensity-modulated radiation therapy (CRT).
Methods
One hundred one pediatric patients were enrolled on a phase II single-institution protocol beginning in 1998 (n = 76) or followed a similar non-protocol treatment plan (n = 25). Surgery was individualized. CRT (54 Gy) was administered using a 1.0-cm or ≤0.5-cm clinical target volume margin. Patients underwent baseline and serial evaluation of the hypothalamic-pituitary axis.
Results
The 10-year cumulative incidence (CI) of growth hormone deficiency (GHD) was 68.42% (±11.27) for black patients and 94.23% (±3.57) for white patients (P = .0286). The CI of thyroid-stimulating hormone deficiency (TSHD) was 70.94% (±8.44) at 10 years for non-shunted patients and 91.67% (±10.40) at 6 years for shunted patients (P = .0260). The CI of TSHD was 100% (±14.29) at 4 years for those with diabetes insipidus (DI) and 71.36% (±8.86) at 10 years for those without DI (P = .0008). The 10-year CI of adrenocortical hormone deficiency was 70.00% (±16.15) for those with DI and 48.39% (±9.19) for those without DI (P = .0080). The 10-year CI of LH/FSH deficiency was 43.33% (±9.32) age <7 years, 61.29% (±9.11) aged 7-10 years, and 78.95% (±6.38) age ≥10 years (P < .0001). BMI was significantly greater prior to CRT in white patients with DI (P = .0004) and preexisting GHD (P = .0275).
Conclusions
Hormone deficiencies are common in pediatric patients with craniopharyngioma and are associated with host, tumor, and treatment factors. Understanding the incidence and time to onset may facilitate intervention and patient selection for treatment.
Keywords: craniopharyngioma, endocrinopathy, outcomes, pediatrics, radiotherapy
Key Points.
Most patients with craniopharyngioma experience endocrinopathy.
Time to onset of endocrinopathy after treatment for craniopharyngioma can be estimated.
Tumor, host, and treatment factors contribute to endocrinopathy.
Importance of the Study.
Endocrinopathy is prevalent in children and adolescents diagnosed and treated for craniopharyngioma. Understanding the incidence of time to onset of clinically significant endocrinopathy associated with radiation therapy is important information that may be used to guide clinical care, design interventions, and comparing new treatment strategies. We retrospectively evaluated endocrinopathy in a large cohort of pediatric patients treated for craniopharyngioma using conformal and intensity-modulated radiation therapy and followed for 10 years. Tumor (hydrocephalus), host (race), and surgical (diabetes insipidus) factors contribute to endocrinopathy and obesity observed after radiation therapy and should be considered when developing patient-specific treatment plans. Effort should be made to reduce invasive interventions and understand the long-term impact of endocrinopathy on health and psychosocial outcomes.
Craniopharyngioma is a locally aggressive tumor that uniformly arises in the sellar and suprasellar regions of the intracranial compartment. Due to its proximity to the hypothalamus and pituitary, its presentation often includes endocrinopathy. The principal treatments for craniopharyngioma, surgery, and irradiation rarely improve tumor-acquired deficits and may contribute to preexisting conditions. A primary goal of treatment is to spare the patient from further harm and complications that may be life-threatening or impact long-term quality of life.1 The hypothalamic-pituitary axis (HPA) is at the center of the targeted volume when planning radiation therapy for most patients and often receives the same dose prescribed to the tumor. Endocrinopathy is an expected outcome of high-dose irradiation of the HPA; however, the incidence and time to onset of abnormalities in hormone secretion and important clinical and treatment factors associated with HPA dysfunction are not well defined. Additional detail could be used to refine strategies for intervention or to design new treatments.
We recently reported on the long-term disease control and incidence of severe complications in children, adolescents, and young adults treated for craniopharyngioma with photon-based conformal and intensity-modulated radiation therapy (CRT) (manuscript submitted). We showed that CRT results in excellent tumor control rates and overall survival, as well as a low incidence of severe complications, including vasculopathy, necrosis, and secondary malignancies.2 Less severe and more common complications that impact daily living and growth and development were not included. Herein, we present the endocrine-related outcomes for our CRT cohort with 10 years of follow-up data. The results provide new information about HPA dysfunction, factors leading to HPA dysfunction and obesity, and the incidence and time to onset of hormone replacement therapy after treatment of this rare brain tumor.
