Abstract
Background: The discovery of vitamin C (ascorbic acid) is related to the ancient history of persistent research on the origins of the haemorrhagic disease scurvy. Vitamin C is an important nutrient that aids in a variety of biological and physiological processes. Scientists have been researching the function of vitamin C in the prevention and ailment of sepsis and pneumonia for decades. This has created a potential platform for applying these results to individuals suffering from severe coronavirus infection (COVID-19). Vitamin C's ability to activate and enhance the immune system makes it a promising treatment in the present COVID-19 pandemic. Vitamin C also aids in the activation of vitamin B, the production of certain neurotransmitters, and the transformation of cholesterol into bile acids. Hence, vitamin C is used for the treatment of many diseases. Aim: This review highlights the Vitamin C investigations that are performed by various researchers on patients with COVID 19 infection, the clinical studies and their observations. The authors have additionally updated information on the significance of vitamin C insufficiency, as well as its relevance and involvement in diseases such as cancer, wound healing, iron deficiency anaemia, atherosclerosis and neurodegenerative disorders. Here, we discuss them with the references. Methods: The method used in order to perform literature search was done using SciFinder, PubMed and ScienceDirect. Results: There is a potential role of vitamin C in various diseases including neurodegenerative disorders, COVID-19 and other diseases and the results are highlighted in the review with the help of clinical and preclinical data. Conclusion: More research on vitamin C and the undergoing clinical trials might prove a potential role of vitamin C in protecting the population from current COVID-19 pandemic.
Keywords: Vitamin C, scurvy, antioxidant, neurodegenerative, COVID-19
Introduction
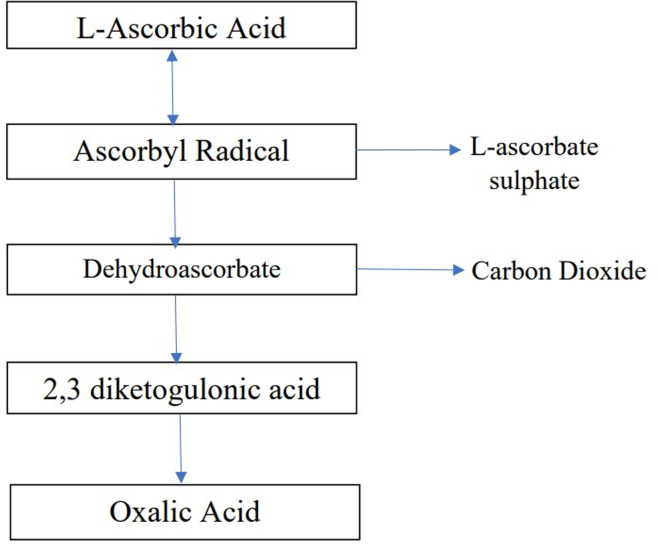
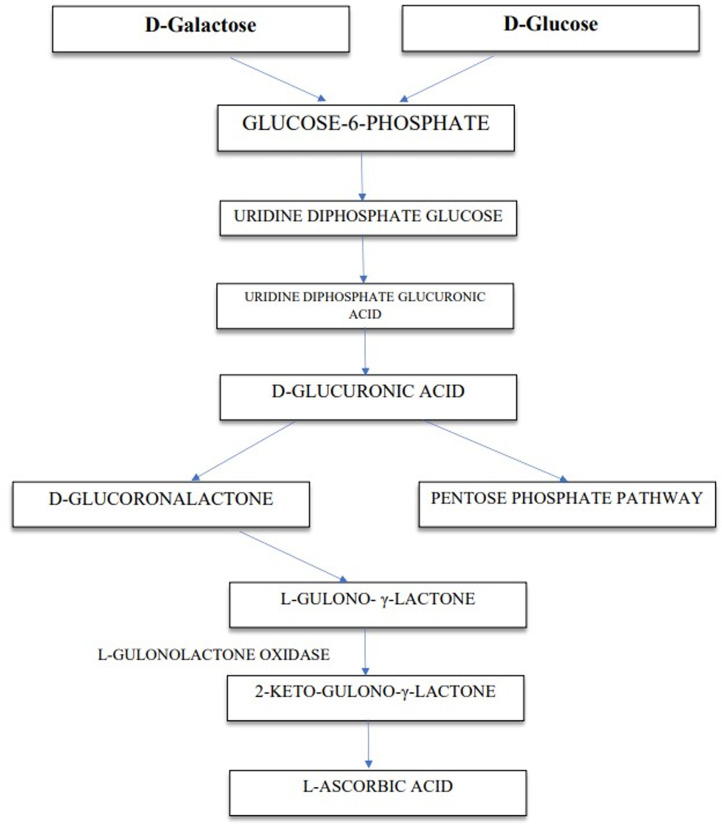
Micronutrients are essential for the immune system's proper functioning and play an important part in supporting health and nutritional well-being (Cámara et al., 2021). Vitamins are necessary for the human body for several biochemical and physiological activities. Vitamins are categorised according to their solubility: (1) water soluble such as B and C complexes and (2) fat-soluble vitamins A, D, E and K (Chambial et al., 2013). Vitamin C is a water-soluble, thermolabile vitamin that is necessary for human health. Vitamin C is found in two forms in living organisms as ascorbic acid (AA)-reduced form and dehydroascorbic acid-oxidised form (Kocot et al., 2017b). The biosynthesis and catabolism of vitamin C is shown in Figures 1 and 2. Despite rising public concern over the malnutrition of micronutrients in low-income and middle-income countries which leads to bad health, greater morbidity and death rates, the worldwide status and prevalence of vitamin C deficiency has not been fully documented (Rowe and Carr, 2020). Humans like the majority of animals do not possess the enzyme L-gluconolactone oxidase which is accountable for vitamin C production, hence, enough vitamin C intake is critical (Langlois and Lamontagne, 2019). This enzyme's deficiency is the product of a mutation that happened about 40 million years ago (Nishikimi et al., 1994). Many regulatory authorities of the countries have decided to increase the recommended dose of vitamin C intake in their countries as a result of growing evidence that increasing the vitamin C intake improves long-term health outcomes (Dickinson et al., 2009; Khan and Iqbal, 2006).
Figure 1.
Catabolism of ascorbic acid.
Figure 2.
Biosynthesis of L-ascorbic acid in animals.
Vitamin C is necessary for a variety of physiological activities. It contributes to the production and metabolism of tyrosine, tryptophan and folic acid as well as the hydroxylation of proline, glycine, lysine, carnitine and catecholamines (Chambial et al., 2013). Vitamin C is a required nutrient for human life as well as a cofactor in the production of collagen (Caritá et al., 2020). It makes cholesterol conversion to bile acids easier, lowering blood cholesterol levels. Vitamin C has various applications in chronic diseases. Vitamin C is a potent antioxidant that contributes to the maintenance of good health and illness prevention. Vitamin C also helps to prevent sepsis-related coagulation problems by lowering pro-inflammatory responses, improving the function of the epithelial barrier, improving alveolar fluid clearance and reducing pro-inflammatory responses (Bharara et al., 2016). Patients who are critically ill and who are suffering from sepsis or multiple organ failure do have lower amounts of vitamin C. They require around 20 to 30 times the amount of vitamin C required by the average population (Carr et al., 2017).
The review highlights the promising role of vitamin C in various diseases such as COVID-19, central nervous system (CNS) disorder to name a few. The method adopted in order to gather information were from different search engines including SciFinder, Sciencedirect and PubMed. This different search engines were used to find out the appropriate literature regarding vitamin C, COVID-19, CNS disorders and other diseases using keywords like vitamin C deficiency, CNS disorders, cardiovascular diseases, ascorbic acid, immunity, toxicity, mechanism of action and clinical trials. The Introduction section covers the various aspects of vitamin C with citation of 15 articles. The mechanism of action and role of vitamin C in immunity covers the citation of 28 articles. The section of vitamin C and toxicity part covers the citation of 19 articles. The treatment of critically ill patients consists of citation of seven articles. The disease part unravels the role of vitamin C in various diseases contains citation of 91 articles. The nutritional strategies of vitamin C in various drug delivery systems consist of citation of five articles.
This review attempts to summarise diverse shreds of evidence in the context of therapeutic roles of vitamin C in various clinical aspects like scurvy, and CNS disorders to name a few. For example, scurvy is a syndrome, caused due to insufficiency of vitamin C for a long time defined by the weakening of the collagenous structure which leads to poor wound healing and impaired immunity (Sunil Kumar et al., 2017). Vitamin C deficiency is also reported in smokers, alcoholics as well as mentally ill patients (Lewis et al., 1998). According to research, vitamin C deficiency is linked to the development of neurological diseases such as Huntington's, Parkinson's, Alzheimer's, multiple sclerosis, and amyotrophic lateral sclerosis, as well as mental disorders like anxiety, depression and schizophrenia (Kocot et al., 2017a). Vitamin C treatment is likely to enhance outcomes in patients with COVID-19 infection, based on findings from clinical trials of people with pneumonia and sepsis, as well as preliminary observational and interventional investigations of patients with COVID-19 infection (Carr and Rowe, 2020). Globally, we observe the presence of low vitamin C in most of the common health conditions. Various studies from basic science to the clinical application of vitamin C have reported to have strong association with vitamin C in acute and chronic conditions. In this article, focus is to discuss the potentials of vitamin C in COVID-19 and many other diseases.
Mechanism of action of vitamin C
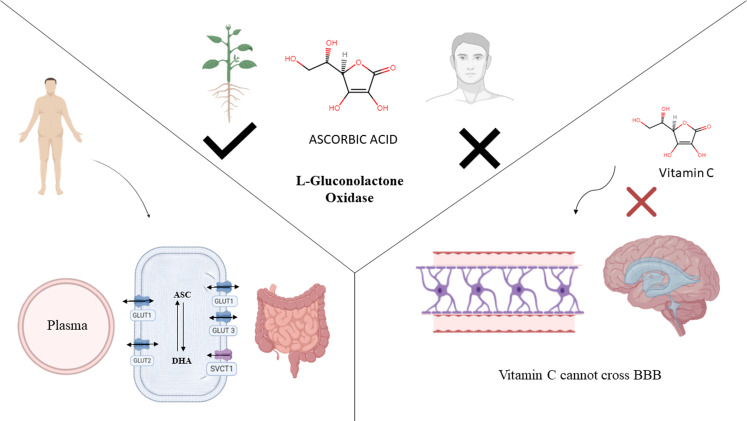
Vitamin C is a co-substrate for a variety of enzymes that are essential for the body's optimal functioning. The mechanism of action of vitamin C is depicted in Figure 3. Vitamin C is predominantly converted to its ionised form ascorbate (ASC) at physiological pH. Because of their poor hydrophobicity, ASC and dehydroascorbate (DHA) have to rely on interactions with the transporter molecules which are anchored in the cell membrane to cross biological membranes. Sodium ascorbate co-transporter 1 (SVCT1) and SVCT2, both are the glycoproteins that are responsible for the transportation of ASC into the cell, which are recognised as SVCTs (Daruwala et al., 1999). SVCT1 is only present within epithelial cells where the amount of ASC transported is more than that of the amount which is required by the cells (Liang et al., 2001). Enterocytes can take up ASC and DHA via the SVCT and glucose transporters (GLUTs), respectively (Malo and Wilson, 2000). Although they have different cellular sites, enterocytes express GLUT1, GLUT2 and GLUT3 (Harris et al., 1992; Helliwell et al., 2000; Mesonero et al., 1994). The apical brush-border membrane contains GLUT3, while the basolateral membrane has GLUT1 and GLUT2 which are located on both sides of the membrane. Vitamin C is thought to enter the bloodstream via endothelial discontinuities and circulate with the blood throughout the body, where it is usually identified as the ASC anion (Wilson, 2005). Both ASC, as well as DHA, are absorbed through the length of the small intestine (duodenum, jejunum and ileum) (Malo and Wilson, 2000; Mellors et al., 1977; Stevenson, 1974). ASC is absorbed differently than glucose, with ASC being faster absorbed in almost the distal segments of the small intestine and absorbed in lesser amounts in proximal portions. The jejunum absorbs DHA better, while the ileum's distal parts absorb only a small quantity (Malo and Wilson, 2000). Several glucose transporters have showed that DHA competes with glucose for transfer (Corpe et al., 2013; Vera et al., 1993). DHA is transported by facilitated diffusion, which allows it to travel along the concentration gradient. DHA is converted to ASC as soon as it crosses through the membrane, therefore, this gradient is maintained (Asard et al., 2005; Hughes and Maton, 1968; Rumsey et al., 2000; Washko et al., 1993). It was established that vitamin C transport is satiable, concentration, energy, temperature, as well as dependent on sodium and is mediated by two distinct components. The blood–brain barrier prevents ASC from entering the brain because it is not permeable to ASC and lacking the SVCT2 expression (Agus et al., 1997; García et al., 2005; Qiao and May, 2008). On the other hand due to the expression of GLUT1, DHA easily passes through the blood–brain barrier (Agus et al., 1997; Farrell et al., 1992). Renal glomeruli filters and remove ASC from the blood and excrete it into the urine (Friedman et al., 1940). Consequently, a considerable part of vitamin C is being reabsorbed throughout proximal tubules, depending on the individual's vitamin C status (Bowers-Komro and McCormick, 1991; Rose, 1986).
