Abstract
Importance
The benefit of an electronic support system for the prescription and adherence to oral anticoagulation therapy among patients with atrial fibrillation (AF) and atrial flutter at heightened risk for of stroke and systemic thromboembolism is unclear.
Objective
To evaluate the effect of a combined alert intervention and shared decision-making tool to improve prescription rates of oral anticoagulation therapy and adherence.
Design, Setting, and Participants
A prospective single arm study of 939 consecutive patients treated at a large tertiary healthcare system.
Exposures
An electronic support system comprising 1) an electronic alert to identify patients with AF or atrial flutter, a CHA2DS2-VASc score ≥ 2, and not on oral anticoagulation and 2) electronic shared decision-making tool to promote discussions between providers and patients regarding therapy.
Main Outcomes and Measures
The primary endpoint was prescription rate of anticoagulation therapy. The secondary endpoint was adherence to anticoagulation therapy defined as medication possession ratio ≥ 80% during the 12 months of follow-up.
Results
Between June 13, 2018 and August 31, 2018, the automated intervention identified and triggered a unique alert for 939 consecutive patients with AF or atrial flutter, a CHA2DS2-VASc score ≥2 who were not on oral anticoagulation. The median CHA2DS2-VASc score among all patients identified by the alert was 2 and the median untreated duration prior to the alert was 495 days (interquartile range 123-1,831 days). Of the patients identified by the alert, 345 (36.7%) initiated anticoagulation therapy and 594 (63.3%) did not: 68.7% were treated with a non-Vitamin K antagonist oral anticoagulant (NOAC), 22.0% with warfarin, and 9.3 % combination of NOAC and warfarin. Compared with historical anticoagulation rates, the electronic alert was associated with a 23.6% increase in anticoagulation prescriptions. The overall 1-year rate of adherence to anticoagulant therapy was 75.4% (260/345).
Conclusion and Relevance
An electronic automated alert can successfully identify patients with AF and atrial flutter at high risk for stroke, increase oral anticoagulation prescription, and support high rates of adherence.
Introduction
Professional society guidelines recommend chronic oral anticoagulation to reduce the risk of stroke and systemic thromboembolism in patients with atrial fibrillation (AF) or atrial flutter.1 Despite this recommendation, contemporary registries show that up to 31% of patients with new- onset AF who are at moderate to high risk for stroke are not prescribed oral anticoagulation therapy.2 Moreover, adherence to therapy among patients prescribed oral anticoagulants is variable. Prior studies have demonstrated that less than 50% of patients are adherent to anticoagulation in the first year following a new diagnosis of AF.3 Computerized electronic alert systems have been shown to increase short-term anticoagulation utilization in hospitalized patients with AF.4 However, long-term data in an ambulatory population are lacking as are data on medication adherence. We sought to prospectively evaluate the use of a computerized electronic support system that combined an alert and shared decision-making tool to facilitate oral anticoagulation treatment discussions in patients with AF. We hypothesized that an electronic resource that provided clinical decision support and promoted discussions between physicians and patients would translate into increased rates of oral anticoagulation prescription and adherence to anticoagulation therapy among patients with AF.
Methods
Study Design
This was a systematic quality improvement intervention with 1-year follow up that evaluated the effect of an electronic derived clinical reminder that identified patients with AF or atrial flutter at high risk for stroke or systemic embolism who were not receiving guideline directed anticoagulation, coupled with an educational shared decision making tool.1 The enrollment took place between June 13, 2018 and August 31, 2018, and then patients were followed over the ensuing year to determine 1) prescription rates for oral anticoagulation in untreated anticoagulation eligible patients with AF or atrial flutter and 2) long-term adherence to oral anticoagulation in these patients (Figure 1). This study was approved by the Durham VA Medical Center Institutional Review Board, which waived informed consent.
Figure 1. Study flow chart.
