Abstract
Purpose
To evaluate the short-term safety of a nonmetallic twinkle marker and compare its conspicuity at color Doppler US with that of standard breast biopsy clips and radioactive seeds by using B-mode US in axillary lymph nodes.
Materials and Methods
This prospective study (November 2020–July 2021) of participants with node-positive breast cancer who completed chemotherapy involved placing a twinkle marker at the time of preoperative radioactive seed localization. A five-point scoring system (1 = easiest, 5 = most difficult) was used to rate the ease of identifying the clip, seed, and twinkle marker on postlocalization sonograms, mammograms, specimen radiographs, and gross pathologic specimens. Descriptive statistics were used.
Results
Eight women (mean age, 57 years ± 16 [SD]) were enrolled. The median scores for US conspicuity of each device were 3.9 (range, 3.7–5.0) for the radioactive seed, 2.4 (range, 1.0–5.0) for the clip, and 2.0 (range, 1.0–4.3) for the twinkle marker. In six of eight participants, the twinkle marker was the most identifiable at US. The seeds, clips, and twinkle markers were scored “very easy” to identify on seven of eight postlocalization mammograms. The surgeon retrieved all eight twinkle markers 1–3 days after localization. In all 16 interpretations, the seeds, clips, and twinkle markers were rated as very easy to identify on specimen radiographs. The clip was the most difficult device to identify at pathologic examination in all participants, and the twinkle marker was the easiest to identify in seven of eight participants.
Conclusion
This pilot study demonstrates that the safety and ease of US detection of a twinkling tissue marker may be comparable to a biopsy clip.
Keywords: Ultrasonography, US-Doppler, Breast, Localization, Surgery
Clinical trial registration no. NCT04674852
© RSNA, 2022
Keywords: Ultrasonography, US-Doppler, Breast, Localization, Surgery
Summary
A nonmetallic twinkle marker placed in the axillary lymph node of women with node-positive breast cancer was safe and identifiable at US and may be comparable to conventional biopsy clips.
Key Points
■ In a prospective, first-in-human clinical trial, a twinkle marker made of polymethyl methacrylate was safely placed and retrieved in all participants with node-positive breast cancer, without complications.
■ In six of eight participants, the twinkle marker was identified more easily at US than were a conventional biopsy clip and radioactive seed.
■ The twinkle marker was easily identified at postlocalization mammography, specimen radiography, and pathologic examination.
Introduction
Neoadjuvant systemic therapy has become more common in patients with lymph node–positive breast cancer, with complete response rates of 10%–70% based on the biologic subtype (1,2). Early attempts to perform a sentinel lymph node (SLN) biopsy alone and avoid a complete lymph node dissection following neoadjuvant chemotherapy (NAC) were associated with a false-negative rate (FNR) of greater than 10%; that is, the SLN is negative for cancer, but other axillary lymph nodes have cancer (3–5). A commonly performed method to decrease the FNR is to place a clip in the positive lymph node prior to initiating NAC and retrieve the clipped node at SLN surgery. In a large cooperative group trial, identifying the clipped lymph node reduced the FNR by 50%, from 14% to 7% (6). Caudle et al (7) coined the phrase targeted axillary dissection when targeting the clipped node for surgical removal. Targeted axillary dissection performed in conjunction with SLN surgery was reported to have an FNR of 2.4% in the Caudle et al series (7), and this method is incorporated into the National Comprehensive Cancer Network guidelines (8). Localization of the clipped node, using one of the currently available markers, is most commonly performed under US guidance before surgery.
Unfortunately, when the axillary lymph nodes normalize following NAC, they are often no longer ultrasonographically conspicuous. US detection of the current clips on the market is challenging, especially in a previously treated axilla that has had an excellent clinical response to systemic therapy, with the marker failing to be detected at US approximately 25% of the time (9,10).
There remains an unmet clinical need for simple, consistent conspicuity of the biopsy clip at US, particularly several months after placement. Our research team has developed a marker (PCT patent application no. US2020/051844, U.S. patent application no. 17/398 778) that takes advantage of a US phenomenon called twinkling artifact that occurs at color Doppler flow imaging, first described in 1996 by Rahmouni et al (11). The twinkle marker is made of biologically inert polymethyl methacrylate (PMMA), approved by the U.S. Food and Drug Administration for off-label use, and has an excellent patient safety profile. The precise cause of color Doppler twinkling remains elusive, but surface roughness and cavitation have been described as possible origins of the twinkling artifact (11–18).