Figure 3.
(a) Humans cannot synthesise vitamin C due to lack of L-gluconolactone oxidase enzyme. Plants can synthesise ascorbate due to presence of L-gluconolactone oxidase enzyme. (b) Mechanism of action of vitamin C and (c) ascorbate molecule is impermeable to blood–brain barrier and lacks SVCT2 expression. ASC: ascorbate; DHA: dehydroascorbate; GLUT: glucose transporter; SVCT: sodium ascorbate co-transporter.
Role of vitamin C in immunity
The immune system is made up of a complex network of specialised cells, tissues, organs, proteins and chemicals that protect the host from foreign pathogens (Kellie and Al-Mansour, 2017). It is separated into epithelial barriers, as well as cellular and humoral components of innate (non-specific) and acquired (specific immunity) (Kellie and Al-Mansour, 2017). For more than a half century of study and research, vitamin C has been proven to be crucial in various areas of the immune system, most notably immune cell activity. Vitamin C is a water-soluble antioxidant found in extracellular fluid and cell cytoplasm, where it performs a variety of functions, including immune homoeostasis (De la Fuente et al., 2020). As it is quickly utilised during the defensive activities, there is high concentration of vitamin C in leukocytes which gets decreased during infections and stress. Vitamin C supplementation in the form of diet or gram doses has been demonstrated to improve neutrophil chemotactic capacity in healthy volunteers (Bozonet et al., 2015). In another study, the intake of diet rich with vitamin C, vitamin E and others showed improvement in the peritoneal leukocyte activities, restoring their redox equilibrium, and extended the life span of the animals in prematurely aged mice (Alvarado et al., 2006). Vitamin C's anti-histamine impact, according to Johnston et al. (1992), is linked to increased chemotaxis. After phagocytosis and microbial annihilation, neutrophils go into a process called cell apoptosis, which involves planned cell death. This procedure aids macrophages in phagocytosis and evacuation of dead neutrophils from inflammatory sites, allowing inflammation to be resolved faster and tissue damage to be reduced. Vitamin C is thought to prevent the oxidant-sensitive caspase-dependent apoptotic pathway after neutrophil activation. In vitro, macrophages did not phagocyte these vitamin C-deficient neutrophils, and they remained in inflammatory sites in vivo. Furthermore, giving vitamin C to sick animals leads to decrease in the amount of neutrophils in the lungs (Vissers and Wilkie, 2007).
Vitamin C deficiency
Because of random genetic alterations that happened throughout evolution, humans had lost their capacity to generate ASC in their livers (Nishikimi and Yagi, 1991). The polymorphism of single nucleotide in the SLC23A1 and SLC23A2 genes, which have been associated to human illnesses, are used to study the impact of genetic variants on vitamin C pharmacokinetics (Granger and Eck, 2018; Padayatty and Levine, 2016). Reduced renal reabsorption was determined to be the strongest proof for numerous common single nucleotide polymorphisms (SNPs) of SLC23A1 lowering circulating vitamin C levels (Corpe et al., 2010; Michels et al., 2013; Monteiro-grillo, 1990). Crohn's disease (Amir Shaghaghi et al., 2014), non-Hodgkin lymphoma (Skibola et al., 2008), pre-term birth (Erichsen et al., 2006) and severe periodontitis (De Jong et al., 2014) all have been linked to SLC23A1 polymorphisms. Sodium-dependent transporter 1 (SVCT-1) is the primary regulator of vitamin C absorption, and it is quickly saturated, resulting in low vitamin C absorption per meal (Savini et al., 2008). More vitamin C is lost when AA gets converted to dehydroascorbic acid, which can then be later converted to AA or undergoes further oxidative breakdown (Washko et al., 1993). Several studies have revealed the relationship between the lifestyle-related diseases as well as vitamin C deficiency. In animal research, vitamin C deprivation is linked to reduced brain growth and hippocampal function. The recommended dietary allowance of vitamin C is depicted in Table 1.
Table 1.
RDAs of vitamin C.
| Category | RDA per day |
|---|---|
| Infants (underage of 6 months) | 40 mg |
| Infants (underage of 12 months) | 50 mg |
| Children (underage of 8 years) | 25 mg |
| Children (underage of 13 years) | 45 mg |
| Male(13–17 years) | 75 mg |
| Female(13–17 years) | 65 mg |
| Adult male | 90 mg |
| Adult female | 75 mg |
| Pregnant women | 85 mg |
| Lactating women | 120 mg |
| Smokers | Requires 35 mg/day more vitamin C as compared to non-smokers |
RDA: recommended dietary allowance.
Toxicity
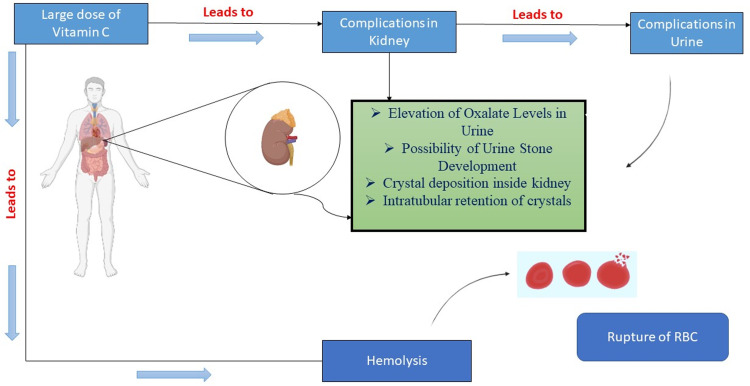
There are several complaints for vitamin C when it is consumed in high doses, such as diarrhoea and intestinal distension or gas (Ohno et al., 2009). Vitamin C at large levels has also been proven to have the following outcomes; for increase in calcium, iron and manganese urinary excretion (Ohno et al., 2009); to enhance iron absorption; to enhance urinary oxalate or uric acid levels, for a small segment of population; and to change the several standard laboratory values. In individuals with the no prior symptoms or indications of renal impairment, evidence shows that the intravenous vitamin C is unlikely to cause any type of damage (Casciari et al., 2005). The people who are suffering from glucose-6-phosphate insufficiency, the administration of vitamin C intravenously or high oral dose of vitamin C can be a cause of haemolysis (Levine et al., 1999). According to Campell and Jack, one patient died from significant tumour necrosis and haemorrhaging followed by the initial dose of the ASC (intravenous). As a result, it is recommended that treatment should begin with the modest dose and be administered via a gradual drip infusion (Campbell and Jack, 1979). It's important to remember that, when taken in large dosages, vitamin C is a drug with adverse effects, just like any other. In the human body, vitamin C is partially converted to oxalate (Hellman and Burns, 1958). As per Figure 4, vitamin C elevates oxalate levels in urine in a dose-dependent manner, raising concerns about its safety and urinary stone development is a possibility. In the setting of hyperoxaluria, there could be the supersaturation of calcium oxalate which will be resulting in the formation of crystal nuclei in the urine. The crystals can pass swiftly in the healthy individuals but intratubular retention is thought to occur in areas of injured and tubular epithelium that regenerates, it is where the molecules with potential binding capacity of crystal are expressed (Cossey et al., 2013; Verkoelen and Verhulst, 2007). Patients who are suffering from sepsis, higher dose of vitamin C may be beneficial, so there should be a careful risk–benefit analysis of its extended administration in patients who are suffering from COVID-19 as well as kidney dysfunction and it should be carried on case-to-case basis (Fontana et al., 2020).
Figure 4.
Toxicity of vitamin C.
Treatment of critically ill patients
The patients who are critically ill, vitamin C insufficiency is prevalent in them, and patients with septic shock having the lowest levels. This is most likely due to the higher metabolism caused along by septic shock's heightened inflammatory response (Carr et al., 2017). The plasma levels of vitamin C regularly play with the scurvy levels in conditions like sepsis, trauma, after major surgery and burn patients, and in any situation caused or characterised by severe systemic oxidative and inflammatory stress (Carr et al., 2017). The increased oxidative stress which is produced by the during critical illness increases antioxidant consumption and decreases antioxidant recycling, especially vitamin C. While other species boost their vitamin C production in response to stress, humans are not able to enhance the serum levels like other stress hormones. So due to this, the insufficiency exists (Langlois and Lamontagne, 2019). Administration of high-dose intravenous (IV) AA reduces demand for fluid, there is occurrence of wound oedema and weight gain in the initial phase of burn injury while simultaneously improving renal and lung function (Tanaka et al., 2000). Patients in the intensive care unit (ICU) are frequently given enteral nutrition or parenteral nutrition if they cannot get enough enteral nutrition (Yamazaki et al., 2011). The presence or lack of a working intestine, as well as the patient's haemodynamic condition, dictate the route of feeding for the patient – enteral or parenteral (Chan et al., 1999) In the case of patient having fluid restriction and organ failure, nutritional requirements are usually altered (Chan et al., 1999). Enteral and parenteral nutrition, which provide 100 to 200 mg of vitamin C per day on average, are insufficient for critically ill patients. A regular intake of vitamin C of at least 2 to 3 g is likely to be required for these patients (Carr et al., 2017).
