Abstract
In upper urinary tract infections, tubular epithelial cells (TEC) may play a pivotal role in the initiation of the renal inflammatory response. They exert crucial immunological functions such as processing and presentation of foreign antigen, secretion of proinflammatory cytokines (interleukin-6 [IL-6] and tumor necrosis factor alpha) and chemokines (IL-8, MCP-1, ENA-78, and RANTES). Since monolayer cultures are a limited model for polarized tubular epithelial cells, we studied the side-dependent IL-8 secretion of TEC by using cell culture inserts as a basement membrane imitation. Primary cultures of proximal TEC were stimulated with differently fimbriated mutants of Escherichia coli, E. coli LPS, S-fimbria isolates, and IL-1α. IL-8 protein was measured by enzyme-linked immunosorbent assay, and IL-8-like biological activity was tested by measuring elastase release from polymorphonuclear cells in supernatants of the upper and lower compartments. IL-8 mRNA was compared by competitive PCR. IL-8 secretion by TEC into the basolateral environment was significantly higher than secretion into the apical compartment, representing the tubular lumen. However, stimulation of IL-8 secretion by TEC was restricted to IL-1α and was not inducible by E. coli mutants, S fimbriae, or lipopolysaccharide. With this in vitro model of polarized TEC, we show that luminal contact of TEC with uropathogenic E. coli does not result in enhanced IL-8 secretion. The basolaterally directed production of the neutrophil chemotactic factor IL-8 by TEC after stimulation with IL-1α might play an important role in the initiation of inflammatory cell influx into the renal parenchyma.
More than 80% of urinary tract infections in adults are caused by Escherichia coli (31). For E. coli, different factors of virulence, e.g., lipopolysaccharides (LPS), hemolysins, or various types of fimbriae, have been characterized (8). Multiple lines of evidence have emerged concerning the involvement of proximal tubular epithelial cells (TEC) in the renal immune response. These cells have been shown to express major histocompatibility complex (MHC) class II antigens, which are essential for antigen presentation to CD4+ lymphocytes (32) and cellular adhesion molecules crucial for leukocyte migration, e.g., intercellular adhesion molecule-1 (16, 18) and VCAM-1 (6). They are capable of processing and presenting foreign antigen (27) and, besides other cytokines, produce different chemokines, a group of low-molecular-weight cytokines with chemotactic functions. So far, the secretion of RANTES (14), MCP-1 (10), ENA-78 (28), and interleukin-8 (IL-8) (29) has been studied in TEC. Secretion of IL-8 is supposed to be of major relevance for the influx of neutrophils after bacterial contact. In earlier studies we showed that, in contrast to renal carcinoma cells (3), the expression of MHC class II molecules and intercellular adhesion molecule-1 by TEC could not be significantly enhanced by S fimbriae, LPS, or E. coli (21, 23). Furthermore, the secretion of IL-6, tumor necrosis factor alpha, and IL-8 by TEC grown as monolayers could be stimulated by cytokines, but not by S fimbriae, LPS, or E. coli (23). Concerning signalling of TEC directed to the basolateral environment, the in vitro model of monolayer cultures grown on a continuous surface has its limitations. Therefore, we tested whether basolaterally directed IL-8 secretion by TEC differs from luminal secretion and, if so, whether basolaterally directed IL-8 secretion can be stimulated by virulence factors of E. coli.
MATERIALS AND METHODS
Primary cell culture of TEC and electron microscopy.
