Abstract
In the new WHO 2021 Classification of CNS Tumors the chapter “Circumscribed astrocytic gliomas, glioneuronal and neuronal tumors” encompasses several different rare tumor entities, which occur more frequently in children, adolescents, and young adults. The Task Force has reviewed the evidence of diagnostic and therapeutic interventions, which is low particularly for adult patients, and draw recommendations accordingly. Tumor diagnosis, based on WHO 2021, is primarily performed using conventional histological techniques; however, a molecular workup is important for differential diagnosis, in particular, DNA methylation profiling for the definitive classification of histologically unresolved cases. Molecular factors are increasing of prognostic and predictive importance. MRI finding are non-specific, but for some tumors are characteristic and suggestive. Gross total resection, when feasible, is the most important treatment in terms of prolonging survival and achieving long-term seizure control. Conformal radiotherapy should be considered in grade 3 and incompletely resected grade 2 tumors. In recurrent tumors reoperation and radiotherapy, including stereotactic radiotherapy, can be useful. Targeted therapies may be used in selected patients: BRAF and MEK inhibitors in pilocytic astrocytomas, pleomorphic xanthoastrocytomas, and gangliogliomas when BRAF altered, and mTOR inhibitor everolimus in subependymal giant cells astrocytomas. Sequencing to identify molecular targets is advocated for diagnostic clarification and to direct potential targeted therapies.
Keywords: circumscribed astrocytic gliomas, glioneuronal tumors, guideline, neuronal tumors
According to the 2021 WHO Classification,1 circumscribed astrocytic gliomas and glioneuronal and neuronal tumors comprise a heterogeneous group of rare tumors of the Central Nervous System (CNS). These tumors occur in children, adolescents, and young adults,2 but some of them may be occasionally seen even in the elderly. A majority of tumors have an indolent course, with aggressive forms occurring only rarely.
Most of these tumors arise spontaneously. However, neurofibromatosis type 1 (NF1) and tuberous sclerosis (TS) are two distinct neurocutaneous syndromes associated with an increased risk for pediatric low-grade gliomas3–6
The epidemiologic features of the different entities are reported in Table 1.
Table 1.
Epidemiology and clinical features
| Tumor type | Estimated Incidence |
Age | Sex | Location | Clinical presentation |
|---|---|---|---|---|---|
| PA | Common (5% PBT*) |
All ages mostly 0–20 yrs |
No predilection | Cerebellum (mostly in children) and cerebrum | Depending on location |
| HGAP | Rare | Mostly adults | No predilection | All sites, mostly cerebellum | Depending on location |
| PXA | Uncommon (< 0.3% PBT*) |
Children/young adults | No predilection | Temporal | Seizures |
| SEGA** | Rare | Mostly 2nd decade | Slight male prevalence | Foramen of Monro | Intracranial hypertension, seizures |
| Chordoid glioma | Very rare | Mostly adults | Female prevalence | Anterior 3rd ventricle | Hydrocephalus, endocrinal abnormalities, visual field defects |
| Astroblastoma MN1-altered |
Very rare | 3 mos – 40 yrs | Female prevalence | Cerebral hemispheres | Seizures |
| Ganglioglioma | Uncommon (1% PBT*) |
All ages mostly young adults |
Slight male prevalence | Temporal, frontal, parietal | Seizures |
| Gangliocytoma*** | No data | No data | No data | No data | No data |
| DIG/ DIA | Rare | 1–2 years | Slight male prevalence | <1 lobe (frontal and parietal) | Megalocephaly, bulging tense fontanelles |
| DNT | Uncommon (0.033/100 000/y) |
children and young adults | Slight male prevalence | Temporal | Seizures |
| DGONC | Very rare | 9–12 years | No predilection | Cerebral hemispheres (cortical/subcortical) | Depending on location |
| PGNT | Rare | All ages mostly 2nd decade |
No predilection | Frontal, temporal | Headache, seizures |
| RGNT | Rare | Young adults | Female prevalence | 4th ventricle, cerebellum | Intracranial hypertension, cerebellar disturbances |
| Myxoid glioneuronal tumor | Rare | Mostly 2nd–3rd decade | No predilection | Septal nuclei, septum pellucidum, corpus callosum |
Headache, seizures, behavioral disturbances |
| DLGNT | Rare | Mostly children and adolescents | Slight male prevalence | Basal leptomeninges | Intracranial hypertension, hydrocephalus |
| MVNT | Very rare | Mostly adults | Slight male prevalence | Temporal, parietal | Seizures |
| Dysplastic cerebellar gangliocytoma | Rare | Mostly 3rd–4th decade | No predilection | Cerebellar hemispheres | Cerebellar disturbances, intracranial hypertension |
| Central Neurocytoma | Uncommon (0.1–0.5% PBT*) |
All ages mostly 2nd or 3rd decade |
No predilection | Lateral ventricles and septum pellucidum | Intracranial hypertension |
| Extraventricular Neurocytoma | Very rare | Mostly 3rd and 4th decade | No predilection | Frontal, cerebellum | Seizures, headache, focal neurological symptoms |
| Cerebellar liponeurocytoma | Very rare | Adults | No predilection | Cerebellum | Cerebellar disturbances |
*Primary brain tumors.
**Most cases associated with tuberous sclerosis.
***Generally combined with ganglioglioma.
Methods
The European Association of Neuro-Oncology (EANO), the Society for Neuro-Oncology (SNO), and the EUropean Network for RAre CANcers (EURACAN) established a multidisciplinary Task Force to develop Guidelines on “Circumscribed astrocytic gliomas, glioneuronal and neuronal tumors.”
As for previous EANO Guidelines the Task Force reviewed the available English literature through March 2022, classified the scientific evidence into classes I–IV, and developed recommendations at levels A–C according to the European Federation of the Neurological Societies Guidelines.7 When sufficient evidence for recommendations was not available, the Task Force offered advice as a Good Practice Point.
Overall, the extensive heterogeneity and rarity of these tumors have precluded the possibility in the past to conduct well-powered clinical trials to generate evidence-based treatment recommendations for non-surgical modalities, and this is reflected in the General Recommendations drawn by this Task Force.
Clinical Features
Seizures are the main symptom of low-grade neuronal or glioneuronal tumors and occur in 80–100% of patients.8,9 They may represent the unique symptom at onset,10 but also develop in the end-of-life phase in rare malignant tumors.11
A number of tumors cause medically intractable seizures: in this regard, they have been denominated “long-term epilepsy-associated tumors.” (LEATs)12
Other tumors may present with focal neurological deficits, symptoms of raised intracranial pressure related to mass effect or cerebrospinal fluid (CSF) obstruction, or be incidental findings on brain imaging. Overall, there are no specific clinical features for particular tumor types, but rather the clinical presentation is related to the location within the CNS.
One particular, albeit rare, aspect of some pediatric low-grade gliomas (pLGGs) is their propensity to disseminate. Dissemination is more common in younger children and in patients with diencephalic tumors. Although dissemination is a sign of more aggressive behavior, this is not a manifestation of malignant transformation as unlike the adult counterparts they rarely transform.
The clinical features in the presentation of the different entities are reported in Table 1.
Pathology and Molecular Markers
The tumors described in this guideline are based on the 2021 WHO Classification of Central Nervous System Tumors.1 In comparison with the previous 2016 WHO Classification,13 some new entities (eg, high-grade astrocytoma with piloid features, diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters, myxoid glioneuronal tumor, and multinodular and vacuolating neuronal tumor) have been added, while others (in particular “anaplastic ganglioglioma”) have been abrogated.14–18 Tumor diagnosis for many of these lesions may still primarily be based on H&E stained sections and some additional techniques, including silver impregnation for reticulin fibers and Alcian blue for demonstration of mucoid changes. Many tumors of this spectrum have a mixed glial and neuronal differentiation and may thus to some extent express glial markers (typically GFAP) and neuronal markers (eg, synaptophysin) by immunohistochemistry. For some tumors the differentiation is mostly glial (eg, pilocytic astrocytoma, pleomorphic xanthoastrocytoma), for others mostly neuronal (eg, central neurocytoma, gangliocytoma), and for many others mixed (eg, ganglioglioma, dysembryoplastic neuroepithelial tumor, subependymal giant cell astrocytoma, diffuse leptomeningeal glioneuronal tumor). Molecular workup is further important to exclude other types of brain tumors that may mimic tumors of the above spectrum (in particular IDH1 or IDH2 mutations for exclusion of IDH-mutant diffuse gliomas and histone H3 K27M mutation for exclusion of diffuse midline glioma, H3 K27-altered), but also to identify potential therapeutic vulnerabilities for targeted therapies and predictive as well as prognostic biomarkers. A summary of the essential diagnostic criteria for the tumors covered in this guideline is presented in Table 2.
Table 2.