The effects of AA supplementation were first discovered in a number of animal models of sepsis and ischaemia reperfusion. The therapy of AA in a sepsis murine model was responsible for enhanced microvascular perfusion, improved blood flow and blood pressure with dosages ranging from 10 to 200 mg/kg provided either as a preventative measure or after an injury (Secor et al., 2010; Tyml et al., 2008). In septic mice, the AA infusion was responsible for enhancing the blood flow of capillary, the barrier function of microvascular and arteriolar response to vasoconstrictors (Armour et al., 2001; Wu et al., 2004). So, the supplementation of vitamin C is useful for the critically ill patients. One study revealed that despite receiving conventional ICU nutritional care, approximately 70% of the critically ill patients were suffering from hypovitaminosis C, with a substantial percentage of patients having the vitamin C insufficiency (32%). Patients suffering with septic shock cause further vitamin C depletion in the blood, with approximately 90% of patients having hypovitaminosis C and 40% having hypovitaminosis B. Vitamin C deficiency is likely due to increased inflammatory pathway activation in response to infection, as demonstrated by higher C-reactive protein levels in these patients (Carr et al., 2017).
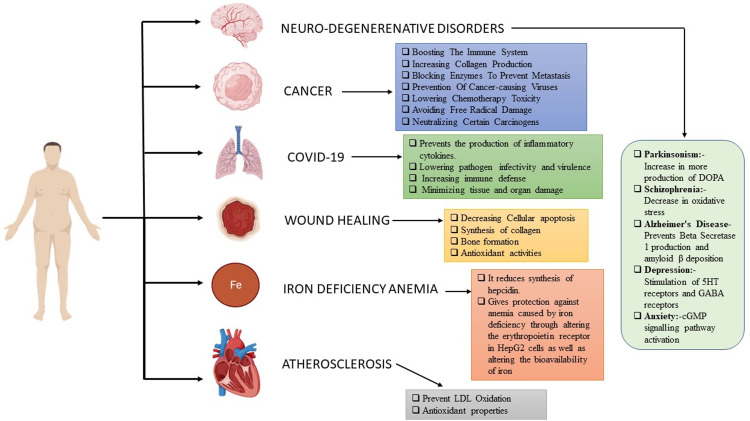
Diseases
The role of vitamin C in various diseases is depicted in Figure 5.
Figure 5.
Various pathways and mechanism through which vitamin C acts in various diseases. 5HT: 5-hydroxytryptamine; cGMP: cyclic guanosine monophosphate; DOPA: dihydroxyphenylalanine; GABA: gamma amino butyric acid; LDL: low-density lipoprotein.
COVID-19
COVID-19 is been reported as one of the most serious public health threats (Zhang et al., 2021). This outbreak began with an animal to human transmission, and severe atypical pneumonia was the immediate cause of mortality (Muscogiuri et al., 2020). The COVID-19 pandemic has caused widespread alarm and action, posing a significant risk to public health. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing COVID-19 results in common cold and has main impact on the respiratory tract and human immune system (Callender et al., 2020). Patients who are aged as well as those with pre-existing respiratory, cardiac or diabetic disease seems to be at a greater risk of rapidly progressing to the severe and critical stages (Weiss and Murdoch, 2020). The search for a coronavirus-specific drug has led to a dead end, with most of the studied medications, including corticosteroids, appearing to be beneficial to patients in addition to normal therapy. Despite the encouraging published results, the vaccination process is taking its time. As a result, there is a pressing need to explore new avenues for potential treatments. When considering the possible function of vitamin C against the novel coronavirus, there are various animal studies that are relevant (Hemilä, 2017). The clinical trials data of vitamin C in COVID-19 is given in Table 2. Vitamin C aids in the downregulation of cytokines, protects the endothelium from oxidative stress, and has significant role in tissue healing, which is especially crucial during COVID-19's critical phase (Holford et al., 2020). Vitamin C also prevents the production of inflammatory cytokines. An immunological hyper-reaction, involving Interleukin-6 (IL-6) and endothelin-1, appears to be driving the progression of COVID-19 pneumonia to respiratory failure. Patients infected with COVID-19 should benefit from vitamin C, as vitamin C has been given the importance of IL-6 in severe COVID-19 and vitamin C's capacity to prevent IL-6 from increasing in a variety of (pro)inflammatory disorders (Mrityunjaya et al., 2020). Vitamin C has long been thought to help sepsis patients retain normal lung function by improving broncho-alveolar function, alveolar fluid clearance, and reduction of neutrophil sequestration. A higher amount of IV vitamin C (10–20 g/day) was successfully administered in 50 patients with severe COVID-19 infection in a recent Chinese study, and the oxygenation index was stabilised, and all of the patients were cured and discharged after a defined length of time (Farjana et al., 2020). Vitamin C studies delayed and eased upper respiratory tract infections that occurred over the course of vitamin C treatment, according to a meta-analysis of 29 randomised trials including 11,306 patients (Hemilä and Chalker, 2020). In addition, studies have shown that combining antiviral, antioxidant and immunomodulatory effects of vitamin C and quercetin are synergistic. As a result, incorporating vitamin C into a dietary supplement can aid to relax and boost the immune system while also acting as an anti-inflammatory and antioxidant against COVID-19 infection (Colunga Biancatelli et al., 2020a). High-dose vitamin C treatment can help patients with viral pneumonia and acute respiratory distress syndrome who have a severe COVID-19 infection by lowering inflammation and pathogen infectivity and virulence, increasing immune defence, minimising tissue and organ damage, and improving overall prognosis (Wessels et al., 2020). Supplementing with vitamin C can drastically minimise the need for corticosteroids, antibiotics and antiviral medications. Vitamin C enhances the innate immune response, which aids in the prevention of viral infections.
Table 2.
List of clinical trials for intervention of vitamin C in COVID-19 infections.
| Serial number | Title | Country | Participants | Interventions | NCT number | Status |
|---|---|---|---|---|---|---|
| 1 | Coronavirus 2019 (COVID-19) – using ascorbic acid and zinc supplementation (COVID A to Z) | The United States | 214 | Ascorbic acid and zinc gluconate | NCT04342728 | Completed |
| 2 | Administration of intravenous vitamin C in novel coronavirus infection (COVID-19) and decreased oxygenation (AVoCaDO) | The United States | 20 | 50 mg/kg L-ascorbic acid of infusion given every 6 h for 4 days (16 total doses) | NCT04357782 | Completed |
| 3 | High-dose vitamin C treatment in critically ill patients with COVID-19 | Turkey | 78 | The daily administration of 6 g of vitamin C intravenously in 4 equal doses every 6 h | NCT04710329 | Completed |
| 4 | Lessening organ dysfunction with vitamin – COVID-19 (lovit-covid) | Canada | 800 | Vitamin C Control: saline or 5% dextrose solution |
NCT04401150 | Recruiting |
| 5 | The effect of melatonin and vitamin C on COVID-19 | The United States | 150 | (1) 10 mg melatonin, at bedtime (2) 1000 mg vitamin C, at bedtime Placebo at bedtime |
NCT04530539 | Recruiting |
| 6 | The study of quadruple therapy zinc, quercetin, bromelain and vitamin C on the clinical outcomes of patients infected with COVID-19 | Saudi Arabia | 60 | 1) Zinc:50 mg 2) Quercetin:500 mg 3) Bromelin:500 mg Vitamin C:1000 mg |
NCT04468139 | Recruiting |
| 7 | Use of ascorbic acid in patients with COVID-19 | Italy | 500 | 10 g* of vitamin C intravenously in addition to conventional therapy | NCT04323514 | Recruiting |
| 8 | Safety study of early infusion of vitamin C for the treatment of novel coronavirus acute lung injury (SAVE EVICT CORONA-ALI) | The United States | 60 | 50 mg/kg intravenous vitamin C infusion every 6 h for up to 96 h | NCT04344184 | Recruiting |
| 9 | A study of hydroxychloroquine, vitamin C, vitamin D and zinc for the prevention of COVID-19 (HELPCOVID19) | The United States | 600 | Experimental: hydroxychloroquine, vitamin C, vitamin D, zinc Placebo: vitamin C, vitamin D, zinc |
NCT04335084 | Recruiting |
| 10 | Role of mega dose of vitamin C in critical patients with COVID-19 infection | Pakistan | 15 | Experimental: the dose was 30 g a day (10 g TDS) for 2 days with standard treatment Placebo: distilled water in the same dose with same standard treatment |
NCT04682574 | Recruiting |
| 11 | International alliance study of therapies to prevent progression of COVID-19 | Australia | 200 | Vitamin C, Hydroxychloroquine Azithromycin Zinc citrate Vitamin D3, vitamin B12 |
NCT04395768 | Recruiting |
| 12 | Vitamin C infusion for the treatment of severe 2019-ncov-infected pneumonia | China | 56 | Experimental: 12g vitamin C was infused in the experimental group twice a day for 7 days Placebo: 50 ml sterile water for injection will be infused in the placebo comparator group twice a day for 7 days |
NCT04264533 | Terminated |
NCT: National Clinical Trial number.
Mechanism of action of vitamin C in COVID-19
With reference to COVID-19, vitamin C has various roles including downregulation of cytokines, endothelium protection from oxidative stress as well as a critical role in tissue repair (May and Harrison, 2013; May and Qu, 2011). The oxidative stress and genes critical to the inflammatory response including IL-1, IL-8, tumour necrosis factor alpha, as well as intercellular adhesion molecule 1 is mediated through the nuclear factor kappa B (NF-κB) pathway activation (Sen and Packer, 1996). Vitamin C causes reduction in the oxidative stress as well as inflammation through activation of NF-κB pathway (Chen et al., 2014). In COVID-19, there is downregulation of interferon type 1 expression (Blanco-Melo et al., 2020). Vitamin C is responsible to upregulate the expression of these important enzymes which acts as a defence against the infection (Colunga Biancatelli et al., 2020b). The concentration of vitamin C is much higher in adrenal glands than in other body organs. Vitamin C is released from the adrenal glands in response to stress and increasing the plasma level of vitamin C (Padayatty et al., 2007). Vitamin C is also responsible for the cortisol production which eventually increases the anti-inflammatory response and cytoprotective effects of glucocorticoids (Barabutis et al., 2017; Kodama et al., 1994).
Clinical trials and nutritional status of vitamin C in COVID-19
The main reason responsible for lung injury in people suffering from COVID-19 is the oxidative stress and the free radicals which are generated by the immune system of the body. The antioxidant status of the patients can be improved by administration of vitamin C (“The Financial Express,” 2020). There are number of studies conducted preclinically on animals which has provided the benefits and proved the efficiency of vitamin C in order to treat infections (Hemilä, 2017). A pilot study was conducted on critically ill patients suffering from sepsis. The patients were administered with IV vitamin C (50 and 200 mg/kg). The results showed that the patients administered with IV vitamin C had lower levels of pro-inflammatory markers and lower scores of Sequential Organ Failure Assessment as compared to the patients administered with placebo (Fowler et al., 2014). Another study titled ‘COVID A to Z’ showed a statistically significant difference in the recovery rate between the vitamin C and usual care arms (P value = 0.025). The time to reduce the symptoms by 50% was 1.2 days less in vitamin C arm in comparison with the usual arm (Hemilä et al., 2021). There are various other studies which do highlight the combination of vitamin C, hydrocortisone and thiamine which had efficient and fruitful results in sepsis (Fujii et al., 2020). There are number of clinical trials that have been announced since the start of the COVID-19 pandemic, so that the vitamin C's therapeutic benefits can be evaluated. The clinical trials are registered under National Institute of Health Clinical Trials in order to evaluate the efficiency of vitamin C in COVID-19 as alone treatment or in combination with other supplements. The randomised controlled trial was conducted in patients suffering from septic shock and the results were compared with the combination of vitamin C (6 g/day), hydrocortisone (200 mg/day) and thiamine (400 mg/day) to hydrocortisone alone. The results revealed that the combination therapy did not affect the duration of the attack but it decreases the Sequential Organ Failure Assessment scores (Fujii et al., 2020). This signifies that vitamin C has a major role in prevention and management of COVID-19. The dose of vitamin C for healthy individuals is 200 mg/day. There is increase in the requirement of dose during infections, that is, 1 to 2 g/day (Abobaker et al., 2020). The clinical trials ongoing may provide clear picture as well as evidence for the role of vitamin C in COVID-19.
