Abstract
Group B streptococci (GBS) are a major cause of severe infection in newborns, pregnant females, and other immunocompromised hosts. Infection often includes septicemia, shock, pneumonia, and respiratory failure. In previous studies, we have reported that GBS induce marked production of tumor necrosis factor alpha (TNF-α) by human mononuclear cells. The present study was designed to measure the production of TNF-α as well as additional cytokines, including interleukin 1β (IL-1β), IL-6, IL-8, IL-12, and gamma interferon (IFN-γ) but also to determine from what cells and at what time point during incubation with GBS that these cytokines are produced. Mixed mononuclear cells were incubated with heat-killed GBS, media alone, or 1 μg of Escherichia coli lipopolysaccharide (LPS). Brefeldin A was added to each sample prior to staining, which prevented the export of cytokines by the Golgi apparatus. The cells were then stained with the appropriate conjugated antibodies and analyzed by using a flow cytometer. Results indicate that intracellular cytokines appear, in almost all cases, simultaneous to or before secreted proteins are detected. In contrast to the response to LPS, where TNF-α, IL-1β, IL-6, and IL-8 appear almost simultaneously, the human monocyte response to GBS results in the production of TNF-α but delayed appearance of IL-1β, IL-6, and IL-8. The lymphocyte response to GBS was also strikingly different from that to LPS in that both secreted IFN-γ and IL-12 was detected, while LPS failed to induce production of these critical cytokines. This suggests an important role for TNF-α, IFN-γ, and IL-12 in GBS pathogenesis and/or immunity.
Group B streptococci (GBS) are a major cause of severe and overwhelming infection in newborns, pregnant females, and other immunocompromised hosts (15). Patients in the neonatal period often present with early onset infection, which includes septicemia, shock, pneumonia, and respiratory failure. Late onset infection, in contrast, commonly is associated with meningitis. Interestingly, early in both types of infection, the inflammatory response at the site of infection is sparse (5). In later stages of the infection, as the number of bacteria multiplies, there is an increase in host defense activity, which includes the release of tumor necrosis factor alpha (TNF-α) and other cytokines (8, 23, 30). Cytokines are soluble proteins that have a significant role in the immunoregulation of immune and inflammatory responses (1, 18). Specifically, they regulate the growth, differentiation, and function of a wide variety of cells and mediate both normal and pathological immune responses. Additionally, they may have both effector and regulatory activities. Cytokines can have multiple functions, target many cellular subsets, and be expressed by diverse cellular subsets (23, 27). TNF-α is a cytokine that is produced by monocytes, macrophages, and other antigen-presenting cells upon stimulation with endotoxin or other bacterial products. It plays an important role in the development of an effective, early inflammatory response. Increased systemic TNF-α concentrations, however, have been correlated with mortality rates for septic shock in both adults and children (3, 28). TNF-α can induce hypotension, tissue injury, and death in animals and is felt to be the major mediator of endotoxin-induced shock (31). In previous studies, it was first reported that GBS induce marked production of TNF-α by human mononuclear cells (24, 33). Thus, although TNF-α likely plays a major role in host resistance to GBS, in excess it probably has a central role in the pathogenesis of profound shock due to these organisms. In the present study, we examined the production of TNF-α and several other members of the proinflammatory cytokine cascade by human mixed mononuclear cells (MMC) following exposure to GBS, as well as the major mediator of gram-negative bacterial septic shock, lipopolysaccharide (LPS). Recently, Jung et al. (17) and Picker et al. (25) adapted a method to detect the intracellular expression of cytokines. This process disrupts intracellular Golgi-mediated transport with drugs, such as monensin or Brefeldin A, allowing the assessment of intracellular cytokine production by various cell types by using flow cytometry. The present study was designed to not only measure the intracellular production of TNF-α, interleukin 1β (IL-1β), IL-6, IL-8, IL-12, and gamma interferon (IFN-γ) but also determine from what cells and at what time points during incubation with GBS these cytokines are being produced or released extracellularly.
(This work was presented in part at the Western Society for Pediatric meeting, Carmel, Calif., February 1998, and at the Society for Pediatric Research meetings, New Orleans, La., May 1998.)