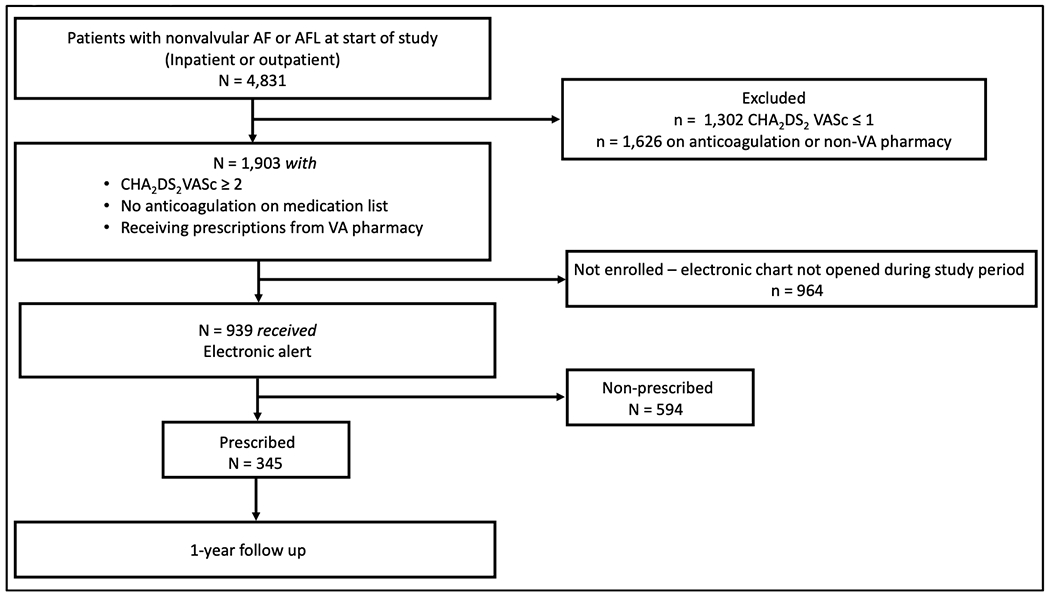
AF = atrial fibrillation; AFL = atrial flutter; VA = Veterans Affairs
Study Population
Subjects were eligible if 1) they received care at the Durham VA Healthcare System (VAHS); 2) had AF or atrial flutter; 3) were at increased risk for stroke and/or systemic thromboembolism, defined as a CHA2DS2-VASc ≥2, and; 4) were not currently prescribed therapeutic oral anticoagulation. Anticoagulation therapy was defined as an active VA prescription for apixaban 2.5 or 5 mg twice daily, dabigatran 75 or 150 mg twice daily, edoxaban 30 or 60 mg daily, rivaroxaban 15 or 20 mg daily, or warfarin (goal International Ratio [INR] of 2-3). Patients were excluded if 1) AF or atrial flutter was in the setting of valvular heart disease, defined as prior prosthetic heart valve replacement or repair and/or severe valvular stenosis or regurgitation; or 2) used a non-VA pharmacy for anticoagulation therapy.
Intervention: electronic alert and shared decision-making tool
We developed a two-part electronic support system that was embedded in the Durham VAHS computerized patient record system (CPRS). The first component of this system was an electronic alert intervention that used International Classification of Diseases, Ninth and Tenth (ICD-9 and 10) codes with billing and prescription records to identify patients with nonvalvular AF or atrial flutter at increased risk for stroke and/or systemic thromboembolism, defined as a CHA2DS2-VASc ≥2 and not on oral anticoagulation. The electronic alert automatically calculated CHA2DS2-VASc scores using baseline characteristics and problem lists obtained from CPRS. The first step of the system would trigger an electronic alert every time the patient’s record was opened, irrespective of who was opening the chart (physician, advanced provider, etc.) or setting (inpatient or outpatient), and recommend providers 1) initiate anticoagulation; 2) refer to cardiology; or 3) document reason for not starting oral anticoagulation. (Figure 2)
Figure 2. CPRS alert.
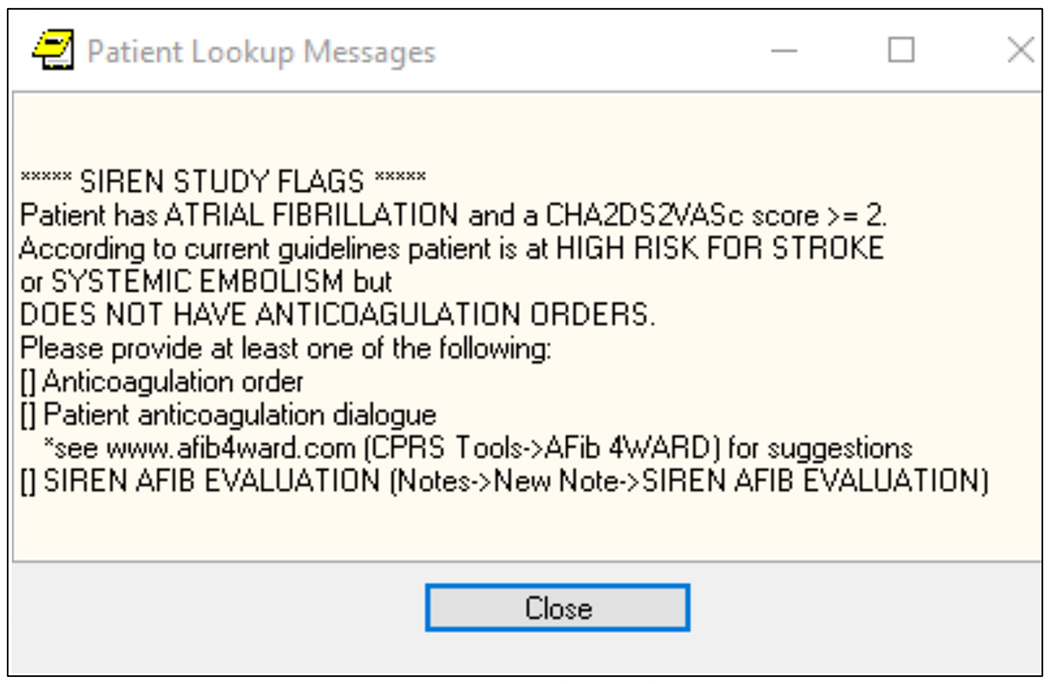
CPRS = computerized patient record system; AFIB = atrial fibrillation
Patients were considered enrolled in the study if the alert was triggered during the 2.5- month enrollment period. However, the alert was activated at every clinical visit during the follow up period until a prescription for anticoagulation was initiated or when a templated AF or atrial flutter note was entered that addressed the reasons for not starting anticoagulation. Enrolled patients were then categorized as prescribed or non-prescribed depending on whether anticoagulation therapy was or was not initiated, respectively.
