Abstract
Hypothyroidism may occur after definitive radiotherapy in rare cases of early glottic carcinoma. However, to the best of our knowledge, no study to date has examined the risk factors for hypothyroidism specifically after definitive radiotherapy in patients with early glottic carcinoma. The present study determined risk factors for hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma. This was a retrospective study that included 73 patients with T1 or T2, N0 glottic squamous cell carcinoma who underwent radiotherapy between June 3, 2009 and December 25, 2020. Demographic and clinical characteristics, including age, sex, tumor stage and pretreatment thyroid volume, were examined to elucidate the clinical risk factors for hypothyroidism. Field size, total prescribed dose and thyroid receiving dose were evaluated as dosimetric risk factors for hypothyroidism. Irradiated underlying thyroid volumes of more than 5, 10, 20, 30, 40, 50, 60 and 65 Gy (V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy and V65Gy) and mean thyroid dose were included as thyroid receiving doses. The median follow-up duration was 61 months (range, 7–150 months). Hypothyroidism was present in 15 (21%) of the 73 patients, including 12 and 3 patients with grade 1 and 2 hypothyroidism, respectively. Among the demographic and clinical factors, sex and pretreatment thyroid volume were significantly associated with hypothyroidism (P=0.007 and P<0.001, respectively). Among the dosimetric factors, the presence of hypothyroidism was significantly associated with V5Gy (P=0.012), V10Gy (P=0.015), V20Gy (P=0.020), V30Gy (P=0.024), V40Gy (P=0.028), V50Gy (P=0.028), V60Gy (P=0.027) and mean thyroid dose (P=0.023). In conclusion, sex, pretreatment thyroid volume and thyroid receiving dose were associated with hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma. Particularly, the receiving dose to the thyroid gland should be reduced in female patients and in those with small thyroid volumes who are at higher risk for hypothyroidism following radiotherapy.
Keywords: glottic carcinomas, hypothyroidism, laryngeal carcinoma, radiotherapy, thyroid gland
Introduction
Radiotherapy is one of the standard treatment methods for patients with early laryngeal carcinoma, although laser therapy and partial laryngectomy can be used for definitive treatment as well (1–3). The goal of treatment is not only to cure cancer, but also to keep the vocal cords with acceptable voice quality, and avoid major treatment-related adverse events (AEs). These goals can be achieved with definitive radiotherapy in most patients with early laryngeal carcinoma. Laryngeal carcinoma is classified in supraglottic, glottic, and subglottic types based on the location, and glottic carcinomas are the most common type (70%). Additionally, most glottic carcinomas are diagnosed in its early stage, accounting for approximately 70% of all cases. Irradiation for early glottic carcinoma is delivered through small radiation portals covering the glottic tumor because the lymph node metastasis in neck is rare. Therefore, AEs are considered mild after radiotherapy for early glottic carcinoma. Nevertheless, serious AEs, including hypothyroidism, may occur after definitive radiotherapy in rare cases of early glottic carcinoma.
Hypothyroidism can be divided into two categories: clinical and subclinical hypothyroidism. Clinical hypothyroidism is a condition of a high thyroid-stimulating hormone (TSH) level in the presence of a free thyroxine (FT4) level lower than the normal range, whereas subclinical hypothyroidism is a condition of a high TSH level in the presence of an FT4 level within the normal range. The fatigue, drowsiness, intolerance of cold, weight gain, constipation, aural changes, and dry skin are common clinical symptoms of hypothyroidism (4). Patients with mild hypothyroidism may have no obvious symptoms, but severe hypothyroidism may increase the incidence of cardiovascular disease and risk of death, including heart failure, atrial fibrillation, and coronary artery disease (5–7).
In our previous study (8), we identified the risk factor for local failure in T1 glottic carcinoma irradiated at the prescribed 66 Gy. However, this study was insufficient to investigate AEs, and risk factors for radiation-related hypothyroidism were unclear. Numerous researchers have examined factors associated with hypothyroidism in patients with head and neck cancers who receive whole-neck radiotherapy. Clinical and demographic factors such as dose-volume parameters (9–11), chemotherapy (12–14), neck surgery (15,16), sex (12,13,17), age (12,18), and follow-up duration (19) are considered risk factors for radiation-induced hypothyroidism. However, no study to date has examined the risk factors for hypothyroidism specifically after definitive radiotherapy in patients with early glottic carcinoma. We therefore conducted a retrospective study to investigate the risk factors associated with hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma given that the knowledge of these factors is important to improve the safety of radiotherapy in these patients.