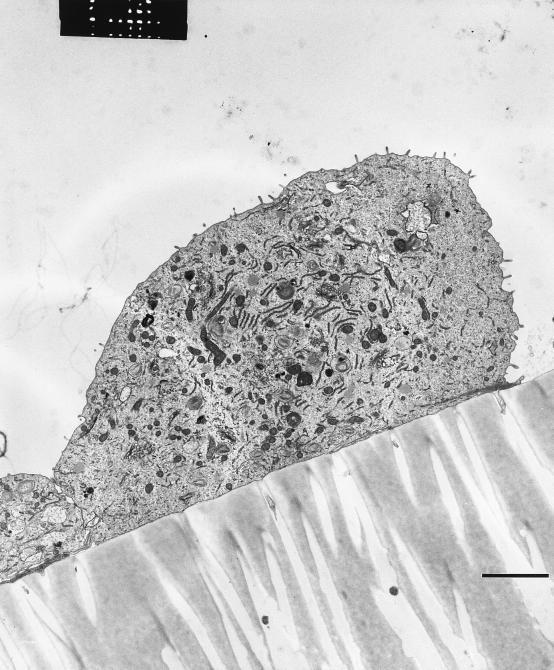
Normal renal tissue was obtained in the local Department of Urology from nephrectomies due to tumors (23). Cells were grown from 1-mm3 pieces of renal cortex in Dulbecco's modified Eagle medium–Ham's F-12 medium (BioWhittaker, Heidelberg, Germany) supplemented with epidermal growth factor (10 ng/ml), insulin-transferrin-sodium-selenite medium supplement (5 mg/liter), hydrocortisone (37.4 μg/liter), 3,3-5-triiodo-l-thyronine (40 mg/liter), penicillin (100 U/ml), streptomycin (100 μg/ml), and 1 M HEPES buffer (15 ml/liter of medium). All supplements were obtained from Sigma, Deisenhofen, Germany, except for penicillin and streptomycin (BioWhittaker). The purity and proximal tubular origin of each cell culture were determined by immunohistochemistry by using anti-cytokeratin (Dianova, Hamburg, Germany), anti-APM (kindly provided by J. E. Scherberich, Frankfurt, Germany), anti-CD68, and anti-factor VIII antibodies (both from Dako, Hamburg, Germany) (21) and by electron microscopy. The ultrastructure of TEC with a microvillus surface is presented in Fig. 1. For electron microscopy, small pieces of the filter membrane covered with TEC were excised and fixed with 2.5% glutaraldehyde and 0.05% CaCl2 in 0.1 mol of cacodylate buffer per liter (pH 7.4) for 2 h at 22°C. After a washing and dehydration, the cells were embedded in araldite. Ultrathin sections were cut on a Reichert Ultracut E and were stained with uranyl acetate and lead citrate by using an ultrastainer (LKB, Bromma, Sweden). Grids were examined with a Phillips EM400 at 60 kV. Only cells in the second to fourth passages were used for this study.
FIG. 1.
Transmission electron micrograph of primary TEC in culture demonstrates polarity and expression of microvilli on the cell surface. Bar, 1 μm.
Mutants of E. coli.
Different mutants of the uropathogenic O6:K15:H31 E. coli 536-21 wild type (536-21wt), kindly provided by J. Hacker, Würzburg, Germany, have been characterized according to their virulence properties (12). The mutant 536-21del shows a spontaneous mutation with lost in vivo virulence, including serum resistance and the production of fimbriae and hemolysin. In order to study the influence of single virulence factors, genes of wild-type fimbriae have been cloned and introduced into the deletion mutant 536-21del as shown in Table 1. Before use, 108 bacteria/ml were fixed in 1.25% glutaraldehyde for 1 h at room temperature to ensure sterile cell culture conditions. In former studies, fixed E. coli has been shown to preserve its stimulating properties (2). S fimbriae were isolated and purified by gradient ultracentrifugation as described recently (23).
TABLE 1.
Mutants of uropathogenic O6:K15:H31 E. coli (wild type) according to their virulence characteristics
| E. coli wild type or mutant | Virulence factor(s) | Abbreviation(s) |
|---|---|---|
| 536-21wt | Hemolysin; serum resistance; S, P, and F1C fimbriae | Hly+; Sr+; Sfa+, Pap+, and F1C+ |
| 536-21del | None | |
| 536-21(pANN801-4) | S fimbriae | Sfa+ |
| 536-21(pRHU845) | P fimbriae | Pap+ |
| 536-21(pPIL110-54) | F1C fimbriae | F1C+ |
Cell stimulation.
A total of 4 × 104 TEC were cultured overnight in culture inserts (Falcon, Heidelberg, Germany) with 0.4-μm pores (1.6 × 106 pores/cm2) and a 0.31-cm2 growth area. On the following day, TEC were stimulated with E. coli mutants (108/ml; fixated in 1.25% glutaraldehyde), IL-1α (1 ng/ml), LPS (1 μg/ml), or S fimbriae (1 μg/ml) on the apical side. The total volume in the upper compartment of the culture was 200 μl after stimulation, and in the lower compartment it was 800 μl. The supernatants in the upper and lower compartments were harvested after 24 to 72 h. After 72 h the viability was >87.5%. Permeability, determined by diffusion of phenol red as described previously (25), was inhibited by a confluent monolayer (4 × 104 cells/insert) by more than 75%. Supernatants were stored at −80°C until examination for IL-8 protein content and neutrophil-directed stimulating activity.
