Abstract
Cardiomyocytes are differentiated heart muscle cells with minimal self-renewal ability. Thus, loss of cardiomyocytes from cardiovascular disease and injury cannot be effectively replenished. Recent studies in animal models have indicated that induction of endogenous cardiomyocyte proliferation is essential for cardiac renewal and that inhibiting the Hippo signaling pathway can stimulate cardiomyocyte proliferation and heart regeneration. Increasing evidence has suggested that cardiomyocyte proliferation requires a permissive microenvironment that consists of multiple cell types. In this review, we summarize recent studies that highlight how the Hippo pathway regulates heart regeneration through cell-autonomous and non–cell-autonomous mechanisms. We also discuss recent translational studies in large animal models that demonstrate the therapeutic potential of targeting the Hippo pathway in the treatment of heart disease.
Keywords: Cardiac regeneration, Hippo pathway, Proliferation, Heart failure, Cell communication, Large animal model
1. Introduction
Heart failure (HF), defined as the inability of the heart to sufficiently pump enough blood to meet the body’s needs, is the leading cause of death globally [1]. Currently, an estimated 38 million patients have HF worldwide, and this number continues to rise [2]. Roughly one-half of the patients with HF die within 5 years of diagnosis. HF is a complicated pathological process initiated by cardiac injury, which arises most commonly from ischemic heart disease associated with coronary vascular disease [3]. Although proven treatment options exist that ameliorate the symptoms of HF, therapeutics that can reverse HF or restore damaged heart tissue are critically needed.
In mammals, the heart has a limited regenerative capacity. After cardiac injury, such as myocardial infarction (MI), millions of cardiomyocytes (CMs) die and are not replenished. To date, there are no direct measures to address this CM loss, which is the central underlying mechanism of ischemic HF. Over the past decade, the possibility of repopulating the heart with new CMs after injury has attracted intense interest. Considerable attention has focused on the Hippo pathway, which is a conserved signaling pathway that regulates organ size during development. Studies have revealed that the Hippo pathway is essential for cardiac development, growth, homeostasis, and regeneration [4–12]. In this review, we summarize recent findings regarding pivotal roles of the Hippo pathway in regulating CM renewal and cell communication between different cardiac cells.
2. Cardiomyocyte renewal state in mammals
Heart regeneration has been widely studied for decades. The key question has gradually shifted from whether CMs can proliferate to whether the proliferative capacity of CMs can be optimized for the treatment of various cardiac diseases involving CM loss. During neonatal life, the mammalian heart undergoes hyperplastic expansion and then hypertrophic growth to accommodate the demand for cardiac output [13]. In mice, studies have shown that neonatal CMs undergo DNA synthesis at a rate of 10% during the first 4 days of life; by day 7, this rate is reduced to 1%, indicating near terminal differentiation [14–18]. Studies of CM division have been performed with multi-isotope labeling [19] and a genetic fate-mapping mouse model called mosaic analysis with double markers (MADM) [20]. In both studies, the rate of CM division was analyzed by using lineage tracing and was shown to significantly decrease with age [19,21]. Genetic lineage tracing by using dual recombinases has provided further evidence ruling out the possibility of noncardiomyocyte-to-cardiomyocyte conversion in postnatal mouse hearts [22,23]. A similar phenomenon was observed in humans. In a highly influential study [24], researchers used carbon dating techniques and mathematical modeling to retroactively analyze 14C isotope integration into the CMs of humans who lived at the time of atmospheric nuclear bomb testing during the cold war [24]. They calculated the self- renewal rates of several human cell types, among which CMs were found to be the least renewable cell type [24,25]. At age 20, the renewal rate of CMs is about 1% per year, which decreases with time to ~0.3% by age 70. Thus, an estimated 40% of CMs are generated throughout a full lifespan, whereas the remaining 60% are originally formed during prenatal development [24,25]. Together, these studies have demonstrated that CM proliferation and division are measurable but rare in mammalian hearts.
3. Injury-induced cardiomyocyte proliferation is essential for neonatal heart regeneration
To better understand the role of CM proliferation in heart regeneration, investigators have studied animal models capable of cardiac regeneration, including amphibians and zebrafish [26,27]. Recent reports have shown that spontaneous cardiac regeneration also occurs in mammalian neonates [18,28]. In either of these contexts, myocardial regeneration is mediated by the compensatory proliferation of preexisting CMs, rather than by stem or progenitor cell populations [29,30]. Studies in zebrafish showed complete cardiac regenerative ability after apical resection, with Cmlc2a- or Gata4-expressing CMs undergoing proliferation and migrating to the injury site to rebuild the damaged myocardium. Interestingly, the cardiogenesis gene Gata4 is mainly expressed in epicardial CMs, suggesting injury-induced CM dedifferentiation [29,30]. Independent studies in mice have revealed that the neonatal mouse heart has the capacity to regenerate. Compared with mice that underwent a sham operation, mice with cardiac injury showed a 5-fold increase in CM proliferation within 7 days after birth (i.e., P7) [18,31]. Lineage tracing experiments performed by using inducible Myh6-MerCreMer mice crossed with Rosa26-lacZ reporter mice showed that the newly generated CMs were primarily derived from preexisting CMs [18,31,32].
Using a transcriptomics approach, Cui et al. [33] compared CMs in a P1 injury versus a P8 injury model and identified an immature CM population (CM4) associated with CM regeneration that was enriched in P1 hearts subject to injury. CM4 is characterized by reduced oxidative metabolism and enhanced cell-cycle activity, along with the increased expression of pro-proliferation and pro-survival factors [33]. These findings suggest that subpopulations of CMs exist in the neonatal heart that maintain an immature gene program and are poised to proliferate in response to injury.
4. Loss of cardiomyocyte proliferation in the adult heart
Because the regenerative window of perinatal murine hearts lasts only a few days, injury in nonregenerative mouse hearts (i.e., age P7 and older) failed to induce CM proliferation, resulting in permanent loss of cardiac muscle [18,31,32]. Similarly, in nonregenerative P7 mice that underwent MI or sham, no difference was observed in the number of phosphorylated histone H3 (pHH3)-positive CMs (pHH3 is a cell cycle marker that is expressed in the G2/M phase, just preceding mitosis) [31]. In another study, CM division detected by using MADM was limited in the hearts of adult mice that underwent MI or a sham operation [20]. Thus, understanding the difference in mechanisms of CM proliferation in neonatal versus adult mouse models is essential for developing therapeutics to induce spontaneous cardiac regeneration in humans.
Although progress has been made, how the heart loses its regenerative ability with age remains poorly understood. Puente et al. [21] reported that the level of reactive oxygen species (ROS) was increased in mice during the first week after birth, which resulted in activation of the DNA damage response in CMs. Reducing ROS level or using compounds to inhibit the DNA damage response extended the window of CM proliferation in the postnatal mouse heart, suggesting that ROS-induced DNA damage plays a role in the transition from the regenerative to the nonregenerative stage in the postnatal heart [21]. Cui et al. [34] recently reported that Nrf1, a stress-responsive transcription factor, regulates proteolysis and redox balance in regenerating CMs. Nrf1 expression is highest in P1 hearts, specifically in regenerating CMs, and decreases with age. In addition, the adeno-associated adenovirus (AAV)-mediated overexpression of Nrf1 in the adult heart reduced infarct area and improved cardiac function after ischemia/reperfusion injury. Transcriptomics and in vitro analyses revealed that Nrf1 can activate ROS scavengers and increase proteasomal activity to maintain an environment suitable for CM survival and renewal after injury [34].
As the heart matures during the perinatal period, it undergoes both structural and metabolic changes needed for optimal cardiac function. The sarcomeres and mitochondria of CMs become highly organized and developed, coinciding with the loss of proliferation capability. After birth, mammalian CMs undergo 1 to 2 rounds of cell division before switching to hypertrophic growth [35], which is accompanied by sarcomere myofibrils becoming further organized. This highly complex structure allows CMs to sustain the cycles of contraction and relaxation that compose each heartbeat, but it becomes a hindrance for cell division. Proliferation requires myofibril disassembly and cytoskeletal reorganization, which is challenging in structured and synchronized CMs.
