Abstract
1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid (DO3A) has been used to prepare 68Ga-labeled probes for the diagnostic counterpart of radiotheranostic applications. While DO3A provides stable complexes with therapeutic radionuclides such as 90Y, 177Lu, and 225Ac, further improvement of the in vivo stability of the Ga-DO3A complex is required. Considering the high stability of an intact Ga-DOTA complex, the stability of Ga complexes of DOTA and DO3A derivatives, including benzyl-DOTA (Bn-DOTA), was evaluated to gain fundamental knowledge for developing the next-generation radiotheranostic probes using 68Ga as a diagnostic counterpart. Following the complexation reaction to prepare 67Ga-labeled DOTA and DO3A derivatives, the stability of the resulting 67Ga-labeled compounds was evaluated in murine plasma and apo-transferrin challenge. [67Ga]Ga-Bn-DOTA produced two isomers, and one of the isomers exhibited the highest stability among the tested complexes. The X-ray crystallography showed that the less stable isomer of Ga-Bn-DOTA suggested an N3O3 coordination geometry, while Ga-DOTA and Ga-Bn-DO3A show N4O2 coordination. To further evaluate the stability, a synthetic somatostatin analogue, [Tyr3]octreotide (TOC), was used as a model peptide, and p-COOH-Bn-DOTA and DO3A were conjugated with TOC to prepare DOTA-Bn-TOC and DOTATOC. [67Ga]Ga-DOTA-Bn-TOC also yielded two isomers with varying stability, and one isomer exhibited significantly higher stability than [67Ga]Ga-DOTATOC both in vitro and in vivo. These findings indicate that para-substituted Bn-DOTA would constitute a suitable chelating agent for developing next-generation radiotheranostic probes, although high-performance liquid chromatography purification is needed. Thus, further chemical modification on the Bn-DOTA molecule is also needed to avoid the formation of a Ga complex with the N3O3 configuration.
Introduction
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) forms kinetically inert complexes with various radiometals, such as 67/68Ga, 111In, 90Y, 177Lu, and 225Ac.1−5 Thus, various radiolabeled probes have been developed by conjugating bifunctional DOTA derivatives with biomolecules6−8 for radiotheranostic applications, which are conducted with the same biomolecules using a pair of radionuclides suitable for imaging and radiotherapy.9 The radiotheranostic platform is expected to help accurately assess patient selection and therapeutic efficacy while facilitating personalized medicine.
Positron emission tomography (PET) is a valuable imaging modality for radiotheranostics. 68Ga is an attractive radiometal for PET because of its availability from a long-lived 68Ge/68Ga generator system, allowing for the use of 68Ga-labeled probes far from cyclotron facilities.10,11 Peptides (including peptidomimetics) accumulate quickly in the target tissue and are rapidly eliminated from the blood, making them suitable for the short half-life (T1/2 = 68 min) of 68Ga. Thus, various [68Ga]Ga-DOTA-based peptide probes have been developed and used for preclinical and clinical applications.12−15 Several [68Ga]Ga-DOTA-based peptide probes have also been used for radiotheranostics in conjunction with therapeutic radiometals, such as 177Lu and 225Ac.16−19 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid (DO3A) derivatives that use one of the carboxylates of DOTA for peptide conjugation have been used in most of these cases. DO3A-conjugated biomolecules labeled with therapeutic radiometals, such as 90Y, 177Lu, and 225Ac, exhibit high in vivo stability.5 However, improved resistance of [68Ga]Ga-DO3A-conjugated biomolecules to apo-transferrin (apoTf) has been needed.20,21 Thus, a DOTA derivative providing a more stable Ga complex would contribute to more accurate diagnostics because the loss of Ga ions from the chelators causes the accumulation in the normal tissues, such as the bone and the spleen.22−24
Compared to the Ga-DO3A derivatives such as DO3AMBu (Figure 1), Ga-DOTA exhibits much higher kinetic inertness,25 indicating that a bifunctional DOTA derivative holding an intact DOTA structure would provide stable Ga complexes.26 One approach for preserving the intact DOTA structure involves the use of 1,4,7,10-tetraazacyclododececane,1-(glutaric acid)-4,7,10-triacetic acid (DOTAGA), which has the 5th carboxylic acid branched from one of the acetate arms of DOTA, allowing for the conjugation with biomolecules to occur. Although a previous study suggested that the in vivo stability of the [68Ga]Ga-DOTAGA-conjugated peptide was lower than that of the 68Ga-labeled peptide prepared with DO3A,27 the details were not investigated. Another approach is to use benzyl-DOTA (Bn-DOTA) derivatives that possess a biomolecule-conjugating moiety, such as amino and isothiocyanate groups, at the para position of the benzyl group elongated from the carbon backbone of DOTA. In addition, steric crowding enhanced the stability of the metal-polyaza polycarboxylate complex when introducing a benzyl group.28 Thus, Bn-DOTA may constitute a promising candidate as a bifunctional DOTA to form a more stable Ga complex than DO3A.