MATERIALS AND METHODS
Ten milliliters of heparinized peripheral blood was obtained from six healthy adult subjects, and MMC were isolated by density-gradient centrifugation (Ficoll-Paque; Pharmacia, Piscataway, N.J.), washed with Hanks balanced salt solution, and diluted with RPMI 1640 culture media with 10% heat-inactivated pooled normal human serum to 106 cells/ml. A 1-ml volume of MMC was incubated with (106/ml) live or heat-inactivated type III GBS (COH1; courtesy of C. Rubens, University of Washington, Seattle, Wash.), culture media alone, or 1 μg of Escherichia coli LPS (Sigma Chemical, St. Louis, Mo.) for 1, 2, 4, 8, 12, 18, 24, and 48 h at 37°C in 5% CO2. Four hours prior to completion of incubation, 20 μl of Brefeldin A (Becton Dickinson, San Jose, Calif.) was added to each incubation tube to inactivate the Golgi apparatus and prevent cytokine secretion. Upon completion of incubation, MMC were centrifuged at 1,600 rpm for 5 min and the excess supernatant was removed. The cells were then treated with normal mouse immunoglobulin G1 (IgG1) as a surface blocking agent and fluorescein isothiocyanate-labeled anti-CD69 (activation marker) monoclonal antibody. Lysing and permeabilization were accomplished by using Becton Dickinson fluorescence-activated cell sorter lysing and permeabilization solutions. The MMC were then processed and surface stained with peridinin chlorophyll protein-labeled anti-CD45 antibodies (a surface marker for monocytes and lymphocytes) or anti-CD14 (a marker for monocytes), fluorescein isothiocyanate-labeled anti-CD69 (an activation marker), and the appropriate intracellular phycoerythrin-conjugated cytokine antibody. The stained MMC were analyzed by flow cytometry. Supernatants from cell cultures which contained extracellular cytokines were analyzed by enzyme-linked immunosorbant assay (Immunotech, Westbrook, Maine). Analysis of the MMC response to activation by type III GBS, culture media alone, or 1 μg of E. coli LPS was accomplished by measuring cytokines both intracellularly and extracellularly in stimulated cells versus unstimulated ones. Initial experiments examining cytokine induction were carried out with living GBS organisms. The results obtained up to 24 h were identical to those obtained with heat-killed GBS, but after this period of time, toxicity was apparent in the cell preparations. For this reason, and because of the comparable results with live and heat-killed GBS, we employed killed bacteria in the majority of these investigations. Reagents were assayed for the presence of endotoxin with the Limulus amebocyte lysate assay (Endotect; Schwarz/Mann Biotech, Cleveland, Ohio), which is sensitive to 0.1 ng/ml, a concentration below that required to stimulate the production of cytokines, and all those utilized were found to be negative.
Extracellular cytokine experiments were carried out at least three times by using peripheral blood from three different healthy adult subjects with duplicate determinations per experiment. (The investigations were approved by the University of Utah Institution Research Board for Human Research. Informed consent was obtained where appropriate for blood sampling.) Results are expressed as means ± standard errors of the means (SEM). The SEM, however, were often so small that they could not be seen in the figures. Intracellular cytokine flow cytometry data were derived from two or three replicates for each of the nine time points. For each time point and replicate, approximately 10,000 cells were analyzed (therefore, standard error was not included in the presentation of the data) and the difference between the geometric means measuring the fluorescent intensity of the positive test cells and those of the control cells was expressed in each figure.