In addition to the rigid sarcomeric cytoskeleton, postnatal metabolism changes also inhibit CM proliferation. Shortly after birth, the metabolic substrate provision changes, which leads to a metabolic switch of CMs from glycolysis to fatty acid oxidation [36,37]. Using an ex vivo three-dimensional cardiac organoid culture system, Mills et al. [38] showed that the switching of metabolic substrate from carbohydrates to fatty acids is a central driver of cardiac maturation, which is accompanied by decreased CM proliferation.
Another potential obstacle for CM proliferation in the adult heart is the change in transcriptional and epigenetic profiles of CMs that occurs beyond the neonatal stage. In a recent study, the authors performed RNA sequencing to examine the transcriptional profiles of CMs and non-CMs from neonatal and adult mouse hearts treated with or without MI [39]. Although injury-responsive genes were identified in regenerative and non-regenerative stages, a regeneration-specific gene profile was not detected. However, in all cardiac cells, the major transcriptional changes observed occurred in the developmental maturation genes from the neonatal stage to adulthood. Further, analysis using the assay for transposase-accessible chromatin sequencing (ATAC-seq) revealed the loss of chromatin accessibility in cell cycle genes. Thus, the change in the epigenetic landscape of CMs during the postnatal maturation phase may be one of the reasons for loss of regenerative capacity. Of note, in this study, cardiac cells were isolated by using enzymatic dissociation and flow cytometry sorting. Therefore, the long isolation process may have altered the physiologic state of the cells, leading to changes in the transcriptional profile that may not fully reflect the transcriptional profile of CMs in vivo [39].
5. Overview of the Hippo signaling pathway
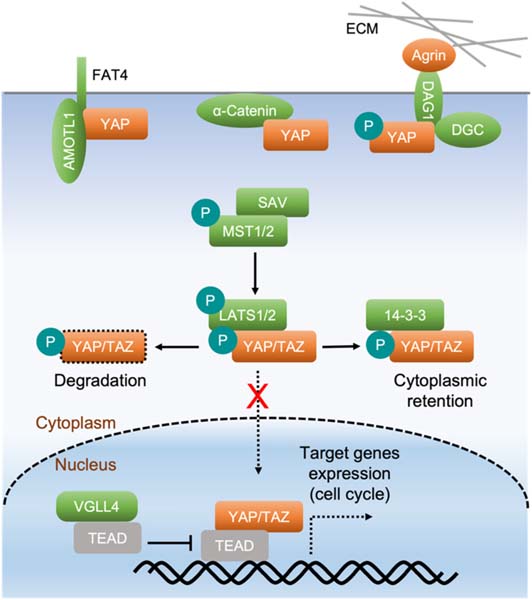
The Hippo pathway is an evolutionarily conserved kinase cascade. In the heart, Hippo pathway activity increases with age, whereas YAP activity decreases [6,9], implying that decreased YAP activity contributes to the loss of regenerative capability in the postnatal heart. In mice, STE20-like kinases 1 and 2 (MST1/2) and their adaptor protein Salvador (SAV1) form an activated complex and phosphorylate the large tumor suppressor homolog 1 and 2 (LATS1/2) and their regulatory protein Mps1-binder-related (MOB) domain kinase activator 1 (MOB1). This kinase cassette further phosphorylates the Hippo pathway’s key effectors Yes-associated protein (YAP)/Transcriptional co-activator with PDZ-binding motif (TAZ), thereby inhibiting the translocation of YAP/TAZ into the nucleus. Non-phosphorylated YAP/TAZ can enter the nucleus, where they function as coactivators with TEA domain (TEAD) transcriptional factors. Together, the YAP/TAZ-TEAD complex induces gene expression programs that favor proliferation and survival [40,41] (Fig. 1).
Fig. 1.
The Hippo pathway regulates cardiomyocyte proliferation. The Hippo pathway is composed of several core components: MST1/2, SAV, and LATS1/2. Kinases MST1/2 interact with their adaptor SAV and phosphorylate and activate kinases LATS1/2 that further interact with and phosphorylate YAP and its analog TAZ. Phosphorylated YAP/TAZ are bound and sequestered by 14–3–3 in the cytoplasm or undergo ubiquitination and degradation. When the Hippo pathway is inactivated, unphosphorylated YAP/TAZ translocate into the nucleus, where they interact with their binding partners, such as TEADs, to regulate the expression of downstream target genes that promote cardiomyocyte proliferation. YAP directly interacts with α-catenin, the FAT4 and AMOTL1 complex, and the dystrophin glycoprotein complex (DGC). This causes YAP cytoplasmic retention at the cell membrane, which regulates YAP activity through a Hippo-independent mechanism. The extracellular matrix (ECM) component Agrin physically interacts with the DGC (DAG1 is a protein of the DGC) and disrupts the YAP-DGC interaction, leading to YAP activation.
6. Hippo pathway function in the regulation of cardiomyocyte proliferation
To determine whether modulating Hippo pathway components or increasing YAP activity in CMs promotes heart regeneration, Heallen et al. [5] deleted Sav1 specifically in embryonic CMs and found evidence of heart expansion during development due to significantly increased CM proliferation. They also deleted other Hippo kinases including Lats2, or Mst1/2, in embryonic CMs and observed phenotypes similar to those in the Sav1 mutant [5]. This study showed for the first time that the Hippo-YAP pathway is a critical regulator of CM proliferation and cardiac organ size. Moreover, Xin et al. [11] showed that the deletion of Yap in embryonic CMs caused lethality in embryos at E10.5 due to hypoplasia of the myocardium. The number of CMs was decreased by nearly 50% due to significantly decreased proliferation. Consistent with this finding, overexpression of the TEAD1 inhibitor VGLL4 decreased CM proliferation in the neonatal mouse heart. VGLL4 interacts with and degrades TEAD1 in postnatal hearts [42]. Similarly, the deletion of miR302–367, which is a negative regulator of the Hippo pathway in CMs, led to decreased CM proliferation and thinning of the ventricular wall. Overexpression of miR302–367 in CMs promoted proliferation and resulted in cardiomegaly [43]. Deletion of Yap in CMs did not affect apoptosis during early heart development, suggesting that YAP regulates cardiac development primarily through its role in proliferation and not through anti-apoptotic functions [9]. Collectively, these studies suggest that the Hippo pathway is essential for restraining CM proliferation during heart development.
The postnatal deletion of Sav1 or Lats1/2 leads to YAP/TAZ activation and stimulates CM proliferation in the heart of 3- to 4-month-old mice [6,7]. Similarly, expression of constitutively active YAP (i.e., YAPS127A, with the substitution of serine 127 with alanine to escape phosphorylation) promotes postnatal CM proliferation [9,10]. Monroe et al. [44] generated a mouse strain in which YAP5SA is conditionally expressed in CMs. YAP5SA harbors five serine-to-alanine mutations that interfere with the LATS1/2-mediated phosphorylation of YAP, resulting in increased nuclear YAP activity. The authors found that YAP5SA expression for 6 days increased the number of CMs by nearly 25% due to uncontrolled CM proliferation. All YAP5SA mice died within 8 days after the induction of YAP5SA due to significantly decreased cardiac chamber size and cardiac output. The proliferative CMs were smaller but with similar ploidy compared with the control CMs, with most CMs being mononuclear [44]. Mononuclear CMs may have better renewal capacity than binuclear CMs, and higher ploidy hampers CM renewal in mice and zebrafish [45,46]. Nuclear RNA sequencing in adult CMs expressing YAP5SA revealed a more primitive and proliferative gene profile than adult control CMs. Genes that were upregulated included genes associated with the cell cycle (eg, Ccna2, Ccnb1, Ccnd1, and Myc) and genes related to cytoskeleton organization, (eg, Tnni3, Tnnt2, Myh6, and Myl2). ATAC-sequencing showed increased chromatin accessibility at TEAD motifs, indicating that YAP5SA alters chromatin structure and induces target gene expression to promote CM proliferation [44]. In support of these findings, a recent study demonstrated that YAP directly interacts with the components of the Switch/sucrose non-fermentable (SWI/SNF) chromatin-remodeling complex [47]. These results indicate that activation of YAP can robustly induce adult CMs to re-enter the cell cycle and proliferate in a cell-autonomous manner.