ELISA for IL-8.
For the measurement of IL-8 protein in the cell culture supernatants, a sandwich-type enzyme-linked immunosorbent assay (ELISA) with IL-8-specific antibodies, developed at the Research Center Borstel (Borstel, Germany), was used. Briefly, wells of U-bottom microassay plates (Dynatech, Denkendorf, Germany) were coated with 10 μg of monoclonal antibody (MAb) 94.1 (raised in BALB/c-mice against recombinant IL-8 [rIL-8] conjugated to myoglobin) per ml in 0.1 M bicarbonate (pH 9) overnight at 4°C. After an extensive washing, all subsequent incubation steps with antigen-containing samples and immunoreagents were performed in dilution buffer (phosphate-buffered saline–Tween–1.5% bovine serum) at 37°C for 1 h. A polyclonal rabbit anti-IL-8 serum, induced by immunization with a synthetic peptide representing the C-terminal part of the IL-8 molecule (residues 54 to 72), was used as a detecting antibody. Peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dianova, Hamburg, Germany) served as a secondary antibody, and development was performed by using o-phenylenediamine–H2O2 as previously described (4). For quantification, a standard of recombinant monocytic IL-8 (rmIL-8; i.e., the 72-residue isoform), produced at the Research Center Borstel, was run in parallel on each assay plate. As determined by solid-phase ELISA and Western blotting, neither MAb 94.1 nor the rabbit anti-IL-8 serum exhibited any cross-reactivity to the IL-8-related chemokines NAP-2, CTAP-III, IP-10, PF-4, and MGSA/GRO.
Neutrophil elastase release assay.
To estimate their contents in IL-8-like biological activity, cell culture supernatants were tested for their capacity to induce the release of lysosomal elastase in suspended, cytochalasin B-pretreated polymorphonuclear neutrophil granulocytes (PMN). The isolation of these granulocytes from the freshly drawn blood of single healthy donors by gradient centrifugation on Ficoll-Hypaque, cell stimulation, and the measurement of released elastase enzymatic activity was performed as previously described (5). A standard of rmIL-8 (see above) was run in parallel to the cell culture samples, and results were expressed as IL-8 activity equivalents. In some experiments, anti-IL-8 MAb (MAb 94.1) at a final concentration of 2 μg/ml, a level sufficient to neutralize the activity of 100 ng of IL-8 per ml, was added to the supernatants (final dilution, 1 in 2) in order to estimate the proportion of IL-8-associated neutrophil-stimulating capacity in the supernatants.
Extraction and reverse transcription of mRNA.
For quantification of IL-8 mRNA, tubular epithelial cells (106) were stimulated with different mutants of E. coli 536-21 or IL-1α (1 ng/ml) for 24 h. Polyadenylated RNA was purified by using a direct mRNA purification kit (Dynal, Hamburg, Germany) according to the manufacturer's protocol. After the final purification step the mRNA was resuspended in a volume of 30 μl. For the reverse transcription, 10 μl of mRNA solution was incubated with 0.5 μg of Oligo-dT12-18-Primer (Pharmacia, Freiburg, Germany) at 70°C for 10 min. Reverse transcription was performed in MMLV-RT-(RNase H−)-Buffer (Gibco, Eggenstein, Germany), 4× 0.5 mM deoxynucleoside triphosphate (dNTP; Pharmacia), 2 mM dithiothreitol (Gibco), 12.5 mU of RNAguard (Pharmacia), 200 U of MMLV-Superscript reverse transcriptase (Gibco), and diethylpyrocarbonate-H2O. The total reaction mixture (20 μl) was then incubated at 37°C for 1 h.
PCR.
As primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), we used 5′-d(AACAGCGACACCCACTCCTC)-3′ (sense) and 5′-d(GGAGGGGAGATTCAGTGTG GT)-3′ (antisense) at an annealing temperature of 67°C, resulting in a 258-bp fragment. All cDNA was normalized for GAPDH expression before competitive PCR. As intron-spanning IL-8 cDNA primers, 5′-d(TGCCAAGGAGTGCTAAAG)-3′ (sense) and 5′-d(TCTCAGCCCTCTTCAA AA)-3′ (antisense) were used at an annealing temperature of 52°C, yielding a product of 219 bp. For quantification of IL-8 cDNA, a competitive segment was generated according to the method of Schmouder et al. (28); it consisted of the same priming sites and base composition as the IL-8 template but was shortened by 119 bp. PCR was performed in a reaction volume of 25 μl. Then, 1.0 μl of cDNA (1:10) was added to a Taq-buffer solution (Gibco) in H2O containing 1.5 mM MgCl2, 1 μM specific primer, 2.5 mM concentrations of each dNTP, and 0.625 U of Taq polymerase (Gibco). For the competitive PCR, 1 μl of the competitive segment in a known dilution was coamplified with 1 μl of IL-8 cDNA. The dilution that yielded the same amount of cDNA as for IL-8 was recorded. Subsequently, 25 (GAPDH) or 30 (IL-8) cycles of PCR were completed (94°C for 1 min, annealing temperature for 1 min, and 72°C for 1 min and 30 s extended by 2 s per cycle and followed by 10 min of elongation at 72°C) in a Biometra thermal cycler. The PCR product was visualized on 2.0% agarose gels in Tris-borate-EDTA buffer stained with ethidium bromide.
Immunohistochemistry.
Serial 6-μm cryostat sections of renal tissue with histopathological diagnosis of acute pyelonephritis were prepared for APAAP staining as described earlier (9). One month before, E. coli (106/ml) was found in the urine culture of this patient. Briefly, sections were fixed with acetone for 10 min and then incubated with polyclonal anti-IL-8 antibody (see above) for 1 h, an intermediate mouse anti-rabbit antibody for 30 min, and rabbit anti-mouse antibody for 30 min. A complex of alkaline phosphatase and monoclonal anti-alkaline phosphatase antibody was added for 30 min. Finally, the sections were stained with new fuchsin and counterstained with Meyer's hematoxylin. The stains were reviewed by a pathologist (S.K.) from the Institute of Pathology, Medical University, Lübeck, Germany.
RESULTS
Time-dependent stimulation of IL-8 secretion by TEC.
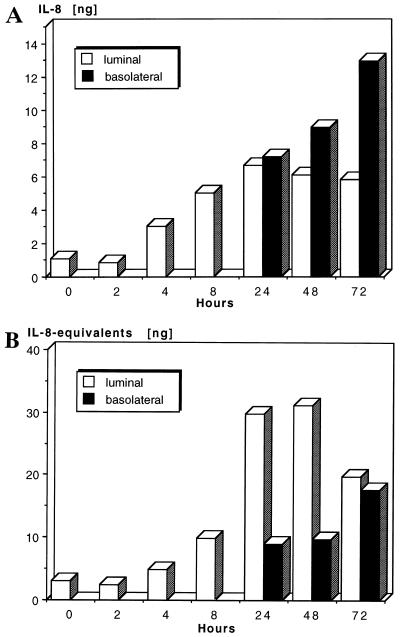
For the detection of IL-8 by ELISA, as well as of IL-8-like biological activity by the neutrophil elastase release assay, the same supernatants, derived from the TEC of three different donors, were used. Stimulation of TEC with IL-1α (1 ng/ml) over a period of 0 to 72 h resulted in increased secretion of IL-8 and IL-8-like activity. Secretion, higher than backround levels, was first detected in the upper compartment, where from 4 h of stimulation on, IL-8 protein and activity levels increased with time, reaching maximal values after 24 h (Fig. 2A and B, respectively). The onset of secretion in the lower compartment, representing the basolateral environment, occurred at 24 h, after which time relatively high IL-8 protein levels, as well as activity levels, were present. Maximal values were obtained after 72 h of stimulation. As measured by ELISA, after the initial luminal IL-8 release the secretion of the chemokine was mainly directed to the basolateral side (Fig. 2A). In contrast, IL-8-like biological activity, as measured by the neutrophil elastase release assay, was at all time points higher in the upper compartment, representing the luminal environment (Fig. 2B). This result suggested that additional neutrophil-directed stimuli, other than IL-8, were also secreted by stimulated TEC. Inhibition studies of neutrophil elastase release with a neutralizing anti-IL-8 MAb revealed that stimulation with upper-compartment supernatants was only partially (the maximum value was 50.1% after 48 h) blocked by anti-IL-8 MAb, whereas stimulation with lower-compartment supernatants could be completely blocked (data not shown). This indicates that the proportion of IL-8 in upper-compartment supernatants is lower than was suggested by elastase release assay.