Diagnostic recommendations for pathological diagnosis
| Tumor type | CNS WHO grade | Essential diagnostic criteria |
|---|---|---|
| PA | 1 | Classic PA histology or a piloid astrocytic neoplasm with a solitary MAPK alteration (eg KIAA1549-BRAF fusion). |
| HGAP | not assigned | Astrocytic glioma with DNA methylation profile of HGAP |
| PXA | 2 or 3 | Astrocytic glioma with pleomorphic tumour cells, including large multinucleated cells, spindle cells, xanthomatous cells, and eosinophilic granular bodies. The presence of a BRAF V600E mutation (or other MAPK alteration), combined with homozygous deletion of CDKN2A/B is typical but not essential for diagnosis. For unresolved lesions, demonstration of typical DNA methylation profile may be helpful. |
| SEGA | 1 | Characteristic SEGA histology and expression of gliale markers and variable expression of neuronal makers. Presence of TSC1/TSC2 mutation or a DNA methylation profile of SEGA is typical but not essential for diagnosis. |
| Chordoid glioma | 2 | Glial tumor with chordoid histology located in the third ventricle. The presence of PRKCA p.D463H mutation or DNA methylation profile of chordoid glioma is typical but not essential for diagnosis. |
| Astroblastoma, MN1-altered | not assigned | Glial neoplasm with astroblastic perivascular pseudorosettes and MN1 alteration. For unresolved lesions demonstration of typical DNA methylation profile. |
| Ganglioglioma | 1 | Low-grade CNS tumor with the combination of the neoplastic ganglion and glial cells. For unresolved lesions presence of BRAF V600E mutation (or other MAPK alteration) or a demonstration of a typical DNA methylation profile. |
| Gangliocytoma | 1 | Irregular groups of large, mature ganglion cells within usually normal appearing neuropil. |
| DIG/DIA | 1 | Astrocytic (DIA) or astrocytic and neuronal (DIG) neuroepithelial tumor with a dominant desmoplastic leptomeningeal component. For unresolved lesions presence of BRAF/RAF1 mutation or fusion without concomitant CDKN2A/B homozygous deletion or demonstration of typical DNA methylation profile. |
| DNT | 1 | Cortical glioneuronal tumour with a specific glioneuronal element. For unresolved lesions presence of aFGFR1gene alteration or demonstration of typical DNA methylation profile. |
| DGONC | Not assigned | Neuroepithelial tumor is composed of oligodendroglioma-like cells forming occasional clusters and expressing OLIG2 and synaptophysin but lacking GFAP expression. Additionally demonstration of atypical DNA methylation profile |
| PGNT | 1 | Biphasic neuroepithelial tumor with pseudopapillary glial structures and neuronal component and presence of a PRKCA gene fusion. For unresolved lesions demonstration of typical DNA methylation profile. |
| RGNT | 1 | Biphasic neuroepithelial tumor with neurocytic and glial component and rosette-like structures and/or perivascular pseudorosettes with synaptophysin expression. For unresolved lesions demonstration of typical DNA methylation profile. |
| Myxoid glioneuronal tumor | 1 | Neuroepithelial tumor located in septum pellucidum, corpus callosum, or periventricular regions composed of oligodendrocyte-like tumor cells surrounded by myxoid stroma. Demonstration of aPDGFRAp.K385L/I dinucleotide mutation and a typical DNA methylation profile are desirable but not essential for diagnosis. |
| DLGNT | Not assigned but likely either 2 or 3 | Neuroepithelial tumor with oligodendroglioma-like morphology and OLIG2 and synaptophysin expression harboring a combined chromosome 1p deletion and a MAPK pathway alteration (typically KIAA1549-BRAF fusion). For unresolved lesions demonstration of typical DNA methylation profile. |
| MVNT | 1 | Mitotically inactive multinodular neuroepithelial tumor composed of neuronal tumor cells with either tumor cell or matrix vacuolation. The tumors show MAPK pathway–activating abnormalities but the demonstration is not essential for diagnosis. |
| Dysplastic cerebellar gangliocytoma | 1 | Neuroepithelial tumor is composed of densely packed ganglionic cells of various sizes surrounded by often normal appearing neuropil with enlargement of cerebellar folia. PTEN mutations or deletions are typical but demonstration is not essential for diagnosis. |
| Central neurocytoma | 2 | Neuroepithelial tumor is composed of monomorphic oligodendroglioma-like cells with synaptophysin expression and intraventricular localization. For unresolved lesions demonstration of typical DNA methylation profile. |
| Extraventricular neurocytoma | 2 | IDH wild-type extraventricular neuroepithelial tumor composed of neurocytic cells with synaptophysin expression. For unresolved lesions demonstration of typical DNA methylation profile. FGFR1 alterations (mostly FGFR1-TACC1fusions) are typical but the demonstration is not essential for diagnosis. |
| Cerebellar liponeurocytoma | 2 | A neuroepithelial tumor is composed of oligodendroglioma-like cells with focal lipoma-like changes, synaptophysin expression, and cerebellar localization. For unresolved lesions demonstration of typical DNA methylation profile. |
Pilocytic astrocytoma (PA) often demonstrates a variable pattern of compact and loosely textured regions (so-called “biphasic pattern”), a varying fractions of bipolar cells with hair-like (so-called “pilocytic”) processes, as well as structures, appearing densely eosinophilic on H&E staining either in the form of fibers (so-called “Rosenthal fibers”) or as eosinophilic granular bodies. Proliferation is typically low but may be substantially higher in children. A solitary mitogen-activated protein kinase (MAPK) pathway alteration (most frequently KIAA1549-BRAF fusion) is typically found.19 ATRX expression is typically retained, whereas its loss should prompt consideration of the newly described class of high-grade astrocytoma with piloid features.15
High-grade astrocytoma with piloid features (HGAP) often shows non-distinct high-grade piloid or glioblastoma-like histological features.15 Alterations of MAPK pathway genes are frequently observed in combination with homozygous CDKN2A/B deletion and/or loss of nuclear ATRX expression. The tumors are defined by a specific DNA methylation profile and currently, DNA methylation profiling is required for the diagnosis. A number of these tumors have lower-grade precursor lesions sometimes dating back many years.
Pleomorphic xanthoastrocytoma (PXA) is a tumor with a wide range of morphology spanning from tumors with prominent pleomorphism and sometimes bizarre cells with multiple nucleoli to more monomorphous and spindle cell-dominated tumors. Intercellular reticulin fiber deposition may be prominent, and many tumors harbor numerous eosinophilic granular bodies. Two WHO grades (CNS WHO 2 or 3) are assigned based on a mitotic count of more than 5 mitoses per 10 microscopic high power fields. However, their prognostic value is the subject to debate. BRAF V600E mutation can be detected in up to 70% of tumors, combined with CDKN2A homozygous deletion in greater than 90%.20
Subependymal giant cell astrocytoma (SEGA) is typically a moderately cellular tumor dominated by polygonal or gemistocytic cells with glassy cytoplasm. Occasional cells may be smaller and more spindle cell-like or much larger with ganglionic-like appearance. Proliferation is typically low.21 Markers typically positive in SEGA are phosphorylated S6 protein (pS6) consistent with mTOR pathway activation.22,23 SEGA is strongly associated with tuberous sclerosis due to germline mutations in either TSC1 or TSC2 genes.24
Chordoid glioma is a well-circumscribed glial neoplasm arising in the anterior third ventricle often composed of cords or clusters of epithelioid cells surrounded by a mucinous stroma and sometimes dense lymphocytic infiltrate. These tumors often express TTF1. Genetically they exhibit a recurrent p.D463H missense mutation in the PRKCA gene.25
Astroblastoma, MN1-altered is a circumscribed glial neoplasm with structural rearrangements of the MN1 gene, most often in the form of a fusion with BEND2. Histologically, the tumors often show variable pseudopapillary growth and astroblastic perivascular pseudorosettes as well as pronounced pericellular or perivascular hyalinization. They need to be molecularly confirmed per the 2021 WHO Classification guidelines, as this provides distinction from other tumor entities that can occasionally demonstrate astroblastoma-like rosettes. Tumors with fusions between MN1 and PATZ1 form a distinct group and should not be confused with Astroblastoma.26
Ganglioglioma is a tumor composed of both neoplastic ganglion and glial cells in varying proportions.27 The tumor cells are a typically intermixed and eosinophilic granular bodies and perivascular lymphocytic infiltrates are common features. The neoplastic ganglion cells typically express synaptophysin, and the neoplastic glial cells typically GFAP. Expression of CD34 by ramified cells, either within the tumor or in the adjacent cortex, is a recurrent finding.28 The BRAF V600E mutation constitutes the most common mutation in gangliogliomas, found in ~20-60% of tumors, with alternative BRAF mutations and fusions, KRAS mutations, NF1 mutations, and RAF1 fusions arising in smaller subsets.29–31 Molecular alterations associated with diffuse gliomas, such as mutations/aberrations in IDH1/2, TP53, and EGFR exclude the diagnosis of ganglioglioma.
Ganglioglioma is defined as a CNS WHO grade 1 tumor by 2021 WHO Classification, while the previous 2016 WHO Classification included an anaplastic variant for those tumors with features such as high mitotic activity and/or microvascular proliferation and/or necrosis, often associated with an aggressive clinical behavior. However, most cases of “anaplastic ganglioglioma” from the previous series lacked molecular analyses to exclude other high-grade gliomas subtypes: in this regard, the 2021 WHO Classification states that “further studies are needed to confirm the existence of anaplastic ganglioglioma”.
Gangliocytoma is a neuroepithelial tumor composed of a pure population of neoplastic mature ganglion cells, and may harbor molecular alterations involving components of the RAS/MAPK signaling pathway.
Desmoplastic infantile ganglioglioma/desmoplastic infantile astrocytoma (DIG/DIA) are benign neoplasms, that may either present as astrocytic neoplasms or more frequently as mixed astrocytic and neuronal neoplasms with a prominent desmoplastic stroma. A genetic alteration activating the MAPK pathway (often BRAF variants or rearrangements other than the canonical p.V600E mutation) is typically present as the solitary oncogenic event.32
Dysembryoplastic neuroepithelial tumor (DNT) shows a multinodular intracortical growth and columns of small oligodendroglia-like cells surrounding axon bundles (so-called “glioneuronal element”). Between these structures a mucoid, alcianophilic matrix may be evident with entrapped normal appearing neurons (so-called “floating neurons”). Proliferation is usually very low. Currently, two major forms of DNT are established (the simple and the complex form). Further forms may exist but are still a matter of debate. They frequently harbor FGFR1 kinase domain tandem duplication or hotspot missense mutations at codons p.N546 or p.K656.33
Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC) is a provisional WHO tumor type composed of neuroepithelial cells often presenting with perinuclear halos (ie, “oligodendroglioma-like”), that occasionally demonstrates focal grouping of nuclei (“clusters”). The tumors frequently harbor monosomy of chromosome 14 and currently, DNA methylation profiling is required for diagnosis.17,18
Papillary glioneuronal tumor (PGNT) is a tumor with the formation of pseudopapillary glial structures and interpapillary neuronal components. Gene-fusions of PRKCA (mostly SLC44A1–PRKCA fusion) are a molecular hallmark of these benign neoplasms.34
Rosette-forming glioneuronal tumor (RGNT) histologically represents a biphasic tumor composed of rosette-forming uniform neurocytes with adjacent glial areas often histologically reminiscent of pilocytic astrocytoma. FGFR1 kinase domain hotspot missense mutations (either at p.N546 or p.K656) are highly characteristic for these benign neoplasms, occurring in conjunction with either PIK3CA or PIK3R1 mutations, frequently with accompanying NF1 or PTPN11 mutations.35,36
Myxoid glioneuronal tumor histologically features a monomorphic oligodendrocyte-like tumor cell population embedded in a prominent myxoid stroma and often admixed “floating” neurons. A dinucleotide mutation at codon p.K385 in the PDGFRA gene is a hallmark for these benign tumors often located in the septum pellucidum or periventricular white matter of the lateral ventricles.16,36
Diffuse leptomeningeal glioneuronal tumor (DLGNT) is composed of diffusely infiltrating oligodendrocyte-like cells and an additional variable neuronal component. The tumors involve the leptomeninges and are frequently initially present spinally. So far, all reported cases harbor a chromosome 1p deletion and many show evidence for an additional activating MAPK pathway alteration, most commonly KIAA1549-BRAF fusion.
Multinodular and vacuolating neuronal tumor (MVNT) is characterized by monomorphous neuronal elements in discrete nodules. MVNT harbor molecular alterations in the RAS/MAPK pathway, most commonly as MAP2K1 exon 2 mutations.37
Dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) histologically presents with a thickening of the cerebellar folia by a population of densely packed dysplastic ganglion cells. Proliferation is typically very low and it has been discussed that these lesions are rather hamartomatous than neoplastic in nature. An association with Cowden syndrome is described, most often presenting with germline mutations in PTEN.38
Central neurocytoma is a typically monomorphous tumor composed of relatively uniform round cells (so-called “neurocytes”) with the occasional formation of cell-free neuropil regions (so-called “neuropil-like islands”). Tumor cells typically express neuronal markers (NeuN, synaptophysin) but staining intensity may be variable. Proliferation is usually low and is likely a prognostic factor for central neurocytoma, but the exact cutoff for prognostication has not been established.