In mice with Yap deletion in CMs, no apparent defects were observed during the first few weeks after birth. However, significant thinning of the myocardium wall was seen at 9 weeks of age, and lethality occurred between 11 and 20 weeks [10]. The combined deletion of Yap and Taz in CMs resulted in lethality by day 1 after birth, suggesting that YAP and TAZ share a redundant role in heart development [10]. Because CMs do not proliferate past P7, the thinning of the myocardium wall after the deletion of Yap/Taz may be attributed to both CM proliferation defects and also increased apoptosis. Indeed, increased apoptosis was evident in the hearts of mice with Yap/Taz deletion [10]. Consistent with this finding, another group showed that CM apoptosis was increased in Yap- deficient mouse hearts, and the knockout of a single allele of Yap in CMs resulted in impaired cardiac function after MI [12]. However, these findings are inconsistent with those of von Gise, et al. [9], who showed that the loss of Yap does not affect CM apoptosis during development, possibly indicating a stage-dependent role of Yap in CMs. Together, these studies suggest that YAP, in addition to promoting proliferation, also may have a role in protecting CMs from apoptosis although further studies are required.
7. The Hippo pathway in the regulation of cardiac regeneration in the context of injury
Analysis of human ischemic and nonischemic HF samples revealed increased Hippo pathway activity when compared with control donor samples [7], suggesting that the Hippo pathway can be targeted as a potential therapy for heart injury and disease. The role of the Hippo pathway in heart regeneration has been tested in several different models. Heallen et al. [6]. examined the capacity for heart regeneration in mice after apical resection and after MI at P8, when the heart has completely lost its regenerative ability [6,18]. In both the resection and MI models, the deletion of Sav1 in CMs promoted greater heart regeneration with better heart function and less fibrosis than in control hearts [6]. In an adult mouse model of MI with well-established ischemic HF, Leach et al. [7] showed that the knockout of Sav1 in CMs resulted in the reversal of systolic HF that was not seen in control mice. They also treated mice with AAV9 encoding short hairpin RNA (shRNA) against Sav1, which resulted in recovered heart function and CM proliferation. From a mechanistic standpoint, Leach et al. showed that Park2, a gene associated with mitochondrial quality control [48], is a target of YAP. CMs with Sav1 deletion showed higher mitochondrial content than control CMs, supporting the conclusion that maintenance of mitochondrial function may be essential for cardiac regeneration by protecting “at risk” CMs in the border zone. Similar to YAP, Park2 is required for neonatal cardiac regeneration. The knockout of Park2 in mice at P8 mitigated cardiac regeneration in CMs with Sav1 deletion [7]. Interestingly, Park2 deletion diminished heart function in Sav1 knockout mice, but the fibrosis was still resolved [7]. Because mitochondria produce energy for CM contraction, the reduced heart function observed after Park2 deletion may be attributed to insufficient energy supply in CMs.
Using the adult MI mouse model with Sav1 deletion, Morikawa et al. [8] observed increased CM proliferation after Sav1 deletion. Moreover, they found extended CM protrusions in the scar region of Sav1 knockdown hearts compared with control hearts. When they performed YAP chromatin immunoprecipitation sequencing (ChIP-seq) and RNA sequencing of P8 mouse hearts, they observed the upregulation of YAP target genes associated with the cell cycle and cytoskeleton organization in Sav1 knockout hearts. Among those genes that were upregulated were components of the dystrophin-glycoprotein complex (DGC), such as Sgcd and Sntb1 [49,50]. They also found that the DGC is essential for the regulation of CM protrusion in neonatal heart regeneration via a cell- autonomous mechanism [8].
Morikawa et al. [51] further uncovered the connection between the Hippo pathway and the DGC in cardiac regeneration by showing that the DGC directly interacts with YAP and prevents the nuclear localization of YAP. DGC deficiency results in muscular dystrophy, which is usually associated with dilated cardiomyopathy. In mdx mice with the mutation of dystrophin, the combined knockout of Sav1 resulted in higher YAP activity than in Sav1 single knockout mice. Double knockout mice were also resistant to dystrophin deficiency–induced fibrosis due to increased YAP activity [51]. In line with this study, Bassat et al. [4] discovered that Agrin, a component of the extracellular matrix (ECM), interacts with the DGC and disassociates the YAP/DGC complex, thereby releasing YAP to the nucleus and promoting CM proliferation. Since Agrin is a component of the extracellular matrix and is expressed by endothelial cells, the study by Bassat et al. provides important new insight into the role of the extracellular microenvironment in CM proliferation.
In neonatal mouse hearts still within the window of regenerative capability, the deletion of Yap impaired regeneration after MI at P2 [10]. In injured adult mouse hearts, the heterozygous deletion of one allele of Yap in CMs resulted in increased scar size and decreased heart function compared with controls due to significantly increased CM apoptosis [12]. Conversely, the overexpression of YAP with enhanced activity (YAPS112A, a homolog of human YAPS127A) specifically in CMs improved heart regeneration after MI induced at P7 or P28 in nonregenerative mouse hearts [10]. Moreover, using a doxycycline- inducible YapS127A mouse strain, Lin et al. [52] showed that YAP activation was sufficient to promote heart regeneration in adult mouse hearts.
During the first week after birth, cardiac levels of ROS are increased. ROS can induce the DNA damage response in CMs and suppress cardiac renewal [21]. Shao et al. [53] reported that ROS levels are increased in the hearts of mice with the cardiac-specific deletion of Yap. They also found that YAP directly interacts with forkhead box protein O1 (FOXO1) to induce antioxidant gene expression. Similarly, Tao et al. [54] showed that paired like homeodomain 2 (PITX2) interacts with YAP and induces the expression of antioxidant genes such as Ldha, Ndufb3, and Oxnad1. Deletion of Pitx2 in CMs impaired neonatal heart function recovery and increased scar formation after apical resection or MI, suggesting an essential role for the anti-oxidative response induced by the PITX2-YAP interaction in heart regeneration [53,54]. In addition as noted above, Leach et al. [7] observed increased mitochondrial content in Sav1 knockout CMs. Thus, the mitochondria-derived ROS may be resolved by YAP-induced antioxidant genes. Together, these results suggest that the Hippo pathway regulates both CM proliferation and antioxidation during cardiac regeneration.
8. The non–cell-autonomous role of the Hippo pathway in regulating cell-to-cell communication
In addition to its cell-autonomous role in regulating CM proliferation, the Hippo pathway has been shown to regulate cell-to-cell communication from one cell type to another (Fig. 2). During cardiac regeneration, the re-establishment of new vasculature and fibrosis resolution are critical to repairing damaged heart tissue. Leach et al. [7] showed that the CM-specific knockout of Sav1 not only promoted CM proliferation, but also increased border zone vascularity and reduced fibrosis. These results suggest that CMs may signal to endothelial cells and fibroblasts via a non–cell-autonomous manner during cardiac renewal.
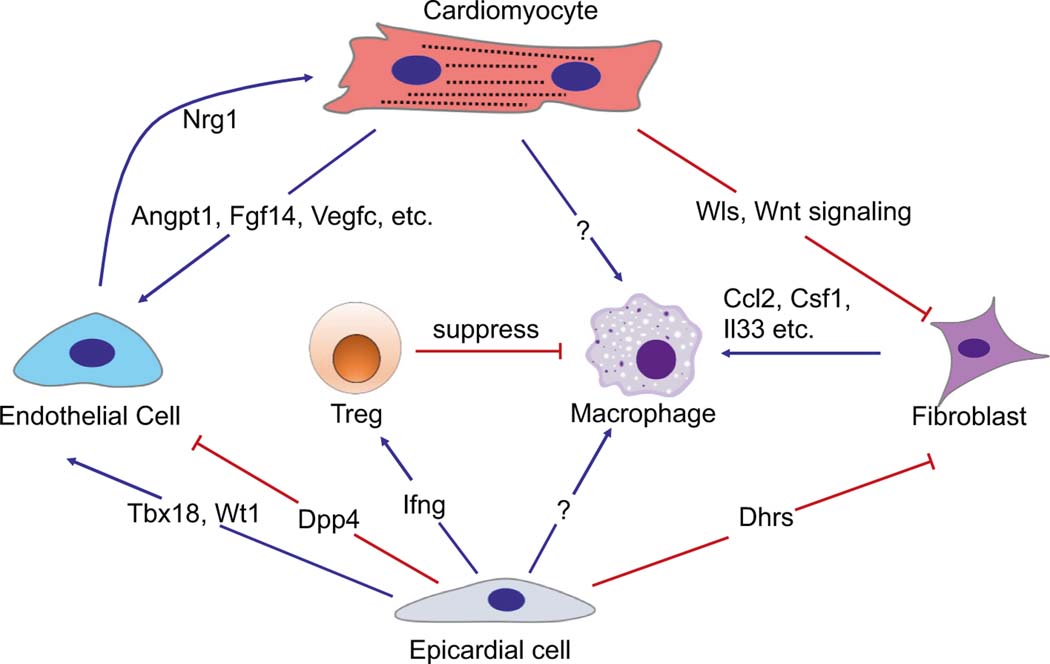
Fig. 2.