Materials and Methods
From May 15, 1998 through January 22, 2014, a total of 101 children and adolescents with craniopharyngioma were treated with photon-based conformal or intensity-modulated radiation therapy at St. Jude Children’s Research Hospital. Most of the patients (n = 76) were enrolled on RT1, a phase II study of image-guided radiation therapy for pediatric CNS tumors and quantification of radiation-related CNS effects.3 The other patients (n = 25) followed a similar non-protocol treatment plan. The protocol was activated on June 10, 1997, and enrolled patients aged 1-25 years. Those included in the current report received diagnoses between May 12, 1995 and August 23, 2013, with only one patient receiving a diagnosis after November 19, 2010. The median age at diagnosis was 8.98 years (range, 3.2-17.63 years). There were 46 female and 55 male patients; 76 were White, 22 were Black, 2 were Hispanic, and 1 was Asian.
The eligibility and treatment details for this cohort have been described elsewhere.2 Briefly, histologic confirmation was not required, and no particular timeline from diagnosis to start of radiation therapy was specified. The prescribed radiation dose was 54.0 Gy at 1.8 Gy per day; 7 patients received 55.8 Gy. The initial clinical target volume (CTV) margin was 1.0 cm and was later reduced to 0.5 cm. The planning target volume (PTV) margin was initially 0.5 cm and was later reduced to 0.3 cm. The RT1 protocol included baseline and serial clinical evaluations for up to 10 years after treatment initiation.
Endocrine evaluations included screening and provocative testing to evaluate the HPA, using previously reported methods.4 The diagnosis and treatment of diabetes insipidus (DI), growth hormone deficiency (GHD), thyroid-stimulating hormone deficiency (TSHD), adrenocorticotropic hormone deficiency (ACTHD), central precocious puberty (CPP), and hypogonadism (luteinizing hormone [LH]/follicle-stimulating hormone deficiency [FSHD]) were recorded. Patients empirically treated for TSHD and ACTHD in the perioperative period underwent a trial wherein replacement therapy was discontinued to confirm the diagnosis. The diagnosis and treatment of these conditions were the events of interest; disease progression, secondary tumor, and death were recorded as competing events if they occurred prior to the events of interest. Cumulative incidence (CI) since craniopharyngioma diagnosis and CI since CRT start date were calculated by using their respective start date and the date of the event or last contact for each event of interest. Gray’s5 method was used to compare CI across groups. The Fine and Gray6 method is used to investigate the association of CI with covariates.
The body mass index (BMI) z-score was calculated by adjusting for age and gender in the patients less than 20 years old. The Centers for Disease Control SAS program was used to calculate BMI z-scores for subjects’ age (>2 and <20 years).7 A SAS Program for the WHO growth charts (ages 0 to <2 years) was used to calculate BMI z-scores for age less than 2 years old.8 BMI was converted to z-score based on the parameters at 20 years for measurements obtain when subjects aged older than 20 years.9 BMI measurements were not included after disease progression or secondary tumor.
The mixed random effect model was used to investigate the change in BMI z-scores over time. The patients having at least two assessments were included in the statistical analyses of longitudinal data. The models included time since CRT and one of the variables of interest at a time to investigate their effects on the changes of BMI z-score over time.
Results
All 101 patients were included in the analysis; no patient was lost to follow-up, and the median follow-up for the 89 survivors was 14.94 years (range, 7.23-21.5 years). At the time of this report, only two patients had been followed up for less than 10 years. Last contact was made within 12 months of the analysis for all patients. The CI curves for GHD, thyroid hormone replacement for TSHD, adrenocorticotropin hormone replacement for ACTHD, sex hormone replacement for LH/FSHD, and treatment for CPP from the start date of CRT and time of diagnosis are presented in Figure 1. The CTV and PTV margins were tested for differences in endocrinopathy after CRT and no associations were identified. The distribution of endocrine variables by race are presented in Table 1.
Fig. 1.
Cumulative incidence (CI) of growth hormone deficiency, thyroid hormone replacement, adrenocorticotropic hormone replacement, sex hormone replacement, and central precocious puberty since conformal radiation therapy (CRT) (upper) and diagnosis (lower) for pediatric patients with craniopharyngioma treated with conformal photon radiation therapy.