Extraventricular neurocytoma is a neuroepithelial tumor with histological characteristics resembling central neurocytoma. Compared to central neurocytomas, extraventricular neurocytomas presents a higher degree of ganglionic differentiation and more frequent glial differentiation. They frequently harbor FGFR1-TACC1 fusions.39
Cerebellar liponeurocytoma shows a prominent neuronal or neurocytic differentiation and may have areas with more glial differentiation. In addition, these tumors often have focal or widespread lipomatous changes. A driving genetic event has not yet been identified but the tumors show a characteristic DNA methylation profile.40
For HGAP, astroblastoma, DGONC, PGNT, and DLGNT, detection of defining molecular alterations has become essential for diagnosis in the 2021 WHO Classification. For many others, the detection of specific molecular alterations is considered desirable, especially because of the emergence of new treatment options but in histologically typical cases the diagnosis may still be made by histology alone (Table 2).
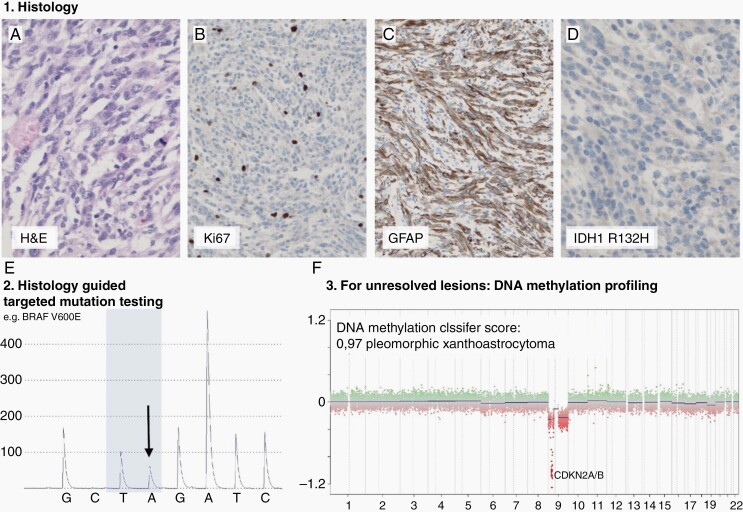
DNA methylation-based tumor classification is recommended for the clarification of histologically unresolved cases for many tumor types of this spectrum.40,41 Furthermore, DNA methylation profiling may be useful to select and streamline specific ancillary molecular testing.42 However, this technology is not yet widely available (Figure 1).
Fig. 1.
Suggested pathological processing of tumors of circumscribed astrocytic gliomas, glioneuronal and neuronal tumors. Diagnostic workup typically starts with histological evaluation of an H&E slide (example of a PXA, (a), followed by immunohistochemical analyses of proliferation markers (b), differentiation markers (eg, GFAP; c) and mutation specific immunohistochemistry (e.g. IDH1 R132H; d). For many tumor types the histological evaluation will guide further molecular testing that may either be desirable or essential for diagnosis (e). In this example the tumor was tested for BRAF because histology represented PXA that frequently harbor BRAF V600E mutation. For cases that are not resolvable by histology (eg, histological features compatible with more than one tumor type) and targeted molecular testing (eg, testing result not clear or testing not established) DNA methylation profiling and consecutive copy number analysis may aid for the classification (f).
Neuroimaging
The imaging features have recently been reviewed43,44 and are summarized in Table 3.
Table 3.
Common neuroimaging features.
| Tumor type | Morphology | Enhancement | Edema/mass effect |
|---|---|---|---|
| PA | Solid/cystic or cystic with nodule Exophytic from brainstem |
Avid enhancement of nodular/solid components | May be marked |
| HGAP | Solid mass | Sometimes ring-enhancement | Present |
| PXA | Cystic cortical tumors adjacent to peripheral leptomeninges May have a reactive dural tail |
Avid nodule ± dural tail | Present |
| SEGA | T1w hypointense, T2w hyperintense | Heterogeneous | Localized. May cause ventricular obstruction |
| Chordoid glioma | Circumscribed ovoid or multi-lobulated mass | Homogeneous | Present |
| Astroblastoma MN1-altered |
Well-demarcated, solid cystic mass | Heterogeneous | Present |
| Ganglioglioma | Variable and non-specific Calcification in approx. 50% |
Enhancing mural nodule Solid mass variable enhancement |
Minimal/moderate |
| Gangliocytoma | Solid hypointense mass on T1W and hyperintense on T2W | Enhancing mural nodule Solid mass variable enhancement |
Minimal/moderate |
| DIG/ DIA | Large cystic lesion with superficial solid portion | Present | Mild |
| DNT | Variable phenotype. Type 1: well-delineated hypointense cyst or polycystic lesion on T1W; type 2: nodular with mixed signal intensity and type 3: iso-or hypointense on T1W Elevated Apparent Diffusion Coefficient (ADC) Calcification in approx. 20% Margin hyperintense on T2w FLAIR |
20%, nodular or ring-like | Usually absent |
| DGONC | hyperintense on T2-FLAIR | Minimal | Absent |
| PGNT | Cystic lesion with an enhancing mural nodule Mixed cystic and solid or cyst with discrete mural nodule Calcification common. Hemorrhage in 10% |
Avid enhancement in the solid component | Minimal edema |
| RGNT | Well-circumscribed hypointense onT1w. Hyperintense on T2w “Green bell pepper sign” Mixed cystic and solid or cyst with a mural nodule Calcification common. Hemorrhage in 10% |
Variable | Mass effect causing ataxia and nystagmus |
| Myxoid glioneuronal tumor | Circumscribed | Absent | Sometimes hydrocephalus |
| DLGNT | Diffuse leptomeningeal infiltration Subpial cysts or pseudocysts |
Leptomeningeal, irregular | May cause CSF flow obstruction. |
| MVNT | Juxtacortical cluster of tiny, well--defined, round or oval T2-w hyperintense nodules without mass effect. Relatively hyperintense on T2-w FLAIR. Highly characteristic appearance | Absent | Absent |
| Dysplastic cerebellar gangliocytoma | Alternating hypo- and hyperintense striations on T1w and T2w images. “tiger striped”/“corduroy” folial pattern Highly characteristic/pathognomonic |
Absent, but venous signal in folia may be seen | Present. May cause 4th ventricular obstruction. |
| Central Neurocytoma | Well circumscribed mass Isointense in T1w and hyperintense on T2w “Soap-bubble” appearance Calcification 50% Vascular flow voids variable low ADC |
Variable and typically heterogeneous | Frequently cause ventricular obstruction |
| Extraventricular Neurocytoma | Well defined T1w hypointense, T2w hyperintense May involve cortex |
Variable heterogeneous | Mass effect |
| Cerebellar Liponeurocytoma | Well-defined T1w hypointense and T2w hyperintense. Foci of T1w hyperintensity due to lipid components | Heterogeneous | Mass effect leads to cerebellar symptoms/CSF obstruction |
Because of the uncommon nature of these tumors, retrospective and limited case series are available.
Although imaging with computer tomography (CT) may play a role in initial detection and is sensitive to calcification and acute hemorrhage, magnetic resonance imaging (MRI) is the mainstay for the evaluation of these lesions. MRI plays a key role in initial diagnosis, therapeutic decision-making, neurosurgical and radiotherapy planning, and surveillance.
No specific imaging guidelines related to these tumor groups have been developed; however, the adoption of generic standardized brain tumor MRI protocols45 is a pragmatic and important step toward improving comparability across centers, and should ideally include susceptibility sensitive MR sequences to detect calcification.
Response evaluation to treatments is becoming of increased importance, especially with the use of targeted agents; however, the rarity of these tumors has precluded dedicated studies thus far.
Most of these entities present heterogeneous components on MRI with the coexistence of non-enhancing and mild or nodular enhancing portions. In the absence of validated criteria, RANO criteria for adult low-grade gliomas46 and RANO criteria for high47 and low48 grade pediatric gliomas are seen to apply to most common tumor entities, such as pilocytic astrocytomas, pleomorphic xanthoastrocytomas, gangliogliomas or central neurocytomas. All these criteria are based on bidirectional (2D) measurements on a single MRI slice, but the future volumetric measurements (3D) could allow a more accurate evaluation of these tumors under treatment.49,50 Clinical factors, such as seizure control (ganglioglioma, PXA, SEGA, etc.) or visual function (optic pathways glioma) should be considered in association with neuroimaging to evaluate response to treatments.
There are also limited data to inform the frequency of imaging surveillance, as highlighted in a systematic review of paediatric and young adult populations,51 and strategies tailored to specific tumor types based on their known characteristics have been proposed.52
Advanced or emerging quantitative imaging methods, such as spectroscopy and perfusion imaging, may provide useful adjuncts to routine structural sequences in individual instances, although imaging signatures lack specificity, and clinical utility has not been established to date in this tumor group.
Functional MRI (fMRI) and diffuse tensor imaging (DTI) tractographic methods also offer the potential for identifying functionally eloquent cortex and fiber tracts for optimum safe resection, while intraoperative imaging is useful to account for brain shift during surgery; however, evidence for an impact on outcome is limited.53
Further prospective studies are required to establish the added value of advanced and intraoperative imaging techniques for stratification, surgical planning, and surveillance.
In general imaging features may be non-specific and overlap with those of diffuse gliomas and other aggressive cerebral neoplasms; however, some are quite characteristic and highly suggestive.
Neuroimaging Findings in Specific Entities
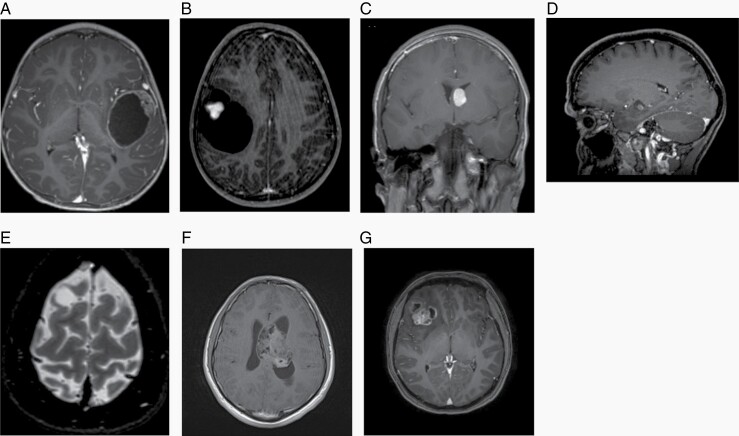
PA is often characterized by a cystic lesion with an enhancing mural nodule (Figure 2A) and most frequently occurs in the cerebellum in children, but in adults is equally common in the supratentorial compartment. Tumors involving the optic pathways are often associated with neurofibromatosis type 1 (NF1) and may be seen as fusiform enhancing masses. PA involving the brainstem is typically exophytic, and extends into the fourth ventricle.