RESULTS
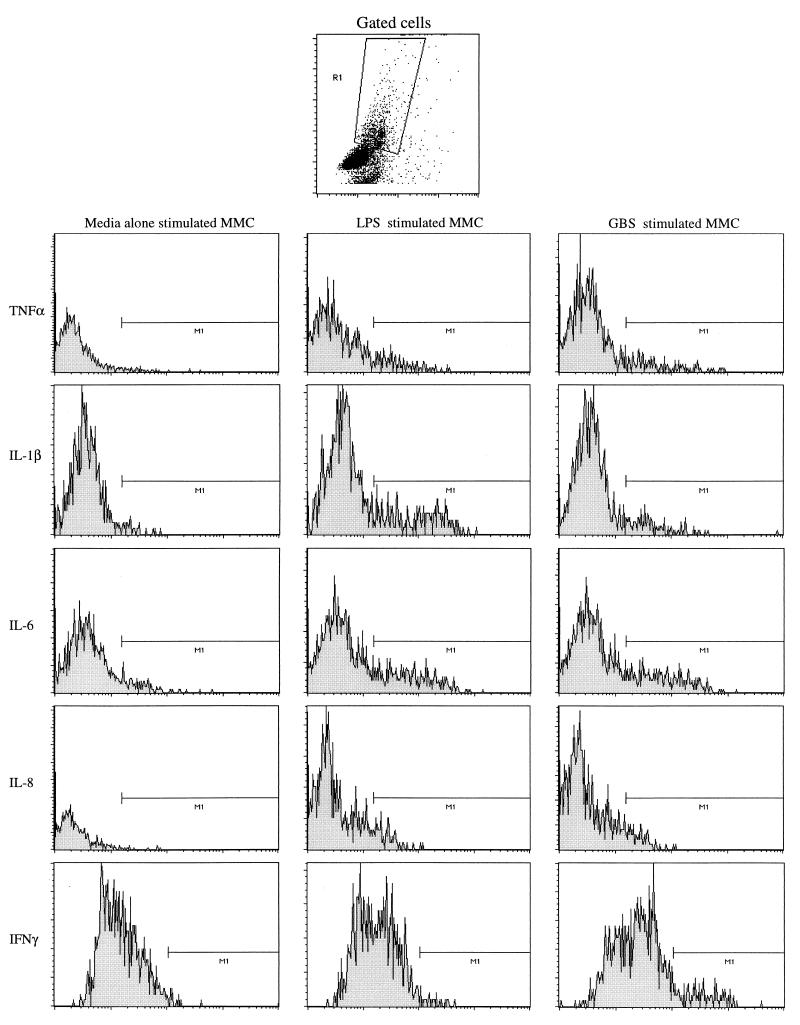
Figure 1 is a representative example of the actual flow cytometry data which are presented in this section. The method of gating that was used to separate the CD45-positive cells from the rest of the MMC is represented at the top of the figure. For each individual cytokine, an example of a histogram of MMC incubated with media alone, LPS-stimulated MMC, and GBS-stimulated MMC is shown.
FIG. 1.
Representative intracellular cytokine flow cytometry data. x axis, number of cells; y axis, fluorescence intensity.
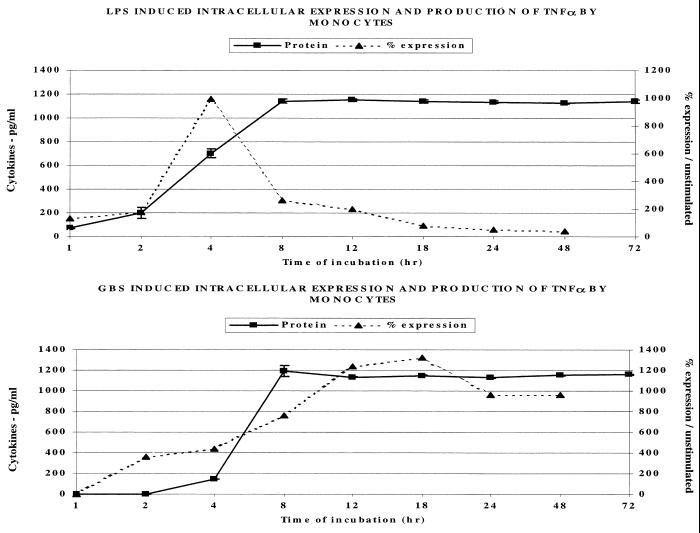
Figure 2 (upper panel) indicates that E. coli LPS in a concentration of 1 μg/ml resulted in a significant increase in the percentage of monocytes expressing intracellular TNF-α following 4 h of incubation. Intracellular expression by electronically gated anti-CD14-labeled monocytes then rapidly declined by 8 h. This was followed by an increase in extracellular TNF-α protein concentration which peaked following 8 h of incubation and plateaued thereafter. In contrast (lower panel), significant intracellular TNF-α was detectable at 2 h following exposure of human MMC to 106 type III GBS per ml. Intracellular TNF-α expression continued to climb, peaking only after 12 to 18 h. Extracellular TNF-α protein rose between 4 and 8 h, plateauing after this time point. As indicated in Materials and Methods, the use of live GBS produced results comparable to those achieved with heat-killed GBS for up to 24 h, but after this point, the use of live organisms resulted in significant toxicity to the mononuclear cells.
FIG. 2.
Effect of LPS (1 μg/ml) and GBS (106/ml) on the intracellular expression and extracellular release of TNF-α protein by human monocytes.