The aims of this phase 0 first-in-human study were as follows: (a) to determine short-term (≤3 days) safety, (b) to determine US conspicuity of the twinkle marker following placement in an axillary lymph node, and (c) to compare the ease of identifying the twinkle marker with that of identifying conventional biopsy clips and the iodine-125 (125I) localization seed by the radiology and the pathology teams.
Materials and Methods
Study Participants
Following institutional review board approval (approval no. 20–002505), we conducted a prospective, crossover phase 0 clinical trial (ClinicalTrials.gov identifier no. NCT04674852) to evaluate the twinkle marker for US conspicuity at the time of preoperative US-guided 125I radioactive seed (Best Medical International) placement for surgical radio-guided localization. Participants provided signed informed consent prior to enrollment. The study was conducted from November 2020 to July 2021. Patients with breast cancer and biopsy-proven metastatic axillary lymph nodes who completed neoadjuvant systemic therapy were eligible if they had normalization of their lymph nodes and were scheduled for seed localization of the clipped node prior to SLN surgery.
Presurgical Procedure
At the time of preoperative radioactive seed localization with a 125I seed, the twinkle marker was manually loaded with the seed into a 15-gauge needle by using standard protocol (Fig 1). The sequence of which was placed first, the twinkle marker or the radioactive seed, was alternated to remove bias based on the order of deployment.
Figure 1:
Twinkling markers. (A) A vial of twinkling markers is next to a vial of nonradioactive seeds. (B) Several twinkling markers placed in US coupling gel with air bubbles are difficult to identify confidently, but the markers demonstrate a conspicuous twinkling signature at (C) color Doppler US.
Following localization of the clipped lymph node with the radioactive seed and twinkle marker, US of the axilla was performed. US conspicuity of the twinkle marker, radioactive seed, and previously placed biopsy clip were scored using a five-point Likert scale scoring system (Fig 2). This score considered the time required from looking at the image to identifying the device, which was captured in one of four 30-second increments on the data collection form by each observer, and a score of 5 was given if the device was not identified. The relative ease (from easiest to hardest) of detecting each device was also recorded. Standard-of-care postlocalization mammograms were acquired for documentation (19), and mammographic detectability of each device was also scored on a five-point scale.
Figure 2:
Data collection form. The five-point Likert scoring scale was used to assess ease of US detection of the seed, the previously placed clip, and the twinkle marker.
Postsurgical Procedure
Standard SLN biopsy and targeted axillary dissection were performed by a single surgeon. Following targeted axillary removal, a standard specimen radiograph was obtained to document removal of the clipped lymph node, as well as removal of the radioactive seed, clip, and twinkle marker. Mammographic conspicuity of the twinkle marker compared with the 125I seed and the previously placed metallic clip was reported using the same five-point scoring system.
Data Collection
Two radiologists placed the marker and completed data collection forms for all participants (C.U.L., cross-sectional imaging fellowship trained with 16 years of breast radiology experience; and G.K.H., cross-sectional imaging fellowship trained with 23 years of breast radiology experience). For US identification of the seed, clip, and twinkle marker, a breast US sonographer (Rebecca Higgins, RT, RDMS, with 27 years of experience as a breast sonographer) also completed data collection forms for all participants. Thus, there were 24 US interpretations for the eight twinkle markers placed. Finally, the ease of identifying all three devices (clip, seed, twinkle marker) was scored by the pathologic analysis technician, and the ease with which they were found was placed in order, with a score of 1 being easiest and 3 being hardest. All scores are reported in median and range, and descriptive statistics were used based on the sample size.
As part of our standard clinical practice, in addition to routine postoperative visits and multidisciplinary follow-up, all participants received a 30-day follow-up nursing phone call from a surgical clinic nurse to assess for any postoperative complications, including surgical site infection, hematoma, seroma, venous thrombotic event, return to the operative theatre, or hospital readmission.