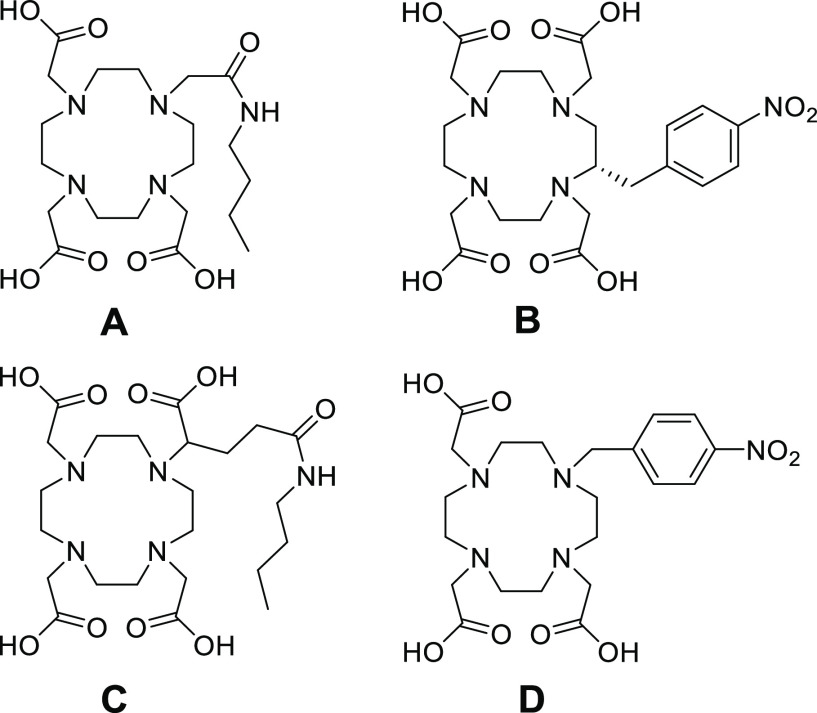
Figure 1.
Chemical structures of model chelators used for the stability experiments; DO3AMBu (A), p-NO2-Bn-DOTA (B), DOTAGAMBu (C), and p-NO2-Bn-DO3A (D).
This study aimed to assess the stability of 67Ga-labeled DOTA derivatives for developing the next-generation radiotheranostic probes that solve the stability problem. We selected Bn-DOTA (p-NO2-Bn-DOTA), DO3A (DO3AMBu), and DOTAGA (DOTAGAMBu), as shown in Figure 1, and an intact DOTA as the DOTA derivatives. We also prepared another DO3A derivative, Bn-DO3A, where one of the carboxylate arms of DOTA was replaced with a para-substituted benzyl group (p-NO2-Bn-DO3A, Figure 1D) to investigate the role played by the benzyl group on the stability of Ga complex. These studies were conducted with 67Ga instead of 68Ga because the longer half-life of 67Ga (3.3 d) is more suitable for the studies. First, the in vitro stability of each 67Ga complex was assessed by incubation in murine plasma and apoTf challenge. Then, the stability of [67Ga]Ga-Bn-DOTA was further assessed after conjugation to a peptide using a newly developed bifunctional Bn-DOTA, p-COOH-Bn-DOTA(tBu)4. Its applicability to the solid-phase peptide synthesis was confirmed by synthesizing the p-COOH-Bn-DOTA-conjugated [Tyr3]octreotide (TOC) analogue, DOTA-Bn-TOC (Figure 2A).29 The stability of [67Ga]Ga-DOTA-Bn-TOC (Figure 2A) was compared with [67Ga]Ga-DOTATOC (Figure 2B). Future perspectives and problems of bifunctional DOTA derivatives providing highly stable 68Ga-labeled probes will be discussed.
Figure 2.
Chemical structures of octreotide analogues; DOTA-Bn-TOC (A) and DOTATOC (B).
Results
Syntheses of 67Ga-Labeled Model Chelators
The synthetic procedures for DOTAGAMBu, DO3AMBu, and p-NO2-Bn-DO3A are described in Schemes S1–S3. All chelators showed a single peak by high-performance liquid chromatography (HPLC) analysis (Figures 3 and S1–S4). After complexation with 67Ga, [67Ga]Ga-DOTAGAMBu, [67Ga]Ga-DO3AMBu, [67Ga]Ga-p-NO2-Bn-DO3A, and [67Ga]Ga-DOTA showed a single radioactivity peak by HPLC analysis (Figures S1–S4). However, two radioactivity peaks were observed for [67Ga]Ga-p-NO2-Bn-DOTA (Figure 3). When the radioactivity peaks were isolated and reanalyzed by HPLC, both complexes of [67Ga]Ga-p-NO2-Bn-DOTA remained unchanged. Two peaks were also observed for nonradioactive Ga complexes of p-NO2-Bn-DOTA at similar retention times. Following MS analysis, the isolated peaks showed identical MS spectra. The results indicated that they were isomers. Each isomer was referred to as isomer A and isomer B hereafter (17.6 and 22.1 min of retention time on HPLC system A, respectively, Figure 3).