Fig. 2.
A. Pilocytic astrocytoma, T1 post-contrast MRI image. B. Pleomorphic xanthoastrocytoma, T1 post-contrast MRI image. C. Subependymal giant cell astrocytoma, T1 post-contrast MRI image. D. Ganglioglioma, T1 post-contrast MRI image. E. DNT, T2-weighted MRI image. F. Central neurocytoma, T1 post-contrast MRI image. G. Extraventricular neurocytoma, T1 post-contrast MRI image.
HGAP is a Solid Tumor with Variable Contrast Enhancement and Edema
PXA commonly shows features seen in some high-grade diffuse gliomas, such as large cystic components, strong enhancement, and perilesional edema (Figure 2B).
SEGA is rarely seen outside the context of Tuberous Sclerosis Complex (TSC)54: the pathognomonic imaging features are related to the location adjacent to the foramina of Monro (Figure 2C).
Choroid glioma is almost exclusively in the anterior part of the third ventricle. On MRI the tumor is usually well-demarcated, solid with a variable cystic component and a homogeneous enhancement.
Astroblastoma, MN1-altered is a well-demarcated, solid/cystic mass with heterogeneous contrast enhancement.
Ganglioglioma (Figure 2D) and gangliocytoma are the most common low-grade epilepsy-associated tumors (LEAT) and MRI appearances are variable.
DIG/DIA is a large cystic lesion with a solid superficial portion and edema.
DNT is the second most common LEAT. These tumors are cortically-based (Figure 2E) and variable in imaging appearance but often show a wedge-shaped morphology extending to the cortex. There is also a growing appreciation of diffuse glioneuronal tumor variants, that lack hallmark features of typical DNT or ganglioglioma, and encompass a spectrum of complex DNTs.55
DGONC is a solid tumor with minimal contrast enhancement and absent edema.
PGNT appears morphologically similar to ganglioglioma on MRI, although has a predilection for deeper temporal structures while ganglioglioma tends to be more cortically based.
RGNT typically involves the 4th ventricle, midbrain, cerebellar vermis, and cerebral aqueduct, although may also be found in cerebellar hemispheres, pineal region, lateral and third ventricles, and hypothalamus.
MGT is a circumscribed tumor without contrast enhancement.
DLGNT involves the surface of the cerebellum, brainstem, and basal cisterns and causes hydrocephalus due to impairment of CSF flow. These tumors may be radiologically mistaken for tuberculous, carcinomatous, or lipomatous meningitis.
MVNT is characterized by juxstacortical multiple nodules without contrast enhancement or edema.
Dysplastic cerebellar gangliocytoma (Lhermitte-Duclos disease) frequently has a pathognomonic MRI appearance, corresponding to the preserved architecture of underlying cerebella folia.
Central neurocytoma typically presents with mass effect and ventricular obstruction (Figure 2F).
Extraventricular neurocytoma (EVN) shows the same characteristics as central neurocytoma (Figure 2G) and may pose MRI problems of differential diagnosis with oligodendroglioma or ganglioglioma.
Cerebellar liponeurocytoma is usually within the posterior fossa, although can occasionally be supratentorial. They variably present focal areas of the lipid-related signal.
For the new tumor class of HGAP neuroimaging has only been presented for single cases.56 The tumors typically present hypo- to isointense on T1-weighted images and hyperintense on T2- weighted MRI images and show heterogeneous contrast enhancement.
Surgery
Surgery is a cornerstone in the management of tumors in children and adults for both symptom and tumor control purposes. Indications for surgery may be epileptic seizures, hydrocephalus, raised intracranial pressure, and/or neurological and neurocognitive deficits. Radiologically demonstrated tumor growth, even if asymptomatic, may also be considered an indication for surgery. In many instances, the treatment goal is not strictly oncological since lesions often are indolent, but present with seizures or focal neurological symptoms and surgery may help to control. The impact of surgery on the various rare CNS tumors is summarized in Table 4.
Table 4.
Impact of surgery and indications for radiotherapy.
| Tumor type | Impact of gross total resection | Consequences of residual tumor |
Indications for radiotherapy |
|---|---|---|---|
| PA | Potential cure Hydrocephalus release Survival benefit over subtotal resection |
Local tumor progression Hydrocephalus Hemorrhage (rare) |
Inoperable or recurrent or aggressive tumors |
| HGAP | unclear due to rarity | Local tumor progression | Completely and incompletely resected tumors |
| PXA (grade 2) |
Potential cure Seizure control |
Local tumor progression Uncontrolled seizures + AEDs increase Malignant transformation |
Recurrent tumors |
| PXA (grade 3) |
Prolonging of survival Reduction of seizure burden |
Local tumor progression Uncontrolled seizures + AEDs increase CSF spread |
Completely and incompletely resected tumors |
| SEGA | Seizure control Potential cure Hydrocephalus release |
Uncontrolled seizures + AEDs increase |
Not indicated |
| Chordoid glioma | unclear due to rarity | Local tumor progression | Unknown (recurrent tumors?) |
| Astroblastoma MN1-altered |
long-term survival | Local tumor progression | Inoperable or recurrent tumors |
| Ganglioglioma | Potential cure Seizure control |
Local tumor progression Uncontrolled seizures + AEDs increase Malignant transformation (5%) |
Incompletely resected tumors or aggressive tumors |
| Gangliocytoma | Potential cure Seizure control |
Uncontrolled seizures + AEDs |
Inoperable or recurrent tumors |
| DIG/ DIA | Long-term tumor control | Local tumor progression | Aggressive tumors |
| DNT | Seizure control Potential cure |
Uncontrolled seizures + AEDs increase |
Aggressive or recurrent tumors |
| DGONC | Potential Cure | Unknown | Unknown |
| PGNT | Longer PFS and OS | Local tumor progression | Unknown |
| RGNT | Time to recurrence Similar between gross Total and subtotal Resection |
Local tumor progression CSF spread (rare) |
Unknown |
| Myxoid glioneuronal tumor | Potential cure | Local tumor progression CSF spread (rare) |
Commonly not indicated |
| DLGNT | Resection not feasible | Diffuse tumor progression | Unknown |
| MVNT | Potential cure Seizure control |
Long stability overtime | Not indicated |
| Dysplastic cerebellar gangliocytoma |
Potential cure | Local tumor progression | Recurrent tumors |
| Central Neurocytoma | Long-term tumor control Hydrocephalus release No survival benefit over subtotal resection + radiotherapy |
Local tumor progression | Incompletely resected or atypical lesions |
| Extraventricular neurocytomas |
survival benefit over subtotal resection seizure control |
Local tumor progression | Incompletely resected or atypical lesions |
| Cerebellar liponeurocytoma |
Long-term survival | Local tumor progression (also late) | Incompletely resected tumors |
Surgical Management of Newly Diagnosed Intracranial Tumors
Surgery is considered the first and crucial step of the standard treatment of most rare CNS tumors. The majority of observational studies demonstrated an association between a higher extent of resection and prolonged tumor control and survival.57–60 Gross-total resection is achievable in many cases, although risks of postoperative deficits linked to a critical location may be an issue,43,61 particularly in central neurocytoma,62 dysplastic gangliocytoma of the cerebellum,63,64 and DIA/DIG.65 Gross total resection for well-circumscribed CNS WHO grade 1 tumors can produce long-term tumor control and even cure. Due to the indolent nature of these entities, long-term control can also be observed in cases with subtotal resection or even when a CSF spread is present. For instance in central neurocytoma, gross total resection has been reported to be associated with better tumor control, but not necessarily improved long-term survival as compared to subtotal resection.58 For tumors CNS WHO grade 2 or higher, there may also be a benefit of gross total resection, associated or not with adjuvant radiotherapy.61
Surgical Management of Tumor-Associated Epileptic Seizures
A favorable seizure outcome was obtained in more than 80% of the cases, allowing for antiepileptic drug therapy withdrawal in more than 25% of adults and children operated on for refractory epilepsy.8,66–68 In patients in whom a discordance between electroclinical data and imaging findings is observed following a comprehensive neurological and epileptological presurgical evaluation, invasive electrophysiological investigations, such as stereotactic electroencephalography, can be useful to tailor the resection.68 A comprehensive systematic review focused on ganglioglioma and DNT reported significantly higher rates of seizure-freedom in patients with less than 1-year duration of epilepsy, and in patients with gross-total resection over subtotal lesionectomy.66 Seizure outcomes did not differ between adults and children, temporal versus extra-temporal location, medically controlled versus refractory seizures, or with the intraoperative use of electrocorticography.66 Extended resection of temporal lobe tumors, with the hippocampectomy and/or corticectomy, may confer additional benefits.66,68
Laser interstitial thermal therapy may have a role in small deep-seated tumors as suggested by the experience in lesional (eg, hypothalamic hamartoma and cavernoma) and non-lesional epilepsy.69,70
Overall, at present there are no long-term data to guide surgical decisions in circumscribed astrocytic gliomas, glioneuronal or neuronal tumors.
Surgical Management of Hydrocephalus
In most patients, tumor-related hydrocephalus can be relieved by tumor resection without the need to address the hydrocephalus more specifically. In case of persistent hydrocephalus despite tumor resection, or in case of unresectable tumor causing hydrocephalus, shunting with or without the septostomy or endoscopic ventriculostomy is useful.
Surgical Management of Tumor-Related Cyst
In patients who present with a symptomatic tumor-related cyst or with a progressive cyst on imaging follow-up and without growth of the other components of the tumor, the cyst can be surgically treated. If one defers the option of simultaneous tumor resection and cyst removal, the cyst can be treated by stereotactic or open surgical puncture, by fenestration of the cyst for communication with cerebral ventricles or subarachnoid spaces, or by a catheter placement either to a reservoir or a shunt from the cyst to the abdomen (ie, a cystoperitoneal shunt).
Surgical Management of Recurrent Tumors
In case of local recurrence or progression, re-resection is to be considered in selected patients to achieve both tumor and symptom control (eg, hydrocephalus or refractory seizures). The impact on survival is not clear from existing literature due to small retrospective studies with strong selection biases and significant heterogeneity.
Radiotherapy
The majority of evidence on the role of radiotherapy derives from retrospective studies and small case series. A summary of current indications of radiotherapy in the various rare CNS tumor types is summarized in Table 4.
CNS WHO Grade 1 Tumors
There are no randomized controlled trials published and evidence on the efficacy of radiotherapy is largely based on retrospective series.
