Abstract
Burkholderia cenocepacia is an opportunistic pathogen isolated from cystic fibrosis patients where it causes infections that are extremely difficult to treat with antibiotics, and sometimes have a fatal outcome. Biofilm is a virulence trait of B. cenocepacia, and is associated with infection persistence and increased tolerance to antibiotics. In biofilms exopolysaccharides have an important role, conferring mechanical stability and antibiotic tolerance. Two different exopolysaccharides were isolated from B. cenocepacia H111 biofilms: a water-soluble polysaccharide rich in rhamnose and containing an L-Man residue, and a water-insoluble polymer made of glucose, galactose and mannose. In the present work, the product encoded by B. cenocepacia H111 bepA-L gene cluster was identified as the water-insoluble exopolysaccharide, using mutant strains and NMR spectroscopy of the purified polysaccharides. It was also demonstrated that the B. cenocepacia H111 wild type strain produces the water-insoluble exopolysaccharide in pellicles, thus underlining its potential importance in in vivo infections.
Keywords: Burkholderia cenocepacia; biofilm; bepA-L gene cluster, exopolysaccharide structure; NMR spectroscopy
Graphical Abstract
1. Introduction
The Burkholderia cepacia complex (Bcc) is a group of more than 20 closely related bacterial species (De Smet et al., 2015). Members of the Bcc complex have been isolated from a wide range of niches such as soil, plants, water, animals and humans (Coenye & Vandamme, 2003). In humans, Bcc bacteria have been associated with severe infections, especially in chronic granulomatous disease (CGD), cystic fibrosis (CF) and infections in immunocompromised patients (Speert, 2002; Mahenthiralingam, Baldwin & Dowson, 2008). Burkholderia cenocepacia and Burkholderia multivorans are the Bcc species most commonly isolated from CF patients (Mahenthiralingam, Baldwin & Dowson, 2008). The infections caused by these bacteria are difficult or impossible to treat with antibiotics, and may be fatal. Biofilm formation is a virulence trait of Bcc strains, and has been associated with the persistence of the infections and the increased tolerance to antibiotics (Caraher, Reynolds, Murphy, McClean, & Callaghan, 2007). In biofilms the bacteria are surrounded by an extracellular matrix where exopolysaccharides (Epols) are of importance for structural stability and antibiotic tolerance (Fazli, McCarthy, Givskov, Ryan & Tolker-Nielsen, 2013; Ciofu & Tolker-Nielsen, 2019; Colvin et al., 2011; Goltermann & Tolker-Nielsen, 2017). Knowledge about the chemical structure of these Epols might lead to the development of new anti-biofilm treatments.
B. cenocepacia H111 is a clinical isolate from a CF patient (Carlier et al., 2014) and is capable of producing different Epols, which were discovered either through purification and structural characterization procedures or through the presence of their gene cluster in the microorganism’s genome. The latter is the case for cellulose, whose gene clusters is well-known (Carlier et al., 2014; Römling & Galperin, 2015), while cepacian (Cescutti et al., 2000) was produced when the strains used in the present study were grown on yeast extract mannitol (YEM) solid medium which is known to stimulate its production (Sage, Linker, Evans & Lessie, 1990). Recently two other Epols were isolated from B. cenocepacia H111 biofilms and fully characterized: H111-SOL, a water-soluble polysaccharide rich in Rhamnose (Rha) residues and containing an L-Mannose (Man) residue (Bellich et al., 2021), and H111-INS, a water-insoluble polymer made of Glucose (Glc), Galactose (Gal) and Mannose (Man) (Bellich et al., 2020) (Fig. 1). In independent investigations, a novel BerA/c-di-GMP regulated exopolysaccharide gene cluster, named bepA-L, was discovered, and its regulation was thoroughly investigated (Fazli et al., 2011; Fazli et al., 2013; Fazli et al., 2017). It was suggested that the product encoded by the bepA-L gene cluster is a major exopolysaccharide that provides structural stability to B. cenocepacia biofilms, and that its production is regulated by c-di-GMP and the transcriptional regulator BerA (Fazli et al., 2011; Fazli et al., 2013). By comparing the gene functions of the bepA-L gene cluster (Fazli et al., 2013) with the known polysaccharides produced by Bcc bacteria (Cuzzi et al., 2014), it was clear that the cluster was responsible for the production of a yet undescribed polymer. We hypothesized that the H111-INS polysaccharide is the product of the bepA-L gene cluster, on the basis of two observations. A previous investigation (Fazli et al., 2013) showed that two B. cenocepacia H111 mutant strains carrying a transposon insertion in the bepA-L gene cluster were unable to produce pellicles. Moreover, the water insolubility of H111-INS Epol may be an essential characteristic for the formation of floating biofilms. In order to establish without doubt which is the product of the bepA-L gene cluster, polysaccharides extracted from pellicles formed by i) a strain over-expressing the transcriptional regulator BerA and ii) two mutant strains carrying a transposon insertion in the bepA-L gene cluster were subjected to chemical and 1D 1H NMR spectroscopy analyses. The product of the bepA-L gene cluster was then identified after comparison with data of the pure polysaccharides (Bellich et al., 2021; Bellich et al., 2020). Moreover, the type of Epols produced by the B. cenocepacia H111 wild type in pellicles were also elucidated. The knowledge about the chemical structure of Epols with an important function in biofilm of pathogenic bacteria might lead to the development of new anti-biofilm treatments.
Fig. 1. Structures of the repeating units of the exopolysaccharides produced by B. cenocepacia H111 isolate.

H111-INS (= Bep) (Bellich et al., 2020), H111-SOL (Bellich et al., 2021), cepacian (Cescutti et al., 2000) and cellulose. In circles: sugars and linkages exclusive of each exopolysaccharide which may be used as markers for each polysaccharide.
2. Materials and methods
2.1. Bacterial strains and pellicle production
Bacterial strains used in the present investigation were: i) B. cenocepacia H111 wild type (H111 WT), a clinical isolate from a cystic fibrosis patient (Huber et al., 2001), ii) the derived ΔbcsB/pBerA strain (Fazli et al., 2011) containing the plasmid pBcam1349 (pBerA), composed of pBBR1MCS2 with the berA gene inserted in the BamHI/XbaI sites and with a deletion of the gene bcal1389 (designated bcsB for Bacterial cellulose synthase subunit B), the first gene in the genetic cluster devoted to cellulose biosynthesis, iii) B. cenocepacia H111 wild type containing the empty plasmid pBBR1MCS2 (WT+pBBR1MCS2) used as control, iv) two mutant strains obtained by transposon insertion in two genes of the 12-gene cluster, gene A and C, and named respectively bepA/pBerA and bepC/pBerA (Fazli et al., 2013).
The three strains H111 WT, ΔbcsB/pBerA and WT+pBBR1MCS2 were grown in 5 mL of Luria Bertani (LB) broth, with kanamycin (50 μg/mL) when needed, for 16 h at 37 °C with shaking. Aliquots of 50 μL of this overnight culture were used to inoculate test tubes containing 5 mL of the same broth; growth was conducted in static conditions at 25 °C and 37 °C, both for 2 and 5 days. All strains formed biofilms as pellicles at the liquid-air interface; the best growth conditions for our purposes were 25 °C for 5 days.