Figure 3.

HPLC chromatograms of p-NO2-Bn-DOTA (yellow) and nonradioactive Ga-p-NO2-Bn-DOTA (blue) and HPLC radiochromatograms of [67Ga]Ga-p-NO2-Bn-DOTA (red), isomer A (green), and isomer B (purple) on system A. [67Ga]Ga-p-NO2-Bn-DOTA exhibited two peaks at retention times of 17.6 min (isomer A) and 22.1 min (isomer B). Each isomer was separated by HPLC and reinjected to HPLC to exhibit a single peak.
In Vitro Stability Assessments of 67Ga-Labeled Model chelators
The in vitro stability experiments were conducted in the presence of plasma or an excess amount of apoTf, either for a short time (3 h) or a long incubation time (24 h), to evaluate the stability of 67Ga complexes clearly. All 67Ga complexes were purified by HPLC to remove free chelators, and the isomers of [67Ga]Ga-p-NO2-Bn-DOTA were simultaneously isolated. The differences in stability were clearly confirmed for apoTf challenge studies (isomer A ≃ [67Ga]Ga-DOTA > [67Ga]Ga-p-NO2-Bn-DO3A > isomer B > [67Ga]Ga-DO3AMBu > [67Ga]Ga-DOTAGAMBu) (Table 1). In the plasma stability study, small but significant differences were partially observed at 3 h incubation, and a similar tendency to an apoTf challenge study was observed at 24 h incubation.
Table 1. Stability of 67Ga-Labeled Model Chelatorsa.
| murine
plasma |
ApoTf (0.1 mM) |
|||
|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | |
| [67Ga]Ga-p-NO2-Bn-DOTA (A) | 97.6 ± 0.3cdef | 95.9 ± 0.1cdef | 95.1 ± 1.2def | 90.3 ± 0.7cdef |
| [67Ga]Ga-p-NO2-Bn-DOTA (B) | 96.2 ± 0.4b | 85.9 ± 0.4bcde | 89.5 ± 0.7bcde | 56.0 ± 0.5bcde |
| [67Ga]Ga-DOTAGAMBu | 96.1 ± 0.4b | 82.2 ± 0.2bcd | 80.0 ± 1.3bcd | 27.5 ± 4.6bcd |
| [67Ga]Ga-DO3AMBu | 96.1 ± 0.6b | 83.9 ± 0.5bc | 87.4 ± 0.5bc | 43.1 ± 1.1bc |
| [67Ga]Ga-p-NO2-Bn-DO3A | 96.7 ± 0.5 | 87.6 ± 0.3b | 93.8 ± 0.6b | 67.5 ± 0.6b |
| [67Ga]Ga-DOTA | 97.5 ± 0.5 | 95.5 ± 0.4 | 96.4 ± 0.2 | 89.6 ± 0.5 |
Results are expressed as mean ± SD (n = 5).
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with [67Ga]Ga-DOTA.
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with[67Ga]Ga-p-NO2-Bn-DO3A.
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with[67Ga]Ga-DO3AMBu.
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with[67Ga]Ga-DOTAGAMBu.
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with[67Ga]Ga-p-NO2-Bn-DOTA (B).
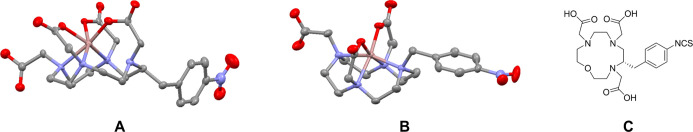
X-ray Structure Analysis of Ga-p-NO2-Bn-DOTA and Ga-p-NO2-Bn-DO3A
To investigate the differences in stability between isomers A and B, we performed X-ray crystallographic analyses of Ga-p-NO2-Bn-DOTA complexes. A single crystal appropriate for X-ray crystallographic analysis was obtained for isomer B. However, we failed to obtain a single crystal of isomer A after several attempts. In the structure of isomer B, a distorted N3O3 octahedral geometry (Figure 4A) was confirmed. Three nitrogen atoms, except an atom next to the carbon atom with the benzyl group, were involved with coordination to the Ga ion, and the carboxylate oxygen atom elongated from the uncoordinated nitrogen atom coordinated to the Ga ion in addition to two oxygen atoms “trans” carboxylates. The coordination bond lengths between the three nitrogen atoms and the Ga ion (2.19–2.24 Å) in isomer B were longer than those in Ga-DOTA (2.09–2.15 Å).25,30 In another peculiarity, isomer B located four nitrogen atoms of the macrocyclic ring in the same plane. On the other hand, Ga-p-NO2-Bn-DO3A showed an N4O2 coordination environment (Figure 4B).
