Abstract
Purpose of Review
The purpose of this review is to describe the main features of the aging chest, studied through different imaging modalities.
Recent Findings
Aging-related changes of the respiratory system are inevitable. Therefore, it is mandatory to be familiar with the para-physiological changes that occurs, in order to avoid inappropriate interpretation of radiological findings that put patients at risk of over or undertreatment.
Summary
The role of the radiologist is fundamental in evaluating aging-related processes affecting the respiratory system and in distinguishing them from frank diseases.
Keywords: Aging chest, Chest CT, Chest X-ray, Digital tomosynthesis, Artificial intelligence
Introduction
Population is aging, with a global life expectancy of around 77.2 years in 2050, as reported by the United Nation Department of Economic and Social Affairs. It is estimated that in 2030 the percentage of population aged 65 years will reach 22% in Europe and Northern America, 19% in Australia/New Zealand, and 16% in Eastern and Southern Asia [1].
Conventionally, an individual older than 65 years of age is defined an elderly [2]. Physiologically, aging involves functional processes and anatomical structures and it is well known that there is a wide spectrum of CT findings that do not represent relevant diseases in elderly asymptomatic patients [3]; however, differentiating between para-physiological changes and pathological features can be particularly challenging in the elderly.
Therefore, knowing aging-related changes is mandatory for a correct interpretation of the images.
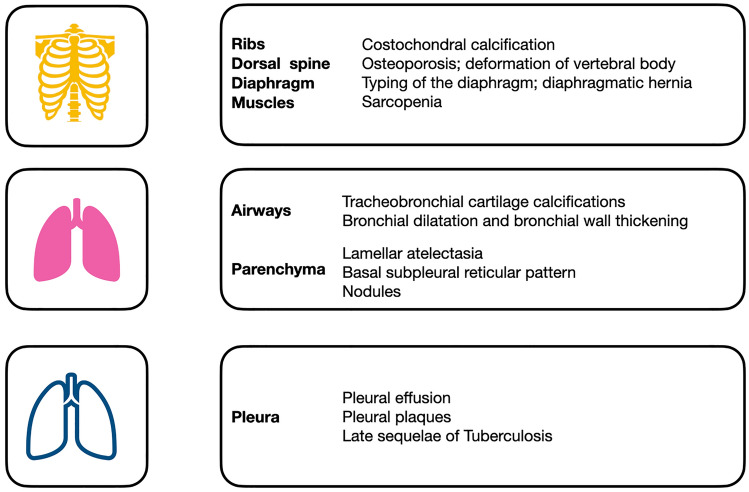
In this review, the main para-physiological changes in the geriatric population will be discussed, with a focus on the possible application of Artificial Intelligence (AI) in this specific area of interest (Fig. 1).
Fig. 1.
Main alterations of thoracic structures in the geriatric population
Main Text
Morphologic Changes of Chest Wall and Thoracic Muscles
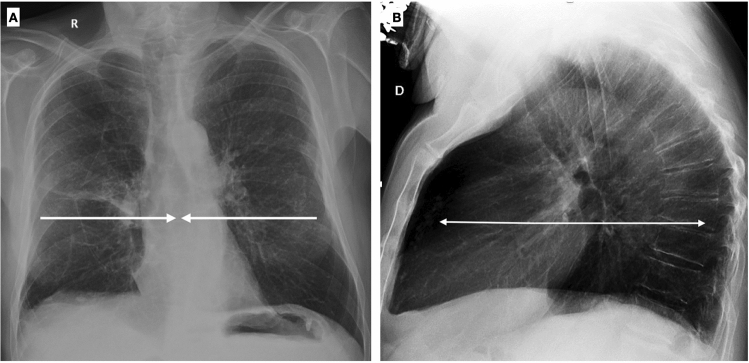
In elderly patients, thorax morphological changes are frequent and need to be known, in order to avoid improper images interpretation. These changes involve the chest wall, thoracic spine, diaphragm, and muscles, with costal cartilage calcification and muscle mass loss representing the most common findings [4]. The chest wall changes in shape and dimension, with an increase of its diameter in the sagittal view and a reduction in the lateral view (Fig. 2A, B).
Fig. 2.
Chest X-ray in postero-anterior (A) and lateral projection (B) shows typical chest wall morphologic changes in shape and dimension: a reduction in the lateral diameter and an increase in the sagittal diameter. On the lateral view a shortening of the thoracic spine is evident, along with the deformation of vertebral bodies and increasing of dorsal kyphosis
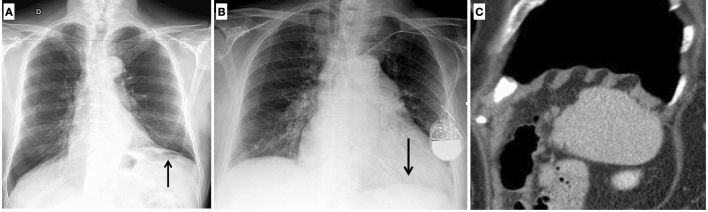
Asymmetry of the lungs may be subsequent to the elevation of the hemidiaphragm, frequently observed after cardiothoracic surgery related to a iatrogenic lesion of the phrenic nerve, or to the lowering of the left hemidiaphragm due to the increase in the heart volume [5]. Moreover, an elevation of the right diaphragm is commonly seen in elderly, an alteration that can be related to the anatomical relationship between the lung and the liver (Fig. 3A, B).
Fig. 3.