The Hippo pathway regulates cell-to-cell communication in the heart. Cell-to-cell communication is essential for cardiac homeostasis and regeneration. In cardiomyocytes, Hippo/YAP induce Wls and Wnt signaling to suppress fibroblast activation in neonatal heart regeneration. Sav1 knockout activates the expression of genes such as Angpt1, Fgf14, and Vegfc in cardiomyocytes [7], which in turn promotes vascularity in the infarcted area. Epicardial cells function as a source of multipotent progenitor cells and paracrine factors essential for cardiac development and repair. YAP regulates epicardial cell proliferation, the epithelial-to-mesenchymal transition, and differentiation into endothelial cells by modulating Wt1 and Tbx18 expression. The Hippo pathway also regulates Dpp4 expression in epicardial cells. Dpp4 proteolyzes both extracellular matrix and matrix- embedded growth factors to modulate endothelial cell migration [123]. Dhrs3 was upregulated in Lats1/2 knockout epicardial cells, which may contribute to impaired fibroblast differentiation by reducing retinoic acid formation and signaling [124]. In epicardial cells, YAP/TAZ induce IFN-γ and recruit T-reg cells to suppress the inflammatory response and fibrosis after myocardial infarction. In fibroblasts, Hippo/YAP induce the expression of cytokines including Ccl2, Csf1, and IL-33 to recruit proinflammatory macrophages. In endothelial cells, YAP induces Nrg1 expression, which is essential for myocardial development [80].
To identify the YAP targets associated with extracellular signaling transduction, Liu et al. [55] examined YAP targets in mouse hearts with CM-specific overexpression of YAP5SA. By combining nuclear RNA sequencing, ATAC-seq, and ChIP-seq datasets, Liu et al. [55] showed that Wnt signaling pathway genes are direct downstream targets of YAP. The expression of Wntless (Wls), which is essential for the trafficking and secretion of Wnt ligands, and several Wnt ligands was significantly upregulated, suggesting a direct connection between the Hippo and Wnt signaling pathways. Using both CRISPR/Cas9 and traditional Cre/LoxP mouse models, we found that Wls is required for neonatal heart regeneration and scar resolution. Using single-cell RNA sequencing, we observed higher expression of noncanonical Wnt ligand genes than canonical Wnt ligand genes in CMs. We also found that Wnt receptors are highly enriched in fibroblast cells, suggesting that noncanonical Wnt signaling from CMs to fibroblasts is essential for cardiac homeostasis and regeneration. Further analysis indicated that impaired heart regeneration was due to excessive cardiac fibrosis after Wls deletion and that noncanonical Wnt signaling from CMs to fibroblasts is essential for inhibiting fibroblast activation in neonatal heart regeneration [55]. This study not only uncovered the role of the Hippo-Wnt gene regulatory network in the cell communication between CMs and fibroblasts, but it revealed noncanonical Wnt signaling from CMs acting as a non–cell- autonomous regulator to suppress cardiac fibroblast activation. In addition to CMs, other cell types also secrete Wnt proteins, such as fibroblasts and epicardial cells. Determining how Hippo regulates Wnt signaling from different sources and how these signals affect heart regeneration and scar resolution requires further study. Additionally, examining the expression and role of each of the 19 Wnt ligands in the heart remains to be done.
The Hippo signaling pathway also plays a role in communication between the epicardium and other cell types. The epicardium covers the outer layer of the heart and functions as a source of multipotent progenitor cells and paracrine factors essential for cardiac development and repair [56–61]. Xiao et al. [62] showed that the Hippo pathway is essential for epicardial cells to differentiate into cardiac fibroblasts. Epicardial Lats1/2 knockout resulted in the expansion of an intermediate cell type between epicardial cells and cardiac fibroblasts. This was accompanied by altered ECM composition and suppressed coronary vessel patterning that occurred in a non–cell-autonomous manner. Consistent with this finding, Singh et al. [63] showed that mice with the epicardial-specific double knockout of Yap/Taz died around E11.5 to E12.5 from cardiovascular hypoplasia due to defects in epicardial cell proliferation, epithelial-to-mesenchymal transition, and fate determination. Using the same WT1-Cre mice, Ramjee et al. [64] deleted Yap/ Taz specifically in epicardial cells and observed profound pericardial inflammation and myocardial fibrosis after MI due to decreased T-reg cell recruitment. In addition, they identified interferon gamma (IFN-γ) as a direct target of Yap/Taz that functions as a T-reg inducer [64–68]. Together, these studies have demonstrated the essential role of the Hippo pathway in the communication of the epicardium with other cells in the heart through non–cell-autonomous mechanisms, including immune cells, endothelial cells, and fibroblasts (Fig. 2).
Cardiac fibroblasts respond to injury by transitioning through different cell states, including resting cardiac fibroblasts, activated fibroblasts, and myofibroblasts. Using Tcf21-Cre mice, Xiao et al. [69] studied Lats1/2 knockout cardiac fibroblasts and observed spontaneous fibroblast proliferation, activation, and transdifferentiation into myofibroblasts. By performing ATAC-seq and CUT&RUN, they identified direct targets of the Hippo pathway associated with fibroblast activation and the inflammatory response. Single-cell RNA sequencing analysis indicated Lats1/2 knockout myofibroblasts recruited myeloid cells through the Csf1-Csf1r axis. Consistent with this, Francisco et al. [70] overexpressed YAP in cardiac fibroblasts by using AAV and observed an increase in fibrosis and the inflammatory response in the heart. In determining the mechanism, they found that YAP occupied the Ccl2 gene and promoted Ccl2 expression. Ccl2 is critical for monocyte and macrophage recruitment, which is in turn essential for cardiac inflammation, remodeling, and regeneration [71–74]. Moreover, Mia et al. [75] reported that YAP/TAZ directly regulate the promoter activity of the profibrotic cytokine interleukin-33 (IL-33) in cardiac fibroblasts. Blocking the IL-33 receptor ST2 by using a neutralizing antibody abrogated the YAP-induced profibrotic response in cardiac fibroblasts. In another study, Del Re et al. [76] reported that the Ras-associated domain family 1 isoform A (RASSF1A), which is an endogenous activator of MST1, inhibits nuclear factor kappa B (NF-κB) and tumor necrosis factor alpha (TNF-α) in cardiac fibroblasts [77,78]. Treating Rassf1a knockout mice with TNF-α antibody decreased transverse aortic constriction–induced fibrosis [76]. Together, these studies show that the Hippo pathway regulates the cell state transition of fibroblasts and the immune response in the context of both homeostasis and injury.
Reports have shown that the Hippo pathway plays a pivotal role in endothelial cells during heart development. Using an endothelial Tie2-Cre mouse, Zhang et al. [79] knocked out YAP in the endothelial and endocardial cells of the developing heart. This conditional mouse strain was embryonic lethal due to vascular abnormalities and impaired heart function. The conditional deletion of YAP not only decreased endothelial cell proliferation, but also impaired the endothelial-to-mesenchymal transition in a cell-autonomous manner [79]. In addition, Artap et al. [80] specifically knocked out YAP/TAZ in endocardial cells using Nfatc1IRES-Cre mice. Consistent with previous findings showing that the coronary endothelium arises from Nfatc1-expressing endocardial cells [81], Artap et al. found that endocardial-specific YAP/TAZ deletion caused early postnatal lethality due to cardiac hypoplasia. They further showed that YAP directly induces the expression of NRG1, which is a secreted factor that plays an essential role in myocardium development [82–84] (Fig. 2).
