Abstract
Fluorine 18 (18F) fluorodeoxyglucose (FDG) PET/CT has shown promise for use in assessing treatment response in patients with bone-only or bone-dominant (BD) metastatic breast cancer (mBC). In this single-institution, prospective single-arm study of 23 women (median age, 59 years [range, 38–81 years]) with biopsy-proven estrogen receptor–positive bone-only or BD mBC about to begin new endocrine therapy between October 3, 2013, and August 3, 2018, the value of early 4-week 18F-FDG PET/CT in predicting progression-free survival (PFS) was evaluated. 18F-FDG PET/CT was performed at baseline, 4 weeks, and 12 weeks. Maximum standardized uptake value (SUVmax) and peak SUV (SUVpeak) were measured for up to five index lesions. The primary end point was PFS. Secondary end points were overall survival (OS) and time to skeletal-related events (tSREs). All end points were compared between responders (reduction of 30% or more in the sum of SUVmax for target lesions) and nonresponders at 4 weeks and 12 weeks. Percentage change from baseline in SUVmax at 4- and 12-week 18F-FDG PET/CT were highly correlated (r = 0.81). At the 4-week time point PET responders had numerically longer PFS (14.2 months vs 6.3 months; P = .53), OS (44.0 months vs 29.7 months; P = .47), and tSRE (27.4 months vs 25.2 months; P = .66) compared with nonresponders, suggesting the clinical utility of 4-week 18F-FDG PET/CT as an early predictor of treatment failure.
Keywords: Breast Cancer, Metastatic Breast Cancer, Bone-Dominant Metastatic Breast Cancer, FDG PET/CT, Estrogen-Receptor Positive Metastatic Breast Cancer
Supplemental material is available for this article.
Clinical trial registration no. NCT04316117
© RSNA, 2022
Keywords: Breast Cancer, Metastatic Breast Cancer, Bone-Dominant Metastatic Breast Cancer, FDG PET/CT, Estrogen-Receptor Positive Metastatic Breast Cancer
Summary
In women with estrogen receptor–positive bone-dominant metastatic breast cancer initiating a new line of endocrine therapy, early 4-week fluorine 18 fluorodeoxyglucose PET/CT was highly correlated with the standard of care 12-week PET/CT.
Key Points
■ Early 4-week fluorine 18 fluorodeoxyglucose (FDG) PET/CT was highly correlated with 12-week FDG PET/CT in terms of percentage changes in maximum standardized uptake values from baseline (r = 0.81).
■ PET responders had numerically longer progression-free survival (14.2 months vs 6.3 months; P = .53) and overall survival (44.0 months vs 29.7 months; P = .47) compared with PET nonresponders at the 4-week time point.
■ These data supported the inclusion of an early 4-week FDG PET/CT time point in the ongoing Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) EA1183 (FEATURE) clinical trial.
Introduction
Despite increasing therapeutic options, breast cancer remains the second leading cause of cancer-related death among women (1). Bone is the most common site of metastasis in breast cancer (2,3), particularly in patients with estrogen receptor (ER)–positive tumors (2). Bone metastases can greatly affect quality of life (4).
In patients with bone-dominant (BD) ER–positive metastatic breast cancer (mBC), endocrine therapy (ET) offers a targeted therapeutic approach with a favorable toxicity profile. However, monitoring therapeutic response remains a challenge. Bone scanning and CT are both confounded by the osteoblastic reaction to successful treatments (5). MRI also has challenges, including expense and length of acquisition time for whole-body scanning. Serum tumor markers, such as cancer antigen 15-3, have reliability concerns, poor sensitivity, and variable tumor antigen expression (6). Developing an objective test to evaluate response to therapy in patients with BD mBC is currently an unmet clinical need.
PET imaging with fluorine 18 (18F) fluorodeoxyglucose (FDG) holds promise in monitoring BD mBC. By imaging tumor glucose metabolism, 18F-FDG PET circumvents the deficiencies of using the osseous reaction to tumor as a proxy for tumor response. A prospective study in women with BD mBC examining 18F-FDG PET at baseline and 4 months after starting a new treatment (including chemotherapy and ET) demonstrated that changes in 18F-FDG uptake predicted time to progression and time to skeletal-related events (tSREs) (7). While novel PET radiotracers are under development, none have demonstrated utility for response assessment. In a 2018 study by Peterson et al (7), 18F-sodium fluoride (NaF) PET was not found to be a useful predictor of time to progression or tSRE; it has been noted that 18F-NaF incorporates into the bone matrix and not the tumor itself (8). In 2020, 18F-fluoroestradiol PET was granted U.S. Food and Drug Administration approval for the detection of ER–positive lesions as an adjunct to biopsy; however, it is not approved for use in assessing response to therapy (9).