On chest X-ray of the elderly, a common finding is the elevation of the left hemidiaphragm related to cardiothoracic surgery (A); on the contrary a lowering of the left hemidiaphragm due to increasing heart volume can be frequently seen as well (B). On sagittal MPR reconstruction, typings of the diaphragm caused by chronic obstructive pulmonary disease should not be misinterpreted as pathological findings (C)
Typing of the diaphragm, typically caused by chronic obstructive pulmonary disease, is a frequent imaging finding in the older adult (Fig. 3C). Large hiatal hernia occurs more frequently in older age, particularly in females, due to the weakened or torn phrenoesophageal membrane [6].
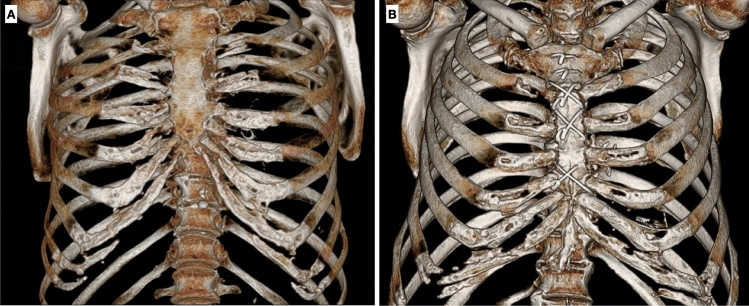
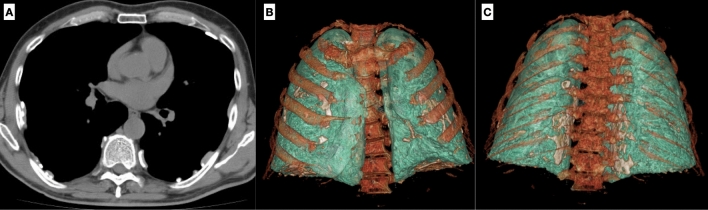
Chondro-sternal ossifications frequently occur in elderly, typically centrally distributed in female and peripheric in male. (Fig. 4).
Fig. 4.
Three-dimensional images of thoracic wall show chondro-sternal ossification, typically located centrally in females (A) and peripherally in males (B)
The so-called “senile osteoporosis” is an age-related process not associated with other diseases, more commonly seen in women. Osteoporosis may lead to a height reduction and deformation of vertebral bodies, resulting in an increase of the kyphosis and in a shortening of the spine. A bridging osteophyte in spondylodiscoarthrosis, costal arthrosic hypertrophy, and bony island can simulate parenchymal opacities on Chest X-ray (CXR), representing the most common cause of doubtful nodular lesion. In these cases, the use of Digital Tomosynthesis (DTS) could be useful to solve the problem.
Aging determines gradual and inevitable changes of functional and structural features of the musculoskeletal system that leads to a loss of volume, leading to a diffuse decrease in attenuation on the CXR of the elderly patient, consequent to the reduced attenuation of fat tissue against muscles, thus simulating emphysema [4]. In addition, along with the structural musculoskeletal changes, a decrease in contractility and elasticity of muscles occurs.
This is physiologically due to the reduction of water content, as well as the increase of connective tissue and lipids. These processes, although their extreme individual variability, depending on genetic, environmental, and lifestyle factors, are significantly reduced in subjects who perform constant physical activity [7]. Several studies have proved the link between sarcopenia and chronic obstructive pulmonary disease (COPD): above all, a systematic review and meta-analysis by Benz et al. showed that the overall presence of sarcopenia in patients with COPD was 21.6%, with a peak of 63% of COPD patients residing in nursing homes [••8]. The combination of sarcopenia and frailty with factors such as impairment of respiratory functions and inactivity are associated with the poorest clinical outcomes [9, 10]. There is a proved vicious circle between sarcopenia and COVID-19: patients with sarcopenia are expected to have increased infection rates and worsen prognoses, as well as COVID-19 itself tends to aggravate sarcopenia because of systemic inflammation and lack of exercise.
The assessment of thoracic muscles may be performed with different imaging methods: in case of sarcopenia, lung ultrasound shows thinning of pectoral muscles, with decreased echogenicity and upheaval of the typical fibrillar appearance [11]. Chest CT allows Hounsfield Unit (HU) average calculation for the evaluation of myosteatosis and measurement of area and perimeter of paraspinal and pectoral muscles [12] (Fig. 5). MRI is superior for the assessment of intramuscular fat because of its better contrast between muscles and fatty tissue [13].
Fig. 5.
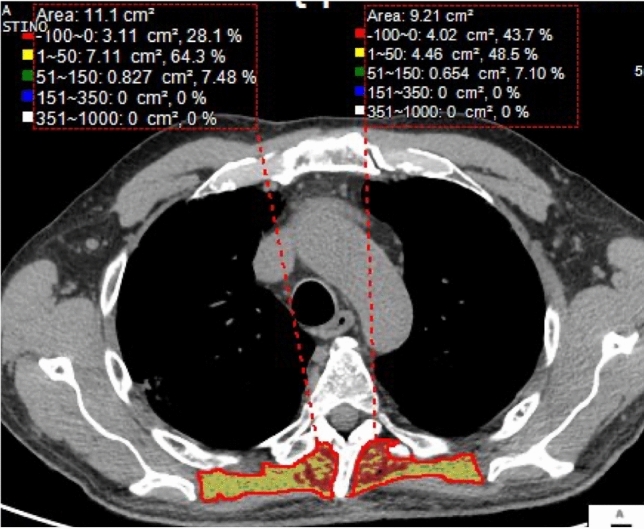
Using commercial software, it is possible to quantify the volume and the density of paraspinal muscles, commonly atrophic in elderly patients
Morphological Changes of the Airways
There is a lack of studies in literature regarding the normal radiological appearance of airways in the elderly [14].
