Abstract
Background
Our aim was to estimate long-term disease control and complications after conformal radiation therapy (CRT) in children and adolescents with craniopharyngioma.
Materials and Methods
Pediatric patients with craniopharyngioma (n = 101) were enrolled on or treated according to a phase II single institutional protocol from 1998. Surgery was individualized, and CRT (54Gy) was administered using a 1.0 cm or 0.5 cm clinical target volume margin. Patients were followed for 10 years by serial MR imaging and MR angiography and a battery of tests to measure the effects of treatment.
Results
Twenty patients had tumor progression. Twelve patients who had tumor progression died due to tumor (n = 6) or complications related to tumor or treatment (n = 6). With a median follow-up of 14.94 years for survivors, the 10 year estimates (±SE) of progression-free survival (PFS), event-free survival (EFS), and overall survival (OS) were 78.84% ± 4.10%, 77.12% ± 4.19%, and 96.02% ± 1.95%, respectively. OS, EFS, and PFS were significantly associated with race, shunt status, and tumor volume. The 10 year cumulative incidence (±SE) of the secondary tumor (1.99% ± 1.40%), secondary malignant tumor (1.0% ± 1.0%), necrosis (1.98% ± 1.39%), vasculopathy (8.47% ± 2.90%), and permanent neurologic deficits (8.28% ± 3.37%) were estimated by competing risk analysis. Three patients required revascularization surgery. Salvage therapy was successful in 13 patients using surgery and radiosurgery.
Conclusions
Limited surgery and CRT using photons results in excellent tumor control. Tumor control and the incidence and severity of complications are associated with host, tumor, and treatment factors.
Keywords: craniopharyngioma, outcomes, pediatrics, radiotherapy
Key Points.
Overall survival is excellent in children with craniopharyngioma treated with RT.
Tumor, host, and radiotherapy factors impact survival and the risk of complications.
Reducing target volume margins and radiation dose exposure should be a priority.
Importance of the Study.
This study describes the long-term results of a cohort of pediatric patients who received limited surgery and conformal radiotherapy (RT) for craniopharyngioma. The majority participated in a phase II clinical trial and the remainder received similar treatment. All patients received 54-55.8Gy focal RT. Overall survival at 10 years was 96%, and 20 patients had tumor progression. Late vasculopathy occurred in 8.5%, neurologic deficits in 8.3%, brainstem necrosis in 2%, and secondary malignant tumor in 1%. Increased risk of mortality was observed for Black patients, those who required CSF shunting, and those with larger target volumes. Five deaths were attributed to initial therapy. Vasculopathy was assessed using cerebral angiography. Female patients were observed to have a higher cumulative incidence of vasculopathy, and a larger planning target volume (PTV) was associated with an increased risk of vasculopathy. Two patients had brainstem necrosis, and 6 developed secondary tumors attributed to radiation therapy.
Craniopharyngioma is a histologically benign, potentially curable, intracranial neoplasm that arises with a bimodal age distribution in children and adults.1 The childhood version is uniformly the adamantinomatous histologic subtype and represents the most common nonglial intracranial neoplasm in children. It is estimated to account for 3.4% of pediatric brain tumors.2 Craniopharyngioma arises along with the craniopharyngeal duct remnant, frequently in the suprasellar region. Endocrinopathy, visual impairment, or neurologic compromise, the last resulting from compression of neurovascular structures or obstruction of cerebrospinal fluid (CSF) pathways, often leads to evaluations that result in the diagnosis. As biologic and molecular features amenable to conventional chemotherapy or targeted agents are lacking, definitive treatment of adamantinomatous craniopharyngioma relies on surgery and radiation therapy (RT).
Understanding that all treatments have associated risks and benefits, there has been a trend to improve patient selection for radical surgery or limited surgery and RT.3 Both approaches result in equivalent rates of overall survival (OS).4 The rate of tumor progression after radical surgery is generally higher than that observed after RT; however, patients who have tumor progression after radical surgery may receive RT and have high rates of disease control.5 The acute and long-term effects of surgery and RT have been compared.6 The concerning acute effects of radical surgery include diabetes insipidus, vision loss, stroke, and rarely peri-operative death; the long term effects have been documented and include reduced performance status and neurocognitive impairment affecting specific domains and quality of life.7 The effects of surgery have been attributed to hypothalamic involvement.8–10 The acute effects of irradiation are self-limiting and include nausea, emesis, and profound fatigue. The long-term effects are more concerning and include vasculopathy, necrosis, and secondary tumors. Both treatments are associated with panhypopituitarism and metabolic syndrome and contribute differentially to fatigue, narcolepsy,11 and hypothalamic obesity.12
We designed a clinical trial to test the ability of conformal radiation therapy (CRT) to safely reduce the targeted volume without affecting the rate or pattern of failure in children with localized brain tumors. Preliminary results documented a high rate of local tumor control and the importance of tumor imaging during treatment.13 The advantages of limiting the prescribed dose to the tumor and a limited margin of surrounding normal tissue provided the rationale for the future use of proton therapy. Proton therapy holds promise to further spare normal tissue exposure and improve outcomes.14 With more than 10 years of follow-up, we present the results from our institutional experience using photon-based CRT. The results include benchmark rates of disease control and selected complications and provide new insights into factors associated with PFS, OS, functional outcomes, and challenges associated with treatment of this rare tumor.