While the first component of the intervention occurred uniformly for every eligible patient, the second component of the electronic support system included optional access to AFIB4WARD.com, which housed an online aid developed by a coalition of academic physicians across various disciplines to help providers and patients engage in shared decision discussions regarding anticoagulation care. Boehringer Ingelheim Pharmaceuticals, Inc partially funded an unbranded initiative to promote medical education with this online aid. The tool was opened at the request of the clinician and opening of the tool was not required. This shared decision-making tool highlighted the following questions and topics: 1) What is AF and how is it related to stroke?; 2) Understanding my risk of stroke; 3) How do I lower my risk of stroke?; and 4) Importance of staying on medications. The support system did not endorse any particular type of anticoagulation but instead focused on the need for anticoagulation in general. We did not track the number of times the decision tool was opened nor obtained any feedback regarding user experience. The online aid was only available during the study period, but the content has been reproduced in its entirety as Figure 3a – 3d.
Figure 3a.

Conversation overview
Figure 3d.

How do I lower my risk of stroke?
Study Characteristics and endpoints
Baseline characteristics, comorbidities, and medications were obtained from the VA Corporate Data Warehouse. Vital status and prescription data was available for all participants throughout study duration. The primary endpoint of this study is the prescription of anticoagulation therapy in patients with AF or atrial flutter at increased risk for stroke and/or systemic thromboembolism – defined as a CHA2DS2-VASc ≥2. Anticoagulation therapy was defined as an active prescription for apixaban 2.5 or 5 mg twice daily, dabigatran 75 or 150 mg twice daily, edoxaban 30 or 60 mg daily, rivaroxaban 15 or 20 mg daily, or warfarin (INR of 2-3). The frequency of prescription for anticoagulation in patients with nonvalvular AF or atrial flutter and a CHA2DS2-VASc score ≥2 was calculated. The anticoagulation prescription rate associated with the intervention was also compared with historical anticoagulation rates among patients receiving care at the Durham VAHS. The historical anticoagulation rates were calculated using the rate of anticoagulation prescription (for a 90-day duration) in patients with 1) nonvalvular AF or atrial flutter, 2) a CHA2DS2-VASc score ≥2, and 3) no anticoagulation in the preceding 90 days one year prior to the present study. The secondary endpoint of this study was adherence to anticoagulation therapy. Participants were defined as either adherent or non-adherent using a validated medication possession ratio.5 The ratio reflects the proportion of days during the time period during which the patient had a sufficient number of pills to fulfill their daily dose. Medication possession was ascertained using pharmacy refill records in VA administrative databases covering all VA facilities nationwide. Patients were defined as adherent if they had medication in their possession for at least 80% of the 12-month follow up period. The adherence rate for the study population was also compared with historical adherence rates. For this comparison the historical population was defined as patients from 06/13/2017 to 09/13/2017 with nonvalvular atrial fibrillation or atrial flutter, CHA2DS2-VASc ≥2, and no anticoagulation in the previous prior 90 days and the study population was defined as patients from 06/13/2018 to 09/13/2018 with nonvalvular atrial fibrillation or atrial flutter, CHA2DS2-VASc ≥2, and no anticoagulation in the previous prior 90 days. Historical adherence rates were calculated using a medication possession of at least 80% of a 12-month follow up period.
Statistical analysis
Baseline demographics, comorbidities, and medications were obtained using (ICD-9 and 10) codes and compared between patients prescribed and not prescribed anticoagulation therapy. Continuous variables were compared using two sample t-test or Wilcoxon rank sum test. Categorical variables were compared using Chi-Square or Fisher’s exact test. A multivariable logistic regression was performed using all of the covariates in Table 1 to assess patient factors association with prescription of anticoagulation therapy. Two-sided p-values were reported, and a p-value <0.05 was considered statistically significant. All analyses were performed using SAS 9.4 for Windows.
Table 1.