Radiotherapy has been rarely employed in patients with WHO grade 1 tumor, either circumscribed astrocytic gliomas or glioneuronal and neuronal tumors. In a series of 348 children, diagnosed with low-grade ganglioglioma and gangliocytoma from 2004 to 2010 using the SEER dataset, Dudley et al 201571 reported a 5-year survival of >95% for the whole population older than 1 year of age, excluding cases not located in the brainstem. Overall survival was similar between patients treated with surgery and those receiving surgery followed by adjuvant radiotherapy; however, only 11 patients received adjuvant radiotherapy. In another review reporting on 402 patients, including 342 with grade 1 gangliogliomas treated with different modalities between 1978 and 2007,72 10-year local control rates were 89% after gross total resection, 90% after gross total resection plus radiotherapy, 52% after subtotal resection and 65% after subtotal resection plus radiotherapy, and 10-year overall survivals were 95%, 95%, 62%, and 74%, respectively. Subgroup analysis of patients with grade 1 ganglioglioma revealed that subtotal resection plus radiotherapy was significantly better than subtotal resection alone for local control but not for overall survival. In contrast, radiotherapy after gross-total resection did improve neither local control nor overall survival. Similar results have been shown in other studies.57,73
Likewise, there are limited data also for other grade 1 tumor, including PA, PGNT, RGNT, and dysplastic gangliocytoma of the cerebellum. Data from a meta-analysis showed that less than 15% of 71 PGNT and less than 5% of 85 RGNT were treated with postoperative radiotherapy, mainly after partial resection or biopsy or in case of CSF spread.43,74–76
CNS WHO Grade 2 Tumors
A review of published studies on central neurocytoma concluded that adjuvant radiotherapy after gross total resection failed to improve overall survival (OS) and progression-free survival (PFS).77 Adjuvant radiotherapy, using doses of 54–59 Gy given in 30 fractions, after subtotal resection significantly improved PFS and OS as compared with subtotal resection alone. Higher radiation doses in the range of 55–60 Gy may result in better 5-year PFS. The value of adjuvant radiotherapy in incompletely resected tumors has been suggested in a recent review.78
The efficacy and safety of stereotactic radiosurgery on central neurocytoma have been reported in several retrospective series with a 5- and 10-year local control in 93%–100% and 87% of patients, respectively, with up to 7% of adverse events.79–82
In a systematic review on 73 patients with liponeurocytoma, treated between 1978 and 2018, a positive impact of postoperative radiotherapy on local control was seen in both patients with complete or incomplete tumor resection.83 Tumor recurrence was seen in 0% and 26% of patients receiving gross-total resection with versus without adjuvant radiotherapy, respectively, and 16.7% and 77.8% of those undergoing incomplete resection with versus without adjuvant radiotherapy, respectively. Conversely, a recent retrospective study on 7 patients with the addition of a pooled analysis of individual patient data did not find any benefit in terms of PFS for the addition of postoperative radiotherapy following gross total resection.84
Among circumscribed astrocytic gliomas, including PXA and chordoid glioma, radiotherapy is usually reserved for growing tumors that have failed prior surgery.
CNS WHO Grade 3 Tumors
The 2021 WHO classification has abolished anaplastic ganglioglioma as a recognized entity. However, there are a number of retrospective studies on the treatment of anaplastic gangliogliomas diagnosed according to the 2016 WHO Classification. These data could be considered when managing patients with gangliogliomas displaying aggressive clinical behavior, although molecular profiling is strongly recommended in such scenarios which may lead to tumor reclassification.
A subgroup analysis on 60 patients with grade 3 tumors among a series of 402 patients with ganglioglioma, treated between 1978 and 2007, suggested that subtotal resection plus adjuvant radiotherapy was significantly superior to subtotal resection alone for local control but not for overall survival.72 Moreover, gross total resection plus radiotherapy resulted in similar local control and survival compared with gross total resection alone. In a series of 58 patients based on the SEER registry, including both adult and pediatric cases, Selvanathan et al 201185 reported a 5-year overall survival of 63% without a significant difference between 21 patients receiving adjuvant radiotherapy and 37 who did not. In another multicentric retrospective study of 43 adult patients with anaplastic gangliogliomas, Terrier et al 201786 reported a 5-year overall survival of 24.9%. Radiotherapy (60 Gy given in 30 daily fractions of 2 Gy), alone or in combination with chemotherapy, had no impact on overall survival; however, a subgroup analysis revealed a trend toward a longer PFS in patients who underwent gross total resection plus adjuvant radiotherapy.
Among circumscribed astrocytic gliomas, adjuvant radiotherapy should be considered for PXA87 and HGAP,56 even if the available information is limited. In some cases of DLGNT with aggressive behavior craniospinal irradiation could be used, especially when failing chemotherapy.88
Medical Treatments in Adults
While surgery and radiotherapy remain the mainstay of treatment, circumstances may arise where chemotherapy is left as the last option. Most commonly, this relates to tumors that have failed prior to surgery and radiation. Most patients receive chemotherapies that have demonstrated some level of efficacy in diffuse gliomas or other primary CNS tumors, extrapolating these regimens to rarer entities and the presumed CNS penetration of drugs. Rarely have trials been conducted in these uncommon tumors, and most of the literature comes from case reports or case series in which typically tumors are described in aggregate, including more often PA, PXA, ganglioglioma, and central neurocytoma.
The most common drug that has been employed in adult patients is temozolomide, given the drug’s benefit in diffuse gliomas as well as its good CNS penetration, and also other chemotherapy agents, such as carboplatin, etoposide, cyclophosphamide.89,90
Bevacizumab, a monoclonal antibody targeting the VEGF-A ligand, may be utilized in the setting of salvage therapy for the control of edema and symptomatic benefit. The use of bevacizumab for the treatment of rare brain tumors is mostly based on case reports and anecdotal experience. The drug could be useful in NF1-associated optic pathways gliomas91 and PA.92 Still is unclear the risk of a rebound of tumor growth upon interruption of bevacizumab in responding patients.
The most innovative therapies for the treatment of rare brain tumors have arisen in the era of next-generation sequencing of tumors and genotype-specific targeted therapies. Somatic alterations in the BRAF gene (BRAF V600E mutation and BRAF fusions) have been repeatedly observed in PXA, PA, and ganglioglioma. In addition to multiple case reports of various agents, a large cohort of patients studied prospectively included gliomas in a large international trial of vemurafenib for BRAF-mutant solid tumors.93 This prospective trial included 7 patients with PXA and 3 patients with ganglioglioma. Amongst the 7 patients with PXA, the best radiographic response included 1 complete response, 2 partial responses, 3 stable diseases, and 1 progressive disease. One patient with a ganglioglioma had a partial response as well. These results have been confirmed in a retrospective study on 28 patients with similar histologies: partial and complete responses accounted for 39% with a median PFS of 18 months.94
The combination of BRAF inhibitor dabrafenib with the MEK inhibitor trametinib to delay the appearance of a treatment resistance has been investigated in the basket trial ROAR, which included lower and higher-grade gliomas.95 In the high-grade glioma cohort (including PXA and 1 anaplastic ganglioglioma), the objective response rate was 33%, while in the low-grade gliomas cohort (including 4 gangliogliomas and 2 PXA) objective response rate was 69%. These data, deriving from an interim analysis of the trial, are promising, also in terms of duration of response and survival.
Additional sequencing efforts have also identified the occurrence of mutations in the neurotrophic tyrosine receptor kinase (NTRK) genes NTRK1, NTRK2, and NTRK3 in a small subset of these tumors96 these mutations are well characterized oncogenic drivers with a class of drugs available for effective targeting. Recently a dramatic response of a STRN- NTRK fused malignant glioneuronal tumor to larotrectinib has been reported.97 Targeting FGFR fusions in IDH wild-type lower-grade astrocytic tumors is a novel avenue of research.98
Medical Treatments in Children
The management of pediatric LGG that cannot be completely resected has evolved considerably over the recent decades.99 While radiotherapy was historically the usual treatment modality for these lesions, increasing awareness of the long-term consequences of radiation has contributed to a progressive deferral or abandonment of radiotherapy, particularly in the younger population. Observation and chemotherapy are increasingly used to avoid or delay radiotherapy. Various protocols of chemotherapy are currently employed, including the combination of carboplatin and vincristine, vinblastine, and the thioguanine/procarbazine/CCNU/vincristine (TPCV) regimen as the most common options.99,100 In most reports on chemotherapy, the majority of children have PAs, whose most common location is the diencephalic/chiasmatic/hypothalamic region, with the brainstem as the second most common site. These treatments are usually administered over a period of 12–18 months. Response to these regimens is difficult to compare across studies due to differences in the evaluation criteria. However, more consistent are the long-term results with 5-year PFS rates in the range of 35%–45% in the non-NF1 population, while PFS in NF1 patients is consistently higher in most studies, at 60%–70%.101,102 Consequently, a majority of LGG patients require several lines of therapy to achieve disease control. Most studies have shown that younger children, children with disseminated tumors, and children with the diencephalic syndrome at diagnosis tend to have a higher rate of progression.101,103,104
Over the last 10 years, several groups have contributed to a better characterization of the molecular landscape of pediatric LGG and have reported a number of additional alterations involving a large majority the RAS/MAPK pathway.19,31,105–107 In addition to the KIAA1549:BRAF fusion and germline NF1 mutations, pediatric LGG can exhibit BRAFV600E mutations, FGFR1/2 alterations (duplications, fusion, or mutations), mutations in MAP2K1, PDGFRA, as well as fusions involving NTRK, ROS1, or ALK. As the large majority harbor alterations of the MAPK pathways, pediatric LGG have been considered as a canonical single pathway disease. The recent study of 1000 pediatric LGG identified driver mutations in 84% of the specimens of which only 4.6% contained alterations in genes with seemingly no direct impact on the RAS/MAPK pathway.31
Thus the introduction of targeted treatments in the management of paediatric LGG has been a major paradigm shift.99 MEK inhibitors and BRAF inhibitors have been successfully used in pediatric LGG patients. A phase II trial of the MEK inhibitor selumetinib has shown high response rates in patients with recurrent NF1-related LGG and patients with recurrent LGG harboring the KIAA1549:BRAF fusion or BRAF V600E mutation.108 A phase I/II trial of the BRAF inhibitor dabrafenib, that enrolled 32 patients with recurrent, refractory, or progressive disease after ≥1 standard therapy, demonstrated meaningful activity with a 1-year PFS of 85%.109 Several trials are ongoing or in development, using a similar design and comparing standard chemotherapy with targeted agents. The Children’s Oncology Group is comparing vincristine and carboplatin to selumetinib in newly diagnosed or previously untreated NF1-associated LGG (https://clinicaltrials.gov/ct2/show/ NCT03871257) or in previously-untreated LGG not associated with BRAF V600E mutations or NF1 (https://clinicaltrials.gov/ct2/show/NCT04166409). LOGGIC is a phase III trial randomizing standard of care chemotherapy (vincristine and carboplatin combination or weekly vinblastine) against targeted therapy in pediatric patients with treatment-naïve pLGG requiring non-surgical management.