Materials and Methods
Patient Characteristics
Between April 1998 and December 2013, 101 children and adolescents ages 3–17 years were treated with photon-based conformal or intensity-modulated radiation therapy at St. Jude Children’s Research Hospital (St. Jude). Pediatric patients (n = 76) were enrolled on a phase II single institutional protocol beginning 1998 or followed (n = 25) a nonprotocol treatment plan. The median follow-up for survivors was 14.94 years (range 7.23–21.5 years). At the time of this report, only two survivors had been followed for less than 10 years. Clinical and treatment factors are presented in Table 1.
Table 1.
Clinical and treatment characteristics
| Variable | N |
|---|---|
| Age | |
| Diagnosis | 7.77 years (1.29–17.52) |
| At start of radiation therapy | 8.98 years (3.20–17.63) |
| Presentation | |
| Headache | 62 |
| Visual disturbance | 17 |
| Growth delay | 9 |
| Emesis | 4 |
| Loss of consciousness | 4 |
| Head injury | 3 |
| Precocious puberty | 2 |
| Gender | |
| Female | 46 |
| Male | 55 |
| Race | |
| Asian/Pacific islander | 1 |
| Black | 22 |
| Hispanic | 2 |
| White | 76 |
| Extraventricular drain | |
| Yes | 23 |
| No | 78 |
| CSF shunt | |
| Yes | 27 |
| No | 74 |
| CTV margin | |
| ≤0.5 cm | 76 |
| 1.0 cm | 25 |
| PTV margin | |
| 0.3 cm | 58 |
| 0.5 cm | 43 |
| Dose | |
| 54Gy | 94 |
| 55.8Gy | 7 |
| Target volumes (mL) | |
| GTV | 12.28(0.42–64.66) |
| CTV | 36.84 (5.20–176.7) |
| PTV | 65.75 (17.31–257.5) |
| Target volume dose coverage (cGy) | |
| D95GTV | 5441 (5298–5740) |
| D95CTV | 5415 (5031–5701) |
| D95PTV | 5364 (5347–5691) |
| Planning and delivery method | |
| Conformal radiation therapy | 84 |
| Intensity-modulated radiation therapy | 17 |
Abbreviations: CSF, cerebrospinal fluid; CTV, clinical target volume; PTV, planning target volume; GTV, gross total volume.
Protocol Enrollment, Treatment, and Follow-up
The RT1 protocol was a phase II study of image-guided RT for pediatric CNS tumors and quantification of radiation-related CNS effects (NCT00187226). The protocol was activated October 6, 1997 and enrolled the first patient with craniopharyngioma on June 4, 1998. Patients diagnosed with craniopharyngioma ages 1–25 years were eligible. Histologic confirmation was not required and patients with a prior history of fractionated irradiation were excluded. Adequate performance status (ECOG 0-3) and informed consent signed by patient, parent, or guardian was required. The study protocol was approved by the institutional review board and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All patients provided written informed consent. “If the patient or their guardian were unwilling to participate in the protocol-specified evaluations, they received identical treatment but were not enrolled on the phase II study.”
Surgery, Radiation Therapy, and Follow-up
Surgery - Protocol accrual relied on referral of patients diagnosed at outside facilities. Referred patients may have undergone radical surgery with subsequent incomplete resection or had progression; most patients were treated with limited surgery. There were 197 tumor directed procedures in 96 patients. This did not include post-catheter placement cyst aspirations, or procedures associated with CSF diversion, intra-cystic chemotherapy or brachytherapy procedures, radiosurgery, radiosurgery, revascularizations, and to treat post-surgical complications or progression. Five patients were treated without tumor directed surgery. Initial surgery was performed at an outside hospital (OSH) in 49 cases. Additional tumor directed procedures were performed in 16 of these patients. In total, 18 of 49 OSH cases were subjected to a single procedure. There were 31 OSH patients who underwent 2–11 tumor directed procedures (median 2 procedures) prior to CRT. Among the 47 patient who underwent their initial tumor directed procedure at our institution, 32 had a single procedure and 15 had multiple procedures. The median was 2 (range, 2–5 procedures). All post-operative patients with residual tumor received immediate post-operative RT at the time of referral. Single tumor directed procedures were performed in 50 patients including 2 patients treated with transsphenoidal resection (tissue assessment was non-diagnostic in both cases), 19 were treated with bur hole procedures that included tumor/cyst decompression and drainage with Ommaya catheter placement in 3 cases, and 29 were treated with craniotomy performed for tumor/cyst decompression, drainage, and resection with Ommaya catheter placement in 4 cases. The extent of these single procedures may be further characterized based on tissue diagnosis and post-operative diabetes insipidus. The procedures were diagnostic in 5/19 bur hole procedure cases and 24/29 craniotomy cases (P-value < 0.001). Five of 29 craniotomy cases were non-diagnostic. Diabetes insipidus resulting from surgery was noted in 1/15 bur hole cases, 17 of 26 craniotomy cases (P-value < 0.001) and was pre-existing is 3 bur hole and 3 craniotomy cases.
CSF Diversion and Interventions during Treatment - A total of 27 patients required shunt placement before or during treatment and immediate post-treatment follow-up and 23 patients required placement of an extra-ventricular (n = 21) or lumbar (n = 2) drain. Cyst enlargement warranting intervention such as adaptive replanning, cyst aspiration, shunt revision, or partial resection occurred in 31 (30.7%) patients. Surgery during RT included partial resection for symptomatic hydrocephalus, facial numbness, and visual impairment.