Characteristics
| Anticoagulation (not prescribed) N=594 | Anticoagulation (prescribed) N=345 | Total N=939 | P-value | |
|---|---|---|---|---|
| Characteristic | ||||
| Age (years) | 0.002 | |||
| Median (25th, 75th percentiles) | 73 (68, 81) | 71 (67, 77) | 72 (68, 80) | |
| Age ≥ 75, n (%) | 262 (44.1) | 112 (32.5) | 374 (39.8) | <.001 |
| Sex, n (%) | 0.227 | |||
| Female | 14 (2.4) | 4 (1.2) | 18 (1.9) | |
| Male | 580 (97.6) | 341 (98.8) | 921 (98.1) | |
| Race, n (%) | 0.061 | |||
| African American | 161 (27.1) | 97 (28.1) | 258 (27.5) | |
| White | 394 (66.3) | 237 (68.7) | 631 (67.2) | |
| Other/Multi-races | 10 (1.7) | 6 (1.7) | 16 (1.7) | |
| Unknown/declined | 29 (4.9) | 5 (1.4) | 34 (3.6) | |
| Untreated duration (days) | <.001 | |||
| Median (25th, 75th percentiles) | 1159 (398, 2436) | 116 (73, 203) | 495 (123, 1831) | |
| Medical history, n (%) | ||||
| Prior Stroke | 109 (18.4) | 71 (20.6) | 180 (19.2) | 0.403 |
| Heart Failure | 240 (40.4) | 154 (44.6) | 394 (42.0) | 0.205 |
| Diabetes Mellitus | 367 (61.8) | 220 (63.8) | 587 (62.5) | 0.545 |
| Hypertension | 564 (94.9) | 337 (97.7) | 901 (96.0) | 0.041 |
| Coronary artery disease | 380 (64.0) | 238 (69.0) | 618 (65.8) | 0.118 |
| Myocardial infarction | 72 (12.1) | 61 (17.7) | 133 (14.2) | 0.018 |
| Creatinine Clearance (mL/min) | 0.253 | |||
| Median (25th, 75th percentiles) | 58.2 (3.4, 322.9) | 61.5 (4.2, 173.1) | 3.4, 322.9 | |
| Peripheral artery disease | 117 (19.7) | 77 (22.3) | 194 (20.7) | 0.339 |
| Alcohol use | 87 (14.6) | 51 (14.8) | 138 (14.7) | 0.955 |
| Prior bleeding episode | 190 (32.0) | 131 (38.0) | 321 (34.2) | 0.062 |
| Hyperlipidemia | 451 (75.9) | 298 (86.4) | 749 (79.8) | <.001 |
| CHA2DS2-VASc score | 0.239 | |||
| 2 | 332 (55.9) | 171 (49.6) | 503 (53.6) | |
| 3 | 173 (29.1) | 106 (30.7) | 279 (29.7) | |
| 4 | 55 (9.3) | 46 (13.3) | 101 (10.8) | |
| 5 | 23 (3.9) | 16 (4.6) | 39 (4.2) | |
| 6 | 11 (1.9) | 6 (1.7) | 17 (1.8) | |
| Medications, n (%) | ||||
| Aspirin | 390 (65.7) | 213 (61.7) | 603 (64.2) | 0.227 |
| P2Y12 inhibitor | 88 (14.8) | 49 (14.2) | 137 (14.6) | 0.798 |
| Thienopyridine | 81 (13.6) | 46 (13.3) | 127 (13.5) | 0.896 |
| Angiotensin converting enzyme inhibitor | 244 (41.1) | 167 (48.4) | 411 (43.8) | 0.029 |
| Angiotensin receptor blocker | 105 (17.7) | 68 (19.7) | 173 (18.4) | 0.438 |
| Aldosterone antagonist | 64 (10.8) | 54 (15.7) | 118 (12.6) | 0.030 |
| Beta blocker | 395 (66.5) | 281 (81.4) | 676 (72.0) | <.001 |
| Loop diuretic | 236 (39.7) | 181 (52.5) | 417 (44.4) | <.001 |
| Potassium sparing diuretic | 70 (11.8) | 59 (17.1) | 129 (13.7) | 0.022 |
| Thiazide diuretic | 96 (16.2) | 71 (20.6) | 167 (17.8) | 0.088 |
| Statin | 404 (68.0) | 302 (87.5) | 706 (75.2) | <.001 |
| Calcium channel blocker | 220 (37.0) | 150 (43.5) | 370 (39.4) | 0.0515 |
| Nonsteroidal anti-inflammatory drug | 87 (14.6) | 46 (13.3) | 133 (14.2) | 0.578 |
| Insulin | 129 (21.7) | 104 (30.1) | 233 (24.8) | 0.004 |
| Metformin | 153 (25.8) | 108 (31.3) | 261 (27.8) | 0.067 |
| Sulfonyl urea | 82 (13.8) | 62 (18.0) | 144 (15.3) | 0.088 |
| Other oral hypoglycemic | 30 (5.1) | 16 (4.6) | 46 (4.9) | 0.777 |
| Digoxin | 31 (5.2) | 21 (6.1) | 52 (5.5) | 0.575 |
| Direct acting vasodilator | 126 (21.2) | 97 (28.1) | 223 (23.7) | 0.016 |
| Proton pump inhibitor | 270 (45.5) | 177 (51.3) | 447 (47.6) | 0.084 |
| Histamine H2 receptor antagonist | 64 (10.8) | 31 (9.0) | 95 (10.1) | 0.381 |
Results
Baseline characteristics
At the start of our study on June 13, 2018, a total of 4,831 Veterans with AF or atrial flutter were registered for care at the Durham VAHS. Of these, 1,302 (27.0%) had a CHA2DS2-VASc score ≤ 1 and 1,626 (33.6%) were already on anticoagulation or received medications from a non-VA pharmacy. The remaining patients, 1,903 (39.4%) were eligible for our study. Between June 13, 2018 to August 31, 2018, the intervention identified and triggered a unique alert for 939 (49.3%) consecutive patients meeting the inclusion criteria of AF or atrial flutter, a CHA2DS2-VASc score ≥2, and who were not on oral anticoagulation. Of these patients 842 (89.6%) had AF and 217 (23.1%) atrial flutter – not exclusively for both diagnoses, as overall 120 (12.8%) had both AF and atrial flutter. The remaining 964 (20.0%) patients never had their electronic record opened during the 2.5-month enrollment period, and therefore were not included. (Figure 1)
The median age of the overall study cohort was 72 [inter-quartile range (IQR) 68-80], with 39.8% being ≥ 75 years. The majority of the study population was male (98.1%) of which 67.2% were White, 27.5% African American, 1.7% other, and 3.6% unknown. The median CHA2DS2- VASc score among all patients identified by the alert was 2 and the median untreated duration was 495 days (IQR 123-1831). With respect to medical history, high rates of hypertension (96.0%), hyperlipidemia (79.8%), coronary artery disease (65.8%), diabetes mellitus (62.5%), heart failure (42.0%), prior bleeding event (34.2%), and stroke (19.2%) were observed. (Table 1)
Alert delivery
The electronic alert was primarily triggered (98.2%) in the outpatient setting. The median time to prescription from first alert was 11 days (IQR 3-63). Figure 4 The use of opt out clauses to document reason for not initiating anticoagulation was only implemented 5 times.