2.2. Pellicles production for sugar analysis and High-Resolution Magic Angle Spinning NMR spectroscopy
Aliquots of 50 μL of an overnight ΔbcsB/pBerA culture were used to inoculate 16 test tubes containing 5 mL of LB broth with kanamycin (50 μg/mL), and growth was conducted in static conditions at 25°C. Four pellicles at each day 2, 3, 4, and 5 of the growth were collected separately, centrifuged at 8600xg for 5 min, washed thrice with water, and freeze-dried. One pellicle of each day of growth was hydrolysed with 2 M trifluoroacetic acid (TFA) for 1 h at 125°C, derivatized to alditol acetates, and subjected to gas liquid chromatography (GLC) analysis for the identification of neutral sugars (Albersheim, Nevins, English & Karr, 1967). The other pellicles were used for recording High-Resolution Magic Angle Spinning (HR-MAS) NMR spectra.
For HR-MAS NMR, whole pellicles collected at day 2, 3, 4, and 5 of the growth were washed thrice with water and freeze-dried. Approximately 50 μL of compact pellet was inserted into a Kel-F disposable insert for a 50 μL volume and subsequently in a 4 mm MAS ZrO2 rotor (Bruker). 10 μL of deuterium oxide (D2O, Sigma-Aldrich) was added. Proton HR-MAS NMR experiments were recorded by a Bruker Avance III 400 MHz spectrometer using a Bruker 4 mm HR-MAS probe. The spectra were recorded at 4500 Hz spin rate at 25°C. The 1H spectra were acquired by a diffusion filter pulse sequence with gradient pulses to remove the low-molecular-mass species free in solution. NMR spectra were collected with 32 k data points over a 10 ppm spectral width. The transmitter was set at the water resonance frequency, which was also used as the reference signal (4.79 ppm). The TopSpin 2.1 software package (Bruker) was used for data acquisition and processing of all spectra.
2.3. Bacterial growth on solid media and sample preparation for Epols structural characterization
Bacterial strains ΔbcsB/pBerA, bepA/pBerA and bepC/pBerA were spread from a −80 °C stock culture directly onto 4 agar plates each containing the nutrient-yeast extract-glycerol medium (NYG) (0.5% peptone, 0.3% yeast extract, 2% (w/v) glycerol, and 1.5% agar) added with kanamycin (100 μg/mL) and grown for 3 days at 37 °C and 2 days at 25 °C. Strain ΔbcsB/pBerA formed a compact wrinkled film which was peeled off each plate in one piece, gently washed in water followed by centrifugation at 1900xg at 10 °C for 30 min. Strains bepA/pBerA and bepC/pBerA produced a cell layer, with no biofilm characteristics; the layer was scraped off the plates with water and centrifuged at 1900xg at 10 °C for 30 min. The pellets were treated with 0.3 M NaOH for 3 h at 10 °C with shaking followed by centrifugation at 14500xg at room temperature for 15 min. The supernatants were dialysed and lyophilised. 15 mg of ΔbcsB/pBerA extract and 11 mg of bepA/pBerA and bepC/pBerA extracts were dissolved in 0.3 M NaOD and exchanged twice with D2O for recording NMR spectra. For composition analysis about 2 mg of each lyophilised supernatant were hydrolysed and derivatised to alditol acetates prior to GLC analysis.
2.4. Pellicles production by H111-WT and ΔbcsB/pBerA strains for Epols structural characterization
Aliquots of 50 μL of an overnight ΔbcsB/pBerA culture were used to inoculate 10 test tubes containing 5 mL of LB or NYG broth with kanamycin (50 μg/mL), and growth was conducted in static conditions at 25 °C for 5 days. Aliquots of 50 μL of an overnight H111 WT culture were used to inoculate 35 test tubes containing 5 mL of LB or NYG broth, and growth was conducted in static conditions at 25 °C for 5 days. Pellicles were removed very carefully with a Pasteur pipette, collected in one test tube and centrifuged at 3800xg at 10 °C for 10 min; afterwards the pellets were gently washed three times with 0.9 % NaCl, and recovered by centrifugation at 3800xg at 10 °C for 10 min. Pellicles were treated with 0.3 M NaOH (3 mL for ΔbcsB/pBerA and 2 mL for H111 WT) for 2 hours at 10 °C followed by centrifugation at 14550xg at room temperature for 10 min. The supernatant solutions were dialyzed to remove NaOH, recovered by lyophilisation and used for composition and linkage analysis. About 5 mg of each extract were dissolved in 0.3 M NaOD and exchanged twice with D2O for recording NMR spectra.
2.5. Composition and linkage analysis of polysaccharides
Native and permethylated polysaccharides were hydrolysed with 2 M TFA for 1 h at 125 °C. Alditol acetates were prepared as described previously (Albersheim, Nevins, English & Karr, 1967), and the linkage analysis, through derivatization to partially methylated alditol acetates, was performed following the protocol developed by Harris et al., (1984). Integration values of the peak areas of the alditol acetates chromatograms were used to estimate the molar ratios of the sugars, while for the partially methylated alditol acetates the integration values of the peak areas were corrected by the effective carbon response factors (Sweet, Shapiro & Albersheim, 1975). Analytical GLC was performed on a PerkinElmer Autosystem XL gas chromatograph equipped with a flame ionisation detector and using He as the carrier gas. An HP-1 capillary column (Agilent Technologies, 30 m × 0.32 mm × 0.25 μm) was used to separate alditol acetates (temperature program: 3 min at 150 °C, 150–270 °C at 3 °C/min, 2 min at 270 °C), partially methylated alditol acetates (temperature program: 1 min at 125 °C, 125–240 °C at 4 °C/min, 2 min at 240 °C). Gas liquid chromatography coupled to mass spectrometry (GLC-MS) analyses were carried out on an Agilent Technologies 7890 A gas chromatograph coupled to an Agilent Technologies 5975C VL MSD using the same columns and the temperature programs of the GLC analysis.
2.6. NMR spectroscopy
Samples were exchanged twice with 99.9% D2O by lyophilization and then dissolved in 0.6 mL of 99.96% D2O and introduced into a 5 mm NMR tube for data acquisition. 1H NMR spectra were recorded using a 500 MHz VARIAN spectrometer operating at 323 K. For spiking experiments, a known amount of H111-INS Epol was added directly into the NMR tube of the samples of interest (see Supplementary Data). Acetone (diluted 1:100 in D2O) was used as external reference in a coaxial tube and set at 2.225 ppm for 1H ppm.