Results
Participant Characteristics
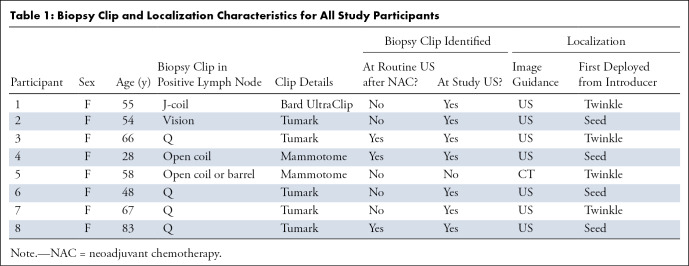
Eight women (mean age, 57 years ± 16 [SD]; range, 28–83 years) were enrolled in the study and included in this analysis. In five of eight (63%) participants, the clip was not seen at routine US after NAC. In one of the eight participants, the clip was not confidently identified at US at localization, and CT localization of the clip was performed (Table 1). In this case, US identification of the seed and twinkle marker could not be performed until after the CT localization was completed.
Table 1:
Biopsy Clip and Localization Characteristics for All Study Participants
Device Conspicuity at Presurgical Imaging
The median scores for ease of identifying the devices via US were 3.9 (range, 3.7–5.0) for the radioactive seed, 2.4 (range, 1.0–5.0) for the clip, and 2.0 (range, 1.0–4.3) for the twinkle marker, with a lower score representing easier identification (Table 2). In six of eight (75%) participants, the twinkle marker was the easiest of the three objects to identify by using US. Figure 3 and Table 3 demonstrate the 24 independent US interpretations on a five-point scale. There were two cases in which ease of identifying the twinkle marker was scored as 4 or 5. In one case, air from CT localization appeared to obscure US identification of the objects (Fig 4), and in the other case, excessive respiratory movement and inability to stay in position secondary to extreme restlessness and agitation complicated the procedure.
Table 2:
Scores for Ease or Difficulty of Visualization at US for All Objects

Figure 3:
Ease or difficulty of visualizing twinkle marker at US, based on independent US interpretations on a five-point Likert scale (see Figure 2 for scale) by three reviewers. The scores for each of the 24 interpretations and the median scores are shown. R1 = reviewer 1 (breast radiologist), R2 = reviewer 2 (breast radiologist), R3 = reviewer 3 (sonographer).
Table 3:
Ease or Difficulty of Finding Twinkle Marker at US

Figure 4:
Air affecting US conspicuity of the seed. (A) The tip of the introducer needle containing the seed and the marker is well-visualized (arrow). (B) As air is introduced at the target site during deployment of the seed and twinkle marker, the margin of the lymph node is not well identified. Moreover, neither the seed nor the twinkle marker are well-visualized at B-mode imaging. (C) At color Doppler US, the twinkle marker is easily identified by its twinkling signature.
All seeds, clips, and twinkle markers were very easy to identify on postplacement localization mammograms (Likert score of 1), except for one case in which multiple surgical clips obscured the field. In this participant, the twinkle marker was the easiest of the three objects to identify. Postlocalization mammography was not performed in one procedure, as the objects were easily confirmed during the CT localization procedure.
Device Conspicuity at Postsurgical Imaging and Pathologic Examination
The surgeon retrieved all eight twinkle markers during the SLN surgery and targeted axillary lymph node procedure. Surgery took place 1–3 days after localization. There were no postoperative 30-day complications. One patient with rheumatoid arthritis, taking methotrexate and prednisone, was admitted on postoperative day 18 for septic arthritis of the knee. This was felt to be unrelated to her breast and axillary procedure. One other patient had contact dermatitis from her surgical dressing, which resolved with conservative management.
Specimen radiographs were obtained and read by both radiologists, for a total of 16 specimen radiograph interpretations. In all 16 interpretations, the ease of visualization of the twinkle marker was scored as 1, considered very easy to visualize compared with background tissue (found in less than 10 seconds). In all participants, the seeds, clips, and twinkle markers were very easy to identify at specimen radiography. In one procedure, the clip was not retrieved and was thought to be lost in the surgical field or suction canister; thus the clip could not be evaluated on the specimen radiograph.