Materials and methods
Patients
A total of 109 consecutive patients with T1 or T2, N0 glottic squamous cell carcinoma were irradiated definitively without concurrent chemotherapy between June 3, 2009 and December 25, 2020 in the study institution. This retrospective study included patients who were evaluated for thyroid function and followed for a minimum of 6 months after radiotherapy. Therefore, 73 of the 109 patients were included in this study. All patients provided written informed consent, and the study was approved by the Institutional Review Board of Tokyo Medical University Hospital (approval no. T2021-0260).
Cancer stage was classified according to the 2016 TNM classification (8th edition, Union for International Cancer Control). This study cohort included 62 male and 11 female patients, and the median age of patient was 72 (range, 47–86) years. The Eastern Cooperative Oncology Group performance status was 0 in 98% of the patients. The cancer stages were T1N0M0 and T2N0M0 in 53 and 20 patients, respectively, and no patients had neck or distant metastases. In the study cohort, 16 patients (22%) had two primary cancers and 3 patients (4%) had three primary cancers.
Radiation treatment
Three-dimensional radiotherapy was planned and performed on patients in the supine position using a shell. As part of the treatment planning, all patients underwent cervical computed tomography (CT) imaging with 2.5-mm slice thickness. Treatment planning was performed using the Eclipse™ treatment planning system (Varian Medical Systems, Palo Alto, CA). In all patients, the standard radiotherapy technique with parallel-opposed lateral fields was used to deliver the photons of a 4-MV X-ray beam for 5 days per week. Irradiation was delivered via local radiation portals, mostly those sized 5–6×5–6 cm, which covered only the glottic tumor. The cervical lymph node chain was not included in the treatment plan. The dose was 66 Gy administered in 33 fractions over 6.6 weeks in patients with T1 glottic carcinoma and 70 Gy administered in 35 fractions over 7 weeks in patients with T2 glottic carcinoma. The patient characteristics and radiotherapy details are summarized in Table I.
Table I.
Characteristics of the patients (n=73) and radiation treatment.
| Variables | Value |
|---|---|
| Sex, n (%) | |
| Male | 62 (85) |
| Female | 11 (15) |
| Median age, years (range) | 72 (47–86) |
| Performance status, n | |
| 0 | 72 |
| 1 | 1 |
| Stage of primary tumors, n (%) | |
| T1 | 53 (73) |
| T2 | 20 (27) |
| Total dose/fractionation, n (%) | |
| 66 Gy/33 Fr | 60 (82) |
| 70 Gy/35 Fr | 13 (18) |
| Field size, n (%) | |
| <6 cm | 36 (50) |
| ≥6 cm | 37 (50) |
Fr, fractionations.
Follow-up procedures
Patients were followed up regularly at 2- to 3-month intervals for the first 2 years after diagnosis and 4- to 6-month intervals thereafter in patients without clinical symptoms. At each follow-up visit, the medical history, physical examination, laryngoscopy, CT scans, and tumor marker assessment were evaluated. Data on radiotoxicity were retrospectively collected from the patient files. Hypothyroidism was defined as the presence of a high TSH level regardless of the FT4 level and was graded as follows according to the Common Terminology Criteria for Adverse Events v. 4.0: grade 1, asymptomatic hypothyroidism requiring clinical or diagnostic observation only; grade 2, symptomatic hypothyroidism requiring medical intervention; grade 3, severely symptomatic hypothyroidism requiring hospitalization; grade 4, life-threatening consequences of hypothyroidism requiring urgent intervention; and grade 5, death.
Assessment of risk factors for hypothyroidism
Age, sex, Tumor stage (T) stage, and pretreatment thyroid volume were evaluated as clinical risk factors for hypothyroidism. Pretreatment thyroid volumes were determined using CT scans obtained during treatment planning for radiotherapy. Field size, total prescribed dose, and thyroid receiving dose were evaluated as dosimetric risk factors for hypothyroidism. Underlying irradiated thyroid volumes of more than 5, 10, 20, 30, 40, 50, 60, and 65 Gy (V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, and V65Gy, respectively) and mean thyroid dose were calculated as thyroid receiving doses.