2.7. Sequence analysis of the exopolysaccharide gene cluster
For the analysis of the eps cluster, we used the available genome sequence of B. cenocepacia H111 uploaded in NCBI database under the accession number GCA_000236215.4. Functional annotation of the genes of the cluster was performed with the tools for sequence analysis COGnitor, InterProScan, FigFam and other integrated in Microscope Platform (Vallenet et al., 2020). For the genes’ identities BLAST (http://www.ncbi.nlm.nih.gov/BLAST) was used. The glycosyltransferases families were determined by using CAZy database (http://www.cazy.org/). Comparative analyses were performed with EasyFig (Sullivan, Petty & Beatson et al., 2011). For comparison purposes the following strains with their GenBank accession number were used: B. cenocepacia K56–2 (LAUA01000014.1); B. cenocepacia VC7848 (CP019668.1); B. cenocepacia F01 (OEOG01000039.1); B. multivorans CGD1 (ACFB01000016.1), B. multivorans CGD2 (ACFC01000001.1) and B. multivorans CGDM2 (ACFD01000001.1).
3. Results
3.1. Production of pellicles and characterization of their Epols content
Initially, we characterised the Epols produced in pellicles formed by the B. cenocepacia H111 derived ΔbcsB/pBerA strain. The ΔbcsB mutation renders the bacteria incapable of producing cellulose, whereas the pBerA plasmid encodes a transcriptional activator of the bep genes (Fazli et al., 2011). The ΔbcsB/pBerA strain therefore overproduces the Burkholderia cenocepacia exopolysaccharide (Bep) but does not produce cellulose, which facilitates Epol purification. The B. cenocepacia H111 wild type (H111 WT), WT/pBBR1MCS2 and ΔbcsB/pBerA strains were grown in static mode at 25 °C for 2 to 5 days to check the conditions suitable for pellicle production. As shown in Fig. S1, all three strains produced biofilms as pellicles at the air-liquid interface after 5 days of growth. As expected, the thickest pellicle was produced by the strain ΔbcsB/pBerA, and hence growth for pellicle production and Epols structural analysis were first conducted using the ΔbcsB/pBerA strain.
Pellicles produced by the ΔbcsB/pBerA strain were collected at day 2, 3, 4, and 5 of growth at 25 °C, and the composition of neutral sugars in whole pellicles was determined by using gas liquid chromatography (GLC) after hydrolysis and derivatization to alditol acetates. Rha, Man, Glc, and Gal were found in all pellicles (Table S1); the relative ratios of Man and Gal stayed constant from day 2 to 5, whereas Rha and Glc decreased with increasing incubation time.
HR-MAS proton NMR spectra were recorded on the four pellicle samples recovered at different time points; a diffusion filter to cut off the small molecules in solution and highlight the components exposed on the surface of pellicles was applied. All HR-MAS proton NMR spectra showed the same type of resonances indicating that there were no substantial changes in the type of macromolecules exposed on the cell surface from day 2 to day 5 of growth. Comparison of the HR-MAS 1H NMR spectra of the pellicle samples with the 1H NMR spectra of H111-SOL Epol recorded in solution (Fig. S2) indicated that most of the 1H resonances, anomeric protons, (4.7–5.5 ppm), ring protons (4.4 – 3.4) and methyl rhamnose (~1.3 ppm), were also present in HR-MAS spectra, thus suggesting that polysaccharides were the major component of the pellicles’ matrices.
3.2. Characterization of Epols produced by the ΔbcsB/pBerA, bepA/pBerA and bepC/pBerA strains grown on solid media
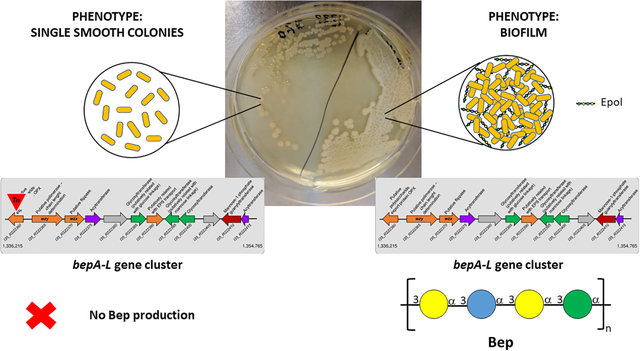
In order to identify the Epol produced by the bepA–L gene cluster proteins (Fazli et al., 2013), the strains ΔbcsB/pBerA, bepA/pBerA and bepC/pBerA were used and grown on solid NYG medium. The bepA and bepC mutations render the bacteria incapable of producing Bep (Fazli et al., 2013). The ΔbcsB/pBerA strain formed wrinkled colonies, while the strains bepA/pBerA and bepC/pBerA produced smooth colonies (Fig. S3). The phenotype of the ΔbcsB/pBerA strain is identical to the phenotype of a wild type B. cenocepacia H111/pBerA strain (data not shown); however, we used the cellulose deficient strain because it facilitated Epol purification. Solubilisation of the polysaccharides was achieved in 0.3 M NaOH. 1H NMR spectra were recorded in 0.3 M NaOD and were compared with those of purified H111-SOL and H111-INS polysaccharides in the same solvent. The anomeric regions of the spectra are reported in Fig. 2 where it can be seen that the ΔbcsB/pBerA strain biosynthesised both H111-SOL and H111-INS Epols, while the bep mutant strains produced only H111-SOL Epol. Since the resonances attributed to the spin systems of H111-INS Epol in the ΔbcsB/pBerA extract did not perfectly match the same signals of the purified H111-INS (Fig. 2A and 2E) due to very small differences in pH, the assignments were confirmed by spiking experiments using a solution of purified H111-INS Epol dissolved in 0.3 M NaOD and the spectra are reported in Fig. S4. Only a rough quantitation of the two exopolysaccharides in the biofilm was obtained by integrating the peaks at 5.25 (H1 of Gal of H111-INS) and 5.13 ppm (H1 of Rha of H111-SOL), since the resonances are very broad and some signals overlap. It was estimated that the bacteria biosynthesised about 83 % (w/w) of the water-insoluble polysaccharide and 17 % (w/w) of the water-soluble one. Monosaccharide composition was achieved by GLC analysis of the alditol acetates derivatives and the data obtained (Table 1) were in very good agreement with the NMR spectroscopy results. In fact, the Epols produced by all three strains contained the same type of sugars, but the ΔbcsB/pBerA extract had Gal, Glc and Man in the molar ratio expected for the H111-INS Epol. On the contrary, the main component of bepA/pBerA and bepC/pBerA Epols was Rha, while only minor amounts of Gal and Glc were detected. These low quantities of sugars can derive from other Epols present in low concentrations, since it is known that B. cenocepacia H111 is able to produce many different polysaccharides (Fig. 1). Therefore, the results obtained showed that H111-INS Epol is not produced by the two bep mutant strains, and thus prove that the bepA-L gene cluster is responsible for the production of the H111-INS Epol, which was therefore renamed Bep for Burkholderia cenocepacia exopolysaccharide. Moreover, as discovered previously (Fazli et al., 2013), Bep is responsible for the wrinkly phenotype and it is necessary for the formation of biofilm under continuous flow conditions and pellicles at the air-liquid interface.
Fig. 2. Identification of Epols produced by ΔbcsB/pBerA, bepA/pBerA and bepC/pBerA strains by means of 1H NMR spectroscopy.