One of the most frequent changes affecting the airways is represented by tracheobronchial cartilage calcifications, which are found in about 40–65% of people aged 60–79 years (Fig. 6). The displacement of the trachea on the right side can be frequently observed, due to the enlargement of the aortic arch.
Fig. 6.
Chest X-ray of a 74-year-old female shows diffuse tracheobronchial calcifications (A), better visualized on coronal MPR reconstruction (B)
Trachea is typically characterized by oval or round shape, non-calcified, with a diameter of 25–27 mm in men and 21–23 mm in women. A tracheal deformity, the so-called “saber-sheath” trachea, is a common finding in the elderlies and is considered a pathognomonic sing of COPD (chronic obstructive pulmonary disease), a feature occurring mostly in men (Fig. 7).
Fig. 7.
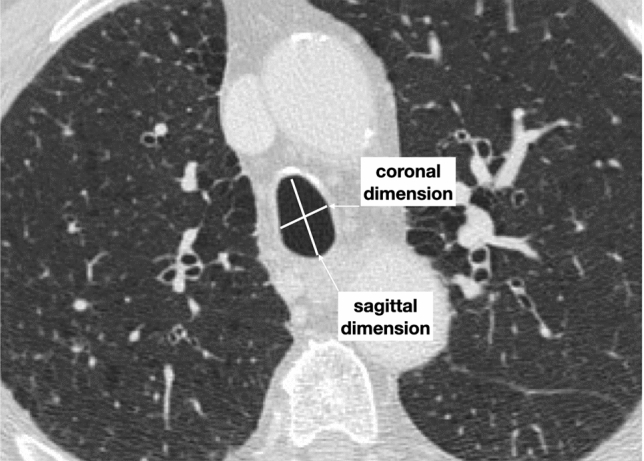
Axial high-resolution CT image in inspiratory phase of a 78-year-old male with a severe COPD shows a saber-sheath trachea characterized by a reduction in coronal diameter with an increase in sagittal diameter (tracheal index < 0.67). Moreover, ossification of tracheal ring can be noticed
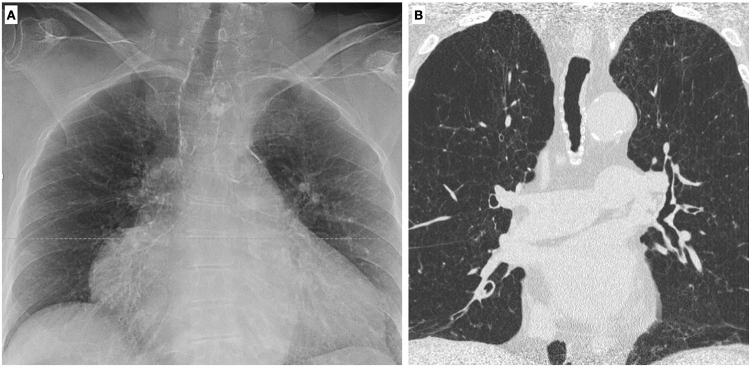
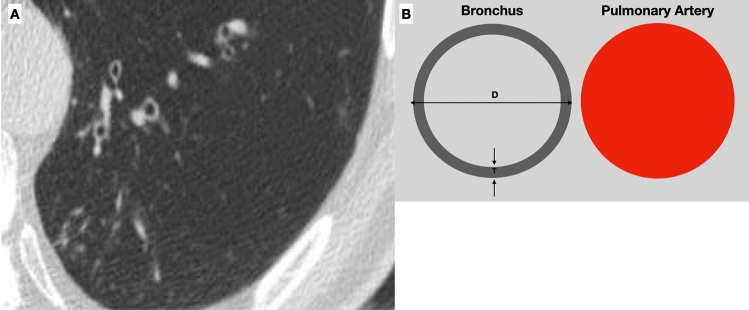
On CT scan it is possible to detect airway structure alterations, such as bronchial wall thickening and bronchial dilatation [15], whose degree correlates with lung function [16]. In asymptomatic elderlies, bronchial dilatation and bronchial wall thickening related to collagen deposition are frequently seen [3] (Fig. 8). Even if Matsuoka et al. reported a significant relationship between bronchoarterial ratio and aging [17], it is still not clear how to interpret these radiological findings; therefore, particular attention should be paid in diagnosing bronchiectasis in asymptomatic elderly patients. Moreover, Lee et al. demonstrated that air trapping is present in more than 50% of asymptomatic subjects older than 61 years [18].
Fig. 8.
Axial high-resolution CT shows bronchial wall thickening and mild dilatation in the lower lobes in an asymptomatic 75-year-old female with normal functional pulmonary tests (A). A normal bronchoarterial ratio requires the bronchus and the adjacent pulmonary artery to have the same diameter (D diameter, T thickness of the bronchial wall) (B)
COPD is a clinically relevant disease, commonly occurring in the elderlies and affecting about 10% of adults over the age of 40. COPD is characterized by airway inflammation and subsequent remodeling, with emphysema and bronchial alterations being the main pulmonary manifestations. COPD is not limited to the lung and extrapulmonary complications are common, including vascular alterations [15].
Nowadays, spirometry tests alone are not adequate to categorize these heterogeneous group of diseases.