Table 1.
Distribution of Endocrine Variables by Race
| Race | ||||||
|---|---|---|---|---|---|---|
| White | Black | Other | ||||
| N | % | N | % | N | % | |
| Desmopressin prior to CRT | ||||||
| No | 36.00 | 47.37 | 11.00 | 50.00 | ||
| Yes | 40.00 | 52.63 | 11.00 | 50.00 | 3.00 | 100.0 |
| Diabetes insipidus | ||||||
| Acquired at surgery | 34.00 | 44.74 | 10.00 | 45.45 | 3.00 | 100.0 |
| No | 42.00 | 55.26 | 12.00 | 54.55 | . | . |
| Pre-RT growth hormone deficiency | ||||||
| No/unknown | 39.00 | 51.32 | 14.00 | 63.64 | 2.00 | 66.67 |
| Yes | 37.00 | 48.68 | 8.00 | 36.36 | 1.00 | 33.33 |
| GH replacement <12 months | ||||||
| No | 46.00 | 60.53 | 11.00 | 50.00 | 1.00 | 33.33 |
| Yes | 22.00 | 28.95 | 1.00 | 4.55 | 1.00 | 33.33 |
| n/a | 8.00 | 10.53 | 10.00 | 45.45 | 1.00 | 33.33 |
| GH replacement <24 months | ||||||
| No | 22.00 | 28.95 | 4.00 | 18.18 | 1.00 | 33.33 |
| Yes | 46.00 | 60.53 | 8.00 | 36.36 | 1.00 | 33.33 |
| n/a | 8.00 | 10.53 | 10.00 | 45.45 | 1.00 | 33.33 |
| GH replacement | ||||||
| No | 8.00 | 10.53 | 10.00 | 45.45 | 1.00 | 33.33 |
| Yes | 68.00 | 89.47 | 12.00 | 54.55 | 2.00 | 66.67 |
| Replaced hormone axes | ||||||
| 0 | 2.00 | 9.09 | ||||
| 1 | 5.00 | 6.58 | 2.00 | 9.09 | ||
| 2 | 5.00 | 6.58 | 2.00 | 9.09 | ||
| 3 | 10.00 | 13.16 | 9.00 | 40.91 | 2.00 | 66.67 |
| 4 | 56.00 | 73.68 | 7.00 | 31.82 | 1.00 | 33.33 |
| Replaced hormone axes + DI | ||||||
| 0 | 2.00 | 9.09 | ||||
| 1 | 5.00 | 6.58 | 2.00 | 9.09 | ||
| 2 | 3.00 | 3.95 | 1.00 | 4.55 | ||
| 2 + DI | 2.00 | 2.63 | 1.00 | 4.55 | ||
| 3 | 8.00 | 10.53 | 4.00 | 18.18 | ||
| 3 + DI | 2.00 | 2.63 | 5.00 | 22.73 | 2.00 | 66.67 |
| 4 | 20.00 | 26.32 | 2.00 | 9.09 | ||
| 4 + DI | 36.00 | 47.37 | 5.00 | 22.73 | 1.00 | 33.33 |
Abbreviations: CRT, conformal and intensity-modulated radiation therapy; DI, diabetes insipidus; GH, growth hormone; RT, radiation therapy.
Diabetes Insipidus
Fifty-four patients had DI diagnosed and were treated with desmopressin prior to radiation therapy. Seven received a diagnosis at presentation, and 47, after surgery. Two patients with surgery-acquired DI discontinued desmopressin after 8.67 years and 11.66 years, respectively. One patient with DI at presentation discontinued desmopressin after 4.48 years. Desmopressin was started 8-647 days after CRT in 6 patients. DI was attributed to surgery for the earlier cases and to cystic tumor progression in the latter.
Growth Hormone Deficiency
A total of 28 patients had GHD diagnosed prior to CRT, including 6 who received a diagnosis on or before their date of craniopharyngioma diagnosis. Among the 73 remaining patients, the 10-year CI of GHD was 87.67% (±3.98). The 10-year CI of GHD was 68.42% (±11.27) for black patients and 94.23% (±3.57) for white patients (P = .0286). Among the 95 patients without GHD at the time of craniopharyngioma diagnosis, the 10-year CI of GHD was 90.66% (±3.11). The 10-year CI of GHD was 71.43% (±10.40) for black patients and 95.83% (±2.59) for white patients (P = .0276) (Figure 2A). The 10-year CI of GHD was 88.59% (±4.01) for non-shunted patients and 96.15% (±5.54) at 4 years for shunted patients (P = .0363).