Figure 4. Days to prescription from first alert trigger.
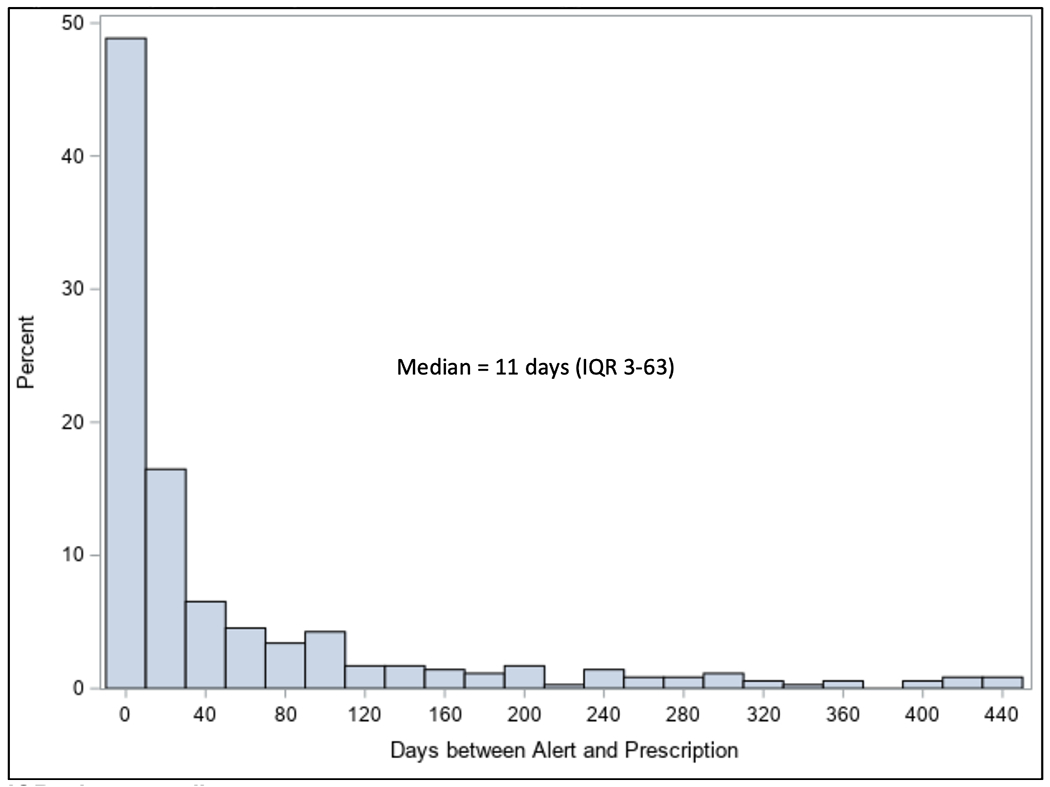
IQR = interquartile range
Endpoints
In total, of the 939 AF or atrial flutter patients with a CHA2DS2-VASc ≥2, and not on anticoagulation identified by the electronic alert, 345 (36.7%) were prescribed anticoagulation therapy (primary endpoint) and 594 (63.3%) were not. The breakdown of anticoagulation therapy prescribed was as follows: 68.7% were treated with NOAC, 22.0% with warfarin, and 9.3% combination of NOAC and warfarin. The distribution of therapy among patients treated with NOAC therapy alone was apixaban (64.5%), rivaroxaban (18.8%), dabigatran (10.7%) and combination of 2 or more NOACs (6%). Comparison of 90-day anticoagulation prescription rates during the study period (22.5%) vs. historical rates (18.2%), found the electronic alert to be associated with a 23.6% (p=0.07) increase in anticoagulation prescriptions (Tables S1–S3 of the Data Supplement). The median age of the prescribed group was 71 years (IQR 67-77) and 73 years (IQR 68-81) for the non-prescribed group. The median CHA2DS2-VASc scores among patients prescribed and not prescribed anticoagulation therapy were both 2. Patients prescribed anticoagulation had higher rates of hypertension, hyperlipidemia, and prior myocardial infarction at baseline compared with patients not initiated on anticoagulation therapy. Further, patients prescribed anticoagulation had higher rates of aldosterone antagonist, beta blocker, calcium channel blocker, diuretic, insulin, and vasodilator use at baseline compared with patients not initiated on anticoagulation. (Table 1)
In regard to the secondary endpoint, the overall adherence rate (medication possession ratio ≥ 80% during the 12 months of follow-up) was 75% (260/345). Adherence rates during the study period (75.4%) were similar to historical rates (72.4%) of adherence among veterans prescribed anticoagulation prior to the study period. Among the 345 patients who initiated anticoagulation therapy, 271 had follow-up greater than 360 days. Of these, 212 (78%) were found to be adherent. Following logistic regression analysis, patients who were older age (Odds Ratio [OR] 0.96; 95% Confidence Interval [CI] 0.94-0.98, p=0.0005) and on aspirin (OR 0.33; 95% CI 0.22-0.50, p<0.0001) were less likely to be prescribed anticoagulation therapy, while those on statin therapy (OR 2.56; 95% CI 1.53-4.29, p=0.004) were more likely to be prescribed anticoagulation therapy. (Table 2)
Table 2.