FIG. 2.
Time kinetics of IL-8 secretion by stimulated TEC. A total of 104 TEC/well were stimulated with IL-1α (1 ng/ml) in cell culture inserts over a period of 2 to 72 h. Secretion toward the upper and lower compartments was recorded. For each time point, supernatants of four wells were pooled and the IL-8 secretion was measured by ELISA (Fig. 2A). The functional activity of IL-8 production was determined by the neutrophil elastase release assay (Fig. 2B). The median of three experiments is shown. Data are given as nanograms of IL-8 protein (ELISA) and IL-8-like biological activity in nanogram IL-8 equivalents (release assay) contained in the total volume of supernatants.
IL-8 secretion after incubation with different strains of E. coli.
For the E. coli mutants used for this study the adherence modalities have been characterized. As recently published (21), S-fimbria-bearing strains showed the strongest adhesion, while the deletion mutant bound the least to primary TEC. In the present study we investigated the influence of E. coli mutants on IL-8 secretion. Stimulation of TEC by the different mutants of E. coli for 24 or 48 h did not result in a significant increase of IL-8 secretion compared to unstimulated controls, as determined by both ELISA and neutrophil elastase release assay (Table 2). Nevertheless, after the stimulation of TEC with IL-1α, IL-8 secretion increased and was preferably directed to the basolateral environment. Furthermore, the IL-8 secretion of peripheral blood mononuclear cells (PBMC) could not be enhanced by E. coli mutants (data not shown).
TABLE 2.
IL-8 production by TEC after stimulation with differently fimbriated mutants of E. coli (108/ml) and IL-1α (1 ng/ml) in cell culture insertsa
| Stimulation | Expt | Secretion of IL-8/total vol (ng) (IL-8 equivalents by release assay [ng]) at:
|
|||
|---|---|---|---|---|---|
| 24 h
|
48 h
|
||||
| Luminal | Basolateral | Luminal | Basolateral | ||
| Control | E1 | 0.22 (0.0) | 0.9 (0.0) | 11.97 (0.92) | 42.96 (2.40) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 536-21wt | E1 | 0.15 (0.0) | 1.32 (0.0) | 10.94 (0.94) | 45.18 (2.64) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 536-21del | E1 | 0.15 (0.0) | 1.08 (0.0) | 11.32 (1.10) | 41.58 (2.64) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E. coli mutants | |||||
| 536-21(pANN801-4) | E1 | 0.17 (0.0) | 1.38 (0.0) | 10.33 (1.22) | 41.40 (3.78) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 536-21(pRHU845) | E1 | 0.18 (0.0) | 1.20 (0.0) | 8.62 (0.82) | 32.28 (3.00) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 536-21(pPIL110-54) | E1 | 0.16 (0.0) | 1.20 (0.0) | 8.12 (0.95) | 34.02 (2.88) |
| E2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| E3 | 0.0 | 0.0 | 0.0 | 0.0 | |
| IL-1α (1 ng/ml) | E1 | 0.96 (0.11) | 3.90 (0.23) | 18.62 (1.48) | 60.72 (5.04) |
| E2 | 6.5 | 8.8 | 10.0 | 12.8 | |
| E3 | 1.5 | 3.2 | 1.68 | 2.40 | |
Supernatants were collected after 24 and 48 h. Cytokine secretion was measured in duplicate by sandwich ELISA, and IL-8-like biological activity (in parentheses) was measured by neutrophil elastase release assay. Experiments (E) with TEC derived from three of six kidneys are shown.
IL-8 secretion after incubation with E. coli S fimbriae and LPS.
S fimbriae were isolated from E. coli HB101(pANN801-4) (23). In previous studies, S fimbriae have been shown to adhere to primary TEC (21) and to induce IL-6 production and ICAM-1 expression by renal carcinoma cells (20). By the same protocol as was used with the E. coli mutants, TEC were incubated with isolates of S fimbriae (1 μg/ml), E. coli LPS (1 μg/ml), and IL-1α (1 ng/ml) for 24 and 48 h. S fimbriae and LPS did not increase IL-8 secretion to either the luminal or the basolateral surface. IL-1α-stimulated IL-8 secretion after 24 and 48 h was again higher in the lower compartment (data not shown). However, incubation of PBMC with S fimbriae and LPS resulted in higher IL-8 production than stimulation with IL-1α (Fig. 3).