Fig. 2.
Cumulative incidence (CI) of growth hormone deficiency (A, upper figure) and growth hormone replacement therapy (B, lower figure) by race since diagnosis for pediatric patients with craniopharyngioma treated with conformal photon radiation therapy.
Growth Hormone Replacement
A total of 14 patients were treated for GHD prior to CRT including 1 treated prior to their craniopharyngioma diagnosis. Among the 87 remaining patients, the 10-year CI of replacement therapy after CRT was 75.86% (±4.66). The 10-year CI of replacement therapy was 52.38% (±11.31) for black and 84.38% (±4.68) for white, respectively (P = .0064). Among the 100 patients not treated for GHD prior to craniopharyngioma diagnosis, the 10-year CI of replacement therapy was 79.05% (±4.14). The 10-year CI of replacement therapy was 54.55% (±11.01) for black and 86.67% (±4.05) for white, respectively (P = .0052) (Figure 2B). There was no association (P = .5148) between disease progression and growth hormone replacement therapy.
Thyroid-Stimulating Hormone Deficiency
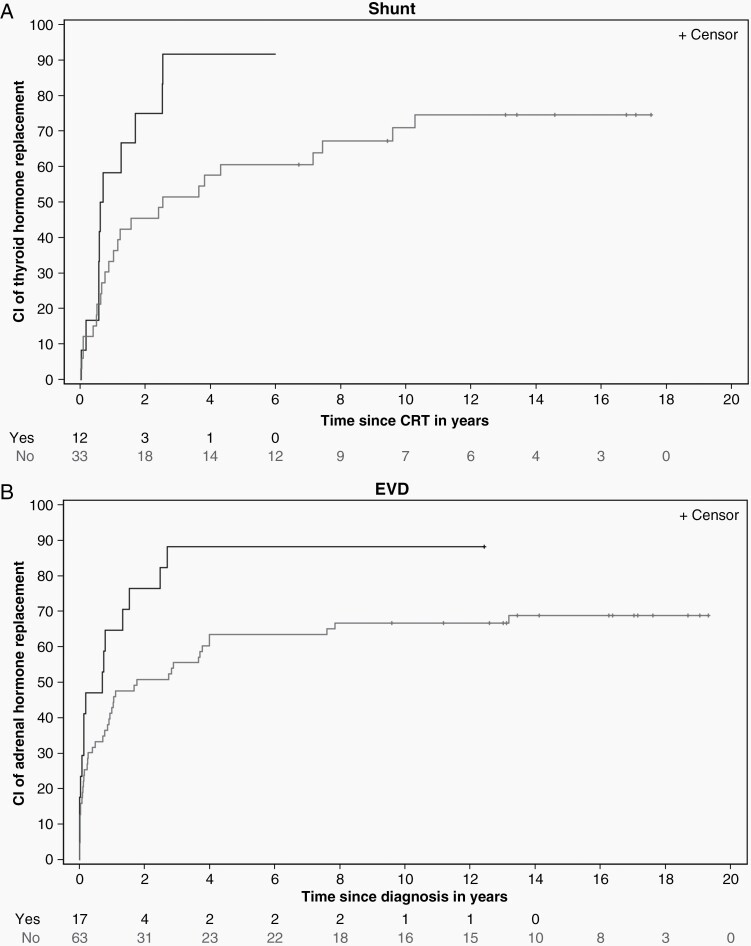
A total of 56 patients were treated for TSHD prior to CRT, including 25 with evidence of TSHD at the time of craniopharyngioma diagnosis. For the 45 remaining patients, the 10-year CI of replacement therapy after CRT was 76.46% (±6.70). The CI of replacement therapy was 70.94% (±8.44) at 10 years for non-shunted patients and 91.67% (±10.40) at 6 years for shunted patients (P = .0260) (Figure 3A). The CI of replacement therapy was 100% (±14.29) at 4 years for those with DI acquired at surgery and 71.36% (±8.86) at 10 years for those without DI (P = .0008) (Figure 4A). Among the 76 patients without evidence of TSHD at the time of craniopharyngioma diagnosis, the 10-year CI of replacement therapy was 86.03% (±4.18). The CI of replacement therapy was 100.00% (±3.45) at 4 years for those with DI acquired at surgery and 75.45% (±7.79) at 10 years for those without DI (P < .0001).