Statistical analysis
The time to hypothyroidism onset after definitive radiotherapy was calculated from the first day of radiotherapy. The relationship of hypothyroidism with age, sex, T stage, field size, and total prescribed dose was calculated using Fisher's exact probability test. The relationship of hypothyroidism with pretreatment thyroid volume, V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, V65Gy, and mean thyroid dose were analyzed using the Mann-Whitney U test. For these factors, the receiving operating characteristic (ROC) curves were generated to determine the cutoff values that yielded the optimal sensitivity and specificity. Univariate logistic regression analyses were performed to evaluate the data using IBM SPSS Statistics 20.0 (SPSS, Armonk, NY, USA). Differences with P-values of <0.05 were considered indicating statistical significance.
Results
Incidence of hypothyroidism
The median follow-up duration was 61 (range, 7–150) months. Hypothyroidism was present in 15 (21%) of the 73 patients and were classified as grades 1 and 2 in 12 and 3 patients, respectively. The median time to hypothyroidism onset after the start of definitive radiotherapy was 16 (range, 3–77) months. Hypothyroidism improved in 6 of the 15 patients in a median of 13 (range, 5–24) months.
Analysis for the demographic and clinical factors with hypothyroidism
The relationships of the demographic and clinical factors with hypothyroidism are summarized in Table II. Briefly, sex and pretreatment thyroid volume were significantly associated with hypothyroidism (P=0.007 and <0.001, respectively). The incidence rates of hypothyroidism were 15 and 55% in male and female patients, respectively, and the incidence of hypothyroidism was 3.67 times higher in female patients than in male patients. The median pretreatment thyroid volumes were 12.3 (range, 4.9-62.8) and 7.0 (4.0-32.5) ml in male and female patients, respectively. The pretreatment thyroid volume was significantly lower in female patients than in male patients (P=0.035). The ROC analysis for hypothyroidism showed that the area under the ROC curve (AUC) for pretreatment thyroid volume was 0.905 [95% confidence interval (CI), 0.833-0.978] (Fig. 1). The cutoff value of pretreatment thyroid volume was 9.50 ml, with a sensitivity of 81.0% and a specificity of 86.7%. The incidence rates of hypothyroidism were 54 and 4.1% for patients with pretreatment thyroid volumes of <9.50 and ≥9.50 ml, respectively. The incidence of hypothyroidism was 13.2 times higher in patients with a pretreatment thyroid volume of <9.50 ml than in those with a pretreatment thyroid volume of ≥9.50 ml.
Table II.
Clinical risk factors associated with hypothyroidism.
| Variables | Without hypothyroidism (n=58) | With hypothyroidism (n=15) | P-value |
|---|---|---|---|
| Sex (male vs. female) | 53 vs. 5 | 9 vs. 6 | 0.007 |
| Age (<75 vs. ≥75 years) | 38 vs. 20 | 7 vs. 8 | 0.149 |
| T stage (T1 vs. T2) | 42 vs. 16 | 11 vs. 4 | 0.610 |
| Pretreatment thyroid volume, ml [median (mean ± SD)] | 13.5 (14.7±8.3) | 6.7 (7.4±2.2) | <0.001 |
Figure 1.
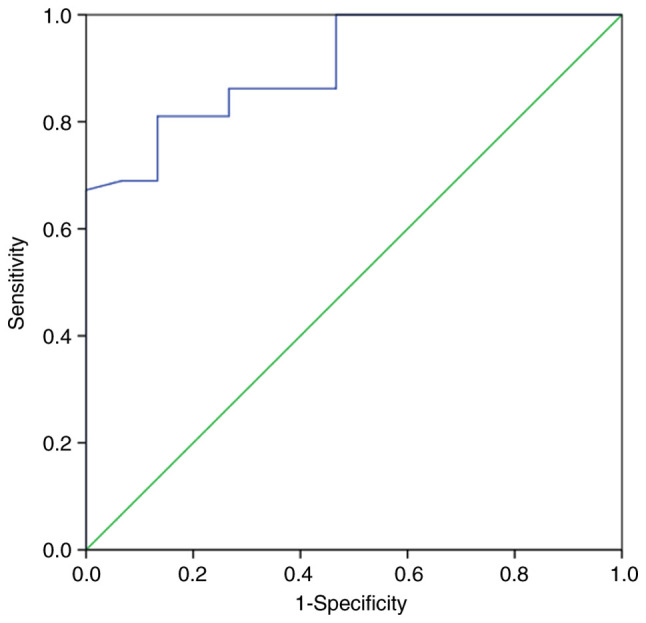
Receiver operating characteristic analysis of the thyroid volume associated with the development of hypothyroidism after radiotherapy for early glottic carcinoma. The area under the receiver operating characteristic curve for pretreatment thyroid volume was 0.905 (95% CI, 0.833-0.978). The cutoff value of pretreatment thyroid volume was 9.50 ml, with a sensitivity of 81.0% and a specificity of 86.7%.