Radiation Therapy - Target volumes were defined using CT and MR performed in the treatment position. The latter was registered to the treatment planning CT. The gross tumor volume (GTV) was the residual tumor or tumor bed; the clinical target volume (CTV) margin was initially a 1.0 cm surrounding the GTV and later reduced to ≤0.5 cm. The planning target volume (PTV) margin was initially a 0.5 cm margin surrounding the CTV and later reduced to 0.3 cm using daily image guidance. The CTV margin was 1.0 cm for 25 patients and ≤0.5 cm for the remainder. The PTV margin was 0.5 cm for 43 patients and 0.3 cm for the remainder. On treatment imaging using MR was performed at weeks 3 and 5 of treatment and weekly after 2004. The median number of MR evaluations during treatment was 4 (range 0-7). IV contrast was used as needed. Replanning was performed when any margin of an enlarging GTV approached the CTV margin. More than one plan was required for 43 (42%) patients. Three planning systems were used during the protocol and collimation included customized Cerrobend apertures and multi-leaf collimation with leaf widths of 1.0 cm, 0.5 cm, and 0.3 cm. Photon beam energy was 6 or 15MV. Conformal and step-and-shoot intensity-modulated RT methods were used. Median (range) volumes and doses were as follows: GTV 12.3 mL (0.4–64.7 mL), CTV 36.8 mL (5.2–176.7 mL), PTV 65.8 mL (17.3–257.5 mL), D95%GTV 5440.9cGy (5298.0–5739.5cGy), D95%CTV 5415.0cGy (5031.3–5700.5cGy), and D95%PTV 5364.4cGy (5350.0–5690.7cGy). Tumor volumes and dosimetric parameters were calculated by the treatment planning system. The protocol prescribed dose was 5400cGy at 180Gy per day, and 7 patients received 5580cGy.
Follow-up, Disease Control, and Survival
Dated from the start of RT, patients were followed every 3 months during the first year, every 6 months through year 5, and annually through year 10. Follow-up included physical and neurologic exam and contrast enhanced MR imaging. MR angiography (MRA) was performed at baseline and annually. Abnormal MRA evaluations were followed by repeat MRA and the addition of MR perfusion imaging. Cerebral angiography was performed for patients with uncompensated perfusion deficits. Vasculopathy was recorded for patients with an abnormal cerebral angiogram. Follow-up endpoints include last imaging study, last clinic visit, or last contact. Patients were followed in the radiation oncology clinic for a minimum for 10 years.
Statistical Analysis
The Cox hazard model was used to investigate the association of OS, event-free survival (EFS), and progression-free survival (PFS) with variables of interest. The log-rank test was used to compare the difference in survival distributions between groups. The level of significance was P < 0.05. Gray’s method15 was used to compare the cumulative incidence of a particular type of failure among different groups in the presence of competing risks. Fine-Gray16 competing risk regression model was used to investigate risk factors associated with cumulative incidence of vasculopathy. OS was calculated from the date of RT start to the date of death or the last contact date. EFS was calculated to the date of disease progression, the date of secondary tumor, date of death not associated with disease progression, or last contact date. PFS was calculated to the date of disease progression, date of death, or last contact date, while the secondary tumor was censored if the date of secondary tumor occurred prior to the date of disease progression. The results from the phase II study have been reported, https://clinicaltrials.gov/ct2/show/NCT00187226?term=RT1&draw=2&rank=6
Results
Tumor Control
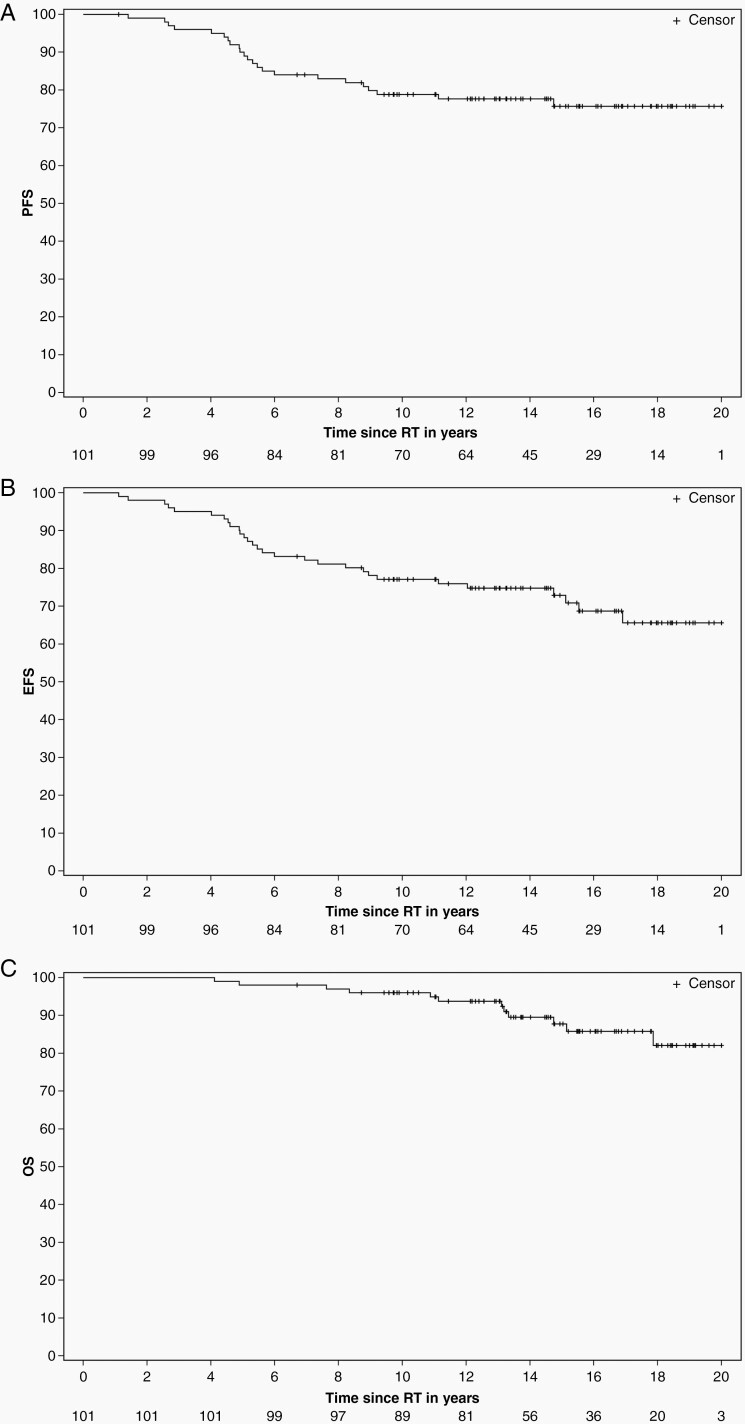
Twenty patients had tumor progression. The median time to progression was 5.15 years (range 1.41–9.21 years). Twelve patients died from tumor progression (n = 6), complications related to tumor or treatment (n = 5) or both (n = 1). The 10 year estimates (±SE) of PFS, EFS, and OS were 78.84% ± 4.10%, 77.12% ± 4.19%, and 96.02% ± 1.95%, respectively (Figure 1). There were no marginal treatment failures.