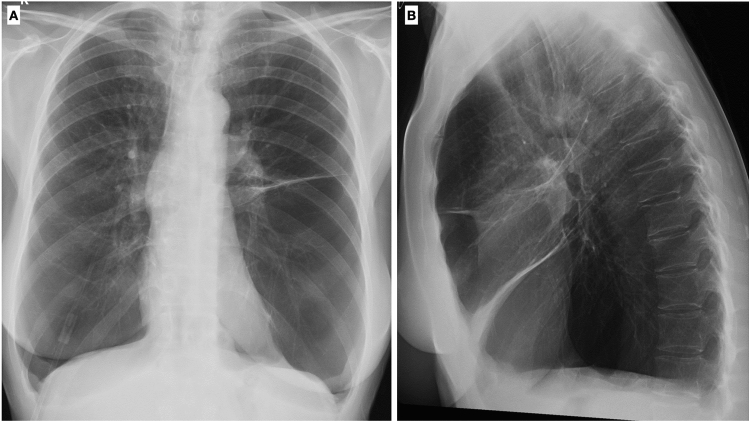
Chest X-ray represents the first imaging modality of choice in patients with known or suspected pulmonary disease. On CXR, indirect signs of pulmonary emphysema may be recognized and are represented by an increase in the radiolucency of the lungs, flattened hemidiaphragms, an increase in the retrosternal airspace (normally < 25 mm), and widening of space ribs (Fig. 9). The only direct sign of emphysema detectable on CXR is the presence of bullae [19]. Extrapulmonary alterations, such as cardiac enlargement, vascular and cardiac valve calcifications, may also be recognized on CXR. Even though CXR is not sensible enough to recognize and properly assess the degree of interstitial involvement and airway alterations, it remains the first step to exclude other causes of lung symptoms.
Fig. 9.
Chest-X-ray in two projections (postero-anterior and latero-lateral) shows a typical example of lung emphysema with pulmonary hyperinflation, flattened hemidiaphragms, and increased lung (A) and retrosternal (B) radiolucency
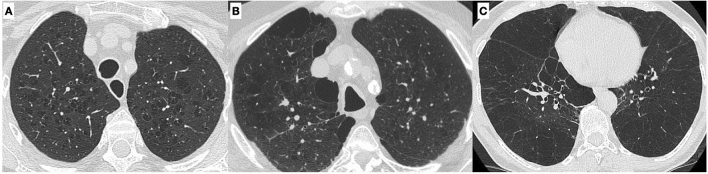
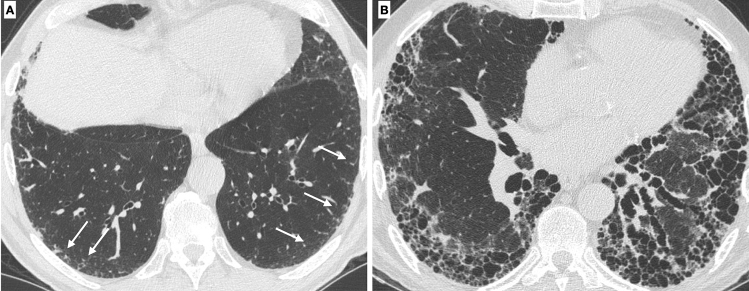
High-resolution CT scan is the gold standard to assess interstitial lung disease. Pulmonary emphysema is the typical smoking-related parenchymal alteration, and, from a pathological point of view, it is defined as the “abnormal permanent enlargement of the airspaces distal to the terminal bronchioles accompanied by destruction of the alveolar wall.” Depending on which part of the acinus is more affected, there are three forms of pulmonary emphysema (centrilobular, paraseptal, and panlobular) that can be easily identified on CT [20] (Fig. 10).
Fig. 10.
Different types of emphysema: centrilobular, in which the central part of the acinus is predominantly affected (A); paraseptal, in which the distal part of the acinus is predominantly involved (B), and panlobular, in which the whole acinus is affected (C)
In elderly patients, para-physiological changes as alveolar dilatation in association with a decrease in vascular structures, the so-called “senile lung,” can simulate an early stage of COPD [5].
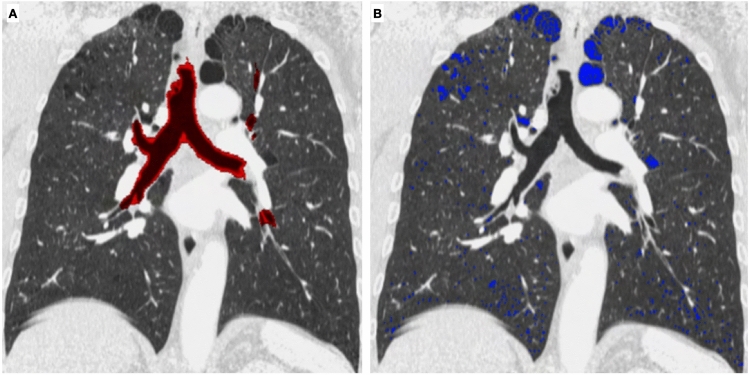
It has to be considered that patients with similar spirometry tests may suffer from different COPD phenotypes (more emphysematous type or more small airway disease involvement) and these different entities can be recognized and quantified by using CT [21] (Fig. 11).
Fig. 11.
Quantitative analysis of airways and pulmonary emphysema can be done with commercial software, in order to quantify the extent of the disease and to differentiate the contribution of small airways and emphysema in COPD (Courtesy of Lucio Calandriello)
Moreover, patients suffering from emphysema may benefit from the quantification of the extent of the disease on CT in order to distinguish which ones can benefit from endoscopy treatment or from surgery [22, 23].
Morphologic Changes of Lung Parenchyma
Interstitial Lung Diseases
Signs of aging include fibrotic changes, such as linear and reticular opacities, which are common findings in the elderly [14, 24].
Copley et al. demonstrated that a basal subpleural reticular pattern not associated with traction bronchiectasis on CT is frequently seen in asymptomatic elderly patients as a normal aging-related finding, independently of the smoking history. In this context, performing a correct differential diagnosis with clinically relevant interstitial lung diseases or interstitial lung abnormalities (ILA) could be particularly challenging [3, 25].