9. The translational potential of Hippo pathway inhibition in a pig model of cardiac renewal
To date, the animal models most commonly used for studying CM renewal have been mice and zebrafish. Although these animal models have provided valuable insights, they have limitations for translational studies because their cardiovascular anatomy and physiologic features are distinctly different from those of the human heart [85,86]. Pig models have become highly valuable in translational cardiovascular research. The physiology, heart size, immune system, and anatomy of the pig heart closely resemble those of the human heart. For example, pig and human hearts have similar contractile indices, determined by using cardiac catheterization measurements [87,88]. Pig and human CMs also share many characteristics in excitation-contraction coupling. Furthermore, similar to human CMs, pig CMs predominantly express β-myosin heavy chain, and stiff N2B and N2BA titin isoforms are both expressed in pig myocytes [87]. Pigs also share similar regional cardiac hemodynamic features with humans [89,90], and both pig and human hearts have reduced contractility after MI that is caused by alterations in Ca2+ handling [86]. Increased Ca2+ sensitivity is also a common feature in pig and human hearts after MI [88].
Velayutham et al. [91] investigated the characteristics of CMs, including cell cycle arrest, nucleation, and hypertrophy in postnatal pigs. Surprisingly, unlike mouse CMs, which lost cell cycle activity within several days after birth, pig CMs showed mitotic activity for up to 2 months of age. This mitotic activity may have resulted from nuclear division (karyokinesis) but not cell division (cytokinesis) and was accompanied by CM hypertrophic growth and multinucleation. At 6 months of age, most pig CMs are multinucleated, with a maximum of 16 nuclei per CM.
Although cell cycle activity is sustained in CMs of 2-month-old pigs, several independent studies have shown that cardiac regenerative ability lasts only up to 2 days after birth [28,92,93]. To examine the translational potential of targeting the Hippo pathway to promote the regeneration of pig hearts, Liu et al. [94] used an AAV9-mediated gene therapy strategy to knockdown the Hippo pathway component Sav1 in CMs. Three-month-old pigs were treated with an angioplasty balloon to induce ischemic/reperfusion injury. Two weeks after MI, NOGA electromechanical mapping was used in combination with a Myostar catheter to detect the infarcted border zone region of the myocardium and deliver AAV9-encoding shRNAs against Sav1 into CMs [94,95]. This noninvasive method resulted in high-efficiency AAV9 viral infection and the nuclear localization of YAP. Compared with the controls, pig hearts injected with AAV9-Sav-shRNA recovered nearly 10% ejection fraction during the three-month follow-up period. In pig hearts that received a higher dosage of the AAV9-Sav-shRNA virus, the ejection fraction was increased by 14%. In the clinical setting, this degree of functional recovery could significantly improve quality of life for patients with MI. We also analyzed CM proliferation by using multiple methods and observed significantly increased cell cycle activity in CMs injected with AAV9-Sav-shRNA. Furthermore, CM dedifferentiation and division was detected in pig hearts injected with AAV9-Sav-shRNA but not in control pig hearts. The effects of AAV9-Sav-shRNA appeared to be CM specific, with no abnormalities or tumor formation detected in other organs such as liver, lung, or kidney during the three-month follow-up period [94]. Notably, the divided CMs had fully matured sarcomere structure and normal gap junction localization, suggesting that the newly formed CMs had coupled and integrated into the myocardium [94].
In a study by Zhao et al. [96], human induced pluripotent stem cell (iPSC)-derived CMs were transplanted into the infarcted hearts of two-month-old pigs. They found that iPSC-derived CMs overexpressing CCND2 could continue to proliferate after being engrafted to the infarcted myocardium. Furthermore, the iPSC-derived CMs secreted exosomes with miRNAs and promoted cardiac regeneration by stimulating the proliferation of pig CMs and endothelial cells while reducing CM apoptosis. In further mechanistic studies, they found that the expression of miR-373–302b is upregulated in exosomes from iPSC- derived CMs overexpressing CCND2. miR-373–302b inhibits the Hippo pathway and activates YAP activity, which in turn promotes cell proliferation and survival [96].
In another study in pigs, Gabisonia et al. [97] injected AAV6 expressing microRNA (miR)-199a into infarcted pig hearts. The known targets of miR-199a include two Hippo pathway inhibitors, TAO kinase 1 [98,99] and the E3 ubiquitin-ligase β-transducing repeat containing protein [100]. Consistent with studies in mice [101–104], they observed CM proliferation, cardiac repair, and reduced scar size in pig hearts one month after AAV6-miR-199a injection. However, the subsequent persistent and uncontrolled expression of miR-199a caused sudden arrhythmic death within two months of viral injection [97]. Similarly, studies in mice have also shown that the constitutive overexpression of YAP or miRNAs in the heart can be deleterious [43,44]. The reason for this may be that miRNAs have hundreds of conserved target genes; thus, negative off-target effects may occur due to miRNA overexpression. Unlike the effect of miRNAs, the knockdown of Hippo pathway components may activate a negative regulation mechanism to constrain the persistent activity of YAP. Indeed, YAP-LATS2 have been shown to form a negative feedback loop [105]. Collectively, these studies support the conclusion that targeting the Hippo pathway to treat heart disease can be done in an effective and safe manner in the translational context of a large animal model.
10. Future directions
Several studies have shown that increasing YAP activity in CMs has the potential to promote cardiac regeneration. However, targeting the Hippo-YAP pathway in a general manner to treat heart disease is impractical because YAP is an oncogene in some tissues [106]. Moreover, the knockout of Hippo pathway components in cardiac fibroblasts causes fibrosis and inflammation, which would impair cardiac regeneration [69]. Thus, activating YAP in a CM-specific manner may be a better strategy for developing treatments for patients with HF. Unlike other cell types, CMs are highly structured cells packed with dense cytoskeleton networks [107,108]. Studies have shown that several cytoskeletal components including α-catenin, and the DGC physically interact with YAP and prevent its nuclear localization in CMs and other cell types [4,7,109–112]. Because the Hippo pathway is involved in sensing mechanical stress [40,113–116], investigating the connection between the CM cytoskeleton and Hippo pathway regulation would be of great interest. Moreover, CMs are the most energy-demanding cells. In the setting of MI, metabolism quickly shifts from mitochondrial oxidative phosphorylation to glycolysis because of CM oxygen deficiency [117–119]. Understanding whether and how the Hippo pathway affects or is affected by metabolism in the context of the post-MI heart requires further study.
Another strategy to circumvent the systemic activation of YAP would be to identify YAP target genes during CM proliferation and directly induce the expression of those YAP target genes to stimulate CM proliferation. Although several studies have shown many YAP target genes to be associated with the cell cycle [8], how YAP regulates the gene regulatory network to induce cell cycle entry and cell division remains poorly understood. In addition, in contrast to fetal and neonatal CMs, mature adult CMs must overcome the structural block to cell division. Dissecting the cell cycle process from entry to division in adult CMs would be essential to developing strategies to stimulate CM proliferation in human patients.
Recent studies have investigated the role of the immune system in CM proliferation, comparing the differences in the cardiac immune response after injury between renewable and nonrenewable models [73,120–122]. These studies highlight subpopulations of macrophages, which are rare in the adult heart, that may facilitate or support CM renewal. Results from other studies have revealed essential roles for YAP in CMs, epicardial cells, and fibroblasts that lead to differential immune cell recruitment and proliferation [44,64,69]. In turn, these myeloid cells signal to other cell types in the microenvironment. Further investigation into the connection between YAP and macrophages and other inflammatory cells in the cardiac microenvironment is warranted.
Acknowledgements
We apologize to researchers whose work is not cited here due to space constraints. Nicole Stancel, PhD, ELS(D) (Texas Heart Institute, Houston, TX, USA) contributed to the editing of the manuscript.
Funding information
This work was supported by the American Heart Association (AHA) Postdoctoral Fellowship (18POST34060186 to S.L.; 903651 to R.G.L.), the AHA Career Development Award (849706 to S.L.), the National Institutes of Health (DE 023177, HL 127717, HL 130804, and HL 118761 to J.F.M.), and the Vivian L. Smith Foundation (to J.F.M.). J.F.M. received support from the LeDucq Foundation’s Transatlantic Networks of Excellence in Cardiovascular Research (14CVD01) and the MacDonald Research Fund Award (16RDM001).
Abbreviations:
- AAV9
adeno-associated virus 9
- ChIP
chromatin immunoprecipitation sequencing
- CM
cardiomyocyte
- DGC
dystrophin-glycoprotein complex
- ECM
extracellular matrix
- FOXO1
forkhead box protein O1
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- IFN-γ
interferon gamma
- IL-33
interleukin-33
- iPSC
induced pluripotent stem cell
- MADM
mosaic analysis with double markers
- MI
myocardial infarction
- MOB
Mps1-binder-related
- NF-κB
nuclear factor kappa B
- PITX2
paired like homeodomain 2
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- SWI/SNF
Switch/sucrose non-fermentable
- AZ
Transcriptional co-activator with PDZ-binding motif
- TEAD
TEA domain
- TNF-α
tumor necrosis factor alpha
- YAP
yes-associated protein
Footnotes
Disclosures
The authors declare no competing interests.