Figure 1.
K-M estimates of progression-free (A), event-free (B), and overall survival (C) after conformal and intensity-modulated photon RT.
Overall Survival
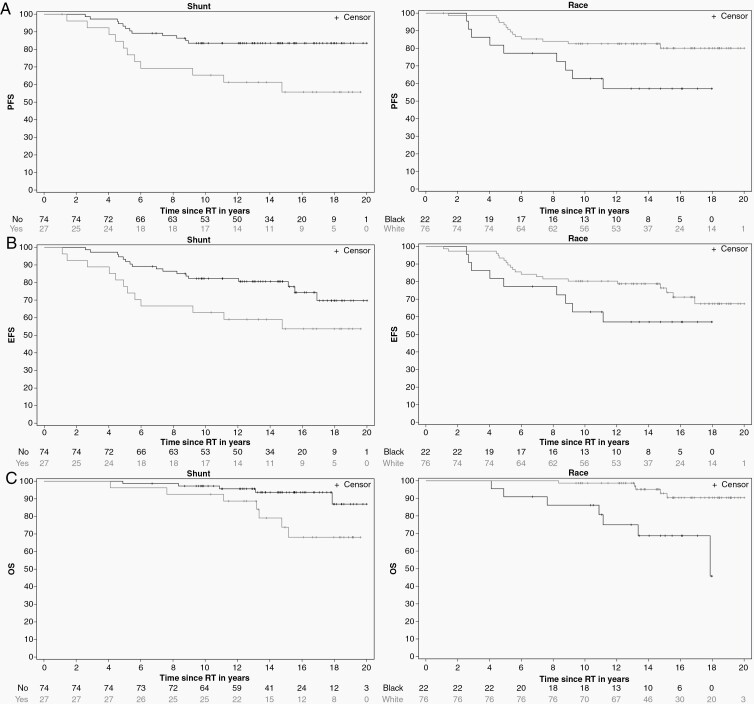
Black patients had a higher risk of death compared to White patients, (HR = hazard ratio of death) HR 6.2826 (95% CI 1.9861,19.8734, P = 0.0018). Shunted patients had a higher risk of death compared to nonshunted patients, HR 3.4946 (95% CI 1.1056,11.0456, P = 0.0331) (Figure 2) and those with larger target volumes had a higher risk of death compared to those with smaller target volumes. The risk of death associated with the GTV was HR 1.0845 (95% CI 1.0497,1.1206, P < 0.0001). Similar associations were found for the CTV and PTV. OS estimates at 10 years were 86.12% (±7.44) for Black and 98.68% (±1.31) for White patients, respectively (P = 0.0003), and 92.59 % (±5.04) for shunted and 97.28% (±1.90) for nonshunted patients, respectively (P = 0.0232). There were strong correlations between shunt and the tumor volumes GTV, CTV, and PTV. Race was not significantly associated with tumor volumes. Shunt was not significantly associated with race. Multiple variable analyses were performed using variables that were not correlated. OS was associated with race (P = 0.0016) and shunt (P = 0.0314). OS was associated with GTV (P = 0.0001) but not race (P = 0.0824). OS was associated with race (P = 0.0387) and CTV (P = 0.0002), and race (P = 0.0270) and PTV (P = 0.0002).
Figure 2.
K-M estimates of progression-free (A), event-free (B), and overall survival (C) after conformal and intensity-modulated photon RT by race and shunt status.
Event-Free Survival
Shunted patients had a higher risk of events, which included progression, secondary tumor, and death, HR 2.2253 (95% CI 1.062,4.6626, P = 0.0341). Estimated EFS at 10 years was 62.96% (±9.29) for shunted and 82.28% (±4.46) for nonshunted patients, respectively (P = 0.0295) (Figure 2). Patients with larger GTV had a higher risk of events, HR 1.0506 (95% CI 1.0295,1.0722, P < 0.0001). Similar associations were found for CTV and PTV. Patients with larger PTV margins had a higher risk of events, HR 1.574 (95% CI 1.0643,2.328, P = 0.0231). For every mL increase in GTV, the risk of an event increased by 5.06%, and for every mm increase in PTV margin, the risk of an event increased by 1.14%. Shunt was not associated with PTV margin. In a multivariable analysis, EFS was associated with PTV margin (P = 0.0421) but not shunt (P = 0.0700).