A focal area of ground glass opacity close to dorsal column osteophytosis is a common imaging finding in elderly patients and it represents an area of dysventilation that should not to be confused with a pathological finding (Fig. 12).
Fig. 12.
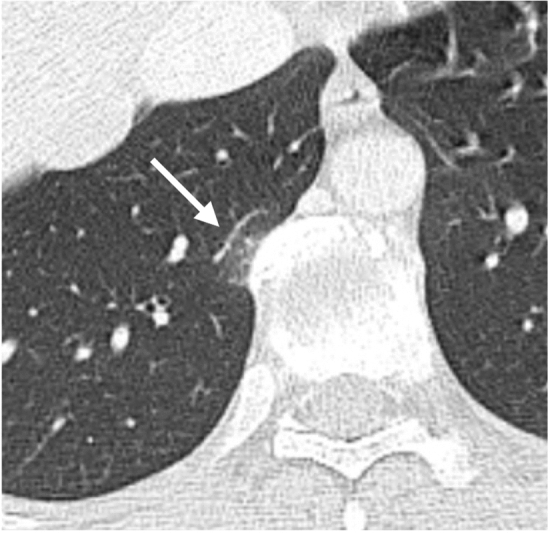
Axial CT scan shows a focal ground glass opacity (arrow) close to an osteophyte of the thoracic spine, representing a focal area of atelectasis
Idiopathic pulmonary fibrosis is a condition related to smoking habit and to pulmonary senescence, rare in individuals younger than 65 years [26]. In order to differentiate age-related interstitial changes from the onset of interstitial lung disease, it is mandatory to evaluate the extension of the interstitial alterations and the longitudinal behavior of the disease and correlate these findings with clinical features. The presence of honeycombing and extensive traction bronchiectasis are suggestive for fibrotic disease rather than age-related changes (Fig. 13) [••27].
Fig. 13.
HRCT demonstrates subpleural basal reticulations in a never-smoker 78-year-old man (arrows) (A). Axial image in a 67-year-old man with a diagnosis of idiopathic pulmonary fibrosis shows diffuse irregular septal thickening, traction bronchiectasis, and honeycombing (B)
Lung Nodules
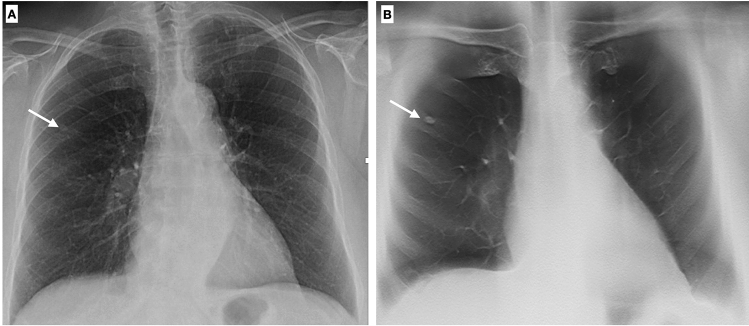
One of the most challenging tasks in thoracic imaging is the detection and characterization of lung nodules, especially on CXR, because of the poor conspicuity of lesions and the overlapping anatomical structures. In the past decades, oblique X-ray projections were usually employed to solve doubtful or equivocal cases, while in recent years DTS has demonstrated to be a useful technique to improve chest lesion diagnosis [28–30] (Fig. 14).
Fig. 14.
Chest X-ray shows a doubtful nodule in the right lung (arrow). DTS demonstrates that this opacity is due to a bony island of sclerosis in the costal arch (enostosis) (B). Enostosis is a typical imaging finding which can mimic a pulmonary opacity in the elderly
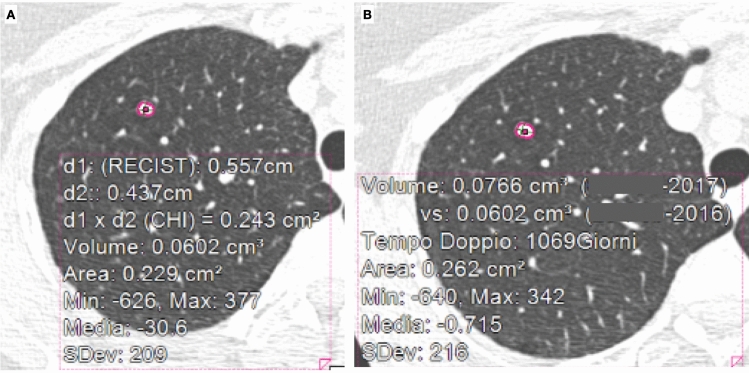
A small solid nodule can be detected on the chest CT of the majority of elderly patients and, due to the increasing numbers of imaging exams, its characterization has become a daily clinical practice problem [5]. There are some specific radiological findings which indicate the probable benign nature of a nodule, such as fat components or pop-corn calcifications. However, the majority of incidentally detected lung nodules on CT scan remain of uncertain nature and need a follow-up CT, according to the Fleischner recommendations for the managing of lung nodules [31]. It is interesting that these guidelines require the quantification of the nodule’s diameter, but also of its volume to classify the risk of malignancy. In addition, considering that a doubling time of more than 500 days for solid nodules has a 98% negative predictive value of malignancy, it is mandatory for radiologists to calculate, as precisely as possible, the volume of the lung nodule [32]. Nevertheless, the limits of manual measurements are well known. In this scenario, artificial intelligence systems, and in particular computer-aided diagnosis (CAD), could be helpful for detection (CADe) and characterization (CADx) of lung nodules on both CXR and CT scan [•33]. A large number of different commercial software can be used to calculate volume and doubling time of nodules to evaluate the probability of malignant growth (Fig. 15).