Based on mTOR pathway hyperactivation, mTOR inhibitors have been investigated in SEGAs. Thus far, everolimus has shown a favorable pharmacokinetic characteristics and activity on both tumor growth and seizures in clinical trials110,111 thus it has been approved by FDA and EMA for SEGAs needing treatment outside of surgery. Following full-dose treatment, a reduced dosage of everolimus for maintenance seems a rational therapeutic option to minimize adverse events while maintaining tumor stability.112 mTOR inhibitors appear to be safe in young children aged less than three years.54
Despite the encouraging results so far, a number of important aspects remain to be further studied in the context of future clinical trials, including the question of the optimal duration of targeted treatment, the introduction of drug holidays as well as the challenge of rapid rebound of tumor growth shortly upon cessation of treatment in a fraction of patients, especially in the context of patients with BRAFV600E mutations.
Whether there is a role for targeted therapies for pediatric LGG harboring less frequent mutations is still not known, and the design of clinical trials for these rare entities is challenging.
General treatment recommendations are reported in Table 5.
Table 5.
General treatment recommendations
| Class of evidence | Level of recommendations | |
|---|---|---|
| Resection is recommended to obtain a histological and molecular diagnosis and should be a gross total resection whenever feasible. When the morbidity can be significant, detailed informed preoperative counseling by a surgeon experienced in performing such surgery is important. | II | B |
| Early postoperative MRI should be performed to evaluate the extent of resection. | n.a. | Good practice point |
| Early operative intervention and gross-total resection are critically important factors in achieving seizure-freedom and should be proposed in patients presenting with seizures. | II | B |
| In children with a developing brain, the indication for an early operative intervention may be even stronger to achieve seizure control and freedom of medications. | IV | Good practice point |
| Use of intraoperative adjuncts in eloquent locations is highly advisable to improve benefit-to-risk ratio. | IV | Good practice point |
| The frequency of surveillance imaging with MRI should be based on the extent of resection (GTR versus non-GTR) and tumor grade/aggressiveness, and the duration be up to 5 years. | IV | Good practice point |
| Repeated surgery in patients with local tumor progression or recurrence should be considered | IV | Good practice point |
| Radiotherapy may have a role for inoperable tumors or following incomplete resection to improve local control depending on tumor grade, size, and location. | III | C |
| No adjuvant radiotherapy is recommended in grade 1 tumors following subtotal or gross total resection. | III | C |
| RT typically 50–54 Gy in 28–30 fractions, should be considered for grade 1 unresectable or large residual/progressive tumors. | IV | C |
| Stereotactic radiosurgery, given at doses of 12–25 Gy in 1–5 fractions, may represent an option for small size recurrent tumors. | IV | C |
| Adjuvant radiotherapy in grade 2 tumors should not be recommended after gross total resection | IV | C |
| The use of adjuvant radiotherapy in patients with incompletely resected aggressive gangliogliomas to improve local tumor control should be considered | IV | C |
| In patients with recurrent tumors who are no longer eligible for local treatments, chemotherapy might be warranted, particularly in patients with a good performance status. | IV | C |
| Consider targeted therapy with BRAF and/or MEK inhibitors in PAs, PXA, and gangliogliomas when BRAF altered. | III | C |
| Consider targeted therapy with the mTOR inhibitor everolimus in SEGAs with pharmacoresistant seizures. | II | B |
| For patients with substantial edema or mass effect resulting in neurologic dysfunction, bevacizumab may be a reasonable treatment. | IV | C |
| Sequencing to identify molecular targets is advocated for diagnostic clarification and to direct potential targeted therapy for incompletely resected or recurrent tumors. | IV | C |
Conclusions and Open Issues
Surgical resection still remains the most important therapeutic option for the majority of circumscribed astrocytic gliomas, glioneuronal, and neuronal tumors, while radiation therapy is commonly reserved for aggressive or recurrent tumors. New basket and umbrella trials, including both adults and children, are being designed to investigate the impact of new targeted agents.
The increasing integration of morphological with molecular data and definition of biologically “clean” entities with the different outcomes will progressively lead to tailored treatments based on molecular pathogenesis. New trial designs, including among others basket trials, are now needed when druggable pathways are identified across different and rare tumor types.
Contributor Information
Roberta Rudà, Department of Neurology, Castelfranco Veneto/Treviso Hospital and Division of Neuro-Oncology, Department of Neuroscience, University of Turin, Turin, Italy.
David Capper, Department of Neuropathology, Charité Universitätsmedizin Berlin, Berlin and German Cancer Consortium (DKTK), Partner Site Berlin, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Adam D Waldman, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh and Department of Brain Science, Imperial College London, United Kingdom.
Johan Pallud, Department of Neurosurgery, GHU-Paris Psychiatrie et Neurosciences, Hôpital Sainte Anne, Paris, France.
Giuseppe Minniti, Radiation Oncology Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy and IRCCS Neuromed (IS), Italy.
Thomas J Kaley, Department of Neurology, Brain Tumor Service, Memorial Sloan Kettering Cancer Center, New York, US.
Eric Bouffet, Division of Paediatric Oncology, The Hospital for Sick Children, University of Toronto, Toronto, Canada.
Ghazaleh Tabatabai, Department of Neurology & Neurooncology, University of Tübingen, German Cancer Consortium (DKTK), DKFZ partner site Tübingen, Germany.
Eleonora Aronica, Department of (Neuro)Pathology, Amsterdam UMC, University of Amsterdam, Amsterdam Neuroscience, Amsterdam and Stichting Epilepsie Instellingen Nederland (SEIN), Heemstede, the Netherlands.
Asgeir S Jakola, Department of Neurosurgery, Sahlgrenska University Hospital, Gothenburg, Sweden. Institute of Neuroscience and Physiology, Department of Clinical Neuroscience, Sahlgrenska Academy, Gothenburg, Sweden.
Stefan M Pfister, Hopp Children´s Cancer Center Heidelberg (KiTZ), Division of Pediatric Neuro-oncology, German Cancer Research Center (DKFZ) and German Cancer Consortium (DKTK), and Department of Pediatric Oncology, Hematology and Immunology, University Hospital Heidelberg, Heidelberg, Germany.
David Schiff, Department of Neurology, Division of Neuro-Oncology, University of Virginia, Charlottesville, US.
Andrew B Lassman, Division of Neuro-Oncology, Department of Neurology and the Herbert Irving Comprehensive Cancer Center, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, NY, US.
David A Solomon, Department of Pathology, University of California, San Francisco, CA, US.
Riccardo Soffietti, Division of Neuro-Oncology, Department of Neuroscience, University and City of Health and Science Hospital, Turin, Italy.
Michael Weller, Department of Neurology, Clinical Neuroscience Center, University Hospital and University of Zurich, Zurich, Switzerland.
Matthias Preusser, Department of Medicine I, Division of Oncology, Medical University of Vienna, Vienna, Austria.
Ahmed Idbaih, Sorbonne Université, Inserm, CNRS, UMR S 1127, Institut du Cerveau et de la Moelle épinière, ICM, AP-HP, Hôpitaux Universitaires La Pitié Salpêtrière - Charles Foix, Service de Neurologie 2-Mazarin, Paris, France.
Patrick Y Wen, Center for Neuro-Oncology, Dana-Farber/Brigham and Women’s Cancer Center; Division of Neuro-Oncology, Department of Neurology, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA, USA.
Martin J van den Bent, Department of Neurology, Brain Tumor Center at Erasmus MC Cancer Institute, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Funding
No Fundings have been received for drawing this guideline
Conflict of interest statement
RR has received honoraria for lectures or advisory boards from Bayer, Novocure, UCB DC reports grants from Novocure, has received honoraria for lectures from the World Federation of Neuro-Oncology Societies (WFNOS), receives royalties for IDH1 R132H -specific antibody from DIANOVA GmbH, receives royalties for BRAF V600E mutation-specific antibody from Roche Diagnostics and holds a patent for a DNA-methylation based method for classifying tumor species. ADW Nothing to declare. JP has received honoraria for lectures or advisory boards from Kyowa Kirin Pharma, Medac, Novocure, and UCB. GM Nothing to declare. TJK Nothing to declare. EB has received Grants (to the Institution) from Roche and BMS for clinical trials and honoraria for the Advisory board from Novartis. GT has served on advisory boards of AbbVie, Bayer, and Boehringer Ingelheim, received consulting fees from AbbVie and Bayer; received speaker fees from Medac and Novocure; received travel grants from Novocure, Medac; received research grants from Roche Diagnostics and Medac. EA has received honoraria for lectures or advisory boards from UCB and Novartis. ASJ Nothing to declare. SMP reports grants from the ITCC-P4 IMI-2 project funded by the EU as well as 10 EFPIA companies (www.itccp4.eu) outside the submitted work; in addition, S.M. Pfister has a patent for EP 16710700 A 20160311 (methylation-based tumor classification) issued. DS has served on advisory boards or data safety monitoring committees for Orbus, Denovo, AstraZeneca, and GlzoSmithKline. ABL in the last 3-years outside the submitted work, reports personal fees and non-financial support from Bioclinica as an expert blinded independent reviewer of clinical and imaging data for a BMS-sponsored trial, personal fees and non-financial support from Forma, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from Orbus, grants and non-financial support from Agios/Servier, grants and non-financial support from Kadmon, grants and non-financial support from VBI Vaccines, grants and non-financial support from Beigene, grants and non-financial support from Oncoceutics/Chimerix, grants and non-financial support from Pfizer, grants and non-financial support from Genentech/Roche, non-financial support from Aeterna Zentaris, personal fees and non-financial support from PER/MJH Holdings, grants and non-financial support from BMS, grants and non-financial support from AbbVie, personal fees and non-financial support from Abbott Molecular, personal fees from Novocure, personal fees from Sapience, personal fees from Vivacitas, non-financial support from American Society of Clinical Oncology (ASCO), grants and non-financial support from Global Coalition for Adaptive Research (GCAR), non-financial support from Matheson Foundation, non-financial support from National Brain Tumor Society (NBTS), personal fees and non-financial support from Society for Neuro-Oncology (SNO), non-financial support from US Food & Drug Administration (FDA), grants from RTOG Foundation, non-financial support from NextSource, non-financial support from DelMar,non-financial support from Corden, non-financial support from Kazia, personal fees from Fondazion AIRC (Italian Foundation for Cancer Research), grants, personal fees and non-financial support from Karyopharm, grants and non-financial support from Semus, personal fees from Elsevier, non-financial support from OligoNation, grants and non-financial support from Novartis, grants, personal fees and non-financial support from QED, grants and personal fees from NIH/NCI. DAS nothing to declare. RS has received honoraria for lectures or advisory boards from Astra Zeneca, Theramex, Puma Technology, Mundipharma. MW has received research grants from Apogenix, Merck, Sharp & Dohme, Merck (EMD), Philogen and Quercis, and honoraria for lectures or advisory board participation or consulting from Adastra, Bayer, Bristol Meyer Squibb, Medac, Merck, Sharp & Dohme, Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen and y-Mabs. MP has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra, Gan & Lee Pharmaceuticals. AI has received, outside the submitted work, research grants from Carthera, Transgene, Sanofi, Air Liquide, Servier, Nutritheragene; honoraria for the advisory board from Leo Pharma, Novocure, and Boehringer Ingelheim Oncology; travel funding from Novocure, Carthera, and Leo Pharma. PYW has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Agios, Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sagimet, Sapience, Servier, Vascular Biogenics, VBI Vaccines and research support from Astra Zeneca/Medimmune, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines. MvdB has received honoraria from AGIOS, Carthera, Genenta, Nerviano, Chimerix, Astra Zeneca, Boehringer Ingelheim.