Figure 4.
X-ray crystal structures of isomer B (A) and Ga-p-NO2-Bn-DO3A (B) and the chemical structure of p-SCN-Bn-oxo-DO3A (C). Hydrogen atoms and solvent molecules are omitted (A,B). Thermal ellipsoids are shown at a 50% probability (A,B).
Radiolabeling of TOC Analogues with 67Ga
The applicability of Bn-DOTA for radiotheranostics using 68Ga as a diagnostic counterpart was evaluated using a model peptide, the p-COOH-Bn-DOTA-conjugated TOC analogue (DOTA-Bn-TOC, Figure 2A). A representative TOC analogue, DO3A-conjugated TOC (DOTATOC, Figure 2B), was used as a reference. [67Ga]Ga-DOTATOC showed a single HPLC peak (Figure 5) at a retention time similar to that of the nonradioactive Ga complex identified with MS (Figure S6). On the other hand, two peaks were observed in the radiochromatogram of [67Ga]Ga-DOTA-Bn-TOC (Figure 5). Two peaks were also observed for nonradioactive Ga complexes of DOTA-Bn-TOC (Figure S5). Each component was isolated under different elution conditions (system C, Figure S5) in high purities (Figure 5). The MS analyses showed identical spectra, indicating that both are isomers (isomer A′: 28.5 min and isomer B′: 29.0 min on HPLC system B) (Figure 5).
Figure 5.
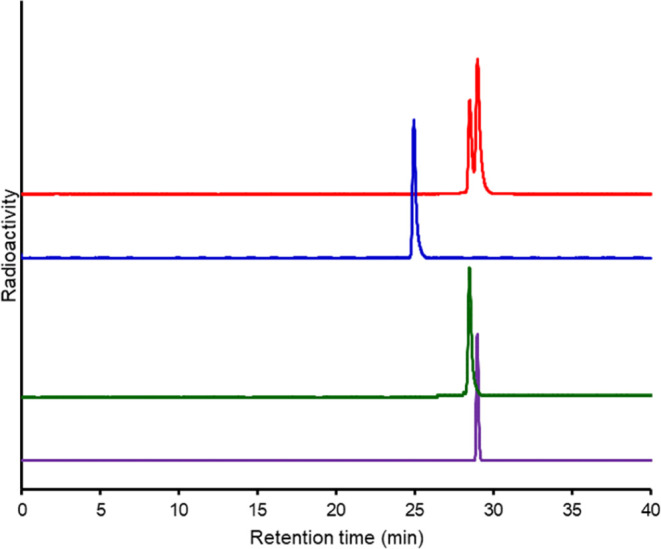
HPLC radiochromatograms of [67Ga]Ga-DOTA-Bn-TOC (red), 67Ga-DOTATOC (blue), and the isolated isomer A′ (green) and isomer B′ (purple) on system B. [67Ga]Ga-DOTA-Bn-TOC exhibited two peaks at retention times of 28.5 min (isomer A′) and 29.0 min (isomer B′). Each isomer was separated by HPLC and reinjected to HPLC to exhibit a single peak. In vitro stability assessment of 67Ga-labeled TOC analogues.
A radiolabeling reaction with a final ligand concentration of 10 μM for 5 min at 95 °C yielded [67Ga]Ga-DOTA-Bn-TOC and [67Ga]Ga-DOTATOC in 97.4 ± 0.5% (isomer A′ + isomer B′) and 95.9 ± 1.5% radiochemical conversions (determined by RP-TLC), respectively. When the ligand concentration was lowered to 3 μM, radiochemical conversions were comparably decreased for both 67Ga-labeled peptides ([67Ga]Ga-DOTA-Bn-TOC in 85.2 ± 18.7% and [67Ga]Ga-DOTATOC in 84.6 ± 15.1%).
The stability of 67Ga-labeled TOC analogues was evaluated after incubation with murine plasma or apoTf, and the results are summarized in Table 2. The same tendency as the results of 67Ga-labeled model chelators was observed (isomer A′ > isomer B′ > [67Ga]Ga-DOTATOC, Table 2).