Progression
Black patients had higher risk of tumor progression compared to White patients, HR 2.5694 (95% CI 1.1113,5.9407, P = 0.0273). Shunted patients had higher risk of tumor progression compared to nonshunted patients, HR 2.9252 (95% CI 1.2901,6.6327, P = 0.0102) (Figure 2). The risk of progression associated with GTV was HR 1.051 (95% CI 1.0285,1.0739, P < 0.0001), and larger PTV margins were associated with a higher risk of tumor progression HR 1.5903 (95% CI 1.0314,2.452, P = 0.0357). Similarly, larger CTV and PTV were associated with a higher risk of progression. PFS estimates at 10 years were 62.78% (±10.47) for Black and 82.62% (±4.38) for White patients, respectively (P = 0.0221), and 65.38% (±9.33) for shunted and 83.57% (±4.34) for nonshunted patients, respectively, (P = 0.0070). PFS was associated with race (P = 0.0448) and shunt (P = 0.0210). PFS was associated with GTV (P < 0.0001) but not race (P = 0.2349). PFS was associated with CTV (P = 0.0016) but not race (P = 0.1377). PFS was associated with PTV (P = 0.0002) but not race (P = 0.0993). When race, shunt, and PTV margin were included in the model, PFS was associated with race (P = 0.0279) and Shunt (P = 0.0431), but not PTV margin (P = 0.0607). The time from initial imaging diagnosis to RT start date was not associated with PFS, EFS, or OS; 50% of patients started RT within 2.28 months of diagnosis.
There was no difference in OS based on sex, age, and RT parameters including target volume margin, dose, treatment delivery type, number of treatment plans, or number of surveillance imaging sessions. There was no difference in EFS based on sex, age, and RT parameters including dose, treatment delivery type, number of treatment plans, or number of surveillance imaging sessions. There was no difference in PFS based on gender, age, and RT parameters including dose, treatment delivery type, number of treatment plans, or number of surveillance imaging sessions.
Salvage Therapy
Salvage therapy was successful in 13 patients given various combinations of surgery and stereotactic radiosurgery. Successful salvage regimens included a single neurosurgery procedure (n = 5), multiple surgery procedures (n = 6), and multiple surgery procedures combined with radiosurgery (n = 2). Among patients with progressive disease, 6 died from progressive disease or complications after additional surgery and one died of a secondary malignant brain tumor, 13.18 years after RT.
Death not Associated with Disease Progression
In addition to the single patient who had disease progression and secondary high-grade glioma, there were 5 other deaths without evidence of tumor progression. Causes of death included opportunistic infection (4.89 years), cerebrovascular disease (11.13 years, 14.74 years), secondary malignant brain tumor (13.11 years), and secondary malignant craniopharyngioma (8.34 years).
Neurologic and Neuropsychologic Sequelae
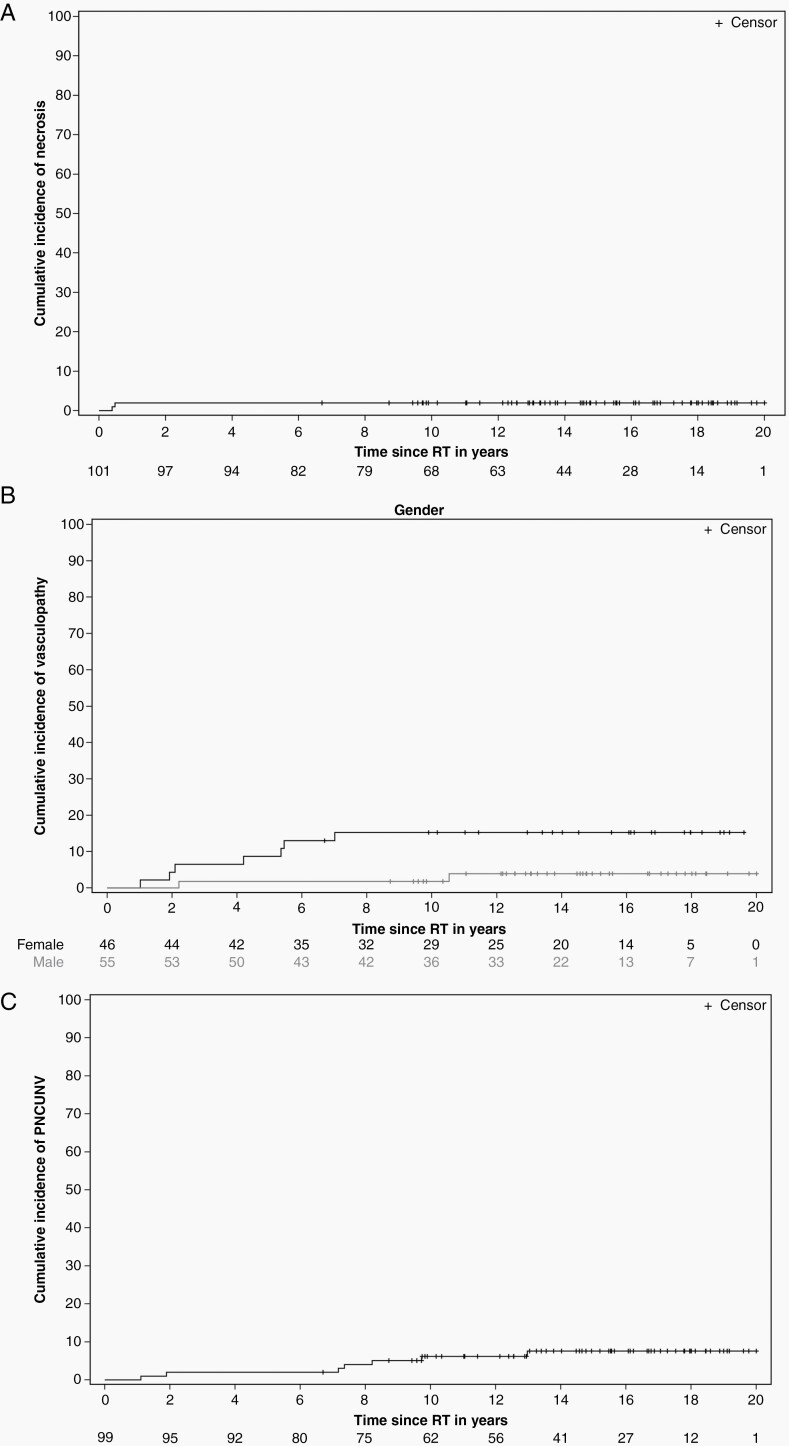
Long-term neurologic and psychologic complications were common and included hypersomnia, attention deficit disorder, depression, anxiety, headache, and seizure disorder. Treatments included stimulants (46.5%), prescription migraine medications (43.5%), and long-term anticonvulsant therapy (19.8%). Four patients developed autonomic dysfunction manifested by neurocardiogenic syncope, temperature dysregulation, and cyclic vomiting. Excluding seizure disorder, the cumulative incidence of a permanent neurologic condition unrelated to vasculopathy and necrosis was 6.17 ± 2.46% at 10 years. (Figure 3)
Figure 3.