Central nervous system
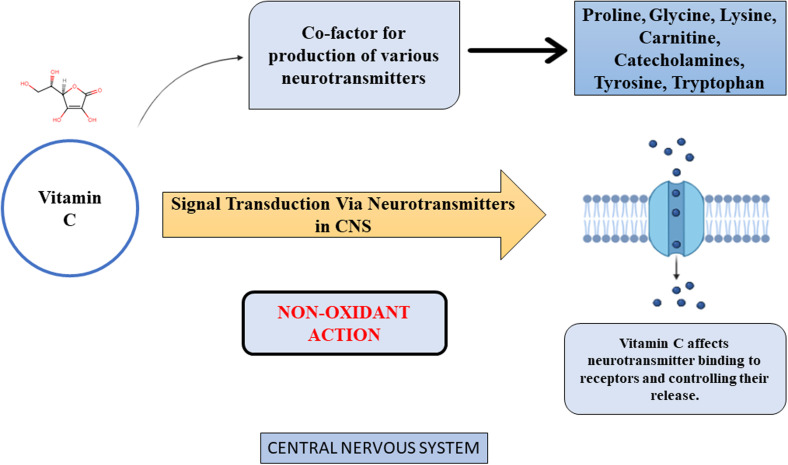
Vitamin C is useful for treatment of wide range of neurological and mental diseases as an adjuvant. High quantities of unsaturated fatty acids and a rapid rate of cell metabolism make the brain a particularly sensitive organ to oxidative stress and free radical activity. As an antioxidant, AA functions by scavenging reactive oxygen and nitrogen species produced during normal cell metabolism. As per Figure 6, vitamin C participates in CNS signal transduction via neurotransmitters, which is a non-antioxidant action. Vitamin C is hypothesised to play a part in this process by affecting neurotransmitter binding to receptors and controlling their release. Furthermore, several neurotransmitters, including catecholamines, require vitamin C as a cofactor in their production.
Figure 6.
Non-oxidant action of vitamin C in central nervous system (CNS).
Anxiety
Anxiety is a natural response that detects and prepares a person for an actual or possible threat (McNaughton and Corr, 2004). Glutamate is an excitatory neurotransmitter that plays a role in anxiety-related behaviour modulation. An elevation in glutamate signalling in the brain is linked to anxious behaviour, whereas a decrease in glutamate signalling reduces anxiety-related behaviour (Swanson et al., 2005). Patients who suffer from anxious types of depression seem to be more prone to experience depression symptoms that are severe, such as exhaustion, guilt and feelings of worthlessness (Goldberg et al., 2014). Furthermore, at a physiological level, AA shields neuronal cells against glutamate-induced excitotoxicity. The importance of internal dietary AA levels for mental wellness is critical. Dianae B. Fraga reported that in diverse animal models of anxiety, the single dose's effects of diazepam (positive control), AA or ketamine, were studied. Mice were given ketamine, AA or diazepam before mice being tested in the open field test, elevated plus maze test, marble burying test and light–dark preference test (Fraga et al., 2018). The anxiogenic and anxiolytic effects of various medications are commonly assessed using the light–dark test (Bourin and Hascoët, 2003; Gallo et al., 2014). The hypothesis behind this study is that mice avoid brightly lit surroundings and engage in spontaneously exploratory action in relation to mild stressors such as novelty and light. In the light–dark preference test, in the light region vitamin C increased latency time and duration, but had no influence on mice's performance in the marble burying test. The findings imply that AA combined with ketamine may have an anxiolytic effect, opening up a new therapy option for anxiety disorders (Fraga et al., 2018). Through cyclic guanosine monophosphate (cGMP)-mediated signalling, nitric oxide (NO) has also been shown to behave as a post-synaptic or pre-synaptic retro-grade messenger (Wang et al., 2005). The phosphorylation of synaptophysin, the protein that induces glutamate release by fusing glutamate-containing granules to the membrane of pre-synaptic nerve terminals, activates the cGMP-dependent protein kinase G pathway (Wang et al., 2005). According to Vaibhav et al., AA, an NO signalling pathway and N-methyl-D-aspartic acid receptor modulator, resulted in an anxiolytic effect in mice. When mice were administered with AA, the amount of nitrite in their brains was reduced. Anxiogenic reactions were generated by pre-treatment with NO donor and sildenafil which were followed by AA treatments (Walia et al., 2019). As a result, modulation of NO signalling was implicated in the action of AA as an anxiolytic in mice. An inherent vitamin C deficiency in SMP30/GNL knockout mice resulted in anxiety and anorexia, according to Koizumi et al. (2016).
Depression
According to World Health Organization, there are millions of people worldwide who are affected by depression. Dopamine, noradrenaline and serotonin neurotransmission disturbances may all play an important role in the illness. Depression is defined by loss of interest and persistent sorrow in the activities you enjoy the most, in addition, for at least 2 weeks, you will be unable to carry out daily activities. In addition, those who suffer from depression are likely to have multiple symptoms such as: loss of energy; changes in the appetite; change of sleeping pattern; worry; poor focus; lack of direction; restlessness; thoughts of worthlessness, regret or hopelessness; a feeling of unworthy, remorse and thoughts of suicide and self-harm (Estimates, 2014). Long-term treatment with a multivitamin that included enough vitamin C enhanced mood in persons with low plasma vitamin C levels (Helmeste and Tang, 1998). Exhaustion and psychosomatic idiosyncrasy can result from a vitamin C deficit (Vahdat Shariatpanaahi et al., 2007). The stimulation of 5-hydroxytryptamine 1A (5-HT1A) and 5-HT2A/2C receptors at post-synaptic sites has also been connected to AA's antidepressant-like activity (Binfaré et al., 2009). Furthermore, a 3-month AA treatment promoted remission of depression symptoms in 50 depressive patients who were experiencing acute depression. Amr et al. (2013) and co-workers hypothesised that AA's antidepressant activity benefits are unrelated to its antioxidant characteristics. Furthermore, investigations have shown that depressed patients have reduced levels of inhibitory neurotransmitter amino butyric acid in their brains, as well as changes in the subunit makeup of its major receptor (gamma amino butyric acid (GABA)-A) (Luscher et al., 2011). Preclinical evidence suggests that current depression medication may help to compensate for GABAnergic deficits (Luscher et al., 2011). AA's antidepressant-like effect may be due to GABA-A receptor activation and maybe the GABA-B receptor inhibition (Rosa et al., 2016). When taken with AA at a sub-effective dose, muscimol (GABA-A agonist) produced an antidepressant-like synergistic effect, but baclofen (GABA-B agonist) reduced AA's antidepressant-like effect (Rosa et al., 2016). Another study conducted in New Zealand in young adult males also suggested the possible relation with the vitamin C status as well as the mood state. The study is cross-sectional, and evidence of causality will require further well-conducted intervention studies. There are several physiological reasons for vitamin C's good influence on mood, including its role in brain function and homoeostasis (Pullar et al., 2018). Antioxidant vitamin intake, according to one study, could be a good dietary choice for preventing depression and anxiety disorders (Farhadnejad et al., 2020).
Parkinsonism
Parkinsonism is a neurodegenerative disease which is marked by tremor, stiffness and bradykinesia in the muscles. Oxidative stress is assumed being the cause of neurodegenerative diseases like Parkinsonism (Niedzielska et al., 2016). Furthermore, this ailment is hyposmia, sleep problems and depression are all linked to non-motor symptoms (Massano and Bhatia, 2012; Schapira et al., 2017). Parkinsonism is marked by the degeneration of dopaminergic neurons in substantia nigra and formation of Lewy bodies within the brain (DeMaagd and Philip, 2015). In investigations on humans, vitamin C deficiency has already been linked with Parkinson's disease (Medeiros et al., 2016; Quiroga et al., 2014; Senarath Yapa, 1992). Vitamin C has been studied extensively in a variety of Parkinson's disease in vivo and in vitro models, and it has been shown to have beneficial effects (Kocot et al., 2017b). The Drosophila model was utilised by Huynh Man Anh et al. to study the duration and dose-dependent effects of vitamin C therapy on Parkinsonism symptoms caused by the knockdown of Drosophila ubiquitin C-terminal hydrolase. In this model, they discovered that vitamin C was found to improve Parkinsonism-like characteristics, which are characterised by neurodegeneration as well as locomotive abnormalities (Man Anh et al., 2019). On the contrary, high dose of AA had deleterious impacts on eating behaviour and motor capacity. Long-term vitamin C medication, for example, may protect against dopaminergic neuron degeneration. Vitamin C can boost dihydroxyphenylalanine synthesis (DOPA). Seitz et al. discovered that incubating the cell line of human neuroblastoma with AA resulted in more production of DOPA in dose-dependent manner. Furthermore, after treatments with AA, tyrosine hydroxylase gene expression increased thrice (Seitz et al., 1998).
Alzheimer's disease
Vitamin C insufficiency has been linked to the neuropathology of Alzheimer's disease as well as normal aging. The neuropathology of Alzheimer's disease is characterised by oxidative stress (Praticò et al., 2002; Praticò and Sung, 2004). In neurodegeneration, disturbance of AA homoeostasis has been well-documented (Covarrubias-Pinto et al., 2015). In the CNS, AA is an important antioxidant that is produced by glial cells within the synaptic cleft and then as an antioxidant defence, neurons absorb it to keep synaptic function as well as neuronal metabolism and in check. The astrocytes and neurons interaction has been discovered to be a key mechanism for AA recycling, as well as a participant in the brain's antioxidative defence (Dringen et al., 1999). Parenteral administration of AA has nootropic characteristics in amyloid protein precursor/presenilin-1 transgenic mice, according to a prior study, without affecting the Alzheimer's like traits of acetylcholinesterase activity, oxidative stress or plaque deposition (Harrison et al., 2009). Dixit et al. (2015) reported that the results of their study conducted imply that as age increases, vitamin C deficiency may accelerate the start of oxidative stress in the brain, as well as play a key role in amyloid production, oligomerization and deposition. Javier et al. reported that during the early asymptomatic phases of Alzheimer's disease, the cerebrovascular response to hypoxia can be altered by vitamin C preventing beta secretase 1 production and amyloid β deposition while lowering the thickness of the cerebrovascular basement membrane (Frontiñán-Rubio et al., 2018).