Authorship statement
RR has coordinated the initiative by assigning the different tasks and pulling together the different sections. RR, DC, ADW, JP, GM, TJK, EB, GT, RS have drawn the first draft of the manuscript as writing group. EA, ASJ, SMP, DS, ABL, DAS, MW, MP, AI, PYW, MJvdB reviewed the first and second drafts. All Authors reviewed and approved the final version of the manuscript
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):1–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khatua S, Gutmann DH, Packer RJ. Neurofibromatosis type 1 and optic pathway glioma: molecular interplay and therapeutic insights. Pediatr Blood Cancer. 2018;65(3):1–7. [DOI] [PubMed] [Google Scholar]
- 4. Cotter JA. An update on the central nervous system manifestations of tuberous sclerosis complex. Acta Neuropathol. 2020;139(4):613–624. [DOI] [PubMed] [Google Scholar]
- 5. Packer RJ, Iavarone A, Jones DTW, et al. Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference. Neuro Oncol. 2020;22(6):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher MJ, Jones DTW, Li Y, et al. Integrated molecular and clinical analysis of low-grade gliomas in children with neurofibromatosis type 1 (NF1). Acta Neuropathol. 2021;141(4):605–617. [DOI] [PubMed] [Google Scholar]
- 7. Brainin M, Barnes M, Baron JC, et al. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces—revised recommendations 2004. Eur J Neurol. 2004;11(9):577–581. [DOI] [PubMed] [Google Scholar]
- 8. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumors: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 9. Thom M, Toma A, An S, et al. One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol. 2011;70(10):859–878. [DOI] [PubMed] [Google Scholar]
- 10. Rudà R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14 Suppl 4(Suppl 4):iv55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zanello M, Pagès M, Roux A, et al. Epileptic seizures in anaplastic gangliogliomas. Br J Neurosurg. 2017;31(2):227–233. [DOI] [PubMed] [Google Scholar]
- 12. Luyken C, Blumcke I, Fimmers R, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurological aspects. Epilepsia. 2003;44(6):822–830. [DOI] [PubMed] [Google Scholar]
- 13. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 14. Huse JT, Edgar M, Halliday J, et al. Multinodular and vacuolating neuronal tumors of the cerebrum: 10 cases of a distinctive seizure-associated lesion. Brain Pathol. 2013;23(5):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reinhardt A, Stichel D, Schrimpf D, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018;136(2):273–291. [DOI] [PubMed] [Google Scholar]
- 16. Solomon DA, Korshunov A, Sill M, et al. Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a recurrent PDGFRA p.K385 mutation and DNT-like methylation profile. Acta Neuropathol. 2018;136(2):339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng MY, Sill M, Sturm D, et al. Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC)—a molecularly defined glioneuronal CNS tumor class displaying recurrent monosomy 14. Neuropathol Appl Neurobiol. 2020;46(5):422–430. [DOI] [PubMed] [Google Scholar]
- 18. Pickles JC, Mankad K, Aizpurua M, et al. A case series of diffuse glioneuronal tumors with oligodendroglioma-like features and nuclear clusters (DGONC). Neuropathol Appl Neurobiol. 2021;47(3):464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones DT, Hutter B, Jager N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nature Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips JJ, Gong H, Chen K, et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019;29(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma M, Ralte A, Arora R, et al. Subependymal giant cell astrocytoma: a clinicopathological study of 23 cases with special emphasis on proliferative markers and expression of p53 and retinoblastoma gene proteins. Pathology 2004;36(2):139–144. [DOI] [PubMed] [Google Scholar]
- 22. Bongaarts A, Giannikou K, Reinten RJ, et al. Subependymal giant cell astrocytomas in tuberous sclerosis complex have consistent TSC1/TSC2 biallelic inactivation, and no BRAF mutations. Oncotarget. 2017;8(56):95516–95529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bongaarts A, van Scheppingen J, Korotkov A, et al. The coding and non-coding transcriptional landscape of subependymal giant cell astrocytomas. Brain. 2020;143(1):131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jansen AC, Belousova E, Benedik MP, et al. Clinical characteristics of subependymal giant cell astrocytoma in tuberous sclerosis complex. Front Neurol. 2019;10:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goode B, Mondal G, Hyun M, et al. A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat Commun. 2018;9(1):810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alhalabi KT, Stichel D, Sievers P, et al. PATZ1 fusions define a novel molecularly distinct neuroepithelial tumor entity with a broad histological spectrum. Acta Neuropathol. 2021;142(5):841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blümcke I, Coras R, Wefers AK, et al. Review: challenges in the histopathological classification of ganglioglioma and DNT: microscopic agreement studies and a preliminary genotype-phenotype analysis. Neuropathol Appl Neurobiol. 2019;45(2):95–107. [DOI] [PubMed] [Google Scholar]
- 28. Blümcke I, Giencke K, Wardelmann E, et al. The CD34 epitope is expressed in neoplastic and malformative lesions associated with chronic, focal epilepsies. Acta Neuropathol. 1999;97(5):481–490. [DOI] [PubMed] [Google Scholar]
- 29. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 30. Pekmezci M, Villanueva-Meyer JE, Goode B, et al. The genetic landscape of ganglioglioma. Acta Neuropathol Commun. 2018;6(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell. 2020;37(4):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang AC, Jones DTW, Abecassis IJ, et al. Desmoplastic infantile ganglioglioma/astrocytoma (DIG/DIA) are distinct entities with frequent BRAFV600 mutations. Mol Cancer Res. 2018;16(10):1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rivera B, Gayden T, Carrot-Zhang J, et al. Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol. 2016;131(6):847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hou Y, Pinheiro J, Sahm F, et al. Papillary glioneuronal tumor (PGNT) exhibits a characteristic methylation profile and fusions involving PRKCA. Acta Neuropathol. 2019;137(5):837–846. [DOI] [PubMed] [Google Scholar]
- 35. Sievers P, Appay R, Schrimpf D, et al. Rosette-forming glioneuronal tumors share a distinct DNA methylation profile and mutations in FGFR1, with recurrent co-mutation of PIK3CA and NF1. Acta Neuropathol. 2019;138(3):497–504. [DOI] [PubMed] [Google Scholar]
- 36. Lucas CG, Gupta R, Doo P, et al. Comprehensive analysis of diverse low-grade neuroepithelial tumors with FGFR1 alterations reveals a distinct molecular signature of rosette-forming glioneuronal tumor. Acta Neuropathol Commun. 2020;8(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pekmezci M, Stevers M, Phillips JJ, et al. Multinodular and vacuolating neuronal tumor of the cerebrum is a clonal neoplasm defined by genetic alterations that activate the MAP kinase signaling pathway. Acta Neuropathol. 2018;135(3):485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dragoo DD, Taher A, Wong VK, et al. PTEN hamartoma tumor syndrome/Cowden syndrome: genomics, oncogenesis, and imaging review for associated lesions and malignancy. Cancers (Basel). 2021;13(13):3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sievers P, Stichel D, Schrimpf D, et al. FGFR1:TACC1 fusion is a frequent event in molecularly defined extraventricular neurocytoma. Acta Neuropathol. 2018;136(2):293–302. [DOI] [PubMed] [Google Scholar]
- 40. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumors. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018;136(2):181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perez E, Capper D. Invited review: DNA methylation-based classification of paediatric brain tumors. Neuropathol Appl Neurobiol. 2020;46(1):28–47. [DOI] [PubMed] [Google Scholar]
- 43. Ajithkumar T, Imbulgoda N, Rees E, et al. Uncommon low-grade brain tumors. Neuro Oncol. 2019;21(2):151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdel Razek AAK, Elsebaie NA, Zamora C, Castillo M. Imaging of neuronal and mixed glioneuronal tumors. J Comput Assist Tomogr. 2020;44(3):356–369. [DOI] [PubMed] [Google Scholar]
- 45. Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 47. Erker C, Tamrazi B, Poussaint TY, et al. Response assessment in paediatric high-grade glioma: recommendations from the response assessment in pediatric neuro-oncology (RAPNO) working group. Lancet Oncol. 2020;21(6):e317–e329. [DOI] [PubMed] [Google Scholar]
- 48. Fangusaro J, Witt O, Hernáiz Driever P, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020 Jun;21(6):e305–e316. [DOI] [PubMed] [Google Scholar]
- 49. Calixto NC, Simão GN, Dos Santos AC, et al. Monitoring optic chiasmatic-hypothalamic glioma volumetric changes by MRI in children under clinical surveillance or chemotherapy. Childs Nerv Syst. 2019;35(1):63–72. [DOI] [PubMed] [Google Scholar]
- 50. Ellingson BM, Kim GHJ, Brown M, et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: evidence from a phase I trial of ivosidenib. Neuro Oncol. 2022;24(5):770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stevens SP, Main C, Bailey S, et al. The utility of routine surveillance screening with magnetic resonance imaging (MRI) to detect tumor recurrence in children with low-grade central nervous system (CNS) tumors: a systematic review. J Neurooncol. 2018;139(3):507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zaazoue MA, Manley PE, Mehdar MA, et al. Optimizing postoperative surveillance of pediatric low-grade glioma using tumor behavior patterns. Neurosurgery. 2020;86(2):288–297. [DOI] [PubMed] [Google Scholar]
- 53. Roessler K, Hofmann A, Sommer B, et al. Resective surgery for medically refractory epilepsy using intraoperative MRI and functional neuronavigation: the Erlangen experience of 415 patients. Neurosurg Focus. 2016;40(3):E15. [DOI] [PubMed] [Google Scholar]
- 54. Northrup H, Aronow ME, Bebin EM, et al. International tuberous sclerosis complex consensus group. Updated international tuberous sclerosis complex diagnostic criteria and surveillance and management recommendations. Pediatr Neurol. 2021;123:50–66. [DOI] [PubMed] [Google Scholar]