Anomeric regions of the 1H NMR spectra of Epols produced by ΔbcsB/pBerA (A), bepA/pBerA (B), and bepC/pBerA (C) strains compared with those of H111-SOL (D) and H111-INS (E) polysaccharides. H1 of the different monosaccharides are indicated; in (A) and (B) H1 resonances belonging to H111-SOL are marked with a black diamond (◆), those belonging to H111-INS with a black circle (●).
Table 1. Composition analysis of the Epols extracted from ΔbcsB/pBerA, bepA/pBerA and bepC/pBerA grown on solid medium.
Quantitation is reported as peaks area ratio relative to Man.
| Monosaccharide | ΔbcsB/pBerA | bepA/pBerA | bepC/pBerA |
|---|---|---|---|
| Rha | 0.6 | 4.2 | 5.2 |
| Man | 1.0 | 1.0 | 1.0 |
| Glc | 1.1 | 0.5 | 0.5 |
| Gal | 2.1 | 0.2 | 0.1 |
3.3. Characterization of Epols in B. cenocepacia H111 wild type strain pellicles
After having demonstrated that the final product of the bepA-L gene cluster is the water-insoluble H111-INS Epol, we wanted to verify if this polymer is also produced by the B. cenocepacia H111 wild type (H111 WT) strain. When grown on solid NYG medium, the H111 WT strain produced a very mucoid cell layer, which is due to cepacian biosynthesis stimulated by glycerol in the NYG medium (Lagatolla et al., 2002). Cepacian gives rise to very viscous water solutions (Sist et al., 2003), making purification of the co-produced polysaccharides mostly unsuccessful. Furthermore, cepacian viscous solutions result in band broadening in NMR spectra and render chemical derivatisation more difficult. Since cepacian is abundantly produced on solid media, Epols produced by the H111 WT strain were extracted from pellicles obtained in LB and NYG liquid media and compared with those produced by the ΔbcsB/pBerA strain in the same growth conditions. Purification of the Epols from the pellicles was kept to a minimum in order to avoid the loss of saccharidic components. 1H NMR spectra were recorded in 0.3 M NaOD, and the anomeric regions of the spectra were compared with those of the purified H111-SOL Epol and Bep recorded in the same experimental conditions (Fig. 3 and 4). The resonances attributed to the spin systems of Bep in the ΔbcsB/pBerA extracts did not perfectly match the same signals of the purified Bep (Fig. 3A and 3D). Therefore, the assignments were confirmed by spiking experiments with a solution of purified Bep dissolved in 0.3 M NaOD and the spectra are reported in Fig. S5. It was concluded that when grown in LB and NYG liquid media, the ΔbcsB/pBerA strain biosynthesised both H111-SOL Epol and Bep, but in different molar ratios (Fig. 3). Due to signals overlapping and broadening, only an approximate estimation of the two polymers was obtained by integrating the peaks at 5.25 (H1 of Gal of H111-INS) and 5.13 ppm (H1 of Rha of H111-SOL). The data showed that in these conditions, the ΔbcsB/pBerA strain produced about 83 % (w/w) and 20 % (w/w) of Bep in NYG and LB media, respectively; the amounts of H111-SOL polymer were estimated 17 % (w/w) and 80 % (w/w) in NYG and LB media, respectively. Pellicles produced by the H111 WT strain were thinner and more fragile than those of the ΔbcsB/pBerA strain (Fig. S1). 1H NMR spectroscopy (Fig. 4) showed that in both media the most abundant Epol was H111-SOL, and after spiking experiments (Fig. S6) it was evident that Bep was produced in both media, although in LB medium it was detected in very small amounts. A quantitative estimate of the two polysaccharides was possible only for the sample obtained in NYG medium, and it was obtained upon integration of NMR peaks as described above: the data showed that the B. cenocepacia H111 WT pellicles contained about 24 % (w/w) and 76 % (w/w) of Bep and H111-SOL Epol, respectively. These quantitative data indicated that nutrients, and thus environment, have a strong influence on Epols production.
Fig. 3. Identification of Epols in pellicles produced by ΔbcsB/pBerA strain by means of 1H NMR spectroscopy.

1H NMR spectra anomeric regions of ΔbcsB/pBerA pellicles extracts produced in NYG (A) and LB (B) media compared with the purified H111-SOL (C) and H111-INS (D) polymers. Spectra were recorded in 0.3 M NaOD at 50 °C and at 500 MHz. In (C) and (D) H1 of the different monosaccharides are indicated; in (A) and (B) H1 resonances belonging to H111-SOL are marked with a black diamond (◆), those belonging to H111-INS with a black circle (●).
Fig. 4. Identification of Epols produced in pellicles by B. cenocepacia H111 wild type strain by means of 1H NMR spectroscopy.

1H NMR spectra anomeric regions of H111 WT pellicles extract produced in NYG (A) and LB (B) media compared with the purified H111-SOL (C) and H111-INS (D) polymers. Spectra were recorded in 0.3 M NaOD at 50 °C and at 500 MHz. In (C) and (D) H1 of the different monosaccharides are indicated; in (A) and (B) H1 resonances belonging to H111-SOL are marked with a black diamond (◆), those belonging to H111-INS with a black circle (●).
Samples extracted from the H111 WT and ΔbcsB/pBerA pellicles formed in LB and NYG media were subjected to neutral sugars composition and linkage analyses, and the data are reported in Tables 2 and 3, respectively. Both composition and linkage analyses are in very good agreement with the results obtained with NMR spectroscopy, confirming that the ΔbcsB/pBerA strain produced both Epols in both media, while the H111 WT strain biosynthesised both Epols only in NYG medium. The quantification of the different Epols was not feasible, because some of the sugars are components of other polysaccharides; for example, 3-linked Glc and 2-linked Rha are also found in cepacian, besides Bep and H111-SOL. Due to the important properties attributed to Bep in biofilm formation, it is very relevant that the B. cenocepacia H111 wild type strain, a cystic fibrosis pathogen, is capable of producing it.
Table 2. Composition in neutral sugars of the Epols extracted from ΔbcsB/pBerA and H111 WT pellicles produced in LB and NYG media.
Quantitation is reported as peaks area ratio relative to Man.
| Monosaccharide | ΔbcsB/pBerA | H111 WT | ||
|---|---|---|---|---|
| LB | NYG | LB | NYG | |
| Rha | 4.0 | 1.3 | 5.3 | 4.4 |
| Man | 1.0 | 1.0 | 1.0 | 1.0 |
| Glc | 0.6 | 1.1 | 0.5 | 1.0 |
| Gal | 0.6 | 2.1 | 0.1 | 1.1 |
Table 3. Linkage analysis of neutral sugars of the Epols extracted from ΔbcsB/pBerA and H111 WT pellicles produced in LB and NYG media.