Multivariable Logistic Regression (prescribed vs. not prescribed anticoagulation)
| Odds Ratio (95% CI) | P-value | |
|---|---|---|
| Characteristic | ||
| Age * | 0.96 (0.94-0.98) | <.001 |
| Sex (reference female) | 3.80 (1.02-14.23) | 0.047 |
| Race (reference Caucasian) | 0.167 | |
| African American | 1.01 (0.67-1.52) | |
| Other/Multi-races | 1.65 (0.40-6.80) | |
| Unknown/declined | 0.32 (0.11-0.92) | |
| Medical history (reference no) | ||
| Prior Stroke | 1.11 (0.69-1.79) | 0.675 |
| Heart Failure | 0.88 (0.58-1.36) | 0.573 |
| Diabetes Mellitus | 0.73 (0.45-1.20) | 0.215 |
| Hypertension | 1.72 (0.57-5.15) | 0.332 |
| Coronary artery disease | 1.48 (0.96-2.28) | 0.075 |
| Myocardial infarction | 1.30 (0.79-2.15) | 0.299 |
| Creatinine Clearance* | 1.01 (1.00-1.01) | 0.094 |
| Peripheral artery disease | 1.05 (0.68-1.62) | 0.818 |
| Alcohol use | 1.19 (0.72-1.97) | 0.488 |
| Prior bleeding episode | 0.92 (0.64-1.33) | 0.663 |
| Hyperlipidemia | 1.37 (0.83-2.26) | 0.211 |
| CHA2DS2-VASc score | 0.459 | |
| 2 | 1.74 (0.45-6.68) | |
| 3 | 1.58 (0.42-5.92) | |
| 4 | 2.51 (0.65-9.68) | |
| 5 | 2.23 (0.49-10.20) | |
| 6 | 1.11 (0.69-1.79) | 0.675 |
| Medications | ||
| Aspirin | 0.35 (0.23-0.52) | <.001 |
| P2Y12 inhibitor | 0.34 (0.08-1.52) | 0.158 |
| Thienopyridine | 2.53 (0.54-11.90) | 0.240 |
| Angiotensin converting enzyme inhibitor | 0.90 (0.61-1.30) | 0.566 |
| Angiotensin receptor blocker | 0.83 (0.52-1.34) | 0.444 |
| Aldosterone antagonist | 0.39 (0.06-2.44) | 0.316 |
| Beta blocker | 1.43 (0.92-2.24) | 0.116 |
| Loop diuretic | 1.48 (0.98-2.24) | 0.061 |
| Potassium sparing diuretic | 3.04 (0.52-17.75) | 0.216 |
| Thiazide diuretic | 1.11 (0.73-1.71) | 0.620 |
| Statin | 2.32 (1.40-3.84) | 0.001 |
| Calcium channel blocker | 1.00 (0.70-1.43) | 0.999 |
| Nonsteroidal anti-inflammatory drug | 0.73 (0.45-1.20) | 0.214 |
| Insulin | 1.42 (0.91-2.20) | 0.119 |
| Metformin | 0.77 (0.48-1.21) | 0.259 |
| Sulfonyl urea | 1.34 (0.81-2.23) | 0.259 |
| Other oral hypoglycemic | 0.86 (0.37-2.04) | 0.738 |
| Digoxin | 1.19 (0.56-2.52) | 0.650 |
| Direct acting vasodilator | 0.97 (0.63-1.49) | 0.881 |
| Proton pump inhibitor | 1.24 (0.86-1.77) | 0.247 |
| Histamine H2 receptor antagonist | 0.80 (0.45-1.41) | 0.438 |
Age and Creatinine Clearance level are continuous variables; the rest are categorical variables.