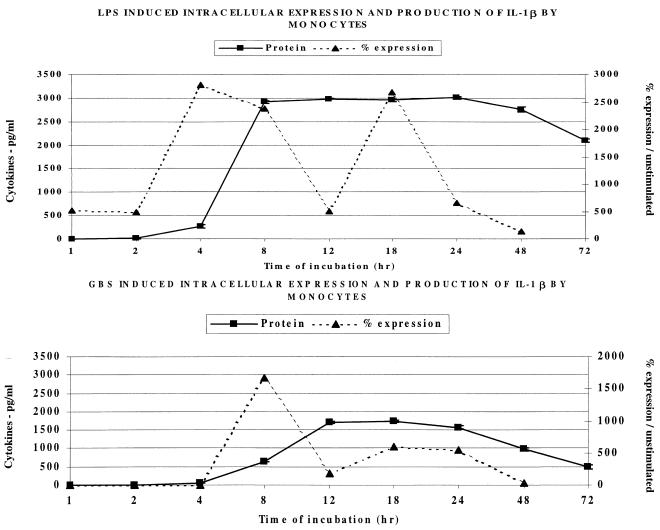
Subsequently, we studied IL-1β production by anti-CD14-labeled monocytes. Figure 3 shows that intracellular expression precedes extracellular production of IL-1β following LPS stimulation, while both intracellular and extracellular IL-1β appear simultaneously after stimulation with GBS. As shown, LPS exposure of mononuclear cells resulted in significant intracellular expression of IL-1β by monocytes appearing at 4 h, with extracellular protein being produced in large quantities between 4 and 8 h. In contrast, intracellular and extracellular IL-1β were not detected until after 8 h of MMC incubation with GBS. Extracellular IL-1β following stimulation with GBS did not peak until after 12 h incubation with human monocytes. Moreover, IL-1β production following GBS stimulation of MMC was more than 1,000 pg/ml less than that with LPS (dose-response experiments had established these were optimal concentrations of both stimulants; data not shown).
FIG. 3.
Effect of LPS (1 μg/ml) and GBS (106/ml) on the intracellular expression and extracellular release of IL-1β by human monocytes.
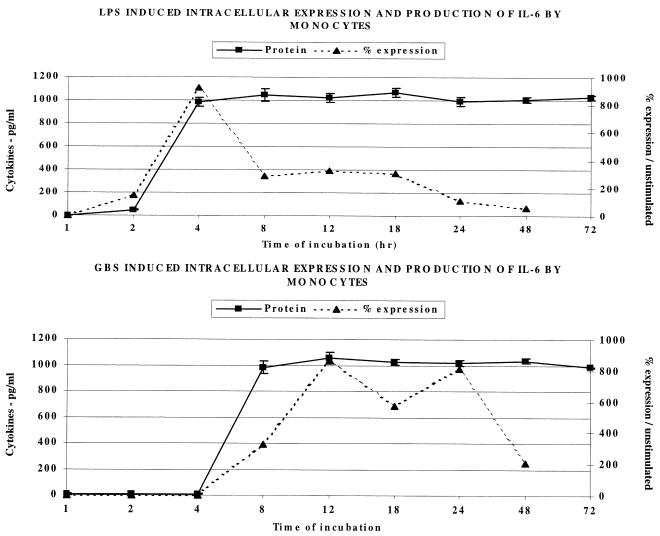
The IL-6 response of anti-CD14-labeled human monocytes to LPS and GBS, as shown in Fig. 4, was similar to that of IL-1β in that the GBS response was delayed, compared to the response to LPS. The peak intracellular and extracellular IL-6 responses to LPS occurred at 4 h, while those to GBS occurred at 8 and 12 h, respectively. Extracellular IL-6 appeared almost simultaneously with intracellular cytokines, perhaps indicating more rapid secretion of this cytokine.
FIG. 4.
Effect of LPS (1 μg/ml) and GBS (106/ml) on the intracellular expression and extracellular release of IL-6 by human monocytes.