Analysis for the dosimetric factors with hypothyroidism
Table III shows the relationship of hypothyroidism with dosimetric factors in the study cohort. The univariate analysis revealed that hypothyroidism was significantly associated with V5Gy (P=0.012), V10Gy (P=0.015), V20Gy (P=0.020), V30Gy (P=0.024), V40Gy (P=0.028), V50Gy (P=0.028), V60Gy (P=0.027), and mean thyroid dose (P=0.023). The ROC analysis revealed that that the AUCs for V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, and mean thyroid dose were 0.711 (95% CI, 0.558-0.865), 0.705 (0.554-0.857), 0.695 (0.541-0.850), 0.690 (0.535-0.845), 0.684 (0.528-0.841), 0.684 (0.531-0.838), 0.686 (0.536-0.846), and 0.692 (0.537-0.847), respectively (Fig. 2). V5Gy had the highest AUC value among all factors related to the thyroid receiving dose. The cutoff value of V5Gy was 45.6%, with a sensitivity of 73.3% and a specificity of 51.3%.
Table III.
Association between dosimetric factors and hypothyroidism.
| Variables | Without hypothyroidism (n=58) | With hypothyroidism (n=15) | P-value |
|---|---|---|---|
| Field size (<6 vs. ≥6 cm) | 28 vs. 30 | 8 vs. 7 | 0.476 |
| Total prescribed dose (66 vs. 70 Gy) | 48 vs. 10 | 12 vs. 3 | 0.531 |
| V 5Gy, % [median (mean ± SD)] | 44.9 (45.2±18.3) | 65.7 (62.0±22.5) | 0.012 |
| V10 Gy, % [median (mean ± SD)] | 38.6 (38.3±17.3) | 53.5 (54.5±23.1) | 0.015 |
| V20 Gy, % [median (mean ± SD)] | 32.8 (33.0±16.4) | 47.0 (48.5±23.2) | 0.020 |
| V30 Gy, % [median (mean ± SD)] | 28.7 (29.4±15.7) | 41.5 (44.2±23.1) | 0.024 |
| V40 Gy, % [median (mean ± SD)] | 25.3 (26.1±15.1) | 36.0 (40.1±22.9) | 0.028 |
| V50 Gy, % [median (mean ± SD)] | 19.8 (22.4±14.2) | 31.3 (35.4±22.6) | 0.028 |
| V60 Gy, % [median (mean ± SD)] | 11.6 (16.1±12.9) | 19.8 (27.2±20.9) | 0.027 |
| V65 Gy, % [median (mean ± SD)] | 1.8 (7.4±10.6) | 9.2 (11.2±11.9) | 0.304 |
| Mean thyroid dose, Gy [median (mean ± SD)] | 19.0 (20.3±9.9) | 27.6 (29.2±13.9) | 0.023 |
Vx Gy, irradiated underlying thyroid volumes of more than × Gy.
Figure 2.
Receiver operating characteristic analysis of the thyroid receiving doses associated with the development of hypothyroidism after radiotherapy for early glottic carcinoma. The AUCs for V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy and V65Gy are shown. (A) V5Gy, 0.711 (95% CI, 0.558-0.865); (B) V10Gy, 0.705 (95% CI, 0.554-0.857); (C) V20Gy, 0.695 (95% CI, 0.541-0.850); (D) V30Gy, 0.690 (95% CI, 0.535-0.845); (E) V40Gy, 0.684 (95% CI, 0.528-0.841); (F) V50Gy, 0.684 (95% CI, 0.531-0.838); (G) V60Gy, 0.686 (95% CI, 0.536-0.846); and (H) mean thyroid dose, 0.692 (95% CI, 0.537-0.847). The AUC value was the highest for V5Gy. The cutoff value of V5Gy was 44.8%, with a sensitivity of 73.3% and a specificity of 50.0%. AUC, area under the receiver operating characteristic curve. Vx Gy, irradiated underlying thyroid volumes of more than × Gy.