Integration values of the peak areas were corrected by the effective carbon response factors (Sweet, Shapiro & Albersheim, 1975). Quantitation is reported as Molar Ratios Relative to 3-Man. Numbers next to sugars indicate the position of glycosidic linkages; t-Hex indicates terminal non-reducing hexose.
| sugar | ΔbcsB/pBerA | H111 WT | ||
|---|---|---|---|---|
| LB | NYG | LB | NYG | |
| 2-Rha | 2.0 | 1.0 | 2.0 | 1.9 |
| 3-Rha | 1.6 | 0.6 | 1.4 | 1.3 |
| t-Glc | - | - | - | 0.1 |
| t-Man | - | 0.6 | - | 0.3 |
| t-Gal | - | 1.1 | - | 0.4 |
| 3-Glc | 0.9 | 1.4 | - | 1.0 |
| 3-Man | 1.0 | 1.0 | 1.0 | 1.0 |
| 3-Gal | 0.6 | 1.5 | - | 0.6 |
| 3,6-Man | - | 0.1 | - | - |
3.4. Determination of the gene functions in the Bep gene cluster
The putative Bep gene cluster of B. cenocepacia H111 responsible for the production and export of the water-insoluble Bep is located in Chromosome 2 (Accession number: NZ_HG938371.1) in the region between positions 1,336,215 to 1,354,765 (Fig. 5A). The gene cluster has a size of 18.5 Kb, and it consists of 12 genes, seven of them in forward position and five of them in the reverse strand (Fig. 5A).
Fig. 5. Organization of the bepA_L gene cluster in different B. cenocepacia isolates.

A) bep gene cluster of B. cenocepacia strain H111 responsible for the production and exportation of the insoluble polysaccharide. B) Comparison with the exopolysaccharide gene cluster of B. cenocepacia J2315 (Accession number: GCA_000009485.1. C) Comparison with other exopolysaccharide clusters found in B. cenocepacia strains: K56–2 (GCA_014357995.1), VC7848 (GCA_001999785.1) and F01 (GCA_900240025.1).
The genes in the cluster encode proteins with characteristic functions involved in polysaccharide biosynthesis, including precursors’ synthesis, polymerization, and transport. Regarding precursors’ synthesis, there is only one gene in the cluster, bepK (I35_RS22410), which encodes mannose 1-P-guanylyltransferase. The other genes necessary for mannose activation, namely glucose 6-phosphate isomerase (I35_RS09535), mannose 6-phosphate isomerase (I35_RS25055) and phosphoglucomutase (I35_RS03815), are located outside the cluster (Fig. S7). The same holds true for the genes required for glucokinase (I35_RS04130), and UTP-glucose 1-P uridylyltransferase (I35_RS06685; I35_RS21125) synthesis, as well as galactose activation (UDP-Glucose 4-Epimerase I35_RS23110; I35_RS25750; I35_RS03795; I35_RS03775) (Fig. S7).
According to CAZy (http://www.cazy.org/), the three glycosyltransferases (GT) in the Bep gene cluster (I35_RS22385, I35_RS22395 and I35_RS22400) belong to GT family 4, which are characterized by a retaining mechanism, in perfect agreement with the α-anomeric configuration of the monosaccharide residues of Bep (Fig. 1). In silico analysis suggests that the gene I35_RS22385 encodes a soluble GT located in the cytoplasm and belonging to the GTB-type superfamily, characterized by using UDP-glucose as substrate; therefore, it was putatively assigned to binding α-d-Glc to C3 of α-d-Gal (α-d-Glcp-(1→3)-α-d-Galp). According to the KEGG database, the protein encoded by the gene I35_RS22395 is involved in binding a mannose residue to position C3 of another sugar; therefore, it is likely involved in the addition of α-d-Man to C3 of α-d-Gal to form α-d-Manp-(1→3)-α-d-Galp. The priming GT was not detected in the cluster, but there are priming GTs elsewhere in the genome. Indeed, downstream of the bep gene cluster, close to the gene berA (I35_RS22455), which encodes the associated transcriptional activator, the gene I35_RS22460 encodes the Undecaprenyl pyrophosphatase: it belongs to pfam02397 and is characterized by transferring galactose to an undecaprenylpyrophosphate (UndPP) molecule (Table 4). The remaining GT (I35_RS22400) could only be tentatively assigned to transferring α-d-Gal on C3 of either α-d-Man or α-d-Glc to form α-d-Galp-(1→3)-α-d-Manp or α-d-Galp-(1→3)-α-d-Glcp, depending on the order of GT-catalysed reactions taking place.
Table 4.
List of genes of B. cenocepacia H111 involved in production, exportation and regulation of the water-insoluble polysaccharide Bep.
| Gene name | Product name | Length nt (aa) | Characterizationd | Locatione | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| BCAM1330 | bepA | EPS I polysaccharide export outer membrane protein EpsA Putative polysaccharide export protein OPX |
1197 (398) |
COG1596 Periplasmic protein involved in polysaccharide export FIG142914 Capsule polysaccharide export protein IPR019554 Soluble_ligand-bd |
Outer Membrane 1 TMH |
|
| BCAM1331 | bepB | Putative tyrosine-protein kinase (with a highly conserved GNVR sequence motif which characterizes this domain) with LPS length determination domain (N terminal) |
2316 (771) |
COG3206 Uncharacterized protein involved in exopolysaccharide biosynthesis FIG138418 Tyrosine-protein kinase Wzc IPR032807 Tyrosine kinase, G-rich domain |
Cytoplasmic Membrane 2 TMH |
|
| BCAM1332 | bepC | Putative membrane protein Putative conserved membrane protein (With permease function - Putative Flippase) |
1425 (474) | COG0477 Permeases of the major facilitator superfamily | Cytoplasmic Membrane 12 TMH |
|
| BCAM1333 | bepD | Putative exopolysaccharide acyltransferase | 1146 (381) |
COG1835 Predicted acyltransferases FIG049994 O-acyltransferase IPR002656 Acyl_transf_3 |
Cytoplasmic Membrane 10 TMH |
|
| BCAM1334 | bepE | Hydrolases of the alpha/beta superfamily | 1812 (603) |
COG1073 Hydrolases of the alpha/beta superfamily FIG037863 Hydrolases of the alpha/beta superfamily IPR029058 Alpha/Beta hydrolase fold |
Unknown | |
| BCAM1335 | bepF | Glycosyltransferase Putatively involved in glucose linkage |
1191 (396) |
COG0438 Glycosyltransferase FIG031322 Glycosyltransferase CAZy_fam GT4 |
Cytoplasmic | |
| BCAM1336 | bepG | Putative exopolysaccharide transporter | 1254 (417) |
COG2244 Membrane protein involved in the export of O-antigen and teichoic acid FIG138592 Membrane protein involved in the export of O-antigen, teichoic acid lipoteichoic acids IPR002797 Polysacc_synth |
Cytoplasmic Membrane 10 TMH |
|
| BCAM1337 | bepH | Glycosyltransferase Putatively involved in mannose linkage |
1089 (362) |
COG0438 Glycosyltransferase FIG102981 Glycosyltransferase IPR001296 Glycosyl transferase, family 1 CAZy_fam GT4 |
Cytoplasmic | |
| BCAM1338 | bepI | Glycosyltransferase | 1155 (384) |
COG0438 – Glycosyltransferase FIG079791 IPR028098 - Glyco_transf_4; Glycosyltransferase subfamily 4-like, N-terminal domain CAZy_fam GT4 |
Unknown | |
| BCAM1339 | bepJ | Thioredoxin reductase | 1446 (481) |
FIG018396 - Thioredoxin reductase IPR039448 - GH87 |
Unknown | |
| BCAM1340 | bepK | Mannose-1-phosphate guanylyltransferase | 1521 (506) |