Cumulative incidence estimates of necrosis (A), vasculopathy (male vs. female) (B) and permanent neurologic conditions unrelated to necrosis or vasculopathy (PNCUNV) (C) and excluding seizure disorders.
Vasculopathy and Brain Necrosis
MRA was performed during the first year of treatment and annually thereafter in all patients. One patient could not be imaged by MRI because of an incompatible aneurysm clip placed during surgery. Forty patients had an abnormal MRA defined as vascular stenosis. The median time to abnormal MRA was 1.57 years, range 0.2–10.85 years. The MRA was abnormal in 15 cases ≤12 months, 22 cases ≤24 months, and 31 cases ≤60 months after the initiation of RT. Seventeen patients with abnormal MRA were evaluated with CT (n = 4) and conventional (n = 13) angiography to investigate neurologic symptoms or imaging findings in these patients. Nine patients had abnormal angiograms. The median time from abnormal MRA to cerebral angiogram was 1.15 years (range 0.04–8.52 years), and median time from radiation therapy to abnormal cerebral angiogram was 1.05 years (range 0.56–4.86 years). Three patients underwent revascularization surgery: encephaloduroarteriosynangiosis was performed in three patients 3.52, 3.69, and 4.24 years after RT. These two patients treated with arteriosynangiosis had perioperative strokes involving middle cerebral circulation and developed permanent deficits. Also, 23 patients were treated with aspirin therapy. The indications included abnormal MRA (n = 15), venous sinus thrombosis (n = 1), transient ischemic attack (n = 1), heart valve replacement (n = 1), dyslipidemia (n = 1), and abnormal cerebral angiography (n = 2), empiric after stroke-like symptoms without imaging correlate (n = 2). The cumulative incidence of vasculopathy was 7.93 ± 2.71% at 10 years. The cumulative incidence of vasculopathy was higher in females (15.28 ± 5.39) than males (1.82 ± 1.82) (P = 0.0415). (Figure 3) Using the Fine-Gray regression model, PTV was significantly associated with vasculopathy HR 1.0095 95% CI 1.0016,1.1076, P = 0.0188). In a multivariable analysis that included marginal variables, vasculopathy was associated with PTV (P = 0.0222) but not sex (P = 0.0776) and D95GTV(P = 0.0677). When sex was removed, vasculopathy was associated with PTV (P = 0.0232) but not D95GTV (P = 0.0812). Vasculopathy was associated with D95CTV (P = 0.0366) and PTV (P = 0.0256) but not sex (P = 0.0713). After removing sex, vasculopathy was associated with PTV (P = 0.0363) but not D95CTV (P = 0.0573).
Two patients had brainstem necrosis 4.67 and 5.75 months after RT. In the first patient, necrosis was observed after cataract surgery in a patient with diabetes mellitus who was noncompliant with his diabetes regimen. In the second patient, necrosis was observed at the site of ischemia after multiple neurosurgical interventions, including resection. Both patients were successfully treated with hyperbaric oxygen and are alive more than 10 years after treatment. The cumulative incidence of necrosis was 1.98 ± 1.39% at 10 years. (Figure 3)
Secondary Tumors
Six patients developed secondary tumors attributed to RT: high-grade glioma (n = 2), meningioma (n = 2), malignant craniopharyngioma, and papillary thyroid carcinoma. Those with high-grade glioma and malignant craniopharyngioma died as noted earlier. Two patients developed second tumors not attributed to RT; one developed osteosarcoma 1.11 years after RT and one patient with type 1 neurofibromatosis developed acoustic neuroma. The cumulative incidence of the secondary tumor was 1.99 ± 1.40% at 10 years. The cumulative incidence of the secondary malignant tumor was 1.00 ± 1.00% at 10 years.