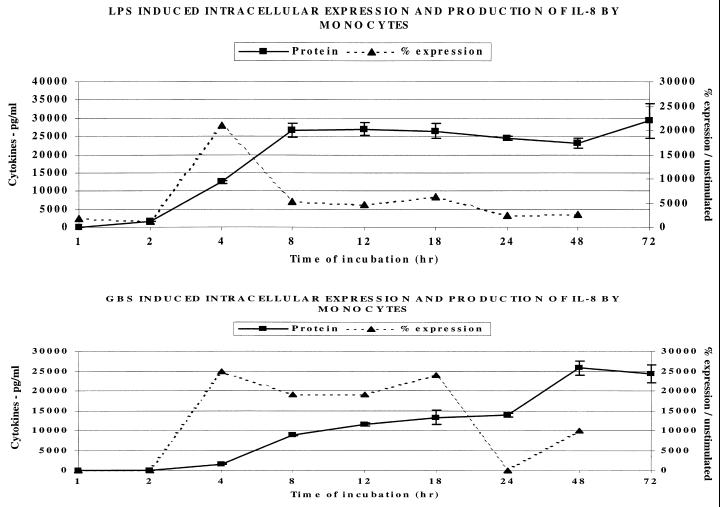
Figure 5 demonstrates that intracellular IL-8 appears after 4 h of incubation with LPS or GBS. In contrast to the LPS response in which extracellular IL-8 peaked at 8 h, the maximal extracellular IL-8 response to GBS was delayed until 48 h.
FIG. 5.
Effect of LPS (1 μg/ml) and GBS (106/ml) on the intracellular expression and extracellular release of IL-8 by human monocytes.
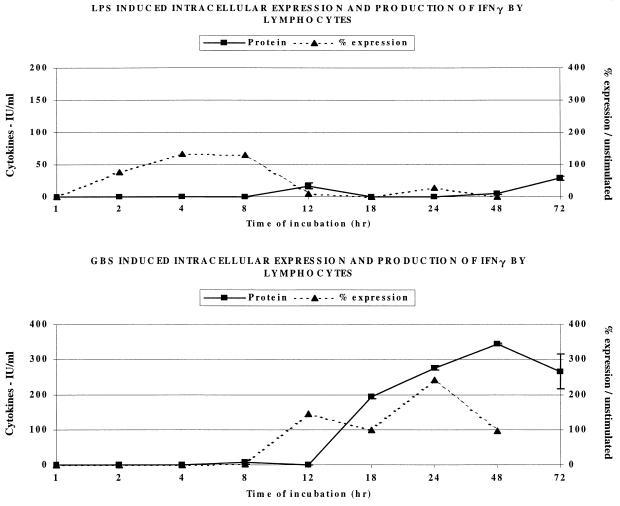
Next, we studied the generation of IFN-γ by the lymphocytes, which are the major cellular source of this cytokine, in human blood. As shown in Fig. 6, following LPS stimulation of lymphocytes, there was significant expression of intracellular IFN-γ by electronically gated anti-CD14-negative anti-CD45-positive lymphocytes at 4 to 8 h but almost no secretion of extracellular cytokine. In contrast, GBS took 12 h to induce intracellular IFN-γ, but this resulted in significant extracellular secretion of this cytokine, peaking after 48 h of incubation.
FIG. 6.
Effect of LPS (1 μg/ml) and GBS (106/ml) on the intracellular expression and extracellular release of IFN-γ by human lymphocytes.
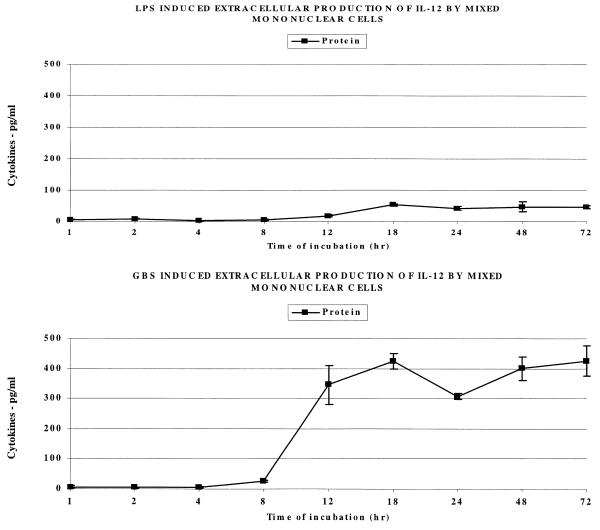
Since IL-12 is known to induce the production of IFN-γ, we next measured the extracellular production of this cytokine by MMC stimulated with LPS or GBS. Labeled antibodies for the detection of intracellular IL-12 were not available to us; thus, only extracellular cytokine measurements were made. As Fig. 7 shows, LPS stimulation of MMC resulted in little to no production of extracellular IL-12 protein. In contrast, GBS caused MMC to secrete significant quantities of IL-12 between 8 and 12 h of incubation. This is at the same time point (8 to 12 h) that intracellular IFN-γ appeared following GBS stimulation, shown in Fig. 6, and prior to the appearance of extracellular IFN-γ between 12 and 18 h. Thus, the extracellular release of IL-12 may have enhanced GBS-induced IFN-γ production and release.