In this study, we sought to evaluate the value of 18F-FDG PET/CT at baseline, 4 weeks, and 12 weeks in women with BD mBC starting a new ET. We examined the potential of such imaging at the 4-week time point to identify early nonresponders as a tool for guiding therapy. We hypothesized that 4-week 18F-FDG PET/CT results would correlate with 12-week results, and that both could predict progression-free survival (PFS).
Materials and Methods
Study Design and Participants
This single-center, prospective, Health Insurance Portability and Accountability Act-compliant cohort study enrolled adult patients 18 years and older with ER–positive mBC and biopsy-proven or imaging evidence of bone metastases, with the majority of disease burden in the bones (although other sites of disease were allowed), and who were starting a new line of ET (any ET and any line of therapy acceptable). Those who had previously undergone treatment with radiation and/or surgery for osseous metastases that would not leave an adequate number of sites to follow were excluded. Participants were enrolled at the University of Pennsylvania from October 3, 2013, through August 3, 2018. The primary exposure in this study was 18F-FDG PET/CT. Participants underwent baseline clinical 18F-FDG PET/CT prior to the start of new ET, 4-week (± 2 weeks) research 18F-FDG PET/CT, and 12-week (+4 weeks/-2 weeks) clinical PET. Participants continued clinical follow-up at approximately monthly intervals (4 weeks ± 2 weeks). Participants and referring physicians were blinded to the 4-week results. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki. Approval was granted by the University of Pennsylvania Institutional Review Board, and written informed consent was obtained from all included participants.
18F-FDG PET/CT Acquisition and Analysis
All 18F-FDG PET/CT imaging was performed using multiple PET/CT scanners at the Hospital of the University of Pennsylvania, an American College of Radiology–accredited facility, and following consensus recommendations (10) with regular cross-calibration to ensure uniformity and reproducibility between scanners. The mean ± standard deviation FDG injected activity was 562 MBq ± 7 (15.2 mCi ± 0.2) with an average uptake time of 61.5 minutes ± 7.8. The 4-week research scanning was performed using an Ingenuity TF scanner (Philips Healthcare), which is also used for clinical imaging. Reconstruction was performed using the list-mode iterative algorithm BLOB-OS-TF (three iterations, 22 subsets). Two subspecialty-trained nuclear radiology fellows conducted blinded review of all studies (K.E.K. and J.G.) under the supervision of a subspecialty-trained nuclear medicine physician with 3 years of experience (A.R.P.). Imaging was anonymized prior to interpretation; no clinical information or other imaging was available for reference. Maximum standardized uptake value (SUVmax) and peak SUV (SUVpeak) of up to five of the most FDG-avid obvious osseous metastases were identified as index lesions on the baseline PET/CT scan using MIM image-viewing software (MIM Encore; MIM Software). Imaging data of index lesions were then collected for subsequent scans. SUVpeak was defined as the maximum average SUV within a 1-cm3 spherical volume of interest in a search area encompassing the tumor. Criteria for quantitative response to treatment included the following baseline uptake thresholds: SUVpeak greater than 1.5× mean liver SUV in participants without hepatic metastases (7) or SUVpeak greater than 2× mean SUV of the blood pool for patients with hepatic metastasis (11). Participants were responders if the sum of their SUVmax or SUVpeak decreased by 30% or more between baseline and follow-up (7,11).
Study End Points
The primary end point was PFS, defined as the time from study enrollment to the date of progression at follow-up imaging (12) or death from any cause. Secondary end points included overall survival (OS) (time from the date of study enrollment until date of death from any cause) and tSRE (time from enrollment to radiation therapy for symptomatic bone metastasis, pathologic fracture, spinal cord compression, surgery to stabilize the skeleton, or hypercalcemia of malignancy). All end points were compared between responders (defined by modified PET Response Criteria in Solid Tumors [PERCIST] criteria as reduction of 30% or more in the sum of SUVmax for target lesions) and nonresponders at 4 weeks and 12 weeks.