Discussion
Craniopharyngioma is a rare pediatric brain tumor with fewer than 150 cases diagnosed each year in the US in individuals under 19 years of age. The infrequent diagnosis of this tumor, the high rate of tumor control after RT, and the need for long-term follow-up to observe enough events associated with tumor progression and death have impacted our ability to identify prognostic factors. Our contemporary photon series with long-term follow-up at a single institution of 101 patients provides new information showing that race, shunt status, and tumor volume have a significant impact on PFS and OS and that PTV and sex are associated with the incidence of vasculopathy. We previously showed, with limited follow-up, that shunt, race, and number of imaging evaluations during treatment were associated with PFS. We showed that CSF shunting is associated with larger tumor volume and postulated that it represented more extensive and aggressive disease.17 We now suggest that the slightly higher incidence of craniopharyngioma in Blacks2 may be linked to biological differences in tumor etiology and requires further investigation. Finally, larger tumor and target volumes, like CSF shunting, indicate more aggressive tumors. Those who treat craniopharyngioma have long held that the volume of the irradiated tumor and other clinical and treatment factors had no impact on outcome, although it was suggested that subtotal resection largely included patients with minimal residual tumor.18
Indeed, the small number of patients included in most institutional reports have led to meta-analysis of similar series.19,20 However, these analyses focused on documenting the equivalence of radical surgery to limited surgery and RT. Although these reports have been valuable and improved patient selection, subgroup analysis of prognostic factors has not been performed due to lack of detailed information.
Investigators from the Necker–Enfants Malades Hospital9 reported on disease control and quality of life outcomes comparing 103 patients from 1984–2002 when radical surgery was the prioritized treatment to 22 patients treated 2002–2004 when the treatment goal was hypothalamic preservation using more limited surgery and irradiation. In the earlier era, 35% of patients received radiotherapy whereas in the later era radiotherapy was administered to 45% of patients. The major findings were lower peri-treatment mortality, improved or stable vision, improved post-operative BMI z-scores and improved health utility index score. They also found that those treated with radical surgery had behavioral problems associated with degree of hypothalamic involvement and were less likely to have normal social development. The results of their research supported their early hypothesis that the quality-of-life outcome correlated with the degree of hypothalamic damage evident on post-operative imaging.8
Craniopharyngioma researchers in Germany10 summarized that OS and quality of life after treatment for craniopharyngioma are impaired based on hypothalamic involvement and by extension concluded that those with hypothalamic involvement should not undergo attempted gross-total resection. Their report did not provide modality specific outcomes for weight, neuropsychological status and quality of life, and psychosocial status, and only 40% (90/223) of patients received radiotherapy.
There is an emerging trend toward noninvasive management of craniopharyngioma.21,22 It has been established that long-term survival is likely and that care should be taken to minimize the sequelae of tumor and treatment. In a review by Yang et al.,23 there was no significant difference in PFS and OS after radical surgery when compared to subtotal resection and adjuvant RT. They concluded that lesions involving the pituitary stalk, hypothalamus, and critical cerebral vasculature may be best treated with intentional subtotal resection and adjuvant CRT. In our experience, disease control after limited surgery and RT yielded tumor progression rates comparable to those offered by gross total resection.24,25 Only 6 patients in our study died from tumor progression. The remaining deaths were due to complications associated with tumor or treatment. Deaths directly attributable to RT are secondary malignant neoplasms and cerebrovascular disease. The latter has multifactorial etiology including endocrinopathy26 and surgery. The contribution of endocrinopathy, adherence to replacement therapy, and the impact of surgery and surgical approach to vasculopathy remain important research questions. We showed that larger tumor volume is a risk factor for vasculopathy. This may be due to stretch induced endothelial dysfunction.27,28 There is increasing concern about the critical transition of these patients to adulthood and their adherence to hormone replacement regimens. The chronicity of problems associated with craniopharyngioma and its treatment parallels those observed for children diagnosed with type 1 diabetes.29 Diabetes insipidus, when not present at the time of diagnosis, is a surgical complication and may be used as a marker of extensive or extended resection.
Investigators in Sweden30 reported on outcomes from a national health registry for those diagnosed between 1997–2011. In this primarily surgical series, where approximately 30% (70/134) of patients were treated with radiotherapy, they found that women more than men, children more than adults, and those with diabetes insipidus more than those with only hypopituitarism or no evidence of hypopituitarism or diabetes insipidus, had higher standardized mortality ratios. And while their outcomes and prior research suggest that patients treated with irradiation are at highest risk of cerebral infarction, they acknowledge that confounders when assessing outcomes based on RT include tumor size and extent. Their report did not include surgery specific outcomes and their database did not include individual patient clinical information such as tumor size, extent, and obesity.
In the months to years following treatment for craniopharyngioma, survivors require close surveillance and strict management of tumor and wide-ranging treatment-related complications including life-threating endocrinopathy, vasculopathy, accelerated cardiovascular disease, and/or nonalcoholic fatty liver disease leading to cirrhosis. Herein, we showed that the PTV was associated with a higher incidence of vasculopathy and that females had a higher incidence than males. The larger planning volume is likely associated with irradiation of all central vessels, a larger volume of the vulnerable cerebral vascular tree, and supports efforts to reduce the targeted volume, overall volume of irradiation, and dose to the uninvolved brain using newer methods of irradiation that spare normal tissues. The finding that females had a higher incidence of vasculopathy than males was unexpected and requires further investigation, including treatment of hormone deficiencies such as hypogonadotropic hypogonadism. Our results suggest that limited surgery and post-operative CRT are associated with excellent rates of tumor control and acceptable toxicity profiles. Further research is required to understand the contribution of host and tumor biology to tumor control and treatment complication rates.
Contributor Information
Drucilla Y Edmonston, Department of Radiation Oncology, University of Tennessee Health Science Center, Memphis, Tennessee, USA.