FIG. 7.
Effect of LPS (1 μg/ml) and GBS (106/ml) on extracellular release of IL-12 by human mononuclear cells. The bars represent the SEM; note that they are often so small that they cannot be seen.
DISCUSSION
The cytokines analyzed in the present study were chosen for their critical role in the development of septic shock due to fulminant infection in both neonates and animal models (13, 30). TNF-α, which is the major mediator of endotoxin shock, is a proinflammatory cytokine that produces fever, metabolic acidosis, capillary leak, and cardiovascular collapse, often leading to systemic hypotension. Group B streptococcal infection also produces a profound septic shock syndrome like that caused by gram-negative endotoxemia, especially in neonates. For this reason, we chose to compare the effects of GBS on MMC to that of E. coli LPS. IL-1β may be induced by TNF-α and serves to potentiate the effects of TNF-α. In addition, IL-1β acts to stimulate the production of acute-phase reactants by the liver and costimulates TH1-cell activation (10). The role of IL-6 in the pathophysiology of septic shock is controversial. High IL-6 levels are associated with acute bacterial infections (6, 14) and endotoxemia (11). IL-6 can block the transcription of proinflammatory cytokines, however, such as IL-1β and TNF-α (reviewed in reference 18). Mancuso and colleagues (20) reported data that are compatible with the hypothesis that IL-6 is involved in negative feedback regulation of plasma TNF-α levels in experimental GBS sepsis and that IL-6 pretreatment can increase survival in animal models of GBS-induced sepsis. IL-6 is also directly responsible for the induction of acute-phase proteins, many of which have anti-inflammatory properties (10, 12). IL-8 stimulates neutrophil and monocyte chemotaxis in nanogram amounts (2). Rowen and colleagues (26) reported that neonatal monocytes have diminished production of IL-8, compared to adult monocytes, in response to both GBS and LPS. This decrease in IL-8 production may contribute to the neonate's poor host response to GBS. IFN-γ is produced by TH1 lymphocytes and natural killer (NK) cells and increases the expression of major histocompatibility complex class I and II molecules and, thus, assists in antigen presentation by appropriate cell types. IFN-γ also is a major activator of neutrophils and macrophages, increases B-cell production of IgG2a, and blocks IL-4-induced class switching to IgE and IgG1 (9). IL-12 induces IFN-γ and has been shown to be critical in mounting an appropriate TH1 type immune response (4, 22). Mancuso and colleagues (19) found that recombinant IL-12 significantly improved survival in animal models of GBS infection. Pretreatment with neutralizing anti-IFN-γ monoclonal antibodies significantly counteracted the beneficial effects of recombinant IL-12. This supports the hypothesis that IFN-γ, in addition to being induced by IL-12, may also partially mediate the activity of IL-12.