The clip was the most difficult device to identify with pathologic examination in all eight participants, and the twinkle marker was the easiest to identify in seven of eight (87.5%) participants. Specifically, the twinkle marker was rated a score of 1 (very easy, found in less than 1 minute) in seven of eight participants and rated a 2 (somewhat easy, found between 1 and 4 minutes) in one participant. On a scale of 1–5, with 4 being “very difficult, specimen radiograph required” and 5 being not identified, the median score the pathology team assigned to finding the twinkle marker, seed, and clip were 1.1, 1.3, and 2.1, respectively (one case with lost clip excluded). Figures 5 and 6 demonstrate an example of imaging for all objects across the spectrum of care.
Figure 5:
Imaging and visualization of devices across the spectrum of care in a 67-year-old woman with right breast invasive ductal carcinoma (estrogen receptor and progesterone receptor negative, HER2/neu positive) and (A) a metastatic right axillary lymph node (arrow). (B) A Tumark Q clip (arrow; Somatex Medical Technologies) is deployed into the positive node at the time of fine-needle aspiration (FNA) and (C) confirmed (arrow) on postclip mammogram on axillary tail view. During iodine-125 (125I) seed localization, neither the seed nor the twinkle marker are conspicuous at (D) B-mode US, but at (E) color Doppler US, the twinkle marker is evident (arrow). (F) Axillary tail view postlocalization mammogram shows successful localization of the Q clip (arrow), and surgical specimen shows the twinkle marker, the Q clip, and the 125I seed. NAC = neoadjuvant chemotherapy.
Figure 6:
Gross specimen. The seed, Turamark Q clip (Somatex Medical Technologies), and twinkling marker are identified in the specimen (arrows).
Discussion
We report a first-in-human study of a nonmetallic twinkle marker that was deployed during US localization of normalized axillary lymph nodes following NAC in participants with breast cancer and was retrieved by the surgeon during SLN biopsy and targeted axillary dissection in all participants. The twinkle marker was rated as the easiest of the three objects to identify in six of eight US procedures, rated as easy to identify (in less than 10 seconds) on all postlocalization mammograms and specimen radiographs, and rated the easiest of the three objects to identify by the pathology team on seven of eight cases.
PMMA is better known as bone cement and is used ubiquitously in orthopedic surgery. Placement in the human body in orthopedics is intended to be permanent and in larger volumes than our application. As a bone cement, or bone replacement, adhesion is its major function, and it is placed under substantial load-bearing conditions. The safety of PMMA primarily depends on its ability to adhere over long periods when put under substantial stress and motion compared with alternative options. Off-label use of PMMA as an imaging marker would have fewer performance concerns.
Despite there being more than 38 types of breast biopsy clips available in the United States (21), localization of normalized lymph nodes by using US after NAC remains a challenge in approximately 25% of cases (9). Localization with CT guidance or tomosynthesis is possible (21,22); however, this is an ionizing imaging technique and is generally less comfortable for the patient, particularly in the axilla. We have developed a marker that takes advantage of the twinkling phenomenon using Doppler color flow imaging and thus is intended to be more conspicuous at US. The average score for ease or difficulty of identifying the twinkle marker (2.0) was similar to the average score for identifying the clip (2.4), which the twinkle marker is meant to replace. In two participants, the twinkle marker was labeled as having a score of 4, meaning it required greater than 90 seconds to identify, or a 5, meaning it was not able to be seen. In one case, the seed and twinkle marker required CT guidance to place, and the air introduced at the time of CT guidance was felt to obscure US visibility of the twinkle marker. Under typical US localization of a clip in preparation for surgery, this air artifact, which creates a visual obstruction to the US waves, would not be in the field. In the other patient, her extreme respiratory motion and difficulty keeping her arm in position limited visualization of the field.
This study took place during the COVID-19 pandemic when patient visits were limited to as few as necessary. As a result, one of the limitations of our study design was that the postchemotherapy US search for the twinkle marker could only be undertaken on the same day that the seed or twinkle marker was deployed. Thus, the US search for the clip was prior to any manipulation of the axillary soft tissue, while attempts to identify the twinkle marker occurred after 15-gauge needle manipulation of the axilla, injecting local anesthesia, which introduced air in two cases. This likely introduced bias in the head-to-head comparison. As this was the first-in-human study, we found it necessary to follow our standard protocol, with clip placement at diagnosis, prior to chemotherapy, and radioactive seed placement after chemotherapy.