Fig. 3.
Cumulative incidence (CI) of thyroid hormone replacement by CSF shunt status since radiation therapy (A, upper figure) and CI of adrenocorticotropic hormone replacement by extraventricular drain (EVD) status since diagnosis (B, lower figure) for pediatric patients with craniopharyngioma treated with conformal photon radiation therapy.
Fig. 4.
Cumulative incidence (CI) of thyroid hormone replacement by diabetes insipidus status since radiation therapy (A, upper figure) and CI of adrenocorticotropic hormone replacement by diabetes insipidus status since diagnosis (B, lower figure) for pediatric patients with craniopharyngioma treated with conformal photon radiation therapy.
Adrenocorticotropic Hormone Deficiency
Fifty patients had diagnosed ACTHD for which they were treated before CRT, including 21 who had evidence of ACTHD at the time of craniopharyngioma diagnosis. For the remaining 51 patients, the CI of replacement therapy after CRT was 54.90% (±7.08) at 10 years. The 10-year CI of replacement therapy was 70.00% (±16.15) for those with DI acquired at surgery and 48.39% (±9.19) for those without DI (P = .0080). Among the 80 who did not have ACTHD before the craniopharyngioma diagnosis, the 10-year CI of replacement therapy was 71.25% (±5.13). The 10-year CI replacement therapy was 66.67% (±6.04) at 10 years for those who did not require an external ventricular drain at the time of surgery and 88.24% (±8.99) for those who required an external ventricular drain to manage hydrocephalus (P = .0153) (Figure 3B). The 10-year CI of replacement therapy was 90.32% (±5.82) for those with DI acquired at surgery and 55.56% (±8.47) for those without DI (P < .0001) (Figure 4B).
Central Precocious Puberty
The 10-year CI of CPP after CRT was 9.90% (±2.99). The 10-year CI of CPP was 0.00% (±0.00) for those with DI acquired at surgery and 24.32% (±7.17) for those without DI (P = .0004).
Gonadotropin Deficiency
Two patients were treated for LH/FSHD prior to CRT. Among the remaining 99, the 10-year CI of treatment for LH/FSHD after CRT was 62.93% (±4.93). The 10-year CI of replacement therapy was 43.33% (±9.32) for those younger than 7 years, 61.29% (±9.11) for those aged 7-10 years, and 78.95% (±6.38) for those aged 10 years or older (P < .0001) (Figure 5A). Among the 101 patients (none had LH/FSHD at craniopharyngioma diagnosis), the 10-year CI of replacement therapy was 60.72% (±4.94). The 10-year CI of replacement therapy was 36.67% (±9.06) for those younger than 7 years, 58.06% (±9.19) for those aged 7-10 years, and 80.83% (±6.66) for those aged 10 years or older (P < .0001) (Figure 5B).
Fig. 5.
Cumulative incidence (CI) of sex hormone replacement therapy for hypogonadotropic hypogonadism by age group since radiation therapy (A, upper figure) and since diagnosis (B, lower figure) for pediatric patients with craniopharyngioma treated with conformal photon radiation therapy.
Body Mass Index
The BMI z-score increased significantly over time. The rate of change was 0.04297 units/year (P < .0001) and was estimated based on 4413 observations in 101 patients. The average patient in this series had a BMI of 1.3106 (±0.0994) at the time of CRT and 1.7403 (±0.0973) 10 years later. Change in BMI z-score by univariate analysis was significant by race, age at CRT, and age at diagnosis. The rate of change difference was 0.0663 per year (P = .0073) for black patients compared to white patients and 0.0072 per year (P = .0038) for each year of age at CRT, and 0.0083 per year (P = .0005) for each year of age at diagnosis. Baseline (time of CRT) and year 10 BMI estimates were, respectively, 1.2878 (±0.2161) and 2.2536 (±0.2081) for black patients, and 1.2936 (±0.1162) and 1.5966 (±0.1097) for white patients. Baseline and year 10 BMI estimates were, respectively, 1.5025 (±0.1505) and 1.6103 (±0.1461) for a 5-year-old patient and 1.0762 (±0.1704) and 1.9003 (±0.1662) for a 15-year-old patient. There was no difference in rate of change comparing patients based on sex, shunt status, DI, pre-RT GHD, and growth hormone replacement therapy within 12 or 24 months of CRT; however, baseline BMI was significantly higher in those with DI whether diagnosed at presentation or after surgery. CRT The difference was 0.6129 units (P = .0018). Baseline BMI was significantly higher in those with DI acquired at surgery. The difference was 0.5138 units (P = .0093). There was no difference in rate of change comparing patients based on the number of replaced axes, with or without considering DI. Those with 4 replaced hormone axes and DI had estimated baseline z-scores 0.7944 units greater than those with ≤2 replaced hormone axes (P = .0104).