von Hunolstein et al. (32) recently reported similar results for extracellular TNF-α, IL-1β, and IL-6. Their blockade experiments with anti-TNF-α antibodies would suggest less induction of IL-1β by TNF-α following such treatment. Our data appear to support their findings that extracellular TNF-α appears within 4 h of incubation with GBS and is followed by the production of IL-1β and IL-6. Our study, in addition to extracellular analysis of the above cytokines, provides an intracellular cytokine profile for monocyte and lymphocyte subsets. The data presented here would suggest that intracellular cytokine production of TNF-α is detectable within 2 h of incubation. de Bont and colleagues (8) have reported increased concentrations of TNF-α and IL-6 during neonatal GBS sepsis, whereas IL-1β appeared to be present in low concentrations only. Their results also suggest that IL-1β, but not TNF-α, plasma concentrations appear to correlate inversely with the development of GBS-induced septic shock. Results of the present study indicate that intracellular cytokines, in response to GBS and LPS stimulation, appear simultaneous to, or prior to, secreted proteins in almost all instances. This is, of course, expected. The monocyte responses to LPS for TNF-α, IL-1β, IL-6, and IL-8 are approximately equivalent in time of appearance and peak expression. In contrast, the TNF-α response to GBS appears first and is followed by a delayed response in IL-1β, IL-6, and IL-8. Lymphocytes stimulated with LPS or GBS demonstrated intracellular IFN-γ, but only GBS-stimulated cells produced extracellular IFN-γ protein. One explanation for this may lie in the ability of GBS to stimulate the production of IL-12, which in turn, enhances IFN-γ translation and secretion. Bacteria and bacterial products interact with polymorphonuclear cells, B cells, NK cells, macrophages, and other antigen-presenting cells to induce the production of IL-12. IL-12, in turn, interacts with these same bacteria and bacterial products to stimulate the cellular production of IFN-γ. D'Andrea and colleagues (7) have shown that neutralizing anti-IL-12 antibody blocks 85% of Staphylococcus aureus Cowan I-stimulated IFN-γ production, while such antibody blocks 50% of the IFN-γ response to poor IL-12 inducers, such as IL-2. Thus, the IL-12–IFN-γ pathway may be a critical component of host resistance to gram-positive bacterial infections. Recently, we have demonstrated that MMC from human neonates fail to transcribe and translate or secrete adequate amounts of both IL-12 and IFN-γ in response to GBS (16). We plan additional studies looking at both intracellular and extracellular cytokine production by neonatal cells in response to these organisms.
The initial appearance of TNF-α intracellularly and extracellularly, as secreted cytokine, suggests that this may be a critical component of the early cytokine cascade in response to GBS. As shown by our in vitro studies, only after this initial rise in TNF-α induced by GBS occurs do other cytokines appear intracellularly or extracellularly. Teti et al. (29) have indicated that treatment of mice with anti-TNF-α murine monoclonal antibody improves survival from GBS infection. In contrast, pretreatment in the same murine model prior to inoculation likely blocks the entire cytokine pathway and actually leads to enhanced lethality. Thus, the kinetics of the response appears to be critical. It is easy to speculate early, when streptococci are beginning to invade tissues, that the local release of TNF-α promotes the influx of inflammatory cells and other antibacterial proteins and factors enhancing host resistance. Later, if organisms are able to multiply and gain access to the systemic circulation, the remainder of the cytokine cascade is triggered, leading to massive release of TNF-α, IL-1β, IL-6, IL-8, and IFN-γ, which act synergistically to promote the septic shock syndrome.
Although the use of anti-TNF monoclonal antibody to treat gram-negative septic shock syndrome has not met with overall success in adults, it is possible that such therapy may have a role in the early therapy of GBS infections in neonates or other similarly immunocompromised hosts by interrupting the cytokine cascade, which we have demonstrated here in vitro. More recently, a recombinant human TNF-α receptor (p75)-antibody Fc fragment fusion protein with TNF-α receptor blocking activity has shown promise in treating conditions such as rheumatoid arthritis (21). We are currently initiating studies to determine the efficacy of this agent in blocking the cytokine cascade in mononuclear cells exposed to GBS and also plan to determine its efficacy in preventing septic shock in an experimental rat model of GBS infection.
ACKNOWLEDGMENTS
We thank Jeannette Rejali for manuscript preparation and editorial assistance.
This research was supported by U.S. Public Health Service grant AI 13150.