UR000000909 - Belongs to the mannose-6-phosphate isomerase type 2 family COG0836 - Mannose-1-phosphate guanylyltransferase COG0662 - Mannose-6-phosphate isomerase FIG118535 - Mannose-1-phosphate guanylyltransferase (GDP) IPR029044 -Nucleotide-diphosphosugar trans |
Cytoplasmic | |
| BCAM1341 | bepL | Acyltransferase | 660 (219) |
COG2153 – acetyltransferase FIG019119 - Acetyltransferase IPR016181 -Acyl_CoA acyltransferase |
Cytoplasmic | |
| BCAM1349 | berA | cAMP-binding proteins-catabolite gene activator and regulatory subunit of cAMP-dependent protein kinases | 720 (259) |
COG0664 - transcriptional regulator, crp fnr family FIG003090 cAMP-binding proteins - catabolite gene activator and regulatory subunit IPR036390 Winged helix DNA-binding domain superfamily; IPR018490 Cyclic nucleotide binding-like; IPR012318 Crp-type HTH domain |
Cytoplasmic | |
| Undecaprenyl-phosphate galactose-phosphotransferase | 1416 (471) |
COG2148 Sugar transferases involved in lipopolysaccharide synthesis FIG007053 Sugar transferases involved in lipopolysaccharide synthesis IPR036291 NAD(P)-bd_dom_sf IPR017475 Exopolysaccharide biosynthesis polyprenyl glycosylphosphotransferase IPR017473 Undecaprenyl-phosphate glucose phosphotransferase, Wca |
Cytoplasmic Membrane 10 TMH |
|||
Gene name according to the
locus tag of GeneBank, and as previously described in references
Characterization according to COGnitor, FigFam and InterProScan
For membrane proteins, the number of TMH (transmembrane helix) domains is indicated
The export system consists of the I35_RS22365 gene that putatively encodes the Wzz protein, a tyrosine kinase with a highly conserved GNVR sequence motif and a lipopolysaccharide length determination domain in the N terminal, characterized by two transmembrane domains; the I35_RS22370 gene that encode a cytoplasmic membrane protein of 12 transmembrane helical (TMH) domains, which putatively corresponds to a flippase (Wzx), and the I35_RS22390 gene that putatively encodes Wzy, the polymerase/chain length determination protein with 10 TMHs domains (Table 4). There is also a polysaccharide export protein OPX in the outer membrane (I35_RS22360). According to these characteristics, the export system putatively belongs to the Wzx/Wzy-dependent pathway (Table 4).
Bioinformatic analysis showed that the Bep gene cluster is not only homologous to that of B. cenocepacia strain J2315, but also to those of other B. cenocepacia strains (Fig. 5B, 5C), as well as of B. multivorans CGD2 (Fig. S8) among others.
4. Discussion
The capacity to form biofilm is recognised as a virulence factor in pathogenic bacteria, because in biofilms, the defences of the host immune system and antimicrobial drugs are unsuccessful in reaching and killing their targets. B. cenocepacia strains are responsible for causing chronic pneumonia in CF and CGD patients, sometimes resulting in fatal outcome. Therefore, many investigations have been devoted to the understanding of the mechanisms involved in biofilm formation by this organism. In this context, the bepA-L gene cluster was described as encoding proteins for the biosynthesis of a polysaccharide that previously was found to be very important for the stability of biofilms grown in flow chambers (Fazli et al., 2013). Moreover, investigations of the molecular mechanisms involved in biofilm formation in B. cenocepacia H111 showed that the process is controlled by four players, c-di-GMP, the transcriptional activators BerB and BerA, and the alternative sigma factor RpoN (σ54), which together regulate the production of the biofilm-stabilizing exopolysaccharide encoded by the bepA-L gene cluster (Fazli et al., 2017). The need of all these regulators can be justified by the energy-intensive polysaccharide biosynthesis process which require tight regulation. By comparing the gene functions of the bepA-L gene cluster with the known polysaccharides produced by Bcc bacteria (Cuzzi et al., 2014), it was clear that the cluster was responsible for production of a yet undescribed polymer. The characterization of the bepA-L gene cluster product required first of all the determination of the polysaccharides present in B. cenocepacia H111 biofilm. For this purpose, we used a strain over-producing the BerA protein, which functions as a positive regulatory protein of the cluster. Two different polysaccharides were found in the biofilm: H111-SOL (Bellich et al., 2021), a water-soluble polysaccharide rich in Rha residues and containing an L-Man residue, a rather rare sugar in nature, and H111-INS (Bellich et al., 2020), a water-insoluble polymer made of Glc, Gal and Man. The use of two strains carrying transposon mutations in the bepA and bepC genes, which render the bacteria incapable of producing Bep (Fazli et al., 2013), demonstrated that the exopolysaccharide encoded by the bepA-L gene cluster is the water-insoluble H111-INS heteropolymer which was, therefore, named Bep.
While it makes sense that an insoluble polysaccharide can provide a good network for a biofilm matrix, especially in the form of a pellicle, there are far less known water-insoluble bacterial polysaccharides than water-soluble ones. Among the former, cellulose, a linear homopolymer of β-(1,4)-Glc, is found in the biofilm matrix of several bacterial species (Serra & Hengge, 201; Limoli et al., 2015). Water-insoluble heteropolysaccharides are better known and more common in the plant and fungal kingdoms than among bacteria. As an example of the latter, a cell-bound polymer composed mainly of N-acetylmannosamine and galactose was reported to be produced by Listeria monocytogenes (Köseoğlu et al., 2015). Production of H111-SOL Epol and Bep by the ΔbcsB/pBerA strain is strongly influenced by the growth medium used. On solid NYG the strain produced 83 % (w/w) of Bep and 17 % (w/w) of H111-SOL, while on solid LB only H111-SOL could be detected (Bellich et al., 2021). The ΔbcsB/pBerA pellicles formed in liquid NYG were found to contain about 83 % (w/w) of Bep and 17 % (w/w) of H111-SOL; an inverse ratio was found for ΔbcsB/pBerA pellicles formed in liquid LB with 20 % (w/w) of Bep and 80 % (w/w) of H111-SOL. Moreover, the state of the medium was relevant only in the case of LB, since Bep was detected only in the pellicles. Because of its relevance in CF, the wild type strain was also investigated for Bep production. On solid NYG the analysis of the product was hampered by the presence of cepacian, while in liquid NYG the strain formed pellicles containing about 24 % (w/w) and 76 % (w/w) of Bep and H111-SOL Epols, respectively. In LB broth Bep could be detected only after spiking experiment since it was present in traces. Finding Bep in the H111 wild type pellicles is of great importance for the possible implications it may have in the in vivo biofilm. These quantitative data also indicated that nutrients, and thus environment, have a strong influence on which Epol is biosynthesised by the wild type strain.