When age at CRT was modeled with other clinical factors, age at CRT and race were significant. BMI change was 0.0056 units per year (P = .0269) per year of age at CRT and 0.0532 units per year (P = .0302) for black patients. Age at CRT and DI had significant and opposite effects on baseline estimates. BMI baseline estimates were 0.0495 units lower per year of age (P = .0424) and those with DI were 0.6448 units higher (P = .0009) compared to those who did not have DI. Additional models that included time after CRT, age at CRT, and race were explored with the clinical factors of DI, preexisting GHD, time to growth hormone replacement therapy, and a number of replaced hormone axes. None of these clinical factors were found to be significant for baseline estimates or change over time. Because of the small number of black patients in each group, the modeling process was limited to white patients and showed that DI, preexisting GHD, and a number of replaced hormone axes were significant for baseline estimates and change over time. Those with DI had higher baseline estimates (0.8052 units, P = .0004) and reduced BMI over time (−0.05532 units/year, P = .0052). Those with preexisting GHD had higher baseline estimates (0.5077 units, P = .0275) and reduced BMI over time (−0.05003 units/year, P = .0113). Those with 4 replaced hormone axes had reduced BMI over time (−0.08315 units/year, P = .0056) compared to those with two or fewer replaced hormone axes. Baseline and year 10 estimates based on age and the presence or absence of DI were, respectively, 1.9168 (±0.2001) and 1.6421 (±0.1917) for a 5-year-old with DI and 1.1116 (±0.1886) and 1.3900 (±0.1793) for a 5-year-old without DI. Baseline and year 10 estimates were, respectively, 1.3168 (±0.2235) and 1.8410 (±0.2133) for a 15-year-old with DI and 0.5116 (±0.2502) and 1.5890 (±0.2419) for a 15-year-old without DI.
Discussion
Conformal radiation therapy was developed nearly 3 decades ago to safely escalate radiation dose and improve local control in difficult-to-treat adult tumors. The goal was to spare normal tissues and reduce the incidence and time to onset of complications associated with radiation therapy. These methods were applied to treat childhood brain tumors and reduce the feared side effects of radiation therapy. We designed a clinical trial to test the ability of conformal radiation therapy to safely reduce the targeted volume without affecting the rate or pattern of failure in children with localized brain tumors. This protocol enabled the enrollment of children with craniopharyngioma and included serial evaluations to study the side effects and long-term outcomes of radiation therapy. The uniform treatment of these patients and follow-up at a single treatment center provided a unique opportunity to research other clinical and treatment factors contributing to the incidence and time to onset of late effects, including endocrinopathy. The key results of this study are the incidence curves calculated from the time of CRT start, and separately, from the time of diagnosis and accounting for endocrinopathies present at the time of diagnosis and competing risks analysis. More importantly, these findings show that factors other than radiation therapy also contribute to hormone deficiencies. These results offer the possibility of improving patient selection, early intervention, and the design of new treatments.
We previously reported the CI of hormone deficiencies in several tumor types, including low-grade glioma.10 The treatment of low-grade glioma closely matches that of craniopharyngioma because radiation therapy is most often administered to diencephalic and optic pathway tumors and encompasses the hypothalamus, which is uniformly irradiated to 54 Gy. Patients with craniopharyngioma may also present with hormone deficiencies. In the series of low-grade glioma treated with CRT,7 GHD was diagnosed in 24% of tested patients, and 12% had CPP. The 10-year CI of treatment for GHD was 48.9%; of TSHD, 64.0%; of ACTHD, 19.2%; and of LH/FSHD, 34.2%. Therefore, endocrinopathy in low-grade glioma closely matched that of our craniopharyngioma series, wherein 20 of 78 patients with low-grade glioma had tumors involving the cerebellum or cerebellar hemispheres.