Schizophrenia
Schizophrenia is a life-altering, chronic illness that causes functional impairment in the social, cognitive and emotional arenas. Positive symptoms (hallucinations and delusions) co-exist with negative symptoms (emotional blunting and apathy) and cognitive impairment (Brown and Roffman, 2014). Negative symptoms and cognitive deficiencies, on the other hand, do not usually react to antipsychotic therapy (Brown and Roffman, 2014). The case reported by Reuven Sandyk was about a patient suffering from schizophrenia since 17. The patient has been profoundly psychotic and has only partially responded to numerous psychotropic medicines. He has had practically total remission of psychotic symptoms since starting vitamin C therapy in 1987, and he is now able to work part-time. Even his relapse in early 1989 was much milder than previous relapses. Finally, his capacity to work part-time after being released from the hospital attests to the significant improvement in his mental state. This demonstrates the usefulness of vitamin C when used as a supplement in the treatment of a subset of persistent schizophrenia patients who do not have responded to the conventional treatments (Sandyk and Kanofsky, 1993). Dakhale et al. also reported that vitamin C supplementation when combined with atypical antipsychotics is responsible for the decrease in oxidative stress and improves schizophrenia outcomes. Forty-five patients took part in the study having schizophrenia. It was an 8-week, non-crossover, prospective, double-blinded trial. The primary finding of this study is that vitamin C supplementation with atypical antipsychotics resulted in a substantial drop in serum malondialdehyde and rise in plasma levels of AA in comparison to placebo with atypical antipsychotics (Dakhale et al., 2005).
Wound healing
AA is responsible for every aspect of wound healing including cellular apoptosis, synthesis of collagen, collagen synthesis and bone formation as well as antioxidant activities (Anderson, 2005). When wounding takes place, there is a drop in tissue and plasma levels. By using high-dose vitamin C supplements early in process of healing appears to be advantageous, especially if people who are already scorbutic (Long et al., 2003). Major difficulties caused by AA deficiency have traditionally been noted in scurvy patients; however, these defining traits are not always present in today's patients. AA deficit is more likely in patients having vascular disease, the elderly, pregnant women, smokers, drug abusers and persons who are underweight. According to the case study, oral vitamin C supplementation increased wound healing in those who had previously suffered from the deficit. The amount of AA in the blood was below than the standard value, that is, 25 µmol/L in 65 of 180 patients throughout a 21-month period (36%). Patients and their clinicians noticed a significant and rapid recovery of vast and intricate wounds after starting supplementation (1000 mg/day) (Bikker et al., 2016). Because exact AA levels can only be determined through laboratory testing, doctors must take a pragmatic approach and be on the watch for any visible indicators of deficit in patients with a case of acute chronic wounds (Moores, 2013).
Iron deficiency anaemia
Iron deficiency anaemia is characterised by a decrease in red blood cells formation as a result of a total body iron deficiency (Brugnara, 2002). It is critical for the structure as well as the function of cells. Dietary iron is made up of both haeme and non-haeme iron. The divalent metal transporter-1 transports non-haeme ferrous iron into enterocytes (Frazer and Anderson, 2005). The basolateral membrane protein ferroportin is responsible for iron exiting enterocytes (Himmelfarb, 2007). Fe3+ is transported by transferrin from the bloodstream to haematopoietic organs and other tissues, including the liver (Graham et al., 2007). Macrophages in the reticuloendothelial system destroy aged red blood cells (Himmelfarb, 2007). Ferroportin is a protein that is responsible for release of iron from macrophages into the bloodstream. Iron is absorbed mostly in the region of duodenum and upper jejunum of intestine, where Fe2+ can be transferred into mucosal epithelial cells of small intestine. AA has shown to boost iron availability and availability from non-haeme iron sources (Hallberg, 1981). Vitamin C reduces synthesis of hepcidin and gives protection against anaemia caused by iron deficiency through altering the erythropoietin receptor in HepG2 cells as well as altering the bioavailability of iron (Chiu et al., 2012). Furthermore, ASC taken orally had a significant positive impact on haematological markers in the anaemic haemodialysis patients (Sirover et al., 2008). Darius et al. reported the ASC's effect on cellular iron absorption from Fe2-Tf and they demonstrate that the physiological ASC concentrations considerably stimulate Tf-dependent iron uptake by variety of human cells (Lane et al., 2013). On contrary, randomised clinical trial reported that the findings call into question the recommendation that vitamin C supplements be taken in conjunction with iron orally in order to boost efficacy and accelerate anaemia recovery. In a moderately acidic environment, iron is absorbed as ferrous salt (Fe2+). Vitamin C is thought to help in calcium absorption. However, based on the findings of this clinical investigation, vitamin C may not be as beneficial as previously assumed (Li et al., 2020).
Atherosclerosis
In the pathophysiology of vascular diseases, the endothelium plays a critical role (Iaccarino et al., 2004; Ross, 1986). Atherosclerosis is a degenerative condition of disease in which the major, as well as medium-sized arteries, are affected which is marked by loss of flexibility of the arterial wall and narrowing of the lumen (Rafieian-Kopaei et al., 2014). Localised thickening of the tunica intima causes the lumen to narrow. Myocardial infarction and ischaemic stroke are the most common symptoms of atherosclerosis. The antioxidant properties of vitamin C give an idea that it may help prevent cardiovascular disease (Moser and Chun, 2016). According to epidemiological data, antioxidant content in fruits and vegetables lowers the incidence of cardiovascular disease (CVD), which could be explained by the antioxidant's role in preventing oxidative alterations to low-density lipoprotein (LDL) (Salvayre et al., 2016). Scavenger receptors recognise oxidised LDL and integrate it into plaque (Li and Mehta, 2005). As a result, vitamin C's ability to prevent LDL oxidation may help to prevent atherosclerosis, potentially lowering CVD risk. Endothelial NO generation has been demonstrated to increase with AA, leading to increased vasodilation and lower blood pressure (d’Uscio et al., 2003). Furthermore, vitamin C may help make plaques more stable if atherosclerosis has been established by preventing vascular smooth muscle cell death (Siow et al., 1999).
Cancer
The debate over vitamin C's anti-cancer capabilities and its usefulness in palliative care has raged on very much (Zasowska-Nowak et al., 2021). Because of the development of resistance by cancer cells as well as toxicity, most chemotherapeutic drugs have restrictions on their usage (Ng et al., 2015). At high therapeutic levels, vitamin C has been demonstrated to have a particular influence on the cytotoxicity of many types of cancer cells (Pawlowska et al., 2019). There are number of processes through which vitamin C prevent and cure cancer. The processes are: boosting the immune system, increasing collagen production, blocking enzymes to prevent metastasis (spreading), prevention of cancer-causing viruses, the deficit of vitamin C in the patients suffering from cancer is treated, post-surgery wound healing in people with cancer improves chemotherapy efficiency, lowering chemotherapy toxicity, avoiding free radical damage and neutralising certain carcinogens (Chambial et al., 2013). Vitamin C's cytotoxic effects have been attributed to its capacity for the generation of reactive oxygen species (ROS) that has been linked to activating p53 which is tumour suppressor protein and the master regulator of DNA repair and death (Liu et al., 2015). Complementary and alternative medicine practitioners routinely administer vitamin C intravenously to treat infections, cancer and tiredness. Vitamin C's bioavailability and anti-cancer properties are known to be influenced by how it is administered in the body. Because high-dose IV has been already demonstrated in various multiple clinical studies to enhance the quality of life and health, it may be considered a palliative treatment (Zasowska-Nowak et al., 2021). SVCT1 does not appear to be important in cancer. According to various studies, SVCT2 plays a significant role in malignancies. The first discovered that when compared to normal cells, breast tumours exhibit greater levels of SVCT2 expression (Roa et al., 2020). Overexpression of this transporter enhanced chemosensitivity to the high dose of AA, resulting in to increase in ROS production as well as cell death (Hong et al., 2013). Cancer cells, on the other hand, become resistant to the therapy when siRNA targeting SVCT2 is used. As a result, SVCT2 may have a role in ASC-mediated cancer cell killing (Hong et al., 2013). Table 3 represents the list of clinical trials for interventions of AA in other disease conditions.
Table 3.
List of clinical trials for interventions of vitamin C (ascorbic acid) in other health conditions.
| Serial number | Title | Study type | Population | Interventions | NCT number | Status | Key findings |
|---|---|---|---|---|---|---|---|
| 1 | Micronutrient to prevent noise-induced hearing loss | Interventional study | 70 participants | Beta-carotene, vitamins C and E, magnesium | NCT00808470 | Completed | Supplement had no effect on noise-induced changes in hearing |
| 2 | Quantitative in vivo biomarkers of oxidative stress in diabetes | Interventional study | 42 participants | Vitamin C | NCT00845130 | Completed | |
| 3 | Prophylaxis to reduce post-operative atrial fibrillation in cardiac surgery | Interventional study | 304 participants | Beta blockers, amiodarone, ascorbic acid |
NCT00953212 | Completed | |
| 4 | ACTS trial | Interventional study | 205 participants | Vitamin C, vitamin B1, hydrocortisone | NCT03389555 | Completed | The combination did not show significant reduction in the SOFA score |
| 5 | Intravenous vitamin C in the treatment of viral infection especially in the treatment of shingles | Interventional study | 68 participants | IV vitamin C | NCT00921934 | Completed | The results showed that there was decline in the pain score which provide evidence that IV vitamin C is beneficial in treatment of viral infection |
| 6 | A study of vitamin C in the treatment of liver cancer to determine if it is safe and effective | Interventional study | 5 participants | Ascorbic acid & sorafenib | NCT01754987 | Completed | The safety data indicates no toxicity |
| 7 | A phase 2 pharmacokinetic study of 6r-bh4 alone or 6r-bh4 with vitamin C in subjects with endothelial dysfunction | Interventional study | 52 participants | Sapropterin dihydrochloride and vitamin C | NCT00532844 | Completed | |
| 8 | The effect of high-dose vitamin C on the liver function in chronic hepatitis patients | Interventional study | 10 participants | High-dose vitamin C | NCT01413360 | Completed | |
| 9 | Liposomal encapsulated vitamin C in complex regional pain syndrome | Interventional study | 66 participants | Liposomal vitamin C versus vitamin C versus placebo |
NCT04204200 | Completed | There was no benefit to patients and no significant difference in time to heal the fracture |
| 10 | Ascorbic acid (vitamin C) infusion in human sepsis | Interventional study | 24 participants | Ascorbic acid | NCT01434121 | Completed | Ascorbic acid significantly reduced the proinflammatory biomarkers C-reactive protein and procalcitonin |
| 11 | Vit C-AF | Interventional study | 20 participants | Vitamin C | NCT03148236 | Completed | High dose of vitamin C is safe and well tolerated at the time of AF ablation and is associated with a blunted rise in C-reactive protein |
| 12 | CITRIS-ALI | Interventional study | 170 participants | Infusion: vitamin C | NCT02106975 | Completed | The infusion of ascorbic acid did not significantly improve the organ dysfunction scores |
| 13 | Study of high-dose vitamin C on outcome in cardiac surgery patients | Interventional study | 57 participants | Drug: ascorbic acid Placebo: 5% Dextrose water or normal saline |
NCT01167569 | Completed | |
| 14 | VCSIP of infant lung infection | Interventional study | 252 participants | Vitamin C | NCT01723696 | Completed | |
| 15 | Does vitamin C reduce finger stiffness after distal radius fractures? | Interventional study | 126 participants | 500 mg vitamin C, 1 pill per day for 6 weeks versus 1 placebo pill for 6 weeks |
NCT02216812 | Completed | There was no difference in the finger stiffness |
| 16 | VICTAS | Interventional study | 501 participants | Vitamin C, thiamine hydrocortisone |
NCT03509350 | Completed | |
| 17 | Vitamin C supplementation intervention | Interventional study | 36 participants | Vitamin C 500 mg versus vitamin C 1000 mg versus placebo | NCT04036110 | Completed | Patients with CHF acute intravenous administration of vitamin C enhances platelet responsiveness to the anti-aggregatory effects of NO donors and improves endothelial function |
ACTS: Ascorbic acid, corticosteroids and thiamine in sepsis; AF: atrial fibrillation, CHF: congestive heart failure, CITRIS-ALI: vitamin C infusion for treatment is sepsis-induced acute lung injury; IV: intravenous; NCT: National Clinical Trial number; NO: nitric oxide; SOFA: Sequential Organ Failure Assessment; VCSIP: vitamin C to decrease effects of smoking in pregnancy; VICTAS: vitamin C, thiamine and steroids in sepsis; Vit C-AF: vitamin C in atrial fibrillation ablation.