Bioinformatic analysis of the bepA-L gene cluster revealed that only one gene, bepK, out of three expected in the cluster, is devoted to the synthesis of activated monosaccharides, and it encodes a protein involved in synthesis of GDP-Man. Glucose and galactose are common monosaccharides, and are constituents of other Epols produced by B. cenocepacia (Fig. 1) and the genes for the biosynthesis of their respective activated precursors are located elsewhere in the genome (Fig. S7). The Bep cluster described here has a high identity (99–100%) with that of B. cenocepacia strains J2315 (Fazli et al., 2013) and K56-2 (Fig. 5C), and not surprisingly, some of the genes within the cluster encoding glycosyltransferases, precursor synthesis, and proteins related to polysaccharide decoration are also found in other B. cenocepacia strains, such as VC748 and F01 with an identity of >73% (Fig. 5C). Comparison of the Bep cluster with the well-known bceI-bceII cluster related to cepacian production and transport (Ferreira, Silva, Oliveira, Cunha & Moreira, 2011) shows homology only with the genes that encode BceA and BceE proteins involved in the activation of mannose and the final stage of export (OPX), respectively, with an identity of 70%. For Bep biosynthesis, a priming glycosyltransferase that catalyses the addition of the first sugar to the lipid carrier was located outside the cluster (I35_RS22460), and its activity was putatively associated with binding galactose to UndPP. This gene shares 43.5% of nucleotide identity with bceB in the cepacian cluster, although the latter binds glucose to the lipid carrier (Videira, Garcia & Sá-Correia, 2005). Genome analysis revealed that the bepA-L cluster is highly conserved in the strains of other Burkholderia species, such as Burkholderia pseudomallei 1026b, with a cluster consisting of 18 genes and 3 gene remnants (Bp1026b-I2907-Bp1026b-I29727, also named as becA-becR, belonging to chromosome I) with a minimum identity of 60%. Moreover, this cluster has homology with Burkholderia mallei ATCC2344 (locus tag from BMA0027 to BMA0048) and Burkholderia thailandensis E264 (BTH_I0520-BTH_I0537) with an identity higher than 60% (Borlee et al., 2017). Likewise, we have also found the Bep cluster in B. multivorans strains CGD1 (BURMUCDG1_4569- BURMUCDG1_4587), CGD2 (BURMUCDG2_5005- BURMUCDG2_5023) and CGDM2 (BURMUCDGM2_4998- BURMUCDGM2_5016) with a minimum identity of 73% (Fig. S8). Although some of the genes belonging to the cluster have inverted direction of transcription, the nucleotide identity is highly conserved, and most importantly, the overall functions of the cluster are maintained (Borlee et al., 2017).
5. Conclusion
The present investigation identified the product of the bepA-L gene cluster in B. cenocepacia H111 as the water-insoluble polysaccharide which was shown (Bellich et al., 2020) to have the following tetrasaccharide repeating unit:
This polysaccharide is named Bep, for Burkholderia cenocepacia exopolysaccharide.
Although Bep was initially isolated from a strain overexpressing a Bep transcriptional activator, it was subsequently found also in biofilms produced by B. cenocepacia H111 wild type strain, thus suggesting its possible functional role in in vivo biofilms.
Bioinformatic analyses evidenced that the bepA-L gene cluster is also present in other B. cenocepacia strains as well as other Burkholderia species, such as B. multivorans, B. mallei, B. pseudomallei and B. thailandensis, thus underlying its potential importance as a constituent of the biofilm matrix of these bacterial pathogens.
Our findings are of interest to the community of researchers who work on understanding the biofilm life style and on finding new targets to combat biofilm-associated infections.
Supplementary Material
Acknowledgments:
The authors thank Bernard Henrissat, Director of Research CNRS, Creator of the CAZy database, for helping in the assignment of the glycosyltransferases family. This work was supported in part by an agreement with Cornell University, under Prime Agreement [R01GM123283] from the National Institute of General Medical Sciences of the National Institutes of Health.
Abbreviations:
- Bcc
Burkholderia cepacia complex
- Bep
Burkholderia cenocepacia exopolysaccharide
- CF
cystic fibrosis
- CGD
chronic granulomatous disease
- Epol
exopolysaccharide
- Gal
galactose
- Glc
glucose
- GLC
gas liquid chromatography
- GLC-MS
gas liquid chromatography coupled to mass spectrometry
- GT
glycosyltransferase
- Man
mannose
- H111-INS
water-insoluble polysaccharide produced by B. cepacia H111
- H111-SOL
water-soluble polysaccharide produced by B. cepacia H111
- H111 WT
B. cenocepacia H111 wild type
- NYG
nutrient-yeast extract-glycerol
- HR-MAS
High-Resolution Magic Angle Spinning
- LB
Luria Bertani
- Rha
rhamnose
- TFA
trifluoroacetic acid
- TMH
transmembrane helical
- UndPP
undecaprenylpyrophosphate
- YEM
yeast extract mannitol
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at …
References
- Albersheim P, Nevins DJ, English PD, & Karr A (1967). A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydrate Research, 5, 340–345. 10.1016/S0008-6215(00)80510-8. [DOI] [Google Scholar]
- Bellich B, Jou IA, Buriola C, Ravenscroft N, Brady JW, Fazli M, Tolker-Nielsen T, Rizzo R, & Cescutti P (2021).The biofilm of Burkholderia cenocepacia H111 contains an exopolysaccharide composed of rhamnose and L-mannose: structural characterization and molecular modelling. Carbohydrate Research, 499, 108231. 10.1016/j.carres.2020.108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellich B, Jou IA, Caterino M, Rizzo R, Ravenscroft N, Fazli M, Tolker-Nielsen T, Brady JW, & Cescutti P et al. (2020). Burkholderia cenocepacia H111 produces a water-insoluble exopolysaccharide in biofilm: structural determination and molecular modelling. International Journal of Molecular Sciences, 21, 1702–1714. https://doi: 10.3390/ijms21051702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlee GI, Plumley BA, Martin KH, Somprasong N, Mangalea MR, Islam MN, Burtnick MN, Brett PJ, Steinmetz I, AuCoin DP, Belisle JT, Crick DC, Schweizer HP, & Borlee BR (2017). Genome-scale analysis of the genes that contribute to Burkholderia pseudomallei biofilm formation identifies a crucial exopolysaccharide biosynthesis gene cluster. PLoS Neglected Tropical Diseases, 11(6), e0005689. 10.1371/journal.pntd.0005689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraher E, Reynolds G, Murphy P, McClean S, & Callaghan M (2007). Comparison of antibiotic susceptibility of Burkholderia cepacia complex organisms when grown planktonically or as biofilm in vitro. European Journal of Clinical Microbiology & Infectious Diseases, 26, 213–216. 10.1007/s10096-007-0256-x. [DOI] [PubMed] [Google Scholar]