Table 2. Stability of [67Ga]Ga-DOTA-Bn-TOC and [67Ga]Ga-DOTATOCa.
| murine
plasma |
ApoTf (0.1 mM) |
|||
|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | |
| [67Ga]Ga-DOTA-Bn-TOC (A′) | 97.4 ± 0.4bc | 96.6 ± 1.0bc | 97.1 ± 0.3bc | 91.9 ± 1.2bc |
| [67Ga]Ga-DOTA-Bn-TOC (B′) | 95.2 ± 0.5 | 86.5 ± 1.5b | 83.3 ± 1.5b | 20.5 ± 0.8b |
| [67Ga]Ga-DOTATOC | 94.4 ± 0.2 | 82.8 ± 0.9 | 66.2 ± 2.2 | 2.9 ± 0.2 |
Results are expressed as mean ± SD (n = 5).
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with [67Ga]Ga-DOTATOC.
Significances determined by Tukey’s test for the one-way ANOVA; p < 0.05 compared with [67Ga]Ga-DOTA-Bn-TOC (B′).
In Vivo Stability Assessment of 67Ga-Labeled TOC Analogues
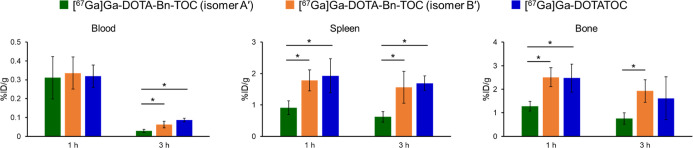
Figure 6 shows the biodistribution of radioactivity in the blood, spleen, and bone after intravenous injection of the two 67Ga-labeled TOC analogues into mice, and the details are shown in Table S1. The isomer A′ showed significantly lower radioactivity levels in normal tissues, such as the spleen (p < 0.05 at 1 and 3 h postinjection) and the bone (p < 0.05 at 1 h postinjection) compared with [67Ga]Ga-DOTATOC. However, no significant differences were observed in the biodistribution between the isomer B′ and [67Ga]Ga-DOTATOC.
Figure 6.
Radioactivity in the blood, spleen, and bone after injection of 67Ga-labeled TOC analogues in ICR mice at 1 h and 3 h postinjection. Significances were determined by Tukey’s test for the one-way ANOVA (*p < 0.05).
Discussion
The size of the DOTA cavity is suitable for binding large cations such as 111In and 90Y, and small Ga prefers NOTA.31−33 However, a DOTA derivative that provides a stable Ga complex for in vivo applications is preferable to radiotheranostic applications. Since intact [67Ga]Ga-DOTA was stable in plasma and against the apoTf challenge, we evaluated Bn-DOTA derivatives for developing next-generation radiotheranostic probes using 68Ga-PET as diagnostic probes.
We first evaluated the gallium complexation reactions with DOTA and DO3A derivatives (Figure 1). DOTA, DO3AMBu, DOTAGAMBu, and p-NO2-Bn-DO3A produced a single Ga complex. However, p-NO2-Bn-DOTA provided two Ga complexes with the same molecular masses (Figures 3 and S8, S9), referring to isomer A and isomer B. The prolonged reaction times to prepare [67Ga]Ga-p-NO2-Bn-DOTA did not change the formation ratios of the two isomers (data not shown). The isolated isomer B did not produce isomer A after heating in the presence of excess p-NO2-Bn-DOTA (data not shown). Thus, isomer B is not a precursor for isomer A, and isomer B remained stable in the reaction solution once formed. X-ray crystallographic analysis of isomer B showed a distorted N3O3 octahedral geometry (Figure 4A). However, Ga complexes of intact DOTA and DO3A derivatives exhibited an N4O2 coordination geometry.25,30,34 Ga-p-NO2-Bn-DO3A also showed an N4O2 coordination geometry (Figure 4B). A prior study suggested that oxo-DO3A (Figure 4C) generated a Ga complex with N3O3 configuration and was unstable in human serum (>80% was decomposed for 1.5 h).35 Taken together, isomer A would possess an N4O2 coordination geometry, although we could not obtain a crystal for X-ray crystallography for isomer A. The small ionic radius of Ga would react with a Bn-DOTA specie preferring N3O3 configuration upon complexation, and the benzene ring would stabilize the resulting complex.
While both [67Ga]Ga-p-NO2-Bn-DO3A and [67Ga]Ga-DO3AMBu possess N4O2 configuration, the former showed higher stability than the latter, indicating the role played by the benzene ring in stabilizing the metal complexes through steric crowding as has been observed with the other radiometal chelates.28 The higher stability of isomer A than [67Ga]Ga-p-NO2-Bn-DO3A also suggests the importance of four carboxylic acids in DOTA to stabilize the Ga complex. On the other hand, [67Ga]Ga-DOTAGAMBu was the least stable among the 6 complexes (Table 1), as observed in an in vivo study.27 Thus, the conjugation to a butylamine through the sidearm carboxylate impaired the inherent ability of DOTA to complex with the metal ion that possesses a small ionic radius for the DOTA cavity.