Statistical Analysis
The trial was designed to have 80% power to detect a hazard ratio of 2.0 for PFS in early responders with a sample size of 85 participants and using a two-sided α of .05. Owing to low accrual secondary to insurance-limited approval for PET/CT, the plan was amended to conduct exploratory survival analyses. All hypothesis tests were two-sided and assumed a type I error rate of .05. Differences in Kaplan-Meier curves for PFS, OS, and tSRE between patients who responded to treatment and those who did not (at both 4 weeks and 12 weeks) were compared and quantitatively assessed using log-rank tests. Multiple testing corrections were not considered owing to the exploratory nature of this study. All analyses were conducted in the R statistical environment (R version 4.1.1; R Foundation for Statistical Computing).
Results
Study Participants
The target accrual was 85 participants; however, a decline in clinical referrals owing to barriers to insurance approval for 18F-FDG PET/CT during the study period limited accrual. Thus, the study enrolled 26 participants. Of these individuals, one withdrew, one did not exhibit uptake at baseline imaging, and one did not undergo protocol-specified scanning. Therefore, 23 participants who underwent all scanning and completed follow-up were evaluable. The median follow-up time was 13.5 months (range, 2.0–44.4 months). Participant clinical characteristics of the total study population as well as by status as a responder or nonresponder at 4-week imaging are presented in Table 1. All analyzed participants had index bone lesions (median of four; maximum of five lesions per participant included). The mean and median SUVmax at baseline were 9.1 and 7.5, respectively.
Table 1:
Participant Demographic and Clinical Characteristics at 4-week Fluorine 18 Fluorodeoxyglucose PET/CT
Correlation between 4-week and 12-week PET/CT
Representative participant cases are shown in Figure 1. We observed a high correlation between percentage change in average SUVmax per participant at 4 weeks and 12 weeks (r = 0.81 [95% CI: 0.61, 0.92]) (Fig 2). Only four of the 23 participants were classified differently at these two time points; three achieved a response at 12 weeks but not at 4 weeks, and one achieved a response at 4 weeks but not at 12 weeks. A similarly high correlation in SUVpeak percentage change was noted (r = 0.79 [95% CI: 0.56, 0.91]) (Fig E1 [supplement]).
Figure 1:
Differences in patient response to therapy on PET/CT scans. (A) Sagittal (top row) and axial (middle row) PET images and axial fused (bottom row) PET/CT images in a 63-year-old woman with multifocal spinal and pelvic osseous metastases. A representative left acetabular osseous metastasis (arrow) demonstrated a 31.2% decrease in maximum standardized uptake value (SUVmax) at 4 weeks and a 43.4% decrease in SUVmax at 12 weeks compared with baseline. This participant progressed in 12.4 months. (B) Sagittal (top row) and axial (middle row) PET images and axial CT and fused PET/CT images (bottom rows) in a 38-year-old woman with multifocal bone-only metastases show a representative right iliac osseous metastatic lesion (arrow), which demonstrated an overall 8% increase in SUVmax at 4 weeks and a 36% increase in SUVmax at 12 weeks. This participant progressed in 2.6 months.
Figure 2:
Percentage change in average maximum standardized uptake value (SUV Max) at 4 weeks versus 12 weeks. The dotted horizontal and vertical lines indicate the treatment response threshold.
Progression-Free Survival
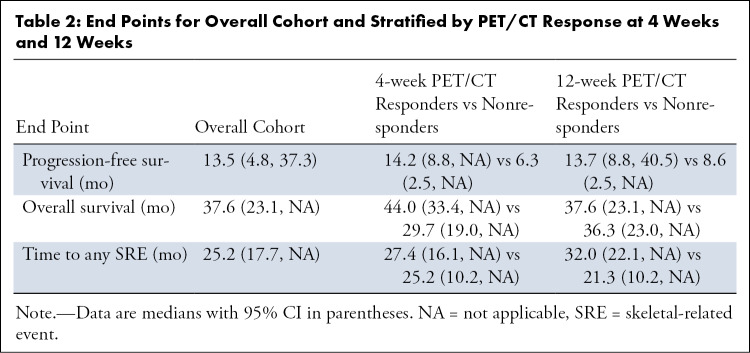
The median PFS for the cohort was 13.5 months (21 observed, two censored). At the 4-week time point, responders by PET/CT SUVmax had a numerically longer median PFS compared with nonresponders (14.2 months vs 6.3 months; P = .53). Results for the 12-week PET/CT were similar to those for the 4-week PET/CT, with participants classified as responders having a numerically longer median PFS than nonresponders (13.7 months vs 8.6 months; P = .69). Similar findings were observed using the parameter SUVpeak (Fig E2 [supplement]). Kaplan-Meier analysis of the 4-week assessment using the parameter SUVpeak did not reach statistical significance (log-rank P = .07) (Fig E3 [supplement]).