- Carlier A, Agnoli K, Pessi G, Suppiger A, Jenul C, Schmid N, Tümmler B, Pinto-Carbo M, & Eberl L (2014). Genome sequence of Burkholderia cenocepacia H111, a cystic fibrosis airway isolate. Genome Announcements 2pii:e00298–14. 10.1128/genomeA.00298-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescutti P, Bosco M, Picotti F, Impallomeni G, Leitão JH, Richau J, & Sá-Correia I (2000). Structural study of the exopolysaccharide produced by a clinical isolate of Burkholderia cepacia. Biochemical Biophysical Research Communications, 273, 1088–1094. 10.1006/bbrc.2000.3059. [DOI] [PubMed] [Google Scholar]
- Ciofu O, & Tolker-Nielsen T (2019). Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents - how P. aeruginosa can escape antibiotics. Frontiers in Microbiology, 10, 114–131. 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye T, & Vandamme P (2003). Diversity and significance of Burkholderia species occupying diverse ecological niches. Environmental Microbiology, 5, 719–729. 10.1046/j.1462-2920.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, & Parsek MR (2011). The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLOS Pathogens, 7(1), e1001264. 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzi B, Herasimenka Y, Silipo A, Lanzetta R, Liut G, Rizzo R, & Cescutti P (2014). Versatility of the Burkholderia cepacia Complex for the biosynthesis of exopolysaccharides: a comparative structural investigation. Plos One, 9, e94372. 10.1371/journal.pone.0094372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet B, Mayo M, Peeters C, Zlosnik JE, Spilker T, Hird TJ, LiPuma JJ, Kidd TJ, Kaestli M, Ginther JL, Wagner DM, Keim P, Bell SC, Jacobs JA, Currie BJ, & Vandamme P (2015). Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov., two novel Burkholderia cepacia complex species from environmental and human sources. International Journal of Systematic and Evolutionary Microbiology, 65, 2265–2271. 10.1099/ijs.0.000251. [DOI] [PubMed] [Google Scholar]
- Fazli M, O’Connell A, Nilsson M, Niehau s K., Dow JM, Givskov M, Ryan RP, & Tolker-Nielsen T (2011). The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Molecular Microbiology, 82, 327–341. 10.1111/j.1365-2958.2011.07814.x. [DOI] [PubMed] [Google Scholar]
- Fazli M, McCarthy Y, Givskov M, Ryan RP, & Tolker-Nielsen T (2013). The exopolysaccharide gene cluster Bcam1330–Bcam1341 is involved in Burkholderia cenocepacia biofilm formation, and its expression is regulated by c-di-GMP and Bcam1349. MicrobiologyOpen, 2(1), 105–122. 10.1002/mbo3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazli M, Rybtke M, Steiner E, Weidel E, Berthelsen J, Groizeleau J, Bin W, Zhi BZ, Yaming Z, Kaever V, Givskov M, Hartmann RW, Eberl L, & Tolker-Nielsen T (2017). Regulation of Burkholderia cenocepacia biofilm formation by RpoN and the c-di-GMP effector BerB. MicrobiologyOpen, 6(4), e00480. 10.1002/mbo3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Silva I, Oliveira V, Cunha R, & Moreira L (2011). Insights into the role of extracellular polysaccharides in Burkholderia adaptation to different environments. Frontiers in Cellular and Infection Microbiology, 1,16. 10.3389/fcimb.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltermann L, & Tolker-Nielsen T (2017). Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrobial Agents and Chemotherapy, 61(4), e02696–16. 10.1128/AAC.02696-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PJ, Henry RJ, Blakeney AB, & Stone BA (1984). An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydrate Research, 127, 59–73. 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Huber B, Riedel K, Hentzer M, Heydorn A, Gotsclich A, Givskov M, Molin S, & Eberl L (2001). The cep quorum-sensing system of Burkholderia cenocepacia H111 controls biofilm formation and swarming motility. Microbiology, 147, 2517–2528. 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- Köseoğlu VK, Heiss C, Azadi P, Topchiy E, Güvener ZT, Lehmann TE, Miller KW, & Gomelsky M (2015). Listeria monocytogenes exopolysaccharide: origin, structure,biosynthetic machinery and c-di-GMP-dependent regulation. Molecular Microbiology, 96(4), 728–743. 10.1111/mmi.12966 [DOI] [PubMed] [Google Scholar]
- Lagatolla C, Skerlavaj S, Dolzani L, Tonin EA, Monti Bragadin C, Bosco M, Bosco M, Rizzo R, Giglio L, & Cescutti P (2002). Microbiological characterisation of Burkholderia cepacia isolates from cystic fibrosis patients. Investigation of the exopolysaccharides produced. FEMS Microbiology Letters, 209, 89–94. 10.1111/j.1574-6968.2002.tb11116.x. [DOI] [PubMed] [Google Scholar]
- Limoli DH, Jones CJ, & Wozniak DJ (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiology Spectrum 3(3) 10.1128/microbiolspec.MB-0011-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahenthiralingam E, Baldwin A, & Dowson CG (2008). Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. Journal of Applied Microbiology, 104, 1539–1551. 10.1111/j.1365-2672.2007.03706.x. [DOI] [PubMed] [Google Scholar]
- Römling U, & Galperin MY (2015). Bacterial cellulose biosynthesis: diversity of operons, subunits, products and functions. Trends in Microbiology, 23(9), 545–557. 10.1016/j.tim.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage A, Linker A, Evans LR, & Lessie TG (1990). Hexose phosphate metabolism and exopolysaccharide formation in Pseudomonas cepacia. Current Microbiology, 20, 191–198. 10.1007/BF02091996. [DOI] [Google Scholar]
- Serra DO, & Hengge R (2019). Extracellular Sugar-Based Biopolymers Matrices. Biologically-Inspired Systems Book 12. In Cohen E, & Merzendorfer H (Eds.), Cellulose in Bacterial Biofilms (pp. 355–392). Switzerland: Springer, Cham. 10.1007/978-3-030-12919-4. [DOI] [Google Scholar]
- Sist P, Cescutti P, Skerlavaj S, Urbani R, Leitão JH, Sá-Correia I, & Rizzo R (2003). Macromolecular and solution properties of cepacian: the exopolysaccharide produced by a strain of Burkholderia cepacia isolated from a cystic fibrosis patient. Carbohydrate Research, 38, 1861–1867. 10.1016/s0008-6215(03)00306-9. [DOI] [PubMed] [Google Scholar]
- Speert DP (2002). Advances in Burkholderia cepacia complex. Paediatric Respiratory Reviews, 3, 230–235. 10.1016/s1526-0542(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, & Beatson SA (2011). Easyfig: a genome comparison visualizer. Bioinformatics, 27(7), 1009–1010. 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DP, Shapiro RH, & Albersheim P (1975). Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydrate Research, 40, 217–225. 10.1016/S0008-6215(00)82604-X. [DOI] [Google Scholar]
- Vallenet D, Calteau A, Dubois M, Amours P, Bazin A, Beuvin M, et al. (2020). MicroScope: an integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Research, 48(D1), D579–589. 10.1093/nar/gkz926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira PA, Garcia AP, & Sá-Correia I (2005). Functional and topological analysis of the Burkholderia cenocepacia priming glucosyltransferase BceB, involved in the biosynthesis of the cepacian exopolysaccharide. Journal of Bacteriology, 187, 5013–5018. 10.1128/JB.187.14.5013-5018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