TOC was then selected as a model peptide to evaluate the stability of Bn-DOTA-derived 67Ga-labeled probes. After conjugation of p-COOH-Bn-DOTA with TOC, [67Ga]Ga-DOTA-Bn-TOC was synthesized by reacting [67Ga]GaCl3. Two isomers were again observed in HPLC analysis of [67Ga]Ga-DOTA-Bn-TOC (Figure 5). The stability estimation showed that isomer A′ possessed the highest stability, followed by isomer B′ and [67Ga]DOTATOC (Table 2). The stability of isomer B′ and [67Ga]Ga-DOTATOC drastically decreased in the apoTf challenge (Table 2) compared to murine plasma. The presence of plasma proteins may have protected the attack of apoTf on the radiometal chelates. The drastic decrease after TOC conjugation (Figure S10) suggests that the TOC molecule may have facilitated the access of apoTf to the chelates through some interactions. Under the conditions, isomer A′ remained stable, which confirms the high stability of isomer A′. In biodistribution studies in mice, isomer A′ exhibited significantly lower radioactivity levels in the spleen and the bone where free radiogallium accumulates (Figure 6).22−24 The lowest radioactivity levels in the blood at 3 h postinjection also support the high stability of isomer A′. When considering clinical studies, radiolabeled peptides would be diluted over 2,000 times upon intravenous injection compared to mice. Thus, the small but significant differences in the in vivo stability observed in mice would be enhanced in the clinical studies. These findings indicate that Bn-DOTA would constitute an appropriate chelating moiety for radiotheranostic applications using 68Ga as a diagnostic counterpart. However, the formation of two Ga isomers with Bn-DOTA presented a new problem to be solved for developing the next-generation radiotheranostic probes. A bifunctional Bn-DOTA providing a single Ga complex with high in vivo stability is needed. The structural modifications of the Bn-DOTA skeleton avoiding the N3O3 configuration upon Ga complexation may constitute a way.36
Conclusions
We evaluated the stabilities of Ga chelates of DOTA derivatives to develop the next-generation of radiotheranostic probes using 68Ga as a diagnostic counterpart. The present study indicates that one isomer of Bn-DOTA provided 67Ga chelate with the highest stability among the tested DOTA and DO3A derivatives shown in Figure 1. Bn-DOTA can be conjugated to peptides of interest using a commercially available p-NH2-Bn-DOTA directly or after converting to a carboxylic acid with succinic anhydride or glutamic anhydride or with p-COOH-Bn-DOTA conducted in this study. However, Bn-DOTA generated two isomers with different stability, which needed HPLC purification after the radiolabeling reaction and decreased the radiochemical yields. The X-ray crystallography indicated that the less stable isomer possesses an N3O3 geometry. Thus, further studies are needed to develop bifunctional Bn-DOTA derivatives that avoid the formation of a Ga complex with an N3O3 configuration.
Methods
General
1H NMR spectra were recorded on a JEOL JNM-ECS 400 spectrometer (JEOL, Tokyo, Japan). Mass spectrometry was performed using an AccuTOF LC-plus (JMS-T100LP, JEOL, Tokyo, Japan). The analytical methods for reversed-phase HPLC (RP-HPLC) are described in detail in the Supporting Information. Radiochemical conversions and in vitro stability were determined by reversed-phase (RP)-TLC (Silica gel 60 RP-18 F254S, Merck, Tokyo, Japan) developed with a mixture of 0.1% aqueous TFA and MeCN with 0.1% TFA (2:3, v/v). [67Ga]GaCl3 was purchased from FUJIFILM RI Pharma (Tokyo, Japan). p-NO2-Bn-DOTA and DOTA were purchased from Macrocyclics (Texas, USA) and Tokyo Chemical Industry (Tokyo, Japan), respectively. DOTA-Bn-TOC and DOTATOC were prepared from the same procedure described previously.29 The syntheses of DOTAGAMBu, DO3AMBu, and p-NO2-Bn-DO3A are described in the Supporting Information. The other chemicals obtained from the commercial sources were of reagent grade or higher and were used without further purification. Data for single crystal X-ray diffraction analyses were collected on a Rigaku R-AXIS RAPID diffractometer using a graphite monochromator with Cu Kα radiation (l = 1.54187 Å). Data collection and reduction were performed using RAPID AUTO. The crystallography structures were solved by direct methods using SHELXL97 and refined with SHELXL97.
Syntheses of Non-Radioactive Gallium-Labeled Model Chelators
A solution of 0.1 M GaCl3 in 0.2 M AcOH–AcONa buffer (pH 5.0) (equivalent molar to each chelator) was added to a solution of chelators (6 mg) in 0.5 mL of 0.2 M AcOH–AcONa buffer (pH 5.0). The mixture was reacted for 25 min at 95 °C. After cooling to room temperature, the mixture was purified by preparative or analytical HPLC. Fractions containing the product were lyophilized to provide nonradioactive Ga complexes.