Fig. 15.
A baseline CT scan shows an incidental lung nodule in the right apex (A); 13 months later a follow-up CT scan was performed (B). By applying a CAD system, the diameter, the volume, and the doubling time can be calculated
In specific cases, lung biopsy or surgery need to be performed to characterize the nature of a lung nodule; however, elderly patients are at higher risk to develop both minor and major complications when undergoing this kind of procedures. Thus, it emerges the need for non-invasive systems to estimate the pre-test probability of malignancy. A recent application of AI systems in characterizing lung nodules is represented by radiomics, a procedure that quantitatively analyzes imaging features to provide information regarding the nature of the nodule, but also to predict the mutational profile of the tumor and to assess the response to therapy or the tumor aggressiveness [34].
Pleural Diseases
Pleural Effusion
Pleural effusion is widely more frequent in elderly patients, since pathological conditions causing an imbalance between fluid production and absorption, like cardiopulmonary disorders, malignant tumors, cirrhosis, inflammatory diseases, or pulmonary fibrosis, strongly develop from older age.
On CXR in standing position, pleural effusion manifests with blunting of the costophrenic and/or the cardiophrenic angle, sometimes with a meniscus and with contralateral mediastinal shift when large amount of fluid is present. However, on account of the fragility of elderly patients, CXR is frequently performed in supine position and pleural effusion may manifests with a reduction of parenchymal transparency only. Therefore, in the supine antero-posterior view, when increased opacity of a hemithorax without obscuration of vascular markings is seen, pleural effusion should be suspected. In addition, adhesions between visceral and parietal pleura in elderly patients can lead to loculated effusion that can simulate a mass [35, 36].
CT is more sensible than x-ray to identify pleural effusion, even with low amount of fluid. CT is useful in recognizing acute hemorrhage, based on its HU values and on the presence of fluid–fluid levels, in differentiating between pleural effusion and empyema and in identifying the underlying causes of pleural effusion (like malignant tumors). Malignant effusions are usually greater than 500 mL and they often represent the first evidence of malignancy. For lung, breast, and ovarian metastases, 92% of pleural effusions are ipsilateral to the primary lesion. These lesions spread via hematogenous dissemination, by pleural invasion (as in T3 bronchogenic cancers), or by direct pleural seeding [35, 37].
Pleural Plaques
Pleural plaques are the main and most frequent benign pleural pathology consequent to asbestos exposure, definitely more frequent in elderly subjects, as long as that they manifest after 15–20 years at least from initial exposure. They are 1–10 mm thick (thicker when developing adjacent to ribs) and predominantly develop in the chest wall, usually in the anterior upper region, or in the diaphragmatic pleura (sparing the costophrenic recesses), while the mediastinal region is rarely affected [35] (Fig. 16).
Fig. 16.
Axial CT image shows bilateral pleural plaques in a 76-year-old male with previous asbestos exposure (A). Three-dimensional reconstructions show the typical distribution of the pleural plaques (B, C)
On CXR, pleural plaques present as focal pleural thickening with a well-defined inner margin, usually bilateral and asymmetrical. Typically, they involve posterolateral regions, along the costal margins and the diaphragm. They are frequently calcified, with punctate, linear, or “cake-like” calcifications. The main differential diagnoses are rib fractures, extra-pleural fat, and pleural tumors, mainly mesothelioma. On CXR, mesothelioma presents with pleural plaques, volume loss of the hemithorax involved, ipsilateral shift of the mediastinum, lymphadenopathy, and pleural effusion [35].
CT allows a better detection and location of pleural plaques, whether they are calcified or not. CT permits to differentiate between pleural plaques and subpleural fat deposits (typically bilateral and symmetrical), between benign pleural plaques and mesothelioma, presenting on CT as a soft tissue attenuation pleural mass or nodulation that penetrates the interlobar fissures and completely encases the lung in advanced stages. CT identifies also mesothelioma spread within the pleura and the local invasion of the chest wall, of the diaphragm and of the lymph nodes [35, 37].
Late Sequelae of Tuberculosis
Nowadays, tuberculosis (TB) is still a worrying cause of death worldwide and it represents a pathology of special interest in the geriatric population; indeed, the elderlies are at higher risk for pulmonary infections, for reactivation of a latent TB (tuberculosis) and for acquisition of a new TB infection [38].
The availability of antibiotics, after the Second World War, has dramatically changed the treatment and prognosis of patients suffering of TB. Therefore, late sequelae of an old TB infection or surgical TB treatment, the last no more in use, are typically seen in the elderlies and are represented by fibrothorax, extra-pleural artificial pneumothorax, and thoracoplasty.
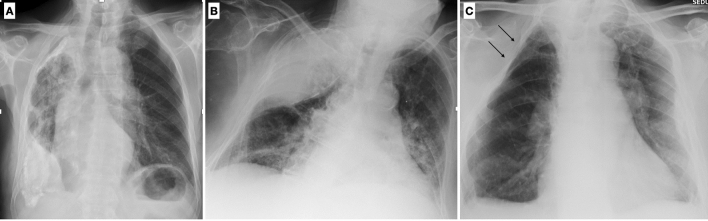
Fibrothorax is defined as fibrosis within the pleural space, occurring after the inflammatory response to a series of pathological conditions, as tuberculosis, thoracic empyema, asbestos-related pleural disease, rheumatoid arthritis, and hemothorax. Fibrothorax is characterized by pleural thickening, which may be calcified, and volume loss of the affected hemithorax (Fig. 17A). The mediastinal pleura is usually spared [39].