In conclusion, this pilot study established proof of principle that the twinkle marker, similar to a standard biopsy clip, is readily detected with US and can be safely placed in the axilla and retrieved at surgery. The twinkle marker is also easily identified on postlocalization mammograms and specimen radiographs and by the pathology team. Identification of foreign material at grossing of specimens at pathologic examination is most dependent on the size of the device. Rigidity of the material and color contrast between the device and natural tissue also influence ease of identification. We are moving forward with prospective clinical efficacy trials involving placement of the twinkle marker prior to neoadjuvant treatment to assess its US conspicuity after being in the human body for 3–6 months. This will also allow a head-to-head comparison of the clip versus seed prior to axillary manipulation.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Monica Kendall, MS, PA, for her efforts in organizing the pathologic analysis retrieval and assessments. The authors would like to acknowledge Rebecca Higgins, RT, RDMS, for her ultrasonographic assessments. The authors would like to acknowledge Sheri Ramaker and Jennifer Krogman for all their efforts with patient accrual.
Supported in part by funding from the Mayo Clinic Center for Individualized Medicine and the Mayo Clinic, Rochester, Department of Surgery.
Data Sharing: Data generated or analyzed during the study are available from the corresponding author upon request.
Disclosures of conflicts of interest: J.W.J. Royalties or licenses from Sorrento Therapeutics; patent co-inventor on a pending patent application for the Twinkling Marker (PCT patent application no. US2020/051844); member of the Melanoma Surgical Advisory Board for Novartis Oncology (May 2020); member of the Corporate Relations Committee for the American Society of Breast Surgeons (voluntary, unpaid position). G.K.H. Patent co-inventor on a pending application on the Twinkling Marker (U.S. patent Application No. 17/398 778). N.B.L. No relevant relationships. M.J.Y. Patent co-inventor on a pending patent application for the Twinkling Marker (PCT patent application no. US2020/051844). A.L.M. Patent co-inventor on a pending patent application for the Twinkling Marker (PCT patent application no. US2020/051844). J.F.G. Patent co-inventor on a pending patent application for the Twinkling Marker (PCT patent application no. US2020/051844); patent co-inventor on a pending application on the Twinkling Marker (U.S. patent Application No. 17/398 778). W.U. U.S. continuation-in-part patent application (17/398 778) filed August 10, 2021 (also incorporates 2019-414 and 2020-192); provisional patent application (62/903 078) filed on September 20, 2019; PCT patent application (PCT/US2020/051844), filed on September 21, 2020 (primary, with 2020-192 as secondary); U.S. patent application (17/762,018) filed on March 18, 2022; European patent application (20786193.1) filed on March 31, 2022; U.S. continuation-in-part patent application (17/398 778) filed on August 10, 2021 (also incorporates 2020-192 and 2021-198). C.U.L. Co–primary investigator on internal grant from the Mayo Clinic Center of Individualized Medicine Imaging Biomarkers Program, no conflicting relationships, activities, or employment with this funding source; patent co-inventor on a pending patent application on the Twinkling Marker (PCT patent Application No. US2020/051844); patent co-inventor on a pending application on the Twinkling Marker (U.S. patent Application No. 17/398 778).
Abbreviations:
- FNR
- false-negative rate
- NAC
- neoadjuvant chemotherapy
- PMMA
- polymethyl methacrylate
- SLN
- sentinel lymph node
References
- 1. Schmid P , Cortes J , Pusztai L , et al . Pembrolizumab for early triple-negative breast cancer . N Engl J Med 2020. ; 382 ( 9 ): 810 – 821 . [DOI] [PubMed] [Google Scholar]
- 2. Yau C , Osdoit M , van der Noordaa M , et al . Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients . Lancet Oncol 2022. ; 23 ( 1 ): 149 – 160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boileau JF , Poirier B , Basik M , et al . Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study . J Clin Oncol 2015. ; 33 ( 3 ): 258 – 264 . [DOI] [PubMed] [Google Scholar]
- 4. Boughey JC , Suman VJ , Mittendorf EA , et al . Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial . JAMA 2013. ; 310 ( 14 ): 1455 – 1461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuehn T , Bauerfeind I , Fehm T , et al . Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study . Lancet Oncol 2013. ; 14 ( 7 ): 609 – 618 . [DOI] [PubMed] [Google Scholar]
- 6. Boughey JC , Ballman KV , Le-Petross HT , et al . Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance) . Ann Surg 2016. ; 263 ( 4 ): 802 – 807 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caudle AS , Yang WT , Krishnamurthy S , et al . Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection . J Clin Oncol 2016. ; 34 ( 10 ): 1072 – 1078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Network. NCC . Breast Cancer 2022. .