Nutritional strategies for delivery of vitamin C
The hydrophilic character as well as the instability of vitamin C has led to its less utilisation. But, during recent years there are various types of studies conducted in order to improve the therapeutic outcome using vitamin C which includes nanoparticles and microparticles (Jang and Lee, 2008), liposomes (Hope et al., 1986), micelles (Sutradhar and Amin, 2014), microemulsion (Sawant et al., 2011) and orally disintegrating films (Garcia et al., 2018). This not only improved the therapeutic efficiency of vitamin C but also the drug targeting (Caritá et al., 2020).
Future perspective
AA is a vital and necessary component of human health. In humans, it is required for a number of physiological activities. Vitamin C is also been found to aid in the transportation of therapeutic agents in the body. Vitamin C has indeed been linked to a number of health advantages which includes antioxidant, anti-atherogenic, anti-carcinogenic and treatment of various neurodegenerative disorders. In recent decades, AA has been thoroughly studied and discovered to be an excellent antioxidant and cofactor in the synthesis of collagen. In preclinical and preliminary trials, vitamin C has been shown to increase organ function amid oxidative stress and inflammation. There are a certain number of ways in which AA reduces inflammation as well as oxidative damage has sparked a surge in interest in its medicinal use. The biggest limitation of the clinical trials of AA is that the study is not conducted on a large number of patients. Smaller groups of studies are conducted which cannot produce the results which can prove something concrete. The patients who are at high risk of COVID-19 suffering from vitamin C deficiency should be encouraged to start supplementation of vitamin C. There are several preclinical and clinical studies reported which highlights the role of vitamin C in COVID-19. There is more data required from the clinical trials which would prove the efficiency of vitamin C in COVID-19. The pending COVID-19 clinical trials may prove the potential benefits of vitamin C in COVID-19.
Conclusion
There is high prevalence of deficiency of vitamin C worldwide. Vitamin C may not be the first-line treatment, but may be considered as a safe, adjuvant as well as inexpensive nutritional supplement therapy for many diseases as mentioned in this review article. The public health sector should take an account of vitamin C deficiency and should do necessary things for the public in order to reduce the patients suffering from deficiency of vitamin C.
Supplemental Material
Supplemental material, sj-png-1-nah-10.1177_02601060221139628 for Unwinding the potentials of vitamin C in COVID-19 and other diseases: An updated review by Nikhil Mehta, Purvi Pokharna and Saritha R Shetty in Nutrition and Health
Acknowledgements
All the figures are partially created with ‘Biorender.com’.
Abbreviations
- 5-HT
5-hydroxytryptamine
- AA
ascorbic acid
- APP/PSEN 1
amyloid protein precursor/presenilin-1;
- ARDS
acute respiratory distress syndrome
- ASC
ascorbate
- cGMP
cyclic guanosine monophosphate
- CVD
cardiovascular disease
- DHA
dehydroascorbate
- DOPA
dihydroxyphenylalanine
- dUCH
Drosophila ubiquitin C-terminal hydrolase
- ET-1
endothelin-1
- GABA
gamma amino butyric acid
- GLUT
glucose transporter
- ICAM-1
intercellular adhesion molecule 1
- ICU
intensive care unit
- IL-1
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- IV
intravenous
- LDL
low-density lipoprotein
- NF-κB pathway
nuclear factor kappa B pathway
- NMDA
N-methyl-D-aspartic acid or N-methyl-D-aspartate
- NO
nitric oxide
- RDA
recommended dietary allowance
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SLC23A
solute carrier family 23 m1
- SNP
single nucleotide polymorphism
- SVCT
sodium ascorbate co-transporter
- TNF-α
tumour necrosis factor alpha
Footnotes
Authors’ contribution: All the authors contributed equally to prepare this article, read and approved the final manuscript.
Consent for publication: All the authors have agreed to submit this paper for publication in Nutrition and Health.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Saritha R Shetty https://orcid.org/0000-0003-2440-5398
Supplemental material: Supplemental material for this article is available online.
References
- Abobaker A, Alzwi A, Alraied AHA. (2020) Overview of the possible role of vitamin C in management of COVID-19. Pharmacological Reports 72(6): 1517–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agus DB, Gambhir SS, Pardridge WM, et al. (1997) Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. Journal of Clinical Investigation 100(11): 2842–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado C, Alvarez P, Puerto M, et al. (2006) Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition (Burbank, Los Angeles County, Calif.) 22(7–8): 767–777. [DOI] [PubMed] [Google Scholar]
- Amir Shaghaghi M, Bernstein CN, Serrano León A, et al. (2014) Polymorphisms in the sodium-dependent ascorbate transporter gene SLC23A1 are associated with susceptibility to Crohn disease. The American Journal of Clinical Nutrition 99(2): 378–383. [DOI] [PubMed] [Google Scholar]
- Amr M, El-Mogy A, Shams T, et al. (2013) Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Nutrition Journal 12(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. (2005) Nutrition and wound healing: The necessity of assessment. British Journal of Nursing (Mark Allen Publishing) 14(19): S30, S32, S34 passim [DOI] [PubMed] [Google Scholar]
- Armour J, Tyml K, Lidington D, et al. (2001) Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. Journal of Applied Physiology 90(3): 795–803. [DOI] [PubMed] [Google Scholar]
- Asard H, May JM, Smirnoff N. (Eds.) (2005) Vitamin C: Functions and Biochemistry in Animal and Plants. 1st ed.Taylor & Francis. https://doi.org/10.4324/9780203500002 [Google Scholar]
- Barabutis N, Khangoora V, Marik PE, et al. (2017) Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest 152(5): 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharara A, Grossman C, Grinnan D, et al. (2016) Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Reports in Critical Care 2016: 8560871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker A, Wielders J, Van Loo R, et al. (2016) Ascorbic acid deficiency impairs wound healing in surgical patients: Four case reports. International Journal of Surgery Open 2: 15–18. [Google Scholar]
- Binfaré RW, Rosa AO, Lobato KR, et al. (2009) Ascorbic acid administration produces an antidepressant-like effect: Evidence for the involvement of monoaminergic neurotransmission. Progress in Neuro-Psychopharmacology and Biological Psychiatry 33(3): 530–540. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181(5): 1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoët M. (2003) The mouse light/dark box test European Journal of Pharmacology 463(1): 55–65. [DOI] [PubMed] [Google Scholar]
- Bowers-Komro DM, McCormick DB. (1991) Characterization of ascorbic acid uptake by isolated rat kidney cells. The Journal of Nutrition 121(1): 57–64. [DOI] [PubMed] [Google Scholar]
- Bozonet SM, Carr AC, Pullar JM, et al. (2015) Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 7(4): 2574–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HE, Roffman JL. (2014) Vitamin supplementation in the treatment of schizophrenia. CNS Drugs 28(7): 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C (2002) A hematologic “gold standard” for iron-deficient states? Clinical Chemistry 48(7): 981–982. 10.1093/clinchem/48.7.981 [DOI] [PubMed] [Google Scholar]
- Callender LA, Curran M, Bates SM, et al. (2020) The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Frontiers in Immunology 11(August): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cámara M, Sánchez-Mata MC, Fernández-Ruiz V, et al. (2021) A review of the role of micronutrients and bioactive compounds on immune system supporting to fight against the Covid-19 disease. Foods (basel, Switzerland) 10(5): 1088. 10.3390/foods10051088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Jack T. (1979) Acute reactions to mega ascorbic acid therapy in malignant disease. Scottish Medical Journal 24(2): 151–153. [DOI] [PubMed] [Google Scholar]
- Caritá AC, Fonseca-santos B, Shultz JD, et al. (2020) Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine: Nanotechnology, Biology, and Medicine 24: 102117. 10.1016/j.nano.2019.102117 [DOI] [PubMed] [Google Scholar]
- Carr AC and Rowe S (2020) The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients 12(11). 10.3390/nu12113286 [DOI] [PMC free article] [PubMed]
- Carr AC, Rosengrave PC, Bayer S, et al. (2017) Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Critical Care 21(1): 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciari JJ, Riordan HD, Miranda-Massari JR, et al. (2005) Effects of high dose ascorbate administration on L-10 tumor growth in guinea pigs. Puerto Rico Health Sciences Journal 24(2): 145–150. [PubMed] [Google Scholar]
- Chambial S, Dwivedi S, Shukla KK, et al. (2013) Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry 28(4): 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, McCowen KC, Blackburn GL. (1999) Nutrition management in the ICU. Chest 115(5 Suppl): 145S–148S. [DOI] [PubMed] [Google Scholar]
- Chen Y, Luo G, Yuan J, et al. (2014) Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators of Inflammation 2014: 426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PF, Ko SY, Chang CC. (2012) Vitamin C affects the expression of hepcidin and erythropoietin receptor in HepG2 cells. Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation 22(3): 373–376. [DOI] [PubMed] [Google Scholar]
- Colunga Biancatelli RML, Berrill M, Catravas JD, et al. (2020a) Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Frontiers in Immunology 11(June): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli RML, Berrill M, Marik PE. (2020b) The antiviral properties of vitamin C. Expert Review of Anti-Infective Therapy 18(2): 99–101. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Eck P, Wang J, et al. (2013) Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. Journal of Biological Chemistry 288(13): 9092–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpe CP, Tu H, Eck P, et al. (2010) Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. The Journal of Clinical Investigation 120(4): 1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossey LN, Rahim F, Larsen CP. (2013) Oxalate nephropathy and intravenous vitamin C. American Journal of Kidney Diseases 61(6): 1032–1035. [DOI] [PubMed] [Google Scholar]
- Covarrubias-Pinto A, Acuña AI, Beltrán FA, et al. (2015) Old things new view: Ascorbic acid protects the brain in neurodegenerative disorders. International Journal of Molecular Sciences 16(12): 28194–28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhale GN, Khanzode SD, Khanzode SS, et al. (2005) Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 182(4): 494–498. [DOI] [PubMed] [Google Scholar]
- Daruwala R, Song J, Koh WS, et al. (1999) Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Letters 460(3): 480–484. [DOI] [PubMed] [Google Scholar]
- De Jong TMH, Jochens A, Jockel-Schneider Y, et al. (2014) SLC23A1 polymorphism rs6596473 in the vitamin C transporter SVCT1 is associated with aggressive periodontitis. Journal of Clinical Periodontology 41(6): 531–540. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Sánchez C, Vallejo C, et al. (2020) Vitamin C and vitamin C plus E improve the immune function in the elderly. Experimental Gerontology 142: 111118. [DOI] [PubMed] [Google Scholar]