References
- [1].Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M, The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries, J. Am. Coll. Cardiol. 63 (12) (2014) 1123–1133. [DOI] [PubMed] [Google Scholar]
- [2].Braunwald E, The war against heart failure: the lancet lecture, Lancet 385 (9970) (2015) 812–824. [DOI] [PubMed] [Google Scholar]
- [3].Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American C. Heart Association Statistics, S. Stroke Statistics, Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation 119 (3) (2009) 480–486. [DOI] [PubMed] [Google Scholar]
- [4].Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E, The extracellular matrix protein agrin promotes heart regeneration in mice, Nature 547 (7662) (2017) 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF, Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size, Science 332 (6028) (2011) 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF, Hippo signaling impedes adult heart regeneration, Development 140 (23) (2013) 4683–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, Martin JF, Hippo pathway deficiency reverses systolic heart failure after infarction, Nature 550 (7675) (2017) 260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, Bai Y, Li W, Willerson JT, Martin JF, Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in hippo-deficient mice, Sci. Signal. 8 (375) (2015) ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT, YAP1, the nuclear target of hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy, Proc. Natl. Acad. Sci. U. S. A. 109 (7) (2012) 2394–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel- Duby R, Olson EN, Hippo pathway effector Yap promotes cardiac regeneration, Proc. Natl. Acad. Sci. U. S. A. 110 (34) (2013) 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN, Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size, Sci. Signal. 4 (196) (2011) ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J, Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury, J. Biol. Chem. 288 (6) (2013) 3977–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Heineke J, Molkentin JD, Regulation of cardiac hypertrophy by intracellular signalling pathways, Nat. Rev. Mol. Cell Biol. 7 (8) (2006) 589–600. [DOI] [PubMed] [Google Scholar]
- [14].Soonpaa MH, Field LJ, Survey of studies examining mammalian cardiomyocyte DNA synthesis, Circ. Res. 83 (1) (1998) 15–26. [DOI] [PubMed] [Google Scholar]
- [15].Walsh S, Ponten A, Fleischmann BK, Jovinge S, Cardiomyocyte cell cycle control and growth estimation in vivo–an analysis based on cardiomyocyte nuclei, Cardiovasc. Res. 86 (3) (2010) 365–373. [DOI] [PubMed] [Google Scholar]
- [16].Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisen J, Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover, Exp. Cell Res. 317 (2) (2011) 188–194. [DOI] [PubMed] [Google Scholar]
- [17].Ahuja P, Sdek P, MacLellan WR, Cardiac myocyte cell cycle control in development, disease, and regeneration, Physiol. Rev. 87 (2) (2007) 521–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA, Transient regenerative potential of the neonatal mouse heart, Science 331 (6020) (2011) 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT, Mammalian heart renewal by pre-existing cardiomyocytes, Nature 493 (7432) (2013) 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R, Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice, Proc. Natl. Acad. Sci. U. S. A. 111 (24) (2014) 8850–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA, The oxygen-rich postnatal environment induces cardiomyocyte cell- cycle arrest through DNA damage response, Cell 157 (3) (2014) 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, Zhao H, Tang J, Ji H, Cai D, Han Z, Han Z, Nie Y, Hu S, Wang QD, Sun R, Fei J, Wang F, Chen T, Yan Y, Huang H, Pu WT, Zhou B, Enhancing the precision of genetic lineage tracing using dual recombinases, Nat. Med. 23 (12) (2017) 1488–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, Yu W, Zhang L, Liu X, Liu K, Tang J, Zhang H, Cai D, Ralf AH, Xu Q, Lui KO, Zhou B, Genetic lineage tracing of nonmyocyte population by dual recombinases, Circulation 138 (8) (2018) 793–805. [DOI] [PubMed] [Google Scholar]
- [24].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J, Evidence for cardiomyocyte renewal in humans, Science 324 (5923) (2009) 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra L, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J, Dynamics of cell generation and turnover in the human heart, Cell 161 (7) (2015) 1566–1575. [DOI] [PubMed] [Google Scholar]
- [26].Oberpriller JO, Oberpriller JC, Response of the adult newt ventricle to injury, J. Exp. Zool. 187 (2) (1974) 249–253. [DOI] [PubMed] [Google Scholar]
- [27].Poss KD, Wilson LG, Keating MT, Heart regeneration in zebrafish, Science 298 (5601) (2002) 2188–2190. [DOI] [PubMed] [Google Scholar]
- [28].Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, Chen WC, Abdurrachim D, Lalic J, Tan RS, Lee TH, Zhang J, Cook SA, Early regenerative capacity in the porcine heart, Circulation 138 (24) (2018) 2798–2808. [DOI] [PubMed] [Google Scholar]
- [29].Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC, Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation, Nature 464 (7288) (2010) 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD, Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes, Nature 464 (7288) (2010) 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA, Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family, Proc. Natl. Acad. Sci. U. S. A. 110 (1) (2013) 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM, Complete cardiac regeneration in a mouse model of myocardial infarction, Aging (Albany NY) 4 (12) (2012) 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cui M, Wang Z, Chen K, Shah AM, Tan W, Duan L, Sanchez-Ortiz E, Li H, Xu L, Liu N, Bassel-Duby R, Olson EN, Dynamic Transcriptional Responses to Injury of Regenerative and Non-regenerative Cardiomyocytes Revealed by Single- Nucleus RNA Sequencing, Dev. Cell 53 (1) (2020) 102–116, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cui M, Atmanli A, Morales MG, Tan W, Chen K, Xiao X, Xu L, Liu N, Bassel-Duby R, Olson EN, Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance, Nat. Commun. 12 (1) (2021) 5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ, Cardiomyocyte DNA synthesis and binucleation during murine development, Am. J. Phys. 271 (5 Pt 2) (1996) H2183–H2189. [DOI] [PubMed] [Google Scholar]
- [36].Girard J, Ferre P, Pegorier JP, Duee PH, Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition, Physiol. Rev. 72 (2) (1992) 507–562. [DOI] [PubMed] [Google Scholar]
- [37].Lopaschuk GD, Jaswal JS, Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation, J. Cardiovasc. Pharmacol. 56 (2) (2010) 130–140. [DOI] [PubMed] [Google Scholar]
- [38].Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE, Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest, Proc. Natl. Acad. Sci. U. S. A. 114 (40) (2017) E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Quaife-Ryan GA, Sim CB, Ziemann M, Kaspi A, Rafehi H, Ramialison M, El-Osta A, Hudson JE, Porrello ER, Multicellular transcriptional analysis of mammalian heart regeneration, Circulation 136 (12) (2017) 1123–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Liu S, Heallen T, Martin JF, The hippo pathway in the heart: pivotal roles in development, disease, and regeneration, Nat. Rev. Cardiol. 15 (11) (2018) 672–684. [DOI] [PubMed] [Google Scholar]
- [41].Yu FX, Zhao B, Guan KL, Hippo pathway in organ size control, Tissue Homeostasis, and Cancer, Cell 163 (4) (2015) 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin Z, Guo H, Cao Y, Zohrabian S, Zhou P, Ma Q, VanDusen N, Guo Y, Zhang J, Stevens SM, Liang F, Quan Q, van Gorp PR, Li A, Dos Remedios C, He A, Bezzerides VJ, Pu WT, Acetylation of VGLL4 regulates hippo-YAP signaling and postnatal cardiac growth, Dev. Cell 39 (4) (2016) 466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM, Morrisey EE, A microRNA-hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice, Sci. Transl. Med. 7 (279) (2015) 279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monroe TO, Hill MC, Morikawa Y, Leach JP, Heallen T, Cao S, Krijger PHL, de Laat W, Wehrens XHT, Rodney GG, Martin JF, YAP Partially Reprograms Chromatin Accessibility to Directly Induce Adult Cardiogenesis In Vivo, Dev. Cell 48 (6) (2019) 765–779, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG, Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish, Dev. Cell 44 (4) (2018) 433–446, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR, Sucov HM, Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration, Nat. Genet. 49 (9) (2017) 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chang L, Azzolin L, Di Biagio D, Zanconato F, Battilana G, Lucon Xiccato R, Aragona M, Giulitti S, Panciera T, Gandin A, Sigismondo G, Krijgsveld J, Fassan M, Brusatin G, Cordenonsi M, Piccolo S, The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ, Nature 563 (7730) (2018) 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dorn GW 2nd, Parkin-dependent mitophagy in the heart, J. Mol. Cell. Cardiol. 95 (2016) 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barton ER, Impact of sarcoglycan complex on mechanical signal transduction in murine skeletal muscle, Am. J. Phys. Cell Phys. 290 (2) (2006) C411–C419. [DOI] [PubMed] [Google Scholar]