The rate of endocrinopathy in our cohort of children and adolescents differs slightly from that in other series that specifically include children with craniopharyngioma because other series tend to include fewer patients, and with a higher proportion treated with radical surgery, which leads to a higher prevalence of endocrinopathy, especially DI. For example, Sarkar et al11 reported a long-term surgical series wherein 16 of 37 children with craniopharyngioma were treated with gross-total resection (GTR) (43.2%).
The large number and diversity of patients in our series, combined with extended follow-up, provide novel statistical findings suggesting that host, tumor, and treatment factors other than radiation therapy contribute to deficits in endocrine function. For example, the higher rate of GHD in white patients suggests differences in radiation sensitivity given that clinical and treatment factors (extent of resection, radiation dose, and volume) and follow-up were the same. Similarly, the higher rate of TSHD in shunted patients provides evidence of the impact of the tumor on the incidence and time to onset of hormone deficiencies. The higher incidence of TSHD in patients with DI acquired at surgery provides evidence of the impact of surgery on the incidence and time to onset of hormone deficiencies. And finally, age at the time of treatment needs to be considered in the follow-up of these patients and the timing of screening for hypogonadism.
As noted in a recent report of one of the largest monocentric European cohorts, among pediatric brain tumor patients, those diagnosed with craniopharyngioma have the highest incidence of panhypopituitarism, highest greatest number of endocrine disorders, are more likely to have obesity at the time of initial evaluation, and the highest BMI after the completion of therapy.12 Their series included 64 patients with craniopharyngioma for whom surgery was the initial treatment in 63 and 47 (74.6%) were eventually treated with radiotherapy. Although no associations were found between demographic and biomedical factors and endocrinopathy and obesity for the different tumor types, suprasellar tumor location was most often associated with endocrinopathy and obesity. For example, obesity (z-score ≥2) was present in 46.2% of patients with suprasellar tumors and highest for this group at the time of last visit.
Newer approaches and less invasive surgery for craniopharyngioma have been proposed to reduce endocrinopathy13 and are supported by evidence from some surgical series14 that show the advantages of transnasal surgery over transcallosal surgery.15 Improved patient selection through advanced imaging of sellar and parasellar tumors16 may be another option to reduce the incidence of hormone deficiencies prior to irradiation, and to identify patients with hypothalamic invasion for whom surgery may be altogether contraindicated.17 Sparing the hypothalamus from radiation exposure in the treatment of craniopharyngioma would be difficult and is unlikely to be a strategy to prevent or reduce the incidence of pituitary hypofunction. Investigators are focused on all points of comparison between treatment modalities given the lack of a demonstrable difference in the use of newer methods of radiation therapy for craniopharyngioma, including proton therapy,18,19 and fractionated stereotactic radiation.20
In summary, the results from our analysis of endocrinopathy in children and adolescents treated with photon CRT provide convincing evidence for early surveillance for pituitary endocrinopathies based on host- and tumor-related factors, including hydrocephalus and its management, and the need to limit the extent of surgery, especially for tumors involving the pituitary stalk or the hypothalamus. Understanding whether the extent of hormone deficiencies and their management contribute to other health and psychosocial effects, such as those observed in patients with localized brain tumors treated with CRT,4 remains an important avenue for future research.
Acknowledgments
The authors are grateful to Christina Bosley, Valerie Carr, Jorden Cunningham, Tina Davis, and Margaret Madey for their help and assistance with this research and to the staff in the Division of Endocrinology and After Completion of Therapy clinic at St. Jude Children’s Research Hospital.
Contributor Information
Thomas E Merchant, Department of Radiation Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Drucilla Y Edmonston, Department of Radiation Oncology, University of Tennessee Health Science Center, Memphis, Tennessee, USA.
Shengjie Wu, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Yimei Li, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Frederick A Boop, Department of Global Pediatric Medicine, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Robert H Lustig, Department of Pediatrics, University of California, San Francisco, California, USA.