REFERENCES
- 1.Aggarwal B B, Puri R K. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Sciences; 1995. Common and uncommon features of cytokines and cytokine receptors: an overview; pp. 3–24. [Google Scholar]
- 2.Baggiolini M P, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 3.Casey R B, Balk R A, Bone R C. Plasma cytokine and endotoxin levels correlate with survival in patients with sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chan S H, Perussia B, Gupta J W, Kobayashi M, Pospisil M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen R D, MacFarlane J L, Taylor N L, Hill H R, Rothstein G. Blood and marrow neutrophils during experimental group B streptococcal infection: quantification of the stem cell, proliferative, storage and circulating pools. Pediatr Res. 1982;16:549–553. doi: 10.1203/00006450-198207000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Damas P, Reuter A, Gysen P, Demonty J, Lamy M, Franchimont P. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit Care Med. 1989;17:975–978. doi: 10.1097/00003246-198910000-00001. [DOI] [PubMed] [Google Scholar]
- 7.D'Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bont E S J, Martins A, van Raan J, Samson G, Fetter W P F, Okken A, de Leij L H. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 plasma levels in neonatal sepsis. Pediatr Res. 1993;33:380–383. doi: 10.1203/00006450-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 9.de Maeyer E, de Maeyer-Guignard J. Interferon γ. Curr Opin Immunol. 1992;4:321–326. doi: 10.1016/0952-7915(92)90083-q. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and the treatment of septic shock syndrome. J Infect Dis. 1991;163:1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Moldawer L L, Marano M, Wei H, Tatter S B, Clarick R H, Santhanam U, Sherris D, May L T, Sehgal P B, Lowry S F. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989;142:2321–2324. [PubMed] [Google Scholar]
- 12.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giradin E, Grau G E, Dayer J-M, Roux-Lombard P, Lambert P-H the J5 Study Group. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1978;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 14.Helfgott D C, Tatter S B, Santhanam U, Clarick R H, Bhardwaj N, May L T, Sehgal P B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989;142:948–953. [PubMed] [Google Scholar]
- 15.Hill H R. Group B streptococcal infections. In: Holmes K K, Mardh P A, Sparling P F, Wiesner P J, Cates W, Lemon S M, Stamm W E, editors. Sexually transmitted diseases. New York, N.Y: McGraw Hill; 1990. pp. 851–861. [Google Scholar]
- 16.Joyner J L, Augustine N H, La Pine T R, Hill H R. Effects of group B streptococci on cord and adult mononuclear cell transcription and translation of IL-12 and IFNγ. Pediatr Res. 1999;45:1546A. [Google Scholar]
- 17.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, Moreno de Alboran I, Gonzalo J A, Martinez C. Immunoregulation by cytokines. Crit Rev Immunol. 1993;13:163–191. [PubMed] [Google Scholar]
- 19.Mancuso G, Cusumano V, Genovese F, Gambuzza M, Beninati C, Teti G. Role of interleukin 12 in experimental neonatal sepsis caused by group B streptococci. Infect Immun. 1997;65:3731–3735. doi: 10.1128/iai.65.9.3731-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancuso G, Tomasello F, Migliardo M, Delfino D, Cochran J, Cook J A, Teti G. Beneficial effects of interleukin-6 in neonatal mouse models of group B streptococcal disease. Infect Immun. 1994;62:4997–5002. doi: 10.1128/iai.62.11.4997-5002.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, Widmer M B, Blosch C M. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 22.Murphy E E, Terres G T, Macatonia S E, Hsieh C S, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994;180:223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 24.Peat E B, Augustine N H, Drummond W K, Bohnsack J F, Hill H R. Effects of fibronectin and group B streptococci on TNF-α production by human culture derived macrophages. Immunology. 1995;84:440–445. [PMC free article] [PubMed] [Google Scholar]
- 25.Picker L J, Singh M K, Zdraveski Z, Treer J R, Waldrop S L, Bergstrasse P R, Maino V C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–1419. [PubMed] [Google Scholar]
- 26.Rowen J L, Smith C W, Edwards M S. Group B streptococci elicit leukotriene B4 and interleukin-8 from human monocytes: neonates exhibit a diminished response. J Infect Dis. 1995;172:420–426. doi: 10.1093/infdis/172.2.420. [DOI] [PubMed] [Google Scholar]
- 27.Street N E, Mosmann T R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan J S, Kilpatrick L, Costarino A T, Jr, Lee S C, Harris M C. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatr. 1992;120:510–515. doi: 10.1016/s0022-3476(05)82476-x. [DOI] [PubMed] [Google Scholar]
- 29.Teti G, Mancuso G, Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993;61:227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teti G, Mancuso G, Tomasello F, Chiofalo M S. Production of tumor necrosis factor-α and interleukin-6 in mice infected with group B streptococci. Circ Shock. 1992;38:138–144. [PubMed] [Google Scholar]
- 31.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey III T J, Zentella A, Albert J D, Shires G T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 32.von Hunolstein C, Totolian A, Alfarone G, Mancuso G, Cusumano V, Teti G, Orefici G. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–4021. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams P A, Bohnsack J F, Augustine N H, Drummond K, Rubens C E, Hill H R. Production of tumor necrosis factor by human cells in vitro and in vivo induced by group B streptococci. J Pediatr. 1993;123:292–300. doi: 10.1016/s0022-3476(05)81706-8. [DOI] [PubMed] [Google Scholar]