- DeMaagd G, Philip A. (2015) Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P & T: A Peer-Reviewed Journal for Formulary Management 40(8): 504–532. https://pubmed.ncbi.nlm.nih.gov/26236139 [PMC free article] [PubMed] [Google Scholar]
- Dickinson N, Macpherson G, Hursthouse AS, et al. (2009) Micronutrient deficiencies in maternity and child health: A review of environmental and social context and implications for Malawi. Environmental Geochemistry and Health 31(2): 253–272. [DOI] [PubMed] [Google Scholar]
- Dixit S, Bernardo A, Walker JM, et al. (2015) Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chemical Neuroscience 6(4): 570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Kussmaul L, Gutterer JM, et al. (1999) The glutathione system of peroxide detoxification is less efficient in neurons than in astroglial cells. Journal of Neurochemistry 72(6): 2523–2530. [DOI] [PubMed] [Google Scholar]
- d’Uscio LV, Milstien S, Richardson D, et al. (2003) Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circulation Research 92(1): 88–95. [DOI] [PubMed] [Google Scholar]
- Erichsen HC, Engel SAM, Eck PK, et al. (2006) Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery. American Journal of Epidemiology 163(3): 245–254. [DOI] [PubMed] [Google Scholar]
- Estimates (2014) Depression and Other Common Mental Disorders Global Health Estimates. WHO. https://apps.who.int/iris/handle/10665/254610
- Farhadnejad H, Neshatbini A, Salehpour A. (2020) Clinical nutrition ESPEN antioxidant vitamin intakes and risk of depression, anxiety and stress among female adolescents. Clinical Nutrition ESPEN 40: 257–262. 10.1016/j.clnesp.2020.09.010 [DOI] [PubMed] [Google Scholar]
- Farjana M, Moni A, Sohag AAM, et al. (2020) Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19. Infection & Chemotherapy 52(4): 461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell CL, Yang J, Pardridge WM. (1992) GLUT-1 glucose transporter is present within apical and basolateral membranes of brain epithelial interfaces and in microvascular endothelia with and without tight junctions. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society 40(2): 193–199. [DOI] [PubMed] [Google Scholar]
- Fontana F, Cazzato S, Giovanella S, et al. (2020) Oxalate nephropathy caused by excessive vitamin C administration in 2 patients with COVID-19. Kidney International Reports 5(10): 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AA.3rd, Syed AA, Knowlson S, et al. (2014) Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. Journal of Translational Medicine 12: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga DB, Olescowicz G, Moretti M, et al. (2018) Anxiolytic effects of ascorbic acid and ketamine in mice. Journal of Psychiatric Research 100: 16–23. [DOI] [PubMed] [Google Scholar]
- Frazer DM, Anderson GJ. (2005) Iron imports. I. Intestinal iron absorption and its regulation. American Journal of Physiology. Gastrointestinal and Liver Physiology 289(4): G631–G635. [DOI] [PubMed] [Google Scholar]
- Friedman GJ, Sherry S, Ralli EP. (1940) The mechanism of the excretion of vitamin C by the human kidney at low and normal plasma levels of ascorbic acid. The Journal of Clinical Investigation 19(5): 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontiñán-Rubio J, Sancho-Bielsa FJ, Peinado JR, et al. (2018) Sex-dependent co-occurrence of hypoxia and β-amyloid plaques in hippocampus and entorhinal cortex is reversed by long-term treatment with ubiquinol and ascorbic acid in the 3 × Tg-AD mouse model of Alzheimer’s disease. Molecular and Cellular Neuroscience 92: 67–81. [DOI] [PubMed] [Google Scholar]
- Fujii T, Luethi N, Young PJ, et al. (2020) Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The VITAMINS randomized clinical trial. JAMA 323(5): 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo I, Rattazzi L, Piras G, et al. (2014) Formyl peptide receptor as a novel therapeutic target for anxiety-related disorders. PLOS ONE 9(12): e114626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MDLA, Salazar K, Millán C, et al. (2005) Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50(1): 32–47. [DOI] [PubMed] [Google Scholar]
- Garcia VAS, Borges JG, Maciel VBV, et al. (2018) Gelatin/starch orally disintegrating films as a promising system for vitamin C delivery. Food Hydrocolloids 79: 127–135. [Google Scholar]
- Goldberg DP, Wittchen HU, Zimmermann P, et al. (2014) Anxious and non-anxious forms of major depression: Familial, personality and symptom characteristics. Psychological Medicine 44(6): 1223–1234. [DOI] [PubMed] [Google Scholar]
- Graham RM, Chua ACG, Herbison CE, et al. (2007) Liver iron transport. World Journal of Gastroenterology 13(35): 4725–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger M, Eck P. (2018) Dietary vitamin C in human health. Advances in Food and Nutrition Research 83: 281–310. [DOI] [PubMed] [Google Scholar]
- Hallberg L. (1981) Bioavailability of dietary iron in man. Annual Review of Nutrition 1: 123–147. [DOI] [PubMed] [Google Scholar]
- Harris DS, Slot JW, Geuze HJ, et al. (1992) Polarized distribution of glucose transporter isoforms in Caco-2 cells. Proceedings of the National Academy of Sciences of the United States of America 89(16): 7556–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP, et al. (2009) Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacology, Biochemistry, and Behavior 93(4): 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck J, et al. (2000) Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. The Biochemical Journal 350(Pt 1): 149–154. https://pubmed.ncbi.nlm.nih.gov/10926838 [PMC free article] [PubMed] [Google Scholar]
- Hellman L, Burns JJ. (1958) Metabolism of L-ascorbic acid-1-C14 in man. The Journal of Biological Chemistry 230(2): 923–930. [PubMed] [Google Scholar]
- Helmeste DM, Tang SW. (1998) The role of calcium in the etiology of the affective disorders. Japanese Journal of Pharmacology 77(2): 107–116. [DOI] [PubMed] [Google Scholar]
- Hemilä H. (2017) Vitamin C and infections. Nutrients 9(4): 2020–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H, Carr A, Chalker E. (2021) Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: Reanalysis of the COVID A to Z randomized clinical trial. Frontiers in Immunology 12(May): 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H, Chalker E. (2020) Vitamin C as a possible therapy for COVID-19. Infection & Chemotherapy 52(2): 222–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelfarb J. (2007) Iron regulation. Journal of the American Society of Nephrology 18(2): 379–381. [DOI] [PubMed] [Google Scholar]
- Holford P, Carr AC, Jovic TH, et al. (2020) Vitamin C—an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients 12(12): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Lee SH, Moon JH, et al. (2013) SVCT-2 in breast cancer acts as an indicator for L-ascorbate treatment. Oncogene 32(12): 1508–1517. [DOI] [PubMed] [Google Scholar]
- Hope MJ, Bally MB, Mayer LD, et al. (1986) Generation of multilamellar and unilamellar phospholipid vesicles. Chemistry and Physics of Lipids 40(2): 89–107. [Google Scholar]
- Hughes RE, Maton SC. (1968) The passage of vitamin C across the erythrocyte membrane. British Journal of Haematology 14: 247–253. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, et al. (2004) AKT Participates in endothelial dysfunction in hypertension. Circulation 109(21): 2587–2593. [DOI] [PubMed] [Google Scholar]
- Jang KI, Lee HG. (2008) Stability of chitosan nanoparticles for L-ascorbic acid during heat treatment in aqueous solution. Journal of Agricultural and Food Chemistry 56(6): 1936–1941. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Martin LJ, Cai X. (1992) Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. Journal of the American College of Nutrition 11(2): 172–176. [PubMed] [Google Scholar]
- Kellie S, Al-Mansour Z. (2017) Overview of the immune system. Micro- and Nanotechnology in Vaccine Development 357: 63–81. [Google Scholar]
- Khan RM, Iqbal MP. (2006) Deficiency of vitamin C in South Asia. Pakistan Journal of Medical Sciences 22(3): 347–355. [Google Scholar]
- Kocot J, Luchowska-Kocot D, Kiełczykowska M, et al. (2017) Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 9(7): 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Kodama T, Murakami M, et al. (1994) Vitamin C infusion treatment enhances cortisol production of the adrenal via the pituitary ACTH route. In Vivo (Athens, Greece) 8(6): 1079–1085. [PubMed] [Google Scholar]
- Koizumi M, Kondo Y, Isaka A, et al. (2016) Vitamin C impacts anxiety-like behavior and stress-induced anorexia relative to social environment in SMP30/GNL knockout mice. Nutrition Research 36(12): 1379–1391. [DOI] [PubMed] [Google Scholar]
- Lane DJR, Chikhani S, Richardson V, et al. (2013) Transferrin iron uptake is stimulated by ascorbate via an intracellular reductive mechanism. Biochimica et Biophysica Acta 1833(6): 1527–1541. [DOI] [PubMed] [Google Scholar]
- Langlois P, Lamontagne F. (2019) Vitamin C for the critically ill: Is the evidence strong enough? Nutrition (Burbank, Los Angeles County, Calif.) 60: 185–190. [DOI] [PubMed] [Google Scholar]
- Levine M, Rumsey SC, Daruwala R, et al. (1999) Criteria and recommendations for vitamin C intake. JAMA 281(15): 1415–1423. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Mcdowell IF, Hawthorne AB. (1998) Vitamin C deficiency in Asian women. Nutrition (Burbank, Los Angeles County, Calif.) 14(2): 231–232. [DOI] [PubMed] [Google Scholar]
- Li D, Mehta JL. (2005) Oxidized LDL, a critical factor in atherogenesis. Cardiovascular Research 68(3): 353–354. [DOI] [PubMed] [Google Scholar]
- Li N, Zhao G, Wu W, et al. (2020) The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia: A randomized clinical trial. JAMA Network Open 3(11): e2023644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WJ, Johnson D, Jarvis SM. (2001) Vitamin C transport systems of mammalian cells. Molecular Membrane Biology 18(1): 87–95. [DOI] [PubMed] [Google Scholar]
- Liu B, Yuan B, Zhang L, et al. (2015) ROS/P38/p53/Puma signaling pathway is involved in emodin-induced apoptosis of human colorectal cancer cells. International Journal of Clinical and Experimental Medicine 8(9): 15413–15422. [PMC free article] [PubMed] [Google Scholar]
- Long CL, Maull KI, Krishnan RS, et al. (2003) Ascorbic acid dynamics in the seriously ill and injured. The Journal of Surgical Research 109(2): 144–148. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. (2011) The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry 16(4): 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo C, Wilson JX. (2000) Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. The Journal of Nutrition 130(1): 63–69. [DOI] [PubMed] [Google Scholar]
- Man Anh HM, Linh DM, Dung VM, et al. (2019) Evaluating dose- and time-dependent effects of vitamin C treatment on a Parkinson’s disease fly model. Parkinson’s Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massano J, Bhatia KP. (2012) Clinical approach to Parkinson’s disease: Features, diagnosis, and principles of management. Cold Spring Harbor Perspectives in Medicine 2(6): a008870–a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Harrison FE. (2013) Role of vitamin C in the function of the vascular endothelium. Antioxidants & Redox Signaling 19(17): 2068–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Qu ZC. (2011) Ascorbic acid prevents oxidant-induced increases in endothelial permeability. BioFactors (Oxford, England) 37(1): 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. (2004) A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews 28(3): 285–305. [DOI] [PubMed] [Google Scholar]