Funding
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), Cancer Center Support [grant No. CA21765-23] from the National Cancer Institute, and by Research Project [grant No. RPG-99-252-01-CCE] from the American Cancer Society.
Conflict of interest statement. The authors report no conflicts of interest concerning the materials or methods used in this study or the findings reported in this paper.
Authorship statement. Concept and design: T.E.M. Data collection: T.E.M. and D.Y.E. Data analysis: S.W. and Y.L. Data interpretation: all authors. Manuscript writing: T.E.M. and D.Y.E. Review, revisions, and approval of final manuscript: all authors.
References
- 1. Muller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75. [DOI] [PubMed] [Google Scholar]
- 2. Edmonston DY, Wu S, Li Y, et al. Limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 protocol. Int J Rad Oncol*Biol*Phys. 2021;111(3):S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merchant TE. A study for image-guided radiation therapy in pediatric brain tumors and side effects. ClinicalTrials.gov Identifier: NCT00187226; 2005. https://clinicaltrials.gov/ct2/show/NCT00187226?term=NCT00187226&draw=2&rank=1
- 4. van Iersel L, van Santen HM, Potter B, et al. Clinical impact of hypothalamic-pituitary disorders after conformal radiation therapy for pediatric low-grade glioma or ependymoma. Pediatr Blood Cancer. 2020;67(12):e28723. [DOI] [PubMed] [Google Scholar]
- 5. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1441–1154. [Google Scholar]
- 6. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):495–509. [Google Scholar]
- 7. Centers for Disease Control and Prevention. Growth chart training a SAS program for the 2000 CDC growth charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm#instructions. Accessed May 1, 2022.
- 8. Centers for Disease Control and Prevention. Growth chart training a SAS program for the WHO growth charts (ages 0 to <2 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm. Accessed May 1, 2022.
- 9. Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (Lond). 2006;30(4):590–594. [DOI] [PubMed] [Google Scholar]
- 10. Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27(22):3691–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarkar S, Chacko SR, Korula S, et al. Long-term outcomes following maximal safe resection in a contemporary series of childhood craniopharyngiomas. Acta Neurochir (Wien). 2021;163(2):499–509. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez Briceno LG, Kariyawasam D, Samara-Boustani D, et al. High prevalence of early endocrine disorders after childhood brain tumors in a large cohort. J Clin Endocrinol Metab. 2022;107(5):e2156–e2166. [DOI] [PubMed] [Google Scholar]
- 13. Lu X, Hang W, Liu H, et al. [Experience in the diagnosis and treatment of the postoperative complications of craniopharyngiomas through expanded endoscopic endonasal transsphenoidal approach]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35(6):505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussein Z, Glynn N, Martin N, et al. Temporal trends in craniopharyngioma management and long-term endocrine outcomes: a multicentre cross-sectional study. Clin Endocrinol (Oxf). 2021;94(2):242–249. [DOI] [PubMed] [Google Scholar]
- 15. Govindarajan V, Luther EM, Morell AA, et al. Perioperative complications in endoscopic endonasal versus transcranial resections of adult craniopharyngiomas. World Neurosurg. 2021;152:e729–e737. [DOI] [PubMed] [Google Scholar]
- 16. Jipa A, Jain V. Imaging of the sellar and parasellar regions. Clin Imaging. 2021;77:254–275. [DOI] [PubMed] [Google Scholar]
- 17. Pascual JM, Prieto R, Rosdolsky M. Craniopharyngiomas primarily affecting the hypothalamus. Handb Clin Neurol. 2021;181(7):75–115. [DOI] [PubMed] [Google Scholar]
- 18. Ravindra VM, Okcu MF, Ruggieri L, et al. Comparison of multimodal surgical and radiation treatment methods for pediatric craniopharyngioma: long-term analysis of progression-free survival and morbidity. J Neurosurg Pediatr. 2021; 28(2):152–159. [DOI] [PubMed] [Google Scholar]
- 19. Li P, Wang J, Axier A, et al. Proton therapy for craniopharyngioma in adults: a protocol for systematic review and meta-analysis. BMJ Open. 2021;11(6):e046043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Astradsson A, Munck Af Rosenschold P, Feldt-Rasmussen U, et al. Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncol. 2017;56(3):415–421. [DOI] [PubMed] [Google Scholar]