Discussion
In the present study, hypothyroidism was present in 15 (21%) of the 73 patients with early glottic carcinoma, including 12 and 3 patients with grade 1 and 2 hypothyroidism, respectively. Our analyses revealed that sex, pretreatment thyroid volume, V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, and mean thyroid dose were significantly associated with hypothyroidism. Several studies reported the risk factors of radiation-induced hypothyroidism in patients with head and neck cancers (9–23). The reported incidence of hypothyroidism after definitive radiotherapy in the neck varies between 23 and 53% (20). In the present study, hypothyroidism developed in 21% of the patients, in agreement with the previous studies. Although numerous groups investigated factors related to radiation-induced hypothyroidism in patients undergoing whole-neck radiotherapy for head and neck cancers, such as nasopharyngeal, oral, and hypopharyngeal cancers, to our knowledge this is the first study examining the risk factors for hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma, because small radiation portals are used to cover only the primary tumor and the cervical lymph node chain is not included in the treatment plan.
We found that sex and pretreatment thyroid volume were significantly associated with hypothyroidism in patients with early glottic carcinoma receiving definitive radiotherapy. Fan et al (12) found that the incidence of hypothyroidism was 2.0 times higher in female patients than in male patients. Rønjom et al (24) found that the median thyroid volume of female patients was significantly lower than that of male patients (13.4 vs. 18.4 cm3, P<0.001). Lertbutsayanukul et al (11) conducted a prospective analysis of 178 patients and reported that a small thyroid volume (<8 cm3) was a risk factor for hypothyroidism based on univariate analysis, similar to the findings of a study by Zhai et al (21), who reported that younger age, female sex, and a smaller thyroid volume were significantly associated with hypothyroidism. Although the relationship between sex and thyroid volume remains to be further elucidated, many studies reveal that female patients are more likely to develop hypothyroidism (22). In the present study, we also found that the pretreatment thyroid volume was significantly lower in female patients than in male patients. We suggest that female patients may be at higher risk for hypothyroidism because of a significantly lower thyroid volume compared with male patients.
Our analyses indicated that V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, and mean thyroid dose were significantly associated with hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma. Several studies elucidated the association between thyroid dose and hypothyroidism in patients with head and neck cancers who were treated with radiotherapy. Sachdev et al (23) reported that V50Gy ≥60% was associated with 6.76 times increased risk of clinical hypothyroidism compared with V50Gy <60%. In a prospective analysis of 135 patients by Zhai et al (21), the univariate analysis indicated that the thyroid mean dose, the minimum dose V30Gy, V35Gy, V40Gy, V45Gy, and V50Gy of the thyroid gland were associated with hypothyroidism and that Dmean, V45Gy, and V50Gy were independent predictors of hypothyroidism after radiotherapy. Altogether, these studies implicate thyroid receiving dose as a risk factor for hypothyroidism in patients with head and neck cancers requiring whole-neck irradiation. In the present study, low dose areas for thyroid, such as V5 Gy, were factors in hypothyroidism. These thyroid doses are lower than the doses in the previous studies. We consider that this is because the large irradiation field such as whole-neck radiotherapy in the previous studies resulted in almost 100% of the low-dose area for thyroid, such as V5Gy, and therefore there was no difference in the low-dose area in each patient. In addition, other thyroid doses that may be factors in hypothyroidism could be found if analyzed using continuous values or values below V5Gy. However, this would increase the volume of data, complicate statistical evaluation, and reduce reliability. Furthermore, V5Gy is clinically small enough in prescribed doses of 66–70 Gy, and evaluation of doses lower than this may not be necessary. In this study, all patients were treated with three-dimensional radiotherapy. Although it is difficult to reduce the thyroid dose with treatment using three-dimensional radiotherapy, it may be possible to reduce the thyroid dose with treatment using Intensity Modulated Radiation Therapy (IMRT) technique. If the thyroid dose cannot be reduced, it is important to explain to the patient the risk of developing hypothyroidism.