Overall Survival
The median OS for the cohort was 37.6 months (13 observed, 10 censored) (Table 2). At the 4-week PET/CT time point, responders had a numerically longer median OS compared with nonresponders (44.0 months vs 29.7 months; P = .47). When assessed at the 12-week PET/CT time point, the median OS between the responders and nonresponders was similar (37.6 months vs 36.3 months; P = .83).
Table 2:
End Points for Overall Cohort and Stratified by PET/CT Response at 4 Weeks and 12 Weeks
Time to Skeletal-related Events
Thirteen patients experienced SREs. The overall median tSRE was 25.2 months. The median tSRE was similar between responders and nonresponders at both 4-week PET/CT (27.4 months vs 25.2 months; P = .66) and 12-week PET/CT (32.0 months vs 21.3 months; P = .27) (Fig 3).
Figure 3:
Kaplan-Meier curves of (A) progression-free survival, (B) overall survival, and (C) time to skeletal-related event of patients in treatment response groups at 4-week (left) and 12-week (right) follow-up. Participants were identified as responders (red) if their average maximum standardized uptake value (SUVmax) decreased by 30% or more between baseline and follow-up. All others were considered nonresponders (teal). Censored participants are represented as vertical lines on the curves.
Discussion
In this study, we demonstrated the utility of FDG PET imaging in patients with BD mBC receiving ET, thus corroborating and extending the results of prior research in this population. The strong correlation between response at 4-week and 12-week FDG PET/CT (r = 0.81) as well as the numerically longer median PFS (14.2 months vs 6.3 months) and OS (44.0 months vs 29.7 months) for responders compared with nonresponders at 4 weeks suggests utility in imaging at this earlier time point, with obvious clinical implications.
Prior studies have explored the role of 18F-FDG PET/CT in the assessment of treatment response in patients with BD mBC (7,13,14). Notably, however, these studies examined response at 8–24 weeks. Based on prior pilot data (15), we hypothesized that imaging as early as 4 weeks might provide an indication of response to ET. The strong correlation observed between percentage changes in FDG uptake (by SUVmax and SUVpeak) at 4 weeks versus 12 weeks in our study corroborates this assertion. Peak uptake provides a robust measure of response in the PERCIST criteria (11), and it has been shown to be predictive of tSRE and time to progression in a similar population of patients with BD mBC (7). The results of our study suggest that the metabolic effects of ET occur sooner (4 weeks) than typical clinical imaging times (12 weeks). Early recognition and discontinuation of futile therapy could have direct patient benefits, including the avoidance of unnecessary toxicity (both symptomatic and financial) from futile therapies.
The main limitation of this study was lack of statistical power to detect significant differences in key clinical end points owing to failure to accrue the planned number of participants. Difficulties obtaining insurance approval of PET/CT for interval imaging in patients with mBC, which is reflective of real-world clinical practice, hampered recruitment. Additionally, our study had a median follow-up time of only 13.5 months, which limited the evaluation of the OS end point; however, although this follow-up time was sufficient for evaluating the primary end point of PFS, as well as the correlation between 4- and 12-week PET. Despite being underpowered, our results suggest an ability for FDG PET to serve as a surrogate predictive marker for PFS, with numerically longer PFS achieved in responders compared with nonresponders; these observations have been corroborated by Peterson et al (7) and Azad et al (14). The early PET time point in this study must be confirmed by further investigation. The data from this study have been used to support the inclusion of a 4-week time point in the ongoing EA1183 (FEATURE) Trial (ClinicalTrials.gov: NCT04316117).
In conclusion, changes in SUVmax and SUVpeak at 4-week PET/CT in patients with BD mBC initiating a new line of ET were highly correlated with changes at 12-week imaging. Responders at 4 weeks had numerically longer PFS and OS compared with nonresponders, which suggests that 4-week PET/CT may be a useful predictor of early response and merits further study in larger prospective trials.
I.M. and K.E.K. contributed equally to this work.
I.M. was partially funded by a National Cancer Institute grant: T32 CA009615-30; Hunger, Maillard (MPI); T32 Cancer Center Research Training Program; D.A.M. received partial support from a Susan G. Komen grant.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: I.M. Part of author's research time spent on coordinating analysis and writing the manuscript was funded by an NCI T32 grant: T32 CA009615-30; Hunger, Maillard (MPI); T32 Cancer Center Research Training Program. This grant lasted from July 1, 2019, to June 30, 2021. K.E.K. No relevant relationships. M.L.M. No relevant relationships. J.G. No relevant relationships. E.S. No relevant relationships. A.R.P. Consulting fees from GE Healthcare, Blue Earth Diagnostics, and Progenics; payment or honoraria for lectures, presentations, speakers bureaus from GE Healthcare and Blue Earth Diagnostics; support for attending meetings and/or travel from GE Healthcare and Blue Earth Diagnostics. D.A.M. Susan G. Komen grant provided partial support for this study; associate editor for Radiology: Imaging Cancer. A.S.C. No relevant relationships.