- Medeiros MS, Schumacher-Schuh A, Cardoso AM, et al. (2016) Iron and oxidative stress in Parkinson’s disease: An observational study of injury biomarkers. PLOS ONE 11(1): e0146129. 10.1371/journal.pone.0146129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors AJ, Nahrwold DL, Rose RC. (1977) Ascorbic acid flux across mucosal border of guinea pig and human ileum. The American Journal of Physiology 233(5): E374–E379. 10.1152/ajpendo.1977.233.5.E374 [DOI] [PubMed] [Google Scholar]
- Mesonero J, Mahraoui L, Matosin M, et al. (1994) Expression of the hexose transporters GLUT1-GLUT5 and SGLT1 in clones of Caco-2 cells. Biochemical Society Transactions 22(3): 681–684. [DOI] [PubMed] [Google Scholar]
- Michels AJ, Hagen TM, Frei B. (2013) Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annual Review of Nutrition 33: 45–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-grillo I. (1990) Letters to the editor. Australian & New Zealand Journal of Psychiatry 24(3): 291–312.2241710 [Google Scholar]
- Moores J. (2013) Vitamin C: a wound healing perspective. British Journal of Community Nursing, Suppl: S6, S8–11. 10.12968/bjcn.2013.18.sup12.s6 [DOI] [PubMed] [Google Scholar]
- Moser MA, Chun OK. (2016) Vitamin C and heart health: A review based on findings from epidemiologic studies. International Journal of Molecular Sciences 17(8): 1328. 10.3390/ijms17081328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrityunjaya M, Pavithra V, Neelam R, et al. (2020) Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Frontiers in Immunology 11(October): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscogiuri G, Barrea L, Savastano S, et al. (2020) Nutritional recommendations for COVID-19 quarantine. European Journal of Clinical Nutrition 74(6): 850–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Meyerhardt JA, Chan AT, et al. (2015) Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. Journal of the National Cancer Institute 107(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzielska E, Smaga I, Gawlik M, et al. (2016) Oxidative stress in neurodegenerative diseases. Molecular Neurobiology 53(6): 4094–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M, Fukuyama R, Minoshima S, et al. (1994) Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. The Journal of Biological Chemistry 269(18): 13685–13688. [PubMed] [Google Scholar]
- Nishikimi M, Yagi K. (1991) Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. The American Journal of Clinical Nutrition 54(6): 1203S–1208S. [DOI] [PubMed] [Google Scholar]
- Ohno S, Ohno Y, Suzuki N, et al. (2009) High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Research 29(3): 809–816. [PubMed] [Google Scholar]
- Padayatty SJ, Doppman JL, Chang R, et al. (2007) Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone. The American Journal of Clinical Nutrition 86(1): 145–149. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. (2016) Vitamin C: The known and the unknown and Goldilocks. Oral Diseases 22(6): 463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowska E, Szczepanska J, Blasiak J. (2019) Pro- and antioxidant effects of vitamin C in cancer in correspondence to its dietary and pharmacological concentrations. Oxidative Medicine and Cellular Longevity 2019: 7286737. 10.1155/2019/7286737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò D, Clark CM, Liun F, et al. (2002) Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Archives of Neurology 59(6): 972–976. [DOI] [PubMed] [Google Scholar]
- Praticò D, Sung S. (2004) Lipid peroxidation and oxidative imbalance: Early functional events in Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD 6(2): 171–175. [DOI] [PubMed] [Google Scholar]
- Pullar JM, Carr AC, Bozonet SM, et al. (2018) High Vitamin C status is associated with elevated mood in male tertiary students. Antioxidants (Basel, Switzerland) 7(7): 91. 10.3390/antiox7070091 [DOI] [PMC free article] [PubMed]
- Qiao H, May JM. (2008) Development of ascorbate transporters in brain cortical capillary endothelial cells in culture. Brain Research 1208: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga MJ, Carroll DW, Brown TM. (2014) Ascorbate- and zinc-responsive parkinsonism. The Annals of Pharmacotherapy 48(11): 1515–1520. [DOI] [PubMed] [Google Scholar]
- Rafieian-Kopaei M, Setorki M, Doudi M, et al. (2014) Atherosclerosis: Process, indicators, risk factors and new hopes. International Journal of Preventive Medicine 5(8): 927–946. [PMC free article] [PubMed] [Google Scholar]
- Roa FJ, Peña E, Gatica M, et al. (2020) Therapeutic use of vitamin C in cancer: Physiological considerations. Frontiers in Pharmacology 11(March): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa PB, Neis VB, Ribeiro CM, et al. (2016) Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacological Reports 68(5): 996–1001. [DOI] [PubMed] [Google Scholar]
- Rose RC. (1986) Ascorbic acid transport in mammalian kidney. The American Journal of Physiology 250(4 Pt 2): F627–F632. [DOI] [PubMed] [Google Scholar]
- Ross R. (1986) The pathogenesis of atherosclerosis – an update. The New England Journal of Medicine 314(8): 488–500. [DOI] [PubMed] [Google Scholar]
- Rowe S, Carr AC. (2020) Global vitamin C status and prevalence of deficiency: A cause for concern? Nutrients 12(7): 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey SC, Daruwala R, Al-Hasani H, et al. (2000) Dehydroascorbic acid transport by GLUT4 in Xenopus oocytes and isolated rat adipocytes. The Journal of Biological Chemistry 275(36): 28246–28253. [DOI] [PubMed] [Google Scholar]
- Salvayre R, Negre-Salvayre A, Camaré C. (2016) Oxidative theory of atherosclerosis and antioxidants. Biochimie 125: 281–296. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Kanofsky JD. (1993) Vitamin C in the treatment of schizophrenia. International Journal of Neuroscience 68(1–2): 67–71. [DOI] [PubMed] [Google Scholar]
- Savini I, Rossi A, Pierro C, et al. (2008) SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 34(3): 347–355. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Vaze O, D’Souza GGM, et al. (2011) Palmitoyl ascorbate-loaded polymeric micelles: Cancer cell targeting and cytotoxicity. Pharmaceutical Research 28(2): 301–308. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, Jenner P. (2017) Non-motor features of Parkinson disease. Nature Reviews. Neuroscience 18(7): 435–450. [DOI] [PubMed] [Google Scholar]
- Secor D, Li F, Ellis CG, et al. (2010) Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Medicine 36(11): 1928–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz G, Gebhardt S, Beck JF, et al. (1998) Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neuroscience Letters 244(1): 33–36. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. (1996) Antioxidant and redox regulation of gene transcription. The FASEB Journal 10(7): 709–720. [DOI] [PubMed] [Google Scholar]
- Senarath Yapa SC. (1992) Detection of subclinical ascorbate deficiency in early Parkinson’s disease. Public Health 106(5): 393–395. [DOI] [PubMed] [Google Scholar]
- Siow RC, Richards JP, Pedley KC, et al. (1999) Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arteriosclerosis, Thrombosis, and Vascular Biology 19(10): 2387–2394. [DOI] [PubMed] [Google Scholar]
- Sirover WD, Siddiqui AA, Benz RL. (2008) Beneficial hematologic effects of daily oral ascorbic acid therapy in ESRD patients with anemia and abnormal iron homeostasis: A preliminary study. Renal Failure 30(9): 884–889. [DOI] [PubMed] [Google Scholar]
- Skibola CF, Bracci PM, Halperin E, et al. (2008) Polymorphisms in the estrogen receptor 1 and vitamin C and matrix metalloproteinase gene families are associated with susceptibility to lymphoma. PLOS ONE 3(7): e2816–e2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson NR. (1974) Active transport of L ascorbic acid in the human ileum. Gastroenterology 67(5): 952–956. [PubMed] [Google Scholar]
- Sunil Kumar BV, Singh S, Verma R. (2017) Anticancer potential of dietary vitamin D and ascorbic acid: A review. Critical Reviews in Food Science and Nutrition 57(12): 2623–2635. [DOI] [PubMed] [Google Scholar]
- Sutradhar KB, Amin ML. (2014) Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnology 2014: 939378. [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, et al. (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Reviews Drug Discovery 4(2): 131–144. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Matsuda T, Miyagantani Y, et al. (2000) Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Archives of Surgery 135(3): 326–331. [DOI] [PubMed] [Google Scholar]
- The Financial Express. (2020) The role of vitamin C in fighting COVID-19 pandemic – the financial express. https://www.financialexpress.com/lifestyle/health/the-role-of-vitamin-c-in-fighting-covid-19-pandemic/2084387/
- Tyml K, Li F, Wilson JX. (2008) Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Critical Care Medicine 36(8): 2355–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat Shariatpanaahi M, Vahdat Shariatpanaahi Z, Moshtaaghi M, et al. (2007) The relationship between depression and serum ferritin level. European Journal of Clinical Nutrition 61(4): 532–535. 10.1038/sj.ejcn.1602542 [DOI] [PubMed]
- Vera JC, Rivas CI, Fischbarg J, et al. (1993) Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364(6432): 79–82. [DOI] [PubMed] [Google Scholar]
- Verkoelen CF, Verhulst A. (2007) Proposed mechanisms in renal tubular crystal retention. Kidney International 72(1): 13–18. [DOI] [PubMed] [Google Scholar]
- Vissers MC and Wilkie RP (2007) Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. Journal of Leukocyte Biology 81(5): 1236–1244. 10.1189/jlb.0806541 [DOI] [PubMed]
- Walia V, Garg C, Garg M. (2019) Nitrergic signaling modulation by ascorbic acid treatment is responsible for anxiolysis in mouse model of anxiety. Behavioural Brain Research 364(February): 85–98. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, et al. (2005) Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron 45(3): 389–403. [DOI] [PubMed] [Google Scholar]
- Washko PW, Wang Y, Levine M. (1993) Ascorbic acid recycling in human neutrophils. Journal of Biological Chemistry 268(21): 15531–15535. [PubMed] [Google Scholar]
- Weiss P, Murdoch DR. (2020) Clinical course and mortality risk of severe COVID-19. Lancet (London, England) 395(10229): 1014–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Rolles B, Rink L. (2020) The potential impact of zinc supplementation on COVID-19 pathogenesis. Frontiers in Immunology 11(July): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JX. (2005) Regulation of vitamin C transport. Annual Review of Nutrition 25: 105–125. [DOI] [PubMed] [Google Scholar]
- Wu F, Wilson JX, Tyml K. (2004) Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radical Biology and Medicine 37(8): 1282–1289. [DOI] [PubMed] [Google Scholar]
- Yamazaki E, Horikawa M, Fukushima R. (2011) Vitamin C supplementation in patients receiving peripheral parenteral nutrition after gastrointestinal surgery. Nutrition (Burbank, Los Angeles County, Calif.) 27(4): 435–439. [DOI] [PubMed] [Google Scholar]
- Zasowska-Nowak A, Nowak PJ, Ciałkowska-Rysz A. (2021) High-dose vitamin c in advanced-stage cancer patients. Nutrients 13(3): 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rao X, Li Y, et al. (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Annals of Intensive Care 11(1): 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-png-1-nah-10.1177_02601060221139628 for Unwinding the potentials of vitamin C in COVID-19 and other diseases: An updated review by Nikhil Mehta, Purvi Pokharna and Saritha R Shetty in Nutrition and Health