FIG. 3.
IL-8 secretion of PBMC (2 × 107/well) either unstimulated or after incubation with IL-1α (1 ng/ml), E. coli LPS (1 μg/ml), or S fimbriae (1 μg/ml) for 24 and 48 h. Cytokine production was determined in duplicate by sandwich ELISA.
Expression of IL-8 mRNA by TEC stimulated with E. coli mutants.
As previously reported by Schmouder et al. (28), we generated a competitive segment for IL-8 cDNA. Unstimulated TEC showed basal expression of IL-8 mRNA (0.1 ng/ml). Incubation of TEC, derived from three different patients, with E. coli mutants did not result in an increase in IL-8 mRNA. However, stimulation with IL-1α enhanced IL-8 mRNA expression of all TEC significantly (Fig. 4).
FIG. 4.
Expression of IL-8 mRNA by TEC after stimulation with E. coli mutants and IL-1α was determined by competitive PCR as described in Materials and Methods. To exclude DNA contamination, negative controls were run without cDNA template. One representative experiment of three is shown.
In vivo detection of IL-8 in pyelonephritis.
To investigate in vivo IL-8 production in bacterial inflammation, we performed immunohistochemical staining with a polyclonal anti-IL-8 antibody on cryostat sections of active pyelonephritis from a patient with clinically significant bacteriuria. As shown in Fig. 5A, IL-8 was found in the tubular epithelium at the site of inflammation. Stains from normal kidney (Fig. 5B), as well as the control without primary antibody, remained negative for IL-8.
FIG. 5.
Immunohistochemical detection of IL-8 on cryostat sections of renal tissue from active pyelonephritis (A) and normal kidney (B). Tubular epithelium in inflammatory areas of the kidney with interstitial nephritis showed a positive reaction with polyclonal anti-IL-8 antibody. The slides were stained with APAAP complex and counterstained with hematoxylin as described in Materials and Methods. Magnification, ×600.
DISCUSSION
The chemoattractant protein IL-8 has been shown to be of major relevance for the influx and transendothelial migration of neutrophils to sites of inflammation (15). In experimental LPS-induced dermatitis and arthritis, as well as in lung reperfusion injury and acute immune complex glomerulonephritis, the administration of anti-IL-8 antibody prevented neutrophil infiltration and subsequent neutrophil-dependent tissue damage (13). Patients with sepsis caused by gram-negative and gram-positive organisms showed elevated serum IL-8 levels, and a correlation between the initial serum IL-8 and fatal outcome was found (11). Furthermore, in patients with acute E. coli pyelonephritis, IL-8 levels were elevated in serum and urine (17).
Besides infiltrating cells of the immune system, e.g., polymorphonuclear cells (7) or macrophages (24), renal mesangial (1, 22) and cortical epithelial (29) cells produce IL-8. In an earlier study we demonstrated that primary cultures of proximal tubular epithelial cells constitutively produce low levels of IL-8 mRNA and protein and can be stimulated with IL-1α for IL-8 secretion. No stimulation after exposure to differently fimbriated E. coli was seen (23). Other investigators described a polarity of renal tubular epithelium cultured on microporous cell culture inserts (26, 30). Recently, Phillips et al. reported that proximal tubular cells secrete transforming growth factor β 1 equally into the apical and basolateral compartments only after basolateral exposure to platelet-derived growth factor in combination with d-glucose (25). Against this background, we stimulated primary tubular epithelial cells on cell culture inserts on the apical side, representing the tubular lumen, with mutants of E. coli, S-fimbria isolates, LPS, and IL-1α. After incubation with IL-1α, an increase of IL-8 production on both the mRNA and protein levels was detected. After initial luminal secretion of IL-8, which might contribute to detection of IL-8 in the urine of patients with pyelonephritis (17), the secretion of IL-8 was preferably directed to the basolateral environment. No increase of IL-8 production was seen after the exposure of TEC to E. coli mutants, S fimbriae, or LPS. However, in mononuclear cells, IL-8 secretion was induceable by LPS and S fimbriae. This result can partially be explained by our recent findings that TEC do not express CD14, the receptor for LPS (23). We conclude that, in vitro, renal proximal TEC secrete IL-8 directed to the basolateral environment after stimulation with IL-1α. In a rat model of acute obstructive pyelonephritis induced by E. coli, increased IL-8 production by the tubular epithelium has recently been demonstrated (19). Accordingly, in human renal tissue from pyelonephritis, TEC at the site of inflammation were also stained by anti-IL-8 antibody, while in cryostat sections of normal kidney no IL-8 was found. However, further investigations will need to elucidate the initiation factors of the immune response after contact of bacteria with the tubular epithelium.