In the present study, there were fewer female than male. All studies should be conducted according to the Sex and Gender Equity in Research (SAGER) guidelines (25). However, the incidence of laryngeal carcinoma is 5 times higher in male than in female (26), and is overwhelmingly more common in male, which inevitably results in fewer female. In addition, it is impossible to adjust for the sex ratio due to the nature of the retrospective study. Importantly, this is the first study to identify that thyroid receiving dose was associated with hypothyroidism even in patients with early glottic carcinoma after definitive radiotherapy, which is delivered using small portals covering only the primary tumor.
A limitation of the present study is the potential selection bias regarding predictive factors, which cannot be ruled out because of the retrospective study design. In addition, due to the nature of the retrospective study, it is possible that some patients may not have regular follow-up. Future prospective studies are warranted to confirm the main study findings.
In conclusion, sex, pretreatment thyroid volume, V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy, and mean thyroid dose were significantly associated with hypothyroidism after definitive radiotherapy in patients with early glottic carcinoma. These findings indicate that caution should be exercised regarding hypothyroidism, which can develop even in patients with early glottic carcinoma who receive definitive radiotherapy through small portals covering only the primary tumor. Particularly, the receiving dose to the thyroid gland should be reduced in female patients and in those with small thyroid volumes who are at higher risk of hypothyroidism.
Acknowledgements
The authors would like to thank radiological technologist Mr. Hideaki Chiba (Department of Radiology, Tokyo Medical University Hospital, Tokyo, Japan) and Dr Yu Tajima (Department of Radiology, Tokyo Medical University Hospital, Tokyo, Japan) for their statistical analysis assistance.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MO, TI, TS and DY conceived the study, and wrote and revised the manuscript. RM, YO, SS and TK reviewed, collected and analyzed the data. KT and KS designed the study and acquired the data. All authors contributed to the writing of the manuscript. MO and TI confirmed the authenticity of all the raw data, and participated in writing and editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by Tokyo Medical University, Institutional Review Board (approval no. T2021-0260; Tokyo, Japan). All patients provided written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 2.Beitler JJ, Johnson JT. Transoral laser excision for early glottic cancer. Int J Radiat Oncol Biol Phys. 2003;56:1063–1066. doi: 10.1016/S0360-3016(03)00412-7. [DOI] [PubMed] [Google Scholar]
- 3.Back G, Sood S. The management of early laryngeal cancer: Options for patients and therapists. Curr Opin Otolaryngol Head Neck Surg. 2005;13:85–91. doi: 10.1097/01.moo.0000156168.63204.70. [DOI] [PubMed] [Google Scholar]
- 4.Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Laurberg P. Gender differences in symptoms of hypothyroidism: A population-based DanThyr study. Clin Endocrinol (Oxf) 2015;83:717–725. doi: 10.1111/cen.12787. [DOI] [PubMed] [Google Scholar]
- 5.Floriani C, Gencer B, Collet TH, Rodondi N. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 Update. Eur Heart J. 2018;39:503–507. doi: 10.1093/eurheartj/ehx050. [DOI] [PubMed] [Google Scholar]
- 6.Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: A review. JAMA. 2019;322:153–160. doi: 10.1001/jama.2019.9052. [DOI] [PubMed] [Google Scholar]
- 7.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okubo M, Itonaga T, Saito T, Shiraishi S, Mikami R, Sakurada A, Sugahara S, Park J, Tokuuye K, Saito K. Predictive factors for local control of early glottic squamous cell carcinomas after definitive radiotherapy. Mol Clin Oncol. 2020;12:541–550. doi: 10.3892/mco.2020.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommat K, Ong WS, Hussain A, Soong YL, Tan T, Wee J, Fong KW. Thyroid V40 predicts primary hypothyroidism after intensity modulated radiation therapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2017;98:574–580. doi: 10.1016/j.ijrobp.