- [50].Goyenvalle A, Seto JT, Davies KE, Chamberlain J, Therapeutic approaches to muscular dystrophy, Hum. Mol. Genet. 20 (R1) (2011) R69–R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF, Dystrophin-glycoprotein complex sequesters yap to inhibit cardiomyocyte proliferation, Nature 547 (7662) (2017) 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT, Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model, Circ. Res. 115 (3) (2014) 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J, A functional interaction between hippo-YAP signalling and FoxO1 mediates the oxidative stress response, Nat. Commun. 5 (2014) 3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, Martin JF, Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury, Nature 534 (7605) (2016) 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu S, Tang L, Zhao X, Nguyen B, Heallen TR, Li M, Wang J, Wang J, Martin JF, Yap promotes noncanonical Wnt signals from cardiomyocytes for heart regeneration, Circ. Res. 129 (8) (2021) 782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero- Martin JM, Dolmatova E, Duffy HS, Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, Pu WT, Adult mouse epicardium modulates myocardial injury by secreting paracrine factors, J. Clin. Invest. 121 (5) (2011) 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R, Olson EN, C/EBP transcription factors mediate epicardial activation during heart development and injury, Science 338 (6114) (2012) 1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, Schneider MD, van den Hoff MJ, Butte MJ, Yang PC, Walsh K, Zhou B, Bernstein D, Mercola M, Ruiz-Lozano P, Epicardial FSTL1 reconstitution regenerates the adult mammalian heart, Nature 525 (7570) (2015) 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lavine KJ, Ornitz DM, Rebuilding the coronary vasculature: hedgehog as a new candidate for pharmacologic revascularization, Trends Cardiovasc. Med. 17 (3) (2007) 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brade T, Kumar S, Cunningham TJ, Chatzi C, Zhao X, Cavallero S, Li P, Sucov HM, Ruiz-Lozano P, Duester G, Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2, Development 138 (1) (2011) 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD, Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations, Circ. Res. 103 (12) (2008) 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xiao Y, Hill MC, Zhang M, Martin TJ, Morikawa Y, Wang S, Moise AR, Wythe JD, Martin JF, Hippo signaling plays an essential role in cell state transitions during cardiac fibroblast development, Dev. Cell 45 (2) (2018) 153–169, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Singh A, Ramesh S, Cibi DM, Yun LS, Li J, Li L, Manderfield LJ, Olson EN, Epstein JA, Singh MK, Hippo signaling mediators yap and Taz are required in the epicardium for coronary vasculature development, Cell Rep. 15 (7) (2016) 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ramjee V, Li D, Manderfield LJ, Liu F, Engleka KA, Aghajanian H, Rodell CB, Lu W, Ho V, Wang T, Li L, Singh A, Cibi DM, Burdick JA, Singh MK, Jain R, Epstein JA, Epicardial YAP/TAZ orchestrate an immunosuppressive response following myocardial infarction, J. Clin. Invest. 127 (3) (2017) 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA, The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology, Immunity 37 (3) (2012) 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ, Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs, J. Clin. Invest. 116 (9) (2006) 2434–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nishikawa H, Kato T, Tawara I, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Old LJ, Shiku H, IFN-gamma controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response, J. Immunol. 175 (7) (2005) 4433–4440. [DOI] [PubMed] [Google Scholar]
- [68].Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M, Effect of IFN-beta therapy on the frequency and function of CD4(+)CD25(+) regulatory T cells and Foxp3 gene expression in relapsing-remitting multiple sclerosis (RRMS): a preliminary study, J. Neuroimmunol. 218 (1–2) (2010) 120–124. [DOI] [PubMed] [Google Scholar]
- [69].Xiao Y, Hill MC, Li L, Deshmukh V, Martin TJ, Wang J, Martin JF, Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis, Genes Dev. 33 (21− 22) (2019) 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Francisco J, Zhang Y, Nakada Y, Jeong JI, Huang CY, Ivessa A, Oka S, Babu GJ, Del Re DP, AAV-mediated YAP expression in cardiac fibroblasts promotes inflammation and increases fibrosis, Sci. Rep. 11 (1) (2021) 10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Noels H, Weber C, Koenen RR, Chemokines as therapeutic targets in cardiovascular disease, Arterioscler. Thromb. Vasc. Biol. 39 (4) (2019) 583–592. [DOI] [PubMed] [Google Scholar]
- [72].Xia Y, Frangogiannis NG, MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy, Inflamm. Allergy Drug Targets 6 (2) (2007) 101–107. [DOI] [PubMed] [Google Scholar]
- [73].Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN, Macrophages are required for neonatal heart regeneration, J. Clin. Invest. 124 (3) (2014) 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shen JZ, Morgan J, Tesch GH, Fuller PJ, Young MJ, CCL2-dependent macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in male mice, Endocrinology 155 (3) (2014) 1057–1066. [DOI] [PubMed] [Google Scholar]
- [75].Mia MM, Cibi DM, Binte Abdul Ghani SA, Singh A, Tee N, Sivakumar V, Bogireddi H, Cook SA, Mao J, Singh MK, Loss of Yap/taz in cardiac fibroblasts attenuates adverse remodeling and improves cardiac function, Cardiovasc. Res. (2021), 10.1093/cvr/cvab205. [DOI] [PubMed] [Google Scholar]
- [76].Del Re DP, Matsuda T, Zhai P, Gao S, Clark GJ, Van Der Weyden L, Sadoshima J, Proapoptotic Rassf1A/Mst1 signaling in cardiac fibroblasts is protective against pressure overload in mice, J. Clin. Invest. 120 (10) (2010) 3555–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH, Im CR, Lee JO, Yonehara S, Lim DS, Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis, Cancer Res. 66 (5) (2006) 2562–2569. [DOI] [PubMed] [Google Scholar]
- [78].Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP, RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network, Curr. Biol. 17 (8) (2007) 700–705. [DOI] [PubMed] [Google Scholar]
- [79].Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, Pu W, Huang X, He L, Cai CL, Camargo FD, Pu WT, Zhou B, Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion, J. Biol. Chem. 289 (27) (2014) 18681–18692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Artap S, Manderfield LJ, Smith CL, Poleshko A, Aghajanian H, See K, Li L, Jain R, Epstein JA, Endocardial hippo signaling regulates myocardial growth and cardiogenesis, Dev. Biol. 440 (1) (2018) 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno- Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ, Zheng D, Lenz J, Baldwin HS, Chang CP, Zhou B, Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling, Cell 151 (5) (2012) 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G, Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor, Nature 378 (6555) (1995) 390–394. [DOI] [PubMed] [Google Scholar]
- [83].Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C, Requirement for neuregulin receptor erbB2 in neural and cardiac development, Nature 378 (6555) (1995) 394–398. [DOI] [PubMed] [Google Scholar]
- [84].Meyer D, Birchmeier C, Multiple essential functions of neuregulin in development, Nature 378 (6555) (1995) 386–390. [DOI] [PubMed] [Google Scholar]
- [85].Spannbauer A, Traxler D, Zlabinger K, Gugerell A, Winkler J, Mester- Tonczar J, Lukovic D, Muller C, Riesenhuber M, Pavo N, Gyongyosi M, Large animal models of heart failure with reduced ejection fraction (HFrEF), Front Cardiovasc. Med. 6 (2019) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, Stienen GJ, Lamers JM, Duncker DJ, Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction, Circ. Res. 95 (11) (2004) e85–e95. [DOI] [PubMed] [Google Scholar]