Fig. 17.
Chest X-ray shows diffuse calcified pleural plaques on the right hemithorax due to the presence of extensive and coarse unilateral calcified pleural thickening (fibrothorax) (A). Chest X-ray shows an extensive opacity of the right upper lung with smooth internal margins and an obtuse angle with the lateral chest wall due to the sequelae of an extra-pleural artificial pneumothorax (B). Chest X-ray of an 83-year-old female shows multiple ribs resection following surgical treatment of TB (thoracoplasty) (C)
Extrapleural artificial pneumothorax was a procedure for the treatment of TB, consisting in the introduction of air into the pleural cavity or between the coverings of the lung, that lead to the collapse of the diseased area and to a complete recovery (Fig. 17B). This procedure was first performed by Tuffier in 1891. Before the use of air to fill the created space, many different substances were used, including paraffin packs; however, these substances could erode the pulmonary tissue, or the chest wall and skin, making this procedure, at least, not a very satisfactory form of treatment [40].
This method is not used anymore since 1960 in Italy.
Thoracoplasty is a surgical procedure that was originally designed to permanently collapse the cavities of pulmonary tuberculosis. It consists in the surgical removal of rib bones from the chest wall (generally 2–3 ribs), resection of the parietal pleura, periosteum, and intercostal muscles (Fig. 17C).
Until supplanted by effective chemotherapy, it was one of several methods used to put the lung to rest, in order to inactivate the disease (41).
Conclusions
Diagnostic imaging has an important role in recognizing para-physiological changes of the respiratory system and to differentiate them from pathological conditions.
In an increasingly older population, Radiologists have to become familiar with chest imaging findings physiologically related to aging, in order to reduce misinterpretations.
Abbreviations
- CXR
Chest X-ray
- CT
Computed tomography
- CAD
Computer-aided diagnosis
- COPD
Chronic obstructive pulmonary disease
- HU
Hounsfield unit
- AI
Artificial intelligence
- DTS
Digital tomosynthesis
- ILA
Interstitial lung abnormalities
- TB
Tuberculosis
Declarations
Conflict of interest
For all authors none were declared.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical collection on Geriatrics.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
- 1.World Population Prospects 2022, Summary of Results. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf.
- 2.Singh S, Bajorek B. Defining 'elderly' in clinical practice guidelines for pharmacotherapy. Pharm Pract (Granada) 2014;12(4):489. doi: 10.4321/S1886-36552014000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copley SJ, Wells AU, Hawtin KE, Gibson DJ, Hodson JM, Jacques AE, et al. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251(2):566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 4.Hochhegger B, Meirelles GS, Irion K, Zanetti G, Garcia E, Moreira J, et al. The chest and aging: radiological findings. J Bras Pneumol. 2012;38(5):656–665. doi: 10.1590/S1806-37132012000500016. [DOI] [PubMed] [Google Scholar]
- 5.Gossner J, Nau R. Geriatric chest imaging: when and how to image the elderly lung, age-related changes, and common pathologies. Radiol Res Pract. 2013;2013:584793. doi: 10.1155/2013/584793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassandro F, Iasiello F, Pizza NL, Valente T, Stefano ML, Grassi R, et al. Abdominal hernias: radiological features. World J Gastrointest Endosc. 2011;3(6):110–117. doi: 10.4253/wjge.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140(1):41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 8.••Benz E, Trajanoska K, Lahousse L, Schoufour JD, Terzikhan N, De Roos E, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28(154). This systematic review highlights the prevalence of sarcopenia in COPD patients. [DOI] [PMC free article] [PubMed]
- 9.Lahousse L, Ziere G, Verlinden VJ, Zillikens MC, Uitterlinden AG, Rivadeneira F, et al. Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Biol Sci Med Sci. 2016;71(5):689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 10.VazFragoso CA, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am J Med. 2012;125(1):79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francisco MJN, Rahal AJ, Vieira FA, Silva PS, Funari MB. Advances in lung ultrasound. Einstein (Sao Paulo) 2016;14(3):443–448. doi: 10.1590/S1679-45082016MD3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryl B, Merrix S, Proud D, Marin A, Byrne A, Duckers J. Are we missing the opportunity to measure muscle mass on computed tomography thorax? J Thorac Imaging. 2021;36(2):W32–W33. doi: 10.1097/RTI.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Shin Y, Huh J, Sung YS, Lee IS, Yoon KH, et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019;20(2):205–217. doi: 10.3348/kjr.2018.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Occhipinti M, Larici AR, Bonomo L, Incalzi RA. Aging airways: between normal and disease. A multidimensional diagnostic approach by combining clinical, functional, and imaging data. Aging Dis. 2017;8(4):471–485. doi: 10.14336/AD.2016.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Washko GR. The role and potential of imaging in COPD. Med Clin N Am. 2012;96(4):729–743. doi: 10.1016/j.mcna.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa M, Nasuhara Y, Onodera Y, Makita H, Nagai K, Fuke S, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, Uchiyama K, Shima H, Ueno N, Oish S, Nojiri Y. Bronchoarterial ratio and bronchial wall thickness on high-resolution CT in asymptomatic subjects: correlation with age and smoking. AJR Am J Roentgenol. 2003;180(2):513–518. doi: 10.2214/ajr.180.2.1800513. [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Chung SY, Yang I, Lee Y, Ko EY, Park MJ. Correlation of aging and smoking with air trapping at thin-section CT of the lung in asymptomatic subjects. Radiology. 2000;214(3):831–836. doi: 10.1148/radiology.214.3.r00mr05831. [DOI] [PubMed] [Google Scholar]