- 9. Hyde B , Geske J , Lee C . Challenges to I-125 seed localization of metastatic axillary lymph nodes following neoadjuvant chemotherapy . J Breast Imaging 2019. ; 1 ( 3 ): 223 – 229 . [DOI] [PubMed] [Google Scholar]
- 10. Nguyen TT , Hieken TJ , Glazebrook KN , Boughey JC . Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges . Ann Surg Oncol 2017. ; 24 ( 10 ): 3011 – 3016 . [DOI] [PubMed] [Google Scholar]
- 11. Rahmouni A , Bargoin R , Herment A , Bargoin N , Vasile N . Color Doppler twinkling artifact in hyperechoic regions . Radiology 1996. ; 199 ( 1 ): 269 – 271 . [DOI] [PubMed] [Google Scholar]
- 12. Cunitz B , Dunmire B , Paun M , et al . Improved detection of kidney stones using an optimized Doppler imaging sequence . IEEE Int Ultrason Symp 2014. ; 2014 : 452 – 455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamzad A , Setarehdan SK . A novel approach for quantification and analysis of the color Doppler twinkling artifact with application in noninvasive surface roughness characterization: an in vitro phantom study . J Ultrasound Med 2014. ; 33 ( 4 ): 597 – 610 . [DOI] [PubMed] [Google Scholar]
- 14. Kamaya A , Tuthill T , Rubin JM . Twinkling artifact on color Doppler sonography: dependence on machine parameters and underlying cause . AJR Am J Roentgenol 2003. ; 180 ( 1 ): 215 – 222 . [DOI] [PubMed] [Google Scholar]
- 15. Khokhlova T , Li T , Sapozhnikov O , Hwang JH . The use of twinkling artifact of Doppler imaging to monitor cavitation in tissue during high intensity focused ultrasound therapy . Proc Meet Acoust 2013. ; 19 ( 1 ): 075034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rokni E , Zinck S , Simon JC . Evaluation of stone features that cause the color Doppler ultrasound twinkling artifact . Ultrasound Med Biol 2021. ; 47 ( 5 ): 1310 – 1318 . [DOI] [PubMed] [Google Scholar]
- 17. Shang M , Sun X , Liu Q , et al . Quantitative evaluation of the effects of urinary stone composition and size on color Doppler twinkling artifact: a phantom study . J Ultrasound Med 2017. ; 36 ( 4 ): 733 – 740 . [DOI] [PubMed] [Google Scholar]
- 18. Sorensen MD , Harper JD , Hsi RS , et al . B-mode ultrasound versus color Doppler twinkling artifact in detecting kidney stones . J Endourol 2013. ; 27 ( 2 ): 149 – 153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jakub J , Gray R . Starting a radioactive seed localization program . Ann Surg Oncol 2015. ; 22 ( 10 ): 3197 – 3202 . [DOI] [PubMed] [Google Scholar]
- 20. Portnow LH , Thornton CM , Milch HS , Mango VL , Morris EA , Saphier NB . Biopsy marker standardization: what’s in a name? AJR Am J Roentgenol 2019. ; 212 ( 6 ): 1400 – 1405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samreen N , Lee CU , Bhatt AA . Nonconventional options for tumor localization in breast and axillary lymph nodes: a pictorial how-to . J Clin Imaging Sci 2018. ; 8 : 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choudhery S , Simmons C , Harper L , Lee CU . Tomosynthesis-guided needle localization of breast and axillary lesions: our initial experience . AJR Am J Roentgenol 2019. ; 212 ( 4 ): 943 – 946 . [DOI] [PubMed] [Google Scholar]