- 55. Al-Hajri A, Al-Mughairi S, Somani A, et al. Pathology-MRI correlations in diffuse low-grade epilepsy associated tumors. J Neuropathol Exp Neurol. 2017;76(12):1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bender K, Perez E, Chirica M, et al. High-grade astrocytoma with piloid features (HGAP): the Charité experience with a new central nervous system tumor entity. J Neurooncol. 2021;153(1):109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Compton JJ, Laack NN, Eckel LJ, et al. Long-term outcomes for low-grade intracranial ganglioglioma: 30-year experience from the Mayo Clinic. J Neurosurg. 2012;117(5):825–830. [DOI] [PubMed] [Google Scholar]
- 58. Zacharoulis S, Morales La Madrid A, et al. Central versus extraventricular neurocytoma in children: a clinicopathologic comparison and review of the literature. J Pediatr Hematol Oncol. 2016;38(6):479–485. [DOI] [PubMed] [Google Scholar]
- 59. Bond KM, Hughes JD, Porter AL, et al. Adult pilocytic astrocytoma: an institutional series and systematic literature review for extent of resection and recurrence. World Neurosurg. 2018;110:276–283. [DOI] [PubMed] [Google Scholar]
- 60. Giordano F, Moscheo C, Lenge M, et al. Neurosurgical treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex: a series of 44 surgical procedures in 31 patients. Childs Nerv Syst. 2020;36(5):951–960. [DOI] [PubMed] [Google Scholar]
- 61. Bartek J, Jr, Dhawan S, Thurin E, et al. Short-term outcome following surgery for rare brain tumor entities in adults: a Swedish nation-wide registry-based study and comparison with SEER database. J Neurooncol. 2020;148(2):281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim JW, Kim DG, Kim IK, et al. Central neurocytoma: long-term outcomes of multimodal treatments and management strategies based on 30 years’ experience in a single institute. Neurosurg. 2013;72(3):407–414. [DOI] [PubMed] [Google Scholar]
- 63. Wang Q, Zhang S, Cheng J, et al. Lhermitte-Duclos disease: clinical study with long-term follow-up in a single institution. Clin Neurol Neurosurg. 2017;162:53–58. [DOI] [PubMed] [Google Scholar]
- 64. Wilson CP, Chakraborty AR, Pelargos PE, et al. Rosette-forming glioneuronal tumor: an illustrative case and a systematic review. Neurooncol Adv. 2020;2(1):vdaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bianchi F, Tamburrini G, Massimi L, Caldarelli M. Supratentorial tumors typical of the infantile age: desmoplastic infantile ganglioglioma (DIG) and astrocytoma (DIA). A review. Childs Nerv Syst. 2016;32(10):1833–1838. [DOI] [PubMed] [Google Scholar]
- 66. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012;53(1):51–57. [DOI] [PubMed] [Google Scholar]
- 67. Southwell DG, Garcia PA, Berger MS, Barbaro NM, Chang EF. Long-term seizure control outcomes after resection of gangliogliomas. Neurosurg. 2012;70(6):1406–1413; discussion 1413–1414. [DOI] [PubMed] [Google Scholar]
- 68. Devaux B, Chassoux F, Landré E, et al. Surgery for dysembryoplastic neuroepithelial tumors and gangliogliomas in eloquent areas. Functional results and seizure control. Neurochir. 2017;63(3):227–234. [DOI] [PubMed] [Google Scholar]
- 69. Le S, Ho AL, Fisher RS, et al. Laser interstitial thermal therapy (LITT): seizure outcomes for refractory mesial temporal lobe epilepsy. Epilepsy Behav. 2018;89:37–41. [DOI] [PubMed] [Google Scholar]
- 70. Satzer D, Tao JX, Issa NP, et al. Stereotactic laser interstitial thermal therapy for epilepsy associated with solitary and multiple cerebral cavernous malformations. Neurosurg Focus. 2020 Apr 1;48(4):E12. [DOI] [PubMed] [Google Scholar]
- 71. Dudley RW, Torok MR, Gallegos DR, et al. Pediatric low-grade ganglioglioma: epidemiology, treatments, and outcome analysis on 348 children from the surveillance, epidemiology, and end results database. Neurosurgery. 2015;76(3):313–9.; discussion 319; quiz 319; discussion 319; quiz 319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rades D, Zwick L, Leppert J, et al. The role of postoperative radiotherapy for the treatment of gangliogliomas. Cancer. 2010;116(2):432–442. [DOI] [PubMed] [Google Scholar]
- 73. Luyken C, Blümcke I, Fimmers R, et al. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101(1):146–155. [DOI] [PubMed] [Google Scholar]
- 74. Ishkanian A, Laperriere NJ, Xu W, et al. Upfront observation versus radiation for adult pilocytic astrocytoma. Cancer. 2011;117(17):4070–4079. [DOI] [PubMed] [Google Scholar]
- 75. Schlamann A, von Bueren AO, Hagel C, et al. An individual patient data meta-analysis on characteristics and outcome of patients with papillary glioneuronal tumor, rosette glioneuronal tumor with neuropil-like islands and rosette forming glioneuronal tumor of the fourth ventricle. PLoS One. 2014;9(7):e101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown PD, Anderson SK, Carrero XW, et al. Adult patients with supratentorial pilocytic astrocytoma: long-term follow-up of prospective multicenter clinical trial NCCTG-867251 (Alliance). Neurooncol Pract. 2015;2(4):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rades D, Fehlauer F. Treatment options for central neurocytoma. Neurology. 2002;59(8):1268–1270. [DOI] [PubMed] [Google Scholar]
- 78. Mahavadi AK, Patel PM, Kuchakulla M, et al. Central neurocytoma treatment modalities: a systematic review assessing the outcomes of combined maximal safe resection and radiotherapy with gross total resection. World Neurosurg. 2020;137:e176–e182. [DOI] [PubMed] [Google Scholar]
- 79. Garcia RM, Ivan ME, Oh T, Barani I, Parsa AT. Intraventricular neurocytomas: a systematic review of stereotactic radiosurgery and fractionated conventional radiotherapy for residual or recurrent tumors. Clin Neurol Neurosurg. 2014;117:55–64. [DOI] [PubMed] [Google Scholar]
- 80. Bui TT, Lagman C, Chung LK, et al. Systematic analysis of clinical outcomes following stereotactic radiosurgery for central neurocytoma. Brain Tumor Res Treat. 2017;5(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hung YC, Lee CC, Yang HC, et al. Stereotactic radiosurgery for central neurocytomas: an international multicenter retrospective cohort study. J Neurosurg. 2020;134(3):1122–1131. [DOI] [PubMed] [Google Scholar]
- 82. Akyoldas G, Samanci Y, Tugcu ES, Peker S. Gamma knife radiosurgery for the treatment of central neurocytoma: a single-institution experience of 25 patients. Neurosurg Rev. 2021;44(6):3427–3435. [DOI] [PubMed] [Google Scholar]
- 83. Gembruch O, Junker A, Mönninghoff C, et al. Liponeurocytoma: systematic review of a rare entity. World Neurosurg. 2018;120:214–233. [DOI] [PubMed] [Google Scholar]
- 84. Zuo P, Sun T, Gu G, et al. Surgical management and clinical outcomes of cerebellar liponeurocytomas—a report of seven cases and a pooled analysis of individual patient data. Neurosurg Rev. 2022;45(2):1747–1757. [DOI] [PubMed] [Google Scholar]
- 85. Selvanathan SK, Hammouche S, Salminen HJ, Jenkinson MD. Outcome and prognostic features in anaplastic ganglioglioma: analysis of cases from the SEER database. J Neurooncol. 2011;105(3):539–545. [DOI] [PubMed] [Google Scholar]
- 86. Terrier LM, Bauchet L, Rigau V, et al. Natural course and prognosis of anaplastic gangliogliomas: a multicenter retrospective study of 43 cases from the French Brain Tumor Database. Neuro Oncol. 2017;19(5):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alshantti K, Nadarajan C, Mijol MM, Mat Zin AA. Anaplastic pleomorphic xanthoastrocytoma: a rare variant of astrocytoma. Cureus. 2022;14(3):e23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aguilera D, Castellino RC, Janss A, et al. Clinical responses of patients with diffuse leptomeningeal glioneuronal tumors to chemotherapy. Childs Nerv Syst. 2018;34(2):329–334. [DOI] [PubMed] [Google Scholar]
- 89. Johnson MO, Kirkpatrick JP, Patel MP, et al. The role of chemotherapy in the treatment of central neurocytoma. CNS Oncol. 2019;8(3):CNS41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gregory TA, Chumbley LB, Henson JW, Theeler BJ. Adult pilocytic astrocytoma in the molecular era: a comprehensive review. CNS Oncol. 2021;10(1):CNS68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Theeler BJ, Ellezam B, Yust-Katz S, et al. Prolonged survival in adult neurofibromatosis type I patients with recurrent high-grade gliomas treated with bevacizumab. J Neurol. 2014;261(8):1559–1564. [DOI] [PubMed] [Google Scholar]
- 92. Wasilewski A, Mohile N. Durable response to bevacizumab in adults with recurrent pilocytic astrocytoma. CNS Oncol. 2018;7(3):CNS26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAFV600-mmutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berzero G, Bellu L, Baldini C, et al. Sustained tumor control with MAPK inhibition in BRAF V600-mutant adult glial and glioneuronal tumors. Neurology. 2021;97(7):e673673–e67e683. [DOI] [PubMed] [Google Scholar]
- 95. Wen PY, Stein A, van den Bent M, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23(1):53–64. [DOI] [PubMed] [Google Scholar]
- 96. Torre M, Vasudevaraja V, Serrano J, et al. Molecular and clinicopathologic features of gliomas harboring NTRK fusions. Acta Neuropathol Commun. 2020;8(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Boyer J, Birzu C, Bielle F, et al. Dramatic response of STRN-NTRK-fused malignant glioneuronal tumor to larotrectinib in adult. Neuro Oncol. 2021;23(7):1200–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim PL. Targeting gene fusions in glioma. Curr Opin Neurol. 2021 Dec 1;34(6):840–847. [DOI] [PubMed] [Google Scholar]
- 99. Packer RJ, Pfister S, Bouffet E, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: a Canadian pediatric brain tumor consortium study. J Clin Oncol. 2016;34(29):3537–3543. [DOI] [PubMed] [Google Scholar]
- 101. Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ater JL, Xia C, Mazewski CM, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gnekow AK, Walker DA, Kandels D, et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma—A final report. Eur J Cancer. 2017;81:206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nature Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qaddoumi I, Orisme W, Wen J, et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131(6):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hargrave DR, Bouffet E, Tabori U, et al. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade glioma: results from a phase I/IIa study. Clin Cancer Res. 2019;25(24):7303–7311. [DOI] [PubMed] [Google Scholar]
- 110. Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. [DOI] [PubMed] [Google Scholar]
- 111. Ebrahimi-Fakhari D, Franz DN. Pharmacological treatment strategies for subependymal giant cell astrocytoma (SEGA). Expert Opin Pharmacother. 2020;21(11):1329–1336. [DOI] [PubMed] [Google Scholar]
- 112. Bobeff K, Krajewska K, Baranska D, et al. Maintenance therapy with everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis—final results from the EMINENTS study. Front Neurol. 2021;12:581102. [DOI] [PMC free article] [PubMed] [Google Scholar]