- 19.Müller NL, Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax. 2002;57(11):982–985. doi: 10.1136/thorax.57.11.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 21.Hackx M, Bankier AA, Gevenois PA. Chronic obstructive pulmonary disease: CT quantification of airways disease. Radiology. 2012;265(1):34–48. doi: 10.1148/radiol.12111270. [DOI] [PubMed] [Google Scholar]
- 22.Fischer AM, Yacoub B, Savage RH, Martinez JD, Wichmann JL, Sahbaee P, et al. Machine learning/deep neuronal network: routine application in chest computed tomography and workflow considerations. J Thorac Imaging. 2020;35(Suppl 1):S21–S27. doi: 10.1097/RTI.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 23.Remy-Jardin M, Faivre JB, Kaergel R, Hutt A, Felloni P, Khung S, et al. Machine learning and deep neural network applications in the thorax: pulmonary embolism, chronic thromboembolic pulmonary hypertension, aorta, and chronic obstructive pulmonary disease. J Thorac Imaging. 2020;35(Suppl 1):S40–S48. doi: 10.1097/RTI.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 24.Ciccarese F, Chiesa AM, Feletti F, Vizioli L, Pasquali M, Forti P, et al. The senile lung as a possible source of pitfalls on chest ultrasonography and computed tomography. Respiration. 2015;90(1):56–62. doi: 10.1159/000430994. [DOI] [PubMed] [Google Scholar]
- 25.Chae KJ, Jin GY, Goo JM, Chung MJ. Interstitial lung abnormalities: what radiologists should know. Korean J Radiol. 2021;22(3):454–463. doi: 10.3348/kjr.2020.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 27.••Ledda RE, Milanese G, Milone F, Leo L, Balbi M, Silva M, et al. Interstitial lung abnormalities: new insights between theory and clinical practice. Insights Imaging. 2022;13(1):6. This reference highlits the importance of ILA recognition and discuss the most recent advancement in understanding this radiological entity. [DOI] [PMC free article] [PubMed]
- 28.Quaia E, Baratella E, Cernic S, Lorusso A, Casagrande F, Cioffi V, et al. Analysis of the impact of digital tomosynthesis on the radiological investigation of patients with suspected pulmonary lesions on chest radiography. Eur Radiol. 2012;22(9):1912–1922. doi: 10.1007/s00330-012-2440-3. [DOI] [PubMed] [Google Scholar]
- 29.Quaia E, Baratella E, Poillucci G, Kus S, Cioffi V, Cova MA. Digital tomosynthesis as a problem-solving imaging technique to confirm or exclude potential thoracic lesions based on chest X-ray radiography. Acad Radiol. 2013;20(5):546–553. doi: 10.1016/j.acra.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Quaia E, Baratella E, Poillucci G, Gennari AG, Cova MA. Diagnostic impact of digital tomosynthesis in oncologic patients with suspected pulmonary lesions on chest radiography. Eur Radiol. 2016;26(8):2837–2844. doi: 10.1007/s00330-015-4104-6. [DOI] [PubMed] [Google Scholar]
- 31.Bueno J, Landeras L, Chung JH. Updated Fleischner Society guidelines for managing incidental pulmonary nodules: common questions and challenging scenarios. Radiographics. 2018;38(5):1337–1350. doi: 10.1148/rg.2018180017. [DOI] [PubMed] [Google Scholar]
- 32.Revel MP, Merlin A, Peyrard S, Triki R, Couchon S, Chatellier G, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol. 2006;187(1):135–142. doi: 10.2214/AJR.05.1228. [DOI] [PubMed] [Google Scholar]
- 33.•Chassagnon G, Vakalopoulou M, Paragios N, Revel MP. Artificial intelligence applications for thoracic imaging. Eur J Radiol. 2020;123:108774. This reference is focused on the use of Artificial Intelligence in thoracic imaging. [DOI] [PubMed]
- 34.Coroller TP, Agrawal V, Huynh E, Narayan V, Lee SW, Mak RH, et al. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J Thorac Oncol. 2017;12(3):467–476. doi: 10.1016/j.jtho.2016.11.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb WR, Higgins CB. Thoracic imaging: pulmonary and cardiovascular radiology. Philadelphia: Lippincott Williams & Wilkins; 2016. [Google Scholar]
- 36.Goodman LR. Felson's principles of chest roentgenology, a programmed text. 5. Amsterdam: Elsevier; 2019. [Google Scholar]
- 37.Dal Pozzo G. Compendio di Tomografia Computerizzata e TC multistrato. Corpo intero: UTET scienze mediche; 2009.
- 38.Caraux-Paz P, Diamantis S, de Wazières B, Gallien S. Tuberculosis in the elderly. J Clin Med. 2021;10(24):5888. doi: 10.3390/jcm10245888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JA, Hong KT, Oh YW, Chung MH, Seol HY, Kang EY. CT manifestations of late sequelae in patients with tuberculous pleuritis. AJR Am J Roentgenol. 2001;176(2):441–445. doi: 10.2214/ajr.176.2.1760441. [DOI] [PubMed] [Google Scholar]
- 40.Mondoni M, Centanni S, Sotgiu G. New perspectives on difficult-to-treat tuberculosis based on old therapeutic approaches. Int J Infect Dis. 2020;92S:S91–S99. doi: 10.1016/j.ijid.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 41.Krasnov D, Krasnov V, Skvortsov D, Felker I. Thoracoplasty for tuberculosis in the twenty-first century. Thorac Surg Clin. 2017;27(2):99–111. doi: 10.1016/j.thorsurg.2017.01.003. [DOI] [PubMed] [Google Scholar]