ACKNOWLEDGMENTS
This study was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 367, B3, and C4.
We also acknowledge the expert technical assistance of C. Pongratz and F. Müller.
REFERENCES
- 1.Abbott F, Ryan J J, Ceska M, Matsushima K, Sarraf C E, Rees A J. Interleukin-1β stimulates human mesangial cells to synthesize and release interleukin-6 and -8. Kidney Int. 1991;40:597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- 2.Agace W, Hedges S, Anderson U, Anderson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agace W W, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect Immun. 1995;63:4054–4062. doi: 10.1128/iai.63.10.4054-4062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt E, Petersen F, Flad H-D. A novel molecular variant of the neutrophil-activating peptide NAP-2 with enhanced biological activity is truncated at the C-terminus: identification by antibodies with defined epitope specificity. Mol Immunol. 1993;30:979–991. doi: 10.1016/0161-5890(93)90123-s. [DOI] [PubMed] [Google Scholar]
- 5.Brandt E, Van Damme J, Flad H-D. Neutrophils can generate their activator neutrophil-activating peptide 2 by proteolytic cleavage of platelet-derived connective tissue-activating peptide III. Cytokine. 1991;3:311–321. doi: 10.1016/1043-4666(91)90499-4. [DOI] [PubMed] [Google Scholar]
- 6.Brockmeyer C, Ulbrecht M, Schendel D, Weiss E H, Hillebrand D, Burckhardt K, Land W, Gokel M J, Riethmüller G, Feucht H E. Distribution of cell adhesion molecules (ICAM-1, VCAM-1, ELAM-1) in renal tissue during allograft rejection. Transplantation. 1993;55:610–615. doi: 10.1097/00007890-199303000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Cassatella M A, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes: the chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol. 1992;148:3216–3220. [PubMed] [Google Scholar]
- 8.Donnenberg M S, Welch R A. Virulence determinants of uropathogenic Escherichia coli. In: Mobley H L T, Warren J W, editors. Virulence determinants of uropathogenic Escherichia coli. Washington, D.C.: American Society for Microbiology; 1996. pp. 136–174. [Google Scholar]
- 9.Erber W N, Pinching A J, Mason D Y. Immunocytochemical detection of T and B cell populations in routine blood smears. Lancet. 1984;i:1042–1046. doi: 10.1016/s0140-6736(84)91451-x. [DOI] [PubMed] [Google Scholar]
- 10.Gesualdo L, Grandaliano G, Ranieri E, Monno R, Montinaro V, Manno C, Schena R P. Monocyte recruitment in cryoglobulinemic membranoproliferative glomerulonephritis: a pathogenetic role for monocyte chemotactic peptide-1. Kidney Int. 1997;51:155–163. doi: 10.1038/ki.1997.19. [DOI] [PubMed] [Google Scholar]
- 11.Hack C E, Hart M, Strack van Schijndel R J M, Eerenberg A J M, Nuijens J H, Thijs L G, Aarden L A. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60:2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker J, Schmidt G, Hughes C, Knapp S, Marget M, Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985;47:434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 14.Heeger P, Wolf G, Neyers C, Sun M J, O'Farrell S, Krensky M, Neilson E G. Isolation and characterization of cDNA from renal tubular epithelium encoding murine RANTES. Kidney Int. 1992;41:220. doi: 10.1038/ki.1992.31. [DOI] [PubMed] [Google Scholar]
- 15.Huber A R, Kunkel S L, Todd R T, Weiss S J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 16.Ishikura H, Takahashi C, Kanagawa K, Hirata H, Imai K, Yoshiki T. Cytokine regulation of ICAM-1 expression on human renal tubular epithelial cells in vitro. Transplantation. 1991;51:1272–1275. doi: 10.1097/00007890-199106000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson S H, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial-virulence-associated traits and renal function. Nephron. 1994;67:172–179. doi: 10.1159/000187923. [DOI] [PubMed] [Google Scholar]