Abbreviations:
- BD
- bone-dominant
- ER
- estrogen receptor
- ET
- endocrine therapy
- FDG
- fluorodeoxyglucose
- mBC
- metastatic breast cancer
- OS
- overall survival
- PERCIST
- PET Response Criteria in Solid Tumors
- PFS
- progression-free survival
- SUVmax
- maximum standardized uptake value
- SUVpeak
- peak SUV
- tSRE
- time to skeletal-related event
References
- 1. Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020 . CA Cancer J Clin 2020. ; 70 ( 1 ): 7 – 30 . [DOI] [PubMed] [Google Scholar]
- 2. Coleman RE , Rubens RD . The clinical course of bone metastases from breast cancer . Br J Cancer 1987. ; 55 ( 1 ): 61 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen AØ , Jacobsen JB , Nørgaard M , Yong M , Fryzek JP , Sørensen HT . Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark . BMC Cancer 2011. ; 11 ( 1 ): 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yong M , Jensen AÖ , Jacobsen JB , Nørgaard M , Fryzek JP , Sørensen HT . Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007) . Breast Cancer Res Treat 2011. ; 129 ( 2 ): 495 – 503 . [DOI] [PubMed] [Google Scholar]
- 5. Hamaoka T , Madewell JE , Podoloff DA , Hortobagyi GN , Ueno NT . Bone imaging in metastatic breast cancer . J Clin Oncol 2004. ; 22 ( 14 ): 2942 – 2953 . [DOI] [PubMed] [Google Scholar]
- 6. Woolf DK , Padhani AR , Makris A . Assessing response to treatment of bone metastases from breast cancer: what should be the standard of care? Ann Oncol 2015. ; 26 ( 6 ): 1048 – 1057 . [DOI] [PubMed] [Google Scholar]
- 7. Peterson LM , O'Sullivan J , Wu QV , et al . Prospective Study of Serial 18F-FDG PET and 18F-Fluoride PET to Predict Time to Skeletal-Related Events, Time to Progression, and Survival in Patients with Bone-Dominant Metastatic Breast Cancer . J Nucl Med 2018. ; 59 ( 12 ): 1823 – 1830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czernin J , Satyamurthy N , Schiepers C . Molecular mechanisms of bone 18F-NaF deposition . J Nucl Med 2010. ; 51 ( 12 ): 1826 – 1829 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Brien SR , Edmonds CE , Katz D , Mankoff DA , Pantel AR . 18F-Fluoroestradiol (FES) PET/CT: review of current practice and future directions . Clin Transl Imaging 2022. ; 10 ( 4 ): 331 – 341 . [Google Scholar]
- 10. Shankar LK , Hoffman JM , Bacharach S , et al . Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials . J Nucl Med 2006. ; 47 ( 6 ): 1059 – 1066 . [PubMed] [Google Scholar]
- 11. Wahl RL , Jacene H , Kasamon Y , Lodge MA . From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors . J Nucl Med 2009. ; 50 ( Suppl 1 ): 122S – 150S . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhauer EA , Therasse P , Bogaerts J , et al . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) . Eur J Cancer 2009. ; 45 ( 2 ): 228 – 247 . [DOI] [PubMed] [Google Scholar]
- 13. Cook GJR , Goh V . Molecular Imaging of Bone Metastases and Their Response to Therapy . J Nucl Med 2020. ; 61 ( 6 ): 799 – 806 . [DOI] [PubMed] [Google Scholar]
- 14. Azad GK , Taylor BP , Green A , et al . Prediction of therapy response in bone-predominant metastatic breast cancer: comparison of [18F] fluorodeoxyglucose and [18F]-fluoride PET/CT with whole-body MRI with diffusion-weighted imaging . Eur J Nucl Med Mol Imaging 2019. ; 46 ( 4 ): 821 – 830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurland BF , Gadi VK , Specht JM , et al . Feasibility study of FDG PET as an indicator of early response to aromatase inhibitors and trastuzumab in a heterogeneous group of breast cancer patients . EJNMMI Res 2012. ; 2 ( 1 ): 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]