Discussion
In a tertiary care Veteran Affairs Health System, we successfully developed and instituted an electronic alert intervention to aid initiation of and adherence to oral anticoagulation in patients with AF or atrial flutter at heightened risk for stroke or systemic thromboembolism. Using ICD-9 and 10 codes in conjunction with billing and VA prescription records, the alert identified 49.3% of the AF/atrial flutter population receiving care at the Durham VA Health System with a CHA2DS2-VASc score ≥2 and not receiving anticoagulation therapy. It is important to note that the remaining at-risk population was not identified by the electronic system because their electronic chart was never opened. The electronic alert intervention resulted in 37.5% of eligible patients being prescribed oral anticoagulation (median untreated time of 1.4 years) and was also associated with high rates of oral anticoagulation adherence, approximately 75%, among patients prescribed therapy during the study period. Compared with historical anticoagulation prescription rates among all AF patients, the intervention was associated with a numerically 23.6% relative increase in anticoagulation prescriptions. Subsequent 12-month adherence to oral anticoagulation was similar among the study population (75.4%) and historical controls (72.4%). The most prescribed anticoagulation therapy was a NOAC in 67% of patients.
The successful use of electronic alerts to improve prescription of pharmacological prophylaxis therapies is well documented among hospitalized patients at high risk for venous thromboembolism.6,7 The use of an electronic alert-based strategy to improve prescription of anticoagulation among hospitalized patients with AF at increased risk for stroke or systemic thromboembolism was recently evaluated in a prospective randomized controlled trial of 458 hospitalized patients. In this aforementioned study, the electronic alert was responsible for nearly tripling the odds of prescriptions for anticoagulation among patients (25.8% alert vs. 9.5% no alert, p = 0.007; OR 3.3; 95% CI 1.92-5.68).4 Our study builds upon previous research by evaluating the efficacy of an electronic alert in a predominantly outpatient population with AF or atrial flutter, which is considered the largest population within health systems at risk for stroke or systemic thromboembolism. We hypothesize the key reason for success of our intervention was its seamless integration into provider workflow. The alert was not only triggered at the time providers accessed a patient’s chart, but also had direct links to the anticoagulation order template within the VA’s EHR. To minimize clinical interruptions, documentation regarding reasoning for not initiating anticoagulation therapy was not mandated. Therefore, insight as to why eligible patients were not prescribed oral anticoagulation is not known.
Evaluation of Medicare claims data has demonstrated that adherence to oral anticoagulation among patients with AF is < 45%.3,8 In the present analysis, both study and historical patients had high 12-month adherence rates (> 70%) to oral anticoagulation. One of the potential reasons for this observed high adherence rate may be due to routine standard patient education provided by VA clinical pharmacy specialists performed at the time of oral anticoagulation prescription. Within the VA Durham VA Health System, all outpatient anticoagulation prescriptions are routed through pharmacists who 1) routinely educate patients upon initiation of anticoagulation therapy. The benefits of using educational interventions to improve treatment of AF was best described in the landmark cluster-randomized trial to IMProve treatment with AntiCoagulanTs in patients with Atrial Fibrillation (IMPACT-AF) trial.9 This study assessed the impact of an educational intervention on oral anticoagulation use among 2,281 patients with AF. The education targeted both providers and patients. Providers were given a systematic review of major societal guidelines for oral anticoagulation via electronic media, while patients received educational brochures, web-based and video materials. This intervention was associated with an increase odds of oral anticoagulation use of 3.28 (95% CI 1.67-6.44, p=0.0002). Among patients who were not on oral anticoagulation at baseline, 48% of the group who received education were still on oral anticoagulation at 1 year, compared to 18% of those who did not (p < 0.0001). A second reason for the increased adherence rates observed in our study may be related to the availability of oral anticoagulation. It is well documented that only 50% of patients with AF receive their medications.3 In the present study, all of the participants received their oral anticoagulation through the VHA which consists of medical centers, outpatients clinics, brick-and-mortar and mail-order (accounting for 80% of all VA prescriptions) pharmacies.10 This national formulary system ensures medication availability and when combined with low drug costs helps make oral anticoagulation therapies readily available to Veterans. At the time of the study approximately 71% of all patients with AF registered for care at the Durham VAHS received their medications via the VA pharmacy system.
There are several limitations to the present study. First, our electronic alert was implemented within the VA CPRS, a health information system developed specifically for the U.S. Department of Veterans Affairs. Therefore, all findings should be interpreted in this context. It is important to note, however, that an open-source version of this EHR system is readily available for free to the public. Second, as previously mentioned, the current study takes place in a setting where the majority of participants receive their care and medications within the same healthcare system. The efficacy of the intervention on prescription and adherence rates may diminish within the public sector where barriers such as access to care and medications are more prevalent. Third, the interaction between providers, patients, and the decision-making tool itself was not directly assessed. Therefore, metrics regarding the tool itself, such as how often it was accessed or ease of use, were not obtained. In order to better evaluate justification for justification for not initiating anticoagulation in high-risk AF and atrial fibrillation cohorts, mandated documentation for closure of the alert component of future iterations of the electronic alert may be considered. Lastly, the use of a CHA2DS2-VASc score of ≥2 as a cutoff for anticoagulation in the present study pre-dates the most recent societal guidelines of ≥2 for men and ≥3 for women.1
Conclusions
Implementation of an electronic alert intervention successfully identified treatment-naïve patients with AF and atrial flutter at high risk for stroke and was associated with an increase in oral anticoagulation prescription and high 1-year adherence rates. The present study reaffirms the use of electronic interventions to improve utilization of guideline recommended therapies among patients with atrial arrhythmias who are increased risk of stroke and/or systemic thromboembolism.
Supplementary Material
Figure 3b.
What is atrial fibrillation and how does it relate to stroke?