- [87].Milani-Nejad N, Janssen PM, Small and large animal models in cardiac contraction research: advantages and disadvantages, Pharmacol. Ther. 141 (3) (2014) 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Stubenitsky R, van der Weerd RW, Haitsma DB, Verdouw PD, Duncker DJ, Cardiovascular effects of the novel Ca2+− sensitiser EMD 57033 in pigs at rest and during treadmill exercise, Br. J. Pharmacol. 122 (7) (1997) 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McCormick ME, Manduchi E, Witschey WR, Gorman RC, Gorman JH 3rd, Jiang YZ, Stoeckert CJ Jr., Barker, Markl M, Davies PF, Integrated regional cardiac hemodynamic imaging and RNA sequencing reveal corresponding heterogeneity of Ventricular Wall shear stress and endocardial transcriptome, J. Am. Heart Assoc. 5 (4) (2016), e003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gyongyosi M, Pavo N, Lukovic D, Zlabinger K, Spannbauer A, Traxler D, Goliasch G, Mandic L, Bergler-Klein J, Gugerell A, Jakab A, Szankai Z, Toth L, Garamvolgyi R, Maurer G, Jaisser F, Zannad F, Thum T, Batkai S, Winkler J, Porcine model of progressive cardiac hypertrophy and fibrosis with secondary postcapillary pulmonary hypertension, J. Transl. Med. 15 (1) (2017) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Velayutham N, Alfieri CM, Agnew EJ, Riggs KW, Baker RS, Ponny SR, Zafar F, Yutzey KE, Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine, J. Mol. Cell. Cardiol. 146 (2020) 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Agnew EJ, Velayutham N, Matos Ortiz G, Alfieri CM, Hortells L, Moore V, Riggs KW, Baker RS, Gibson AM, Ponny SR, Alsaied T, Zafar F, Yutzey KE, Scar formation with decreased cardiac function following ischemia/reperfusion injury in 1 month Old swine, J. Cardiovasc. Dev. Dis. 7 (1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, Hunter JD, Borovjagin AV, Walcott GP, Chen JY, Qin G, Zhang J, Regenerative potential of neonatal porcine hearts, Circulation 138 (24) (2018) 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liu S, Li K, Wagner Florencio L, Tang L, Heallen TR, Leach JP, Wang Y, Grisanti F, Willerson JT, Perin EC, Zhang S, Martin JF, Gene therapy knockdown of hippo signaling induces cardiomyocyte renewal in pigs after myocardial infarction, Sci. Transl. Med. 13 (600) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gyongyosi M, Dib N, Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease, Nat. Rev. Cardiol. 8 (7) (2011) 393–404. [DOI] [PubMed] [Google Scholar]
- [96].Zhao M, Nakada Y, Wei Y, Bian W, Chu Y, Borovjagin AV, Xie M, Zhu W, Nguyen T, Zhou Y, Serpooshan V, Walcott GP, Zhang J, Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial repair in a swine model of myocardial infarction, Circulation 144 (3) (2021) 210–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, Ali H, Braga L, Gorgodze N, Bernini F, Burchielli S, Collesi C, Zandona L, Sinagra G, Piacenti M, Zacchigna S, Bussani R, Recchia FA, Giacca M, MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs, Nature 569 (7756) (2019) 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Plouffe SW, Meng Z, Lin KC, Lin B, Hong AW, Chun JV, Guan KL, Characterization of hippo pathway components by gene inactivation, Mol. Cell 64 (5) (2016) 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Poon CL, Lin JI, Zhang X, Harvey KF, The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-warts-hippo pathway, Dev. Cell 21 (5) (2011) 896–906. [DOI] [PubMed] [Google Scholar]
- [100].Zhao B, Li L, Tumaneng K, Wang CY, Guan KL, A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta- TRCP), Genes Dev. 24 (1) (2010) 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Giacca M, Zacchigna S, Harnessing the microRNA pathway for cardiac regeneration, J. Mol. Cell. Cardiol. 89 (Pt A) (2015) 68–74. [DOI] [PubMed] [Google Scholar]
- [102].Diez-Cunado M, Wei K, Bushway PJ, Maurya MR, Perera R, Subramaniam S, Ruiz-Lozano P, Mercola M, miRNAs that induce human cardiomyocyte proliferation converge on the hippo pathway, Cell Rep. 23 (7) (2018) 2168–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M, Functional screening identifies miRNAs inducing cardiac regeneration, Nature 492 (7429) (2012) 376–381. [DOI] [PubMed] [Google Scholar]
- [104].Aguirre A, Montserrat N, Zacchigna S, Nivet E, Hishida T, Krause MN, Kurian L, Ocampo A, Vazquez-Ferrer E, Rodriguez-Esteban C, Kumar S, Moresco JJ, Yates JR 3rd, Campistol JM, Sancho-Martinez I, Giacca M, Izpisua Belmonte JC, In vivo activation of a conserved microRNA program induces mammalian heart regeneration, Cell Stem Cell 15 (5) (2014) 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, Guan KL, A YAP/TAZ-induced feedback mechanism regulates hippo pathway homeostasis, Genes Dev. 29 (12) (2015) 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Moroishi T, Hansen CG, Guan KL, The emerging roles of YAP and TAZ in cancer, Nat. Rev. Cancer 15 (2) (2015) 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Caporizzo MA, Chen CY, Prosser BL, Cardiac microtubules in health and heart disease, Exp. Biol. Med. (Maywood) 244 (15) (2019) 1255–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB, Prosser BL, Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure, Nat. Med. 24 (8) (2018) 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Vite A, Zhang C, Yi R, Emms S, Radice GL, Alpha-catenin-dependent cytoskeletal tension controls yap activity in the heart, Development 145 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, van Roy F, Radice GL, Alpha-catenins control cardiomyocyte proliferation by regulating yap activity, Circ. Res. 116 (1) (2015) 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ragni CV, Diguet N, Le Garrec JF, Novotova M, Resende TP, Pop S, Charon N, Guillemot L, Kitasato L, Badouel C, Dufour A, Olivo-Marin JC, Trouve A, McNeill H, Meilhac SM, Amotl1 mediates sequestration of the hippo effector Yap1 downstream of Fat4 to restrict heart growth, Nat. Commun. 8 (2017) 14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD, Yap1 acts downstream of alpha-catenin to control epidermal proliferation, Cell 144 (5) (2011) 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chang YC, Wu JW, Wang CW, Jang AC, Hippo signaling-mediated Mechanotransduction in cell movement and Cancer metastasis, Front. Mol. Biosci. 6 (2019) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu S, Martin JF, The regulation and function of the hippo pathway in heart regeneration, Wiley Interdiscip. Rev. Dev. Biol. 8 (1) (2019), e335. [DOI] [PubMed] [Google Scholar]
- [115].Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, Piccolo S, YAP/TAZ link cell mechanics to notch signalling to control epidermal stem cell fate, Nat. Commun. 8 (2017) 15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Halder G, Dupont S, Piccolo S, Transduction of mechanical and cytoskeletal cues by YAP and TAZ, Nat. Rev. Mol. Cell Biol. 13 (9) (2012) 591–600. [DOI] [PubMed] [Google Scholar]
- [117].Honkoop H, de Bakker DE, Aharonov A, Kruse F, Shakked A, Nguyen PD, de Heus C, Garric L, Muraro MJ, Shoffner A, Tessadori F, Peterson JC, Noort W, Bertozzi A, Weidinger G, Posthuma G, Grun D, van der Laarse WJ, Klumperman J, Jaspers RT, Poss KD, van Oudenaarden A, Tzahor E, Bakkers J, Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Doenst T, Nguyen TD, Abel ED, Cardiac metabolism in heart failure: implications beyond ATP production, Circ. Res. 113 (6) (2013) 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC, Myocardial fatty acid metabolism in health and disease, Physiol. Rev. 90 (1) (2010) 207–258. [DOI] [PubMed] [Google Scholar]
- [120].Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL, Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart, Proc. Natl. Acad. Sci. U. S. A. 111 (45) (2014) 16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD, An acute immune response underlies the benefit of cardiac stem cell therapy, Nature 577 (7790) (2020) 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang Z, Cui M, Shah AM, Ye W, Tan W, Min YL, Botten GA, Shelton JM, Liu N, Bassel-Duby R, Olson EN, Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling, Proc. Natl. Acad. Sci. U. S. A. 116 (37) (2019) 18455–18465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT, The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices, Cancer Res. 66 (9) (2006) 4652–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Billings SE, Pierzchalski K, Butler Tjaden NE, Pang XY, Trainor PA, Kane MA, Moise AR, The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development, FASEB J. 27 (12) (2013) 4877–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]