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Lee V, Chan SY, Choi CW, Kwong D, Lam KO, Tong CC, Sze CK, Ng S, Leung TW, Lee A. Dosimetric predictors of hypothyroidism after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol) 2016;28:e52–e60. doi: 10.1016/j.clon.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Lertbutsayanukul C, Kitpanit S, Prayongrat A, Kannarunimit D, Netsawang B, Chakkabat C. Validation of previously reported predictors for radiation-induced hypothyroidism in nasopharyngeal cancer patients treated with intensity-modulated radiation therapy, a post hoc analysis from a phase III randomized trial. J Radiat Res. 2018;59:446–455. doi: 10.1093/jrr/rry036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan CY, Lin CS, Chao HL, Huang WY, Su YF, Lin KT, Tsai IJ, Kao CH. Risk of hypothyroidism among patients with nasopharyngeal carcinoma treated with radiation therapy: A population-based cohort study. Radiother Oncol. 2017;123:394–400. doi: 10.1016/j.radonc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Luo R, Li M, Yang Z, Zhan Y, Huang B, Lu J, Xu Z, Lin Z. Nomogram for radiation-induced hypothyroidism prediction in nasopharyngeal carcinoma after treatment. Br J Radiol. 2017;90:20160686. doi: 10.1259/bjr.20160686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunwoo JB, Herscher LL, Kroog GS, Thomas GR, Ondrey FG, Duffey DC, Solomon BI, Boss C, Albert PS, McCullugh L, et al. Concurrent paclitaxel and radiation in the treatment of locally advanced head and neck cancer. J Clin Oncol. 2001;19:800–811. doi: 10.1200/JCO.2001.19.3.800. [DOI] [PubMed] [Google Scholar]
- 15.Tell R, Lundell G, Nilsson B, Sjödin H, Lewin F, Lewensohn R. Long-term incidence of hypothyroidism after radiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60:395–400. doi: 10.1016/j.ijrobp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Tell R, Sjödin H, Lundell G, Lewin F, Lewensohn R. Hypothyroidism after external radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 1997;39:303–308. doi: 10.1016/S0360-3016(97)00117-X. [DOI] [PubMed] [Google Scholar]
- 17.Ling S, Bhatt AD, Brown NV, Nguyen P, Sipos JA, Chakravarti A, Rong Y. Correlative study of dose to thyroid and incidence of subsequent dysfunction after head and neck radiation. Head Neck. 2017;39:548–554. doi: 10.1002/hed.24643. [DOI] [PubMed] [Google Scholar]
- 18.Diaz R, Jaboin JJ, Morales-Paliza M, Koehler E, Phillips JG, Stinson S, Gilbert J, Chung CH, Murphy BA, Yarbrough WG, et al. Hypothyroidism as a consequence of intensity-modulated radiotherapy with concurrent taxane-based chemotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:468–476. doi: 10.1016/j.ijrobp.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Grande C. Hypothyroidism following radiotherapy for head and neck cancer: Multivariate analysis of risk factors. Radiother Oncol. 1992;25:31–36. doi: 10.1016/0167-8140(92)90192-W. [DOI] [PubMed] [Google Scholar]
- 20.Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: A systematic review. Radiother Oncol. 2011;99:1–5. doi: 10.1016/j.radonc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhai RP, Kong FF, Du CR, Hu CS, Ying HM. Radiation-induced hypothyroidism after IMRT for nasopharyngeal carcinoma: Clinical and dosimetric predictors in a prospective cohort study. Oral Oncol. 2017;68:44–49. doi: 10.1016/j.oraloncology.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Vogelius IR, Bentzen SM, Maraldo MV, Petersen PM, Specht L. Risk factors for radiation-induced hypothyroidism: A literature-based meta-analysis. Cancer. 2011;117:5250–5260. doi: 10.1002/cncr.26186. [DOI] [PubMed] [Google Scholar]
- 23.Sachdev S, Refaat T, Bacchus ID, Sathiaseelan V, Mittal BB. Thyroid V50 highly predictive of hypothyroidism in head-and-neck cancer patients treated with intensity-modulated radiotherapy (IMRT) Am J Clin Oncol. 2017;40:413–417. doi: 10.1097/COC.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 24.Rønjom MF, Brink C, Bentzen SM, Hegedüs L, Overgaard J, Johansen J. Hypothyroidism after primary radiotherapy for head and neck squamous cell carcinoma: Normal tissue complication probability modeling with latent time correction. Radiother Oncol. 2013;109:317–322. doi: 10.1016/j.radonc.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 25.Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: Rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin. 2017;67:31–50. doi: 10.3322/caac.21386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.