Ga-p-NO2-Bn-DOTA
p-NO2-Bn-DOTA (8.75 μmol) was used as the chelator to provide isomer A (1.9 mg, 3.13 μmol, 35.8%) and isomer B (3.4 mg, 5.61 μmol, 64.1%).
Isomer A: 14.5 min of retention time of preparative HPLC on system F and 17.6 min of retention time of analytical HPLC on system A. 1H NMR (D2O): d 2.95–3.82 [24H, m, DOTA, CH2], 4.16 [1H, d, J = 18.8 Hz, DOTA], 7.46 [2H, d, J = 8.0 Hz, CH], 8.12 [2H, d, J = 8.8 Hz, CH]. HR-MS(ESI) calcd. for C23H29N5O10Ga [M – H]–: m/z, 604.11702; found, 604.11551 and m/z, 606.11615; found, 606.11638.
Isomer B: 23.2 min of retention time of preparative HPLC on system F and 22.7 min of retention time of analytical HPLC on system A. 1H NMR (D2O): d 2.69–3.78 [25H, m, DOTA, CH2], 7.40 [2H, d, J = 8.8 Hz, CH], 8.10 [2H, d, J = 8.8 Hz, CH]. HR-MS(ESI) calcd. for C23H29N5O10Ga [M – H]–: m/z, 604.11702; found, 604.11497 and m/z, 606.11615; found, 606.11397.
Ga-DOTAGAMBu
DOTAGAMBu (6.07 μmol) was used as the chelator to provide Ga-DOTAGAMBu (2.8 mg, 4.68 μmol, 77.0%). 26.5 min of retention time of preparative HPLC on system F and 21.6 min of retention time of analytical HPLC on system A. 1H NMR (D2O): δ 0.74 (3H, t, J = 7.4 Hz, CH3), 1.09–1.19 (2H, m, CH2), 1.26–1.33 (2H, m, CH2), 1.89–1.98 (2H, m, CH2), 2.23–2.80 (2H, m, CH2), 3.03 (2H, t, J = 7.0 Hz, CH2), 3.12–3.80 (23H, m, DOTA, CH2). HR-MS(ESI) calcd. for C23H37N5O9Ga [M – H]–: m/z, 596.18471; found, 596.18385 and m/z, 598.18383; found, 598.18545.
Ga-DO3AMBu
DO3AMBu (6.55 μmol) was used as the chelator to provide Ga-DO3AMBu (1.8 mg, 3.42 μmol, 52.2%). 24.3 min of retention time of preparative HPLC on system F and 19.9 min of retention time of analytical HPLC on system A. 1H NMR (D2O): δ 0.73 (3H, t, J = 7.4 Hz, CH3), 1.12–1.22 (2H, m, CH2), 1.31–1.38 (2H, m, CH2), 3.07 (2H, t, J = 7.2 Hz, CH2), 3.24–3.30 (8H, m, DOTA), 3.38–3.44 (4H, m, DOTA), 3.53 (2H, s, DOTA), 3.62 (2H, s, DOTA), 3.77–3.79 (6H, m, DOTA), 3.88–3.94 (2H, m, DOTA). ESI-MS [M – H]–: m/z, 524; found, 524 and m/z, 526; found, 526.
Ga-p-NO2-Bn-DO3A
p-NO2-Bn-DO3A (6.40 μmol) was used as the chelator to provide Ga-p-NO2-Bn-DO3A (3.5 mg, 6.38 μmol, 99.8%). 14.5 min of retention time of preparative HPLC on system F and 16.7 min of retention time of analytical HPLC on system A. 1H NMR (D2O): δ 3.03 [2H, d, J = 14.4 Hz, DOTA], 3.20–3.32 [6H, m, DOTA], 3.38–3.46 [6H, m, DOTA], 3.63 [2H, s, DOTA], 3.78–3.95 [6H, m, DOTA], 4.07 [2H, s, CH2], 7.62 [2H, d, J = 7.2 Hz, aromatic], 8.18 [2H, d, J = 7.6 Hz, aromatic]. HR-MS(ESI) calcd. for C21H29N5O8Ga [M + H]+: m/z, 548.12719; found, 548.12654 and m/z, 550.12632; found, 550.12933.
Ga-DOTA
DOTA (14.8 μmol) was used as the chelator to provide Ga-DOTA (6.9 mg, 14.6 μmol, 98.7%). 8.6 min of retention time of analytical HPLC on system E. 1H NMR (D2O): δ 3.25 (8H, br, CH2), 3.42 (4H, Br, CH2), 3.64 (4H, s, CH2), 3.75 (4H, s, CH2), 3.89 (4H, br, CH2). ESI-MS [M – H]–: m/z, 469; found, 469 and m/z, 471; found, 471.