- 18.Jevnikar A M, Wuthrich R P, Takei F, Xu H-W, Brennan D C, Glimcher L H, Rubin-Kelley V E. Differing regulation and function of ICAM-1 and class II antigens on renal tubular cells. Kidney Int. 1990;38:417–425. doi: 10.1038/ki.1990.221. [DOI] [PubMed] [Google Scholar]
- 19.Kaboré A F, Simard M, Bergeron M G. Local production of inflammatory mediators in an experimental model of acute obstructive pyelonephritis. J Infect Dis. 1999;179:1162–1172. doi: 10.1086/314700. [DOI] [PubMed] [Google Scholar]
- 20.Kreft B, Bohnet S, Carstensen O, Hacker J, Marre R. Differential expression of interleukin-6, intercellular adhesion molecule 1, and major histocompatibility complex class II molecules in renal carcinoma cells stimulated with S fimbriae of uropathogenic Escherichia coli. Infect Immun. 1993;61:3060–3063. doi: 10.1128/iai.61.7.3060-3063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreft B, Placzek M, Doehn C, Hacker J, Schmidt G, Wasenauer G, Daha M R, van der Woude F J, Sack K. S fimbriae of uropathogenic Escherichia coli bind to primary human renal proximal tubular epithelial cells but do not induce expression of intercellular adhesion molecule 1. Infect Immun. 1995;63:3235–3238. doi: 10.1128/iai.63.8.3235-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusner D J, Luebbers E L, Nowinski R J, Konieczkowski M, Sedor J R. Cytokine and LPS-induced synthesis of interleukin-8 from human mesangial cells. Kidney Int. 1991;39:1240–1248. doi: 10.1038/ki.1991.157. [DOI] [PubMed] [Google Scholar]
- 23.Leeker, A., M. Ernst, S. Krüger, K. Sack, and B. Kreft. Lack of cytokine response in human tubular epithelial cells following exposure to Escherichia coli. Submitted for publication.
- 24.Lin C-Y, Huang T-P. Gene expression and release of interleukin-8 by peritoneal macrophages and polymorphonuclear leukocytes during peritonitis in uremic patients on continuous ambulatory peritoneal dialysis. Nephron. 1994;68:437–441. doi: 10.1159/000188304. [DOI] [PubMed] [Google Scholar]
- 25.Phillips A O, Steadman R, Morrisey K, Williams J D. Polarity of stimulation and secretion of transforming growth factor-beta 1 by cultured proximal tubular cells. Am J Pathol. 1997;150:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- 26.Prozialeck W C, Lamar P C. Surface binding and uptake of cadmium (Cd2+) by LLC-PK1 cells on permeable membrane supports. Arch Toxicol. 1993;67:113–119. doi: 10.1007/BF01973681. [DOI] [PubMed] [Google Scholar]
- 27.Rubin-Kelley V, Singer G G. The antigen presentation function of renal tubular epithelial cells. Exp Nephrol. 1993;1:102–111. [PubMed] [Google Scholar]
- 28.Schmouder R L, Strieter R M, Walz A, Kunkel S L. Epithelial-derived neutrophil-activating factor-78 production in human renal tubule epithelial cells and in renal allograft rejection. Transplantation. 1995;59:118–124. doi: 10.1097/00007890-199501150-00021. [DOI] [PubMed] [Google Scholar]
- 29.Schmouder R L, Strieter R M, Wiggins R C, Chensue S W, Kunkel S L. In vitro and in vivo interleukin-8 production in human renal cortical epithelia. Kidney Int. 1992;41:191–198. doi: 10.1038/ki.1992.26. [DOI] [PubMed] [Google Scholar]
- 30.Schramek H, Gstraunthaler G, Willinger C C, Pfaller W. Hyperosmolality regulates endothelin release by Madin-Darby canine kidney cells. J Am Soc Nephrol. 1993;4:206–213. doi: 10.1681/ASN.V42206. [DOI] [PubMed] [Google Scholar]
- 31.Warren J W. Clinical presentation and epidemiology of urinary tract infections. In: Mobley H L T, Warren J W, editors. Urinary tract infections. Molecular pathogenesis and clinical management. Washington, D.C.: American Society for Microbiology; 1996. pp. 3–27. [Google Scholar]
- 32.Wuthrich R P, Glimcher L H, Yui M A, Jevnikar A M, Dumas S E, Kelley V E. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]