Shengjie Wu, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Yimei Li, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Raja B Khan, Department of Neurology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Frederick A Boop, Department of Global Pediatric Medicine, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Thomas E Merchant, Department of Radiation Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Funding
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and the American Cancer Society, Grant Number: RPG-99-252-01-CCE.
Conflict of interest statement. The authors report no conflicts of interest concerning the materials or methods used in this study or the findings reported in this paper.
Authorship statement. Concept and design: D.Y.E. and T.E.M. Data collection: D.Y.E. and T.E.M. Data Analysis: S.W. and Y.L. Data Interpretation: All Authors. Manuscript writing: D.Y.E. and T.E.M. Review, revisions, and approval of final manuscript: All authors.
References
- 1. Momin AA, Recinos MA, Cioffi G, et al. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary. 2021;24(4):517–522. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcus HJ, Rasul FT, Hussein Z, et al. Craniopharyngioma in children: trends from a third consecutive single-center cohort study. J Neurosurg Pediatr. 2019;25(3):242–250. [DOI] [PubMed] [Google Scholar]
- 4. Grewal MR, Spielman DB, Safi C, et al. Gross total versus subtotal surgical resection in the management of craniopharyngiomas. Allergy Rhinol (Providence). 2020;11:2152656720964158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madsen PJ, Buch VP, Douglas JE, et al. Endoscopic endonasal resection versus open surgery for pediatric craniopharyngioma: comparison of outcomes and complications. J Neurosurg Pediatr. 2019;24(3):236–245. [DOI] [PubMed] [Google Scholar]
- 6. Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28(4):E10. [DOI] [PubMed] [Google Scholar]
- 7. Sands SA, Milner JS, Goldberg J, et al. Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J Neurosurg. 2005;103(4 Suppl):302–311. [DOI] [PubMed] [Google Scholar]
- 8. Sainte-Rose C, Puget S, Wray A, et al. Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst. 2005;21(8-9):691–695. [DOI] [PubMed] [Google Scholar]
- 9. Puget S, Garnett M, Wray A, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106(1 Suppl):3–12. [DOI] [PubMed] [Google Scholar]
- 10. Sterkenburg AS, Hoffmann A, Gebhardt U, et al. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro Oncol. 2015;17(7):1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandrell BN, LaRosa K, Hancock D, et al. Predictors of narcolepsy and hypersomnia due to medical disorder in pediatric craniopharyngioma. J Neurooncol. 2020;148(2):307–316. [DOI] [PubMed] [Google Scholar]
- 12. van Iersel L, Brokke KE, Adan RAH, et al. Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar tumors: a systematic review. Endocr Rev. 2019;40(1):193–235. [DOI] [PubMed] [Google Scholar]
- 13. Merchant TE, Kun LE, Hua CH, et al. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 2013;85(4):e187–e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90(2):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. AnnStat. 1988;16(3):1141–1154. http://www.jstor.org/stable/2241622. Accessed August 31, 2021. [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 17. Elliott RE, Hsieh K, Hochm T, et al. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J Neurosurg Pediatr. 2010;5(1):30–48. [DOI] [PubMed] [Google Scholar]
- 18. Stripp DC, Maity A, Janss AJ, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714–720. [DOI] [PubMed] [Google Scholar]
- 19. Clark AJ, Cage TA, Aranda D, et al. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst. 2013;29(2):231–238. [DOI] [PubMed] [Google Scholar]
- 20. Wang G, Zhang X, Feng M, Guo F. Comparing survival outcomes of gross total resection and subtotal resection with radiotherapy for craniopharyngioma: a meta-analysis. J Surg Res. 2018;226:131–139. [DOI] [PubMed] [Google Scholar]
- 21. Muller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75. [DOI] [PubMed] [Google Scholar]
- 22. Fouda MA, Karsten M, Staffa SJ, et al. Management strategies for recurrent pediatric craniopharyngioma: new recommendations. J Neurosurg Pediatr. 2021;22(5):548–555. [DOI] [PubMed] [Google Scholar]
- 23. Yang I, Sughrue ME, Rutkowski MJ, et al. Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28(4):E5. [DOI] [PubMed] [Google Scholar]
- 24. Wen BC, Hussey DH, Staples J, et al. A comparison of the roles of surgery and radiation therapy in the management of craniopharyngiomas. Int J Radiat Oncol Biol Phys. 1989;16(1):17–24. [DOI] [PubMed] [Google Scholar]
- 25. Villani RM, Tomei G, Bello L, et al. Long-term results of treatment for craniopharyngioma in children. Childs Nerv Syst. 1997;13(7):397–405. [DOI] [PubMed] [Google Scholar]
- 26. Boon IS, Perera D, Ayuk J. When Occam’s razor fails: hemipontine infarct on a background of previous surgery and radiotherapy for craniopharyngioma. BMJ Case Rep. 2016;2016:bcr2016215420. doi: 10.1136/bcr-2016-215420. PMID: 27056943; PMCID: PMC4840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Komalavilas P, Luo W, Guth CM, et al. Vascular surgical stretch injury leads to activation of P2X7 receptors and impaired endothelial function. PLoS One. 2017;12(11):e0188069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Girao-Silva T, Fonseca-Alaniz MH, Ribeiro-Silva JC, et al. High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci Rep. 2021;11(1):13493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monaghan M, Helgeson V, Wiebe D. Type 1 diabetes in young adulthood. Curr Diabetes Rev. 2015;11(4):239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab. 2015;100(2):467–474. [DOI] [PubMed] [Google Scholar]