Figure 3c.
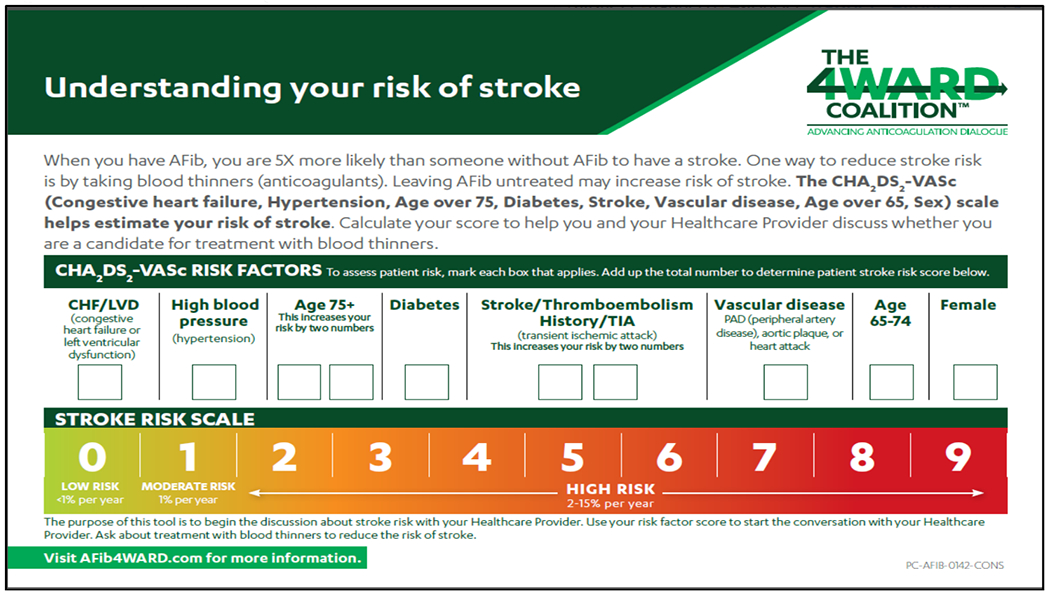
Understanding your risk of stroke
Funding
This work was supported by an unrestricted research grant from Boehringer Ingelheim Pharmaceuticals, Inc. Boehringer Ingelheim Pharmaceuticals, Inc had no role in the study design or data collection, analysis, and interpretation of the study. JAG, CTR, and SVR had full access to the data and had final responsibility for the content of the publication. Dr. Aday was supported by the National Institutes of Health under Award Numbers K12 HL133117 and K23 HL151871.
Disclosures
Dr. JA Gutierrez discloses the following relationships – Consulting: Amgen Inc, Janssen Pharmaceuticals, and Medicure Inc; Research – Veterans Health Administration
Dr. CT Ruff discloses the following relationships – Research grant through institution: Anthos, Boehringer Ingelheim, Daiichi Sankyo, AstraZeneca, National Institutes of Health; Honoraria for scientific advisory boards and consulting: Anthos, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Janssen, Pfizer, Portola. Dr. Ruff is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Roche, The Medicines Company, Zora Biosciences.
Dr. AW Aday discloses the following relationships – Consulting: OptumCare.
L Shihai and P Michaela are employees of Boehringer Ingelheim Pharmaceuticals, Inc. The other authors have no industry relations to disclose.
The author(s) meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no direct compensation related to the developmentofthemanuscript. BoehringerIngelheimPharmaceuticals,Inc.(BIPI)wasgiventhe opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
References
- 1.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg BA, Gao H, Shrader P, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. 2017;194:132–140. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez I, He M, Chen N, Brooks MM, Saba S, Gellad WF. Trajectories of Oral Anticoagulation Adherence Among Medicare Beneficiaries Newly Diagnosed With Atrial Fibrillation. J Am Heart Assoc. 2019;8(12):e011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piazza G, Hurwitz S, Galvin CE, et al. Alert-based computerized decision support for high- risk hospitalized patients with atrial fibrillation not prescribed anticoagulation: a randomized, controlled trial (AF-ALERT). Eur Heart J. 2020;41(10):1086–1096. [DOI] [PubMed] [Google Scholar]
- 5.Siegel D, Lopez J, Meier J. Antihypertensive medication adherence in the Department of Veterans Affairs. Am J Med. 2007;120(1):26–32. [DOI] [PubMed] [Google Scholar]
- 6.Piazza G, Rosenbaum EJ, Pendergast W, et al. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation. 2009;119(16):2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazza G, Anderson FA, Ortel TL, et al. Randomized trial of physician alerts for thromboprophylaxis after discharge. Am J Med. 2013;126(5):435–442. [DOI] [PubMed] [Google Scholar]
- 8.Chen N, Brooks MM, Hernandez I. Latent Classes of Adherence to Oral Anticoagulation Therapy Among Patients With a New Diagnosis of Atrial Fibrillation. JAMA Netw Open. 2020;3(2):e1921357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017;390(10104):1737–1746. [DOI] [PubMed] [Google Scholar]
- 10.McCaughan M Health Policy Brief: Veterans Health Administration. 2017. Published August 10, 2017. Accessed August 12, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