Preparation of the Crystal for X-ray Crystallographic Analysis
Isomer B
A solution of isomer B (1 mg) in water (120 μL) was prepared in a 0.3 mL tube and stored in a 50 mL vial including THF (ca. 5 mL). Standing of the solution for 1 week provided a colorless crystal. A selected crystal was used for the analysis. The crystallographic information file for isomer B can be obtained from Cambridge structure data base with the CCDC no. 2204293.
Ga-p-NO2-Bn-DO3A
A solution of Ga-p-NO2-Bn-DO3A (2 mg) in water (120 μL) was prepared in a 0.3 mL tube and slowly evaporated for 1 week to provide a colorless needle crystal. A selected crystal was used for the analysis. The crystallographic information file for Ga-p-NO2-Bn-DO3A can be obtained from Cambridge structure data base with the CCDC no. 2204295.
Syntheses of Non-Radioactive Gallium-Labeled TOC Analogues
Ga-DOTA-Bn-TOC
GaCl3 was dissolved in a 1 M AcOH–AcONa buffer (pH 3.5) (5 mg/mL) and stood for 5 min at room temperature. A solution of GaCl3 (100 μL) was added to a solution of DOTA-Bn-TOC (50 μL, equivalent molar to Ga). The mixture was reacted for 30 min at 100 °C. After cooling to room temperature, the mixture was analyzed by analytical HPLC. Retention time of analytical HPLC: 28.4 (isomer A′, 50.2%) and 28.9 min (isomer B′, 45.0%) on system B, ESI-MS [M + H + Na]2+: m/z, 823.3; found, 823.2 and [M + H + K]2+: m/z, 831.3; found, 831.2 for both isomers.
Ga-DOTATOC
Ga-DOTATOC was prepared according to the same procedure as Ga-DOTA-Bn-TOC using DOTATOC. 24.4 min of retention time (86.7%) of analytical HPLC on system B. ESI-MS [M + H]+: m/z, 1489.5; found, 1489.6.
Radiolabeling with 67Ga
To a 1 M AcOH–AcONa buffer (pH 4.0) was added the same volume of [67Ga]GaCl3. The mixture was allowed to stand for 5 min at room temperature to provide [67Ga]Ga-acetate solution. The same volume of a solution of chelators (20 μM) or TOC analogues (6 or 20 μM) in 0.1 M AcOH–AcONa buffer (pH 4.0) was added to the solution. A radiolabeling reaction was conducted for 5 min at 95 °C, and the radiochemical conversion was determined by RP-TLC. Then, 67Ga-labeled compounds were subjected to analytical HPLC purification to remove free chelators: 17.6 and 22.1 min of retention time for [67Ga]Ga-p-NO2-Bn-DOTA on system A, 22.4 min of retention time for [67Ga]Ga-DOTAGAMBu on system A, 20.2 min of retention time for [67Ga]Ga-DO3AMBu on system A, 16.9 min of retention time for [67Ga]Ga-p-NO2-Bn-DO3A on system A, 8.8 min of retention time for [67Ga]Ga-DOTA on system D, 23.5 and 25.1 min of retention time for [67Ga]Ga-DOTA-Bn-TOC on system C, and 24.6 min of retention time for [67Ga]Ga-DOTATOC on system B. The collected radioactive fractions were applied to Sep-Pak Plus C18 Cartridge (Nihon waters K.K., Tokyo), washed with water (3 mL), and then eluted with methanol (3 mL) for solvent change. The solution was evaporated to dryness, and the radioactive residue was redissolved in D-PBS(−) (Wako Pure Chemical Industries, Ltd., Osaka). The final solution of each sample was analyzed by analytical HPLC.
Stability Assessment in Murine Plasma
67Ga-labeled chelators or TOC analogues (74 kBq/20 μL) were added to freshly prepared murine plasma (180 μL). The mixture was incubated at 37 °C and 5% CO2. After incubation for 3 and 24 h, a 5 μL aliquot of each sample was drawn, and analyzed by RP-TLC.
Stability Assessment in apoTf Solution
67Ga-labeled chelators or TOC analogues (74 kBq/100 μL) were added to the same volume of 0.2 mM apoTf in 200 mM sodium bicarbonate buffer (pH 7.4). The mixture was incubated at 37 °C and 5% CO2. After incubation for 3 and 24 h, a 5 μL aliquot of each sample was drawn and analyzed by RP-TLC.
Biodistribution
Animal studies were conducted following our institutional guidelines and were approved by the Chiba University Animal Care Committee. Biodistribution studies were performed by intravenous administration of 0.1 mL of D-PBS(−) containing 0.1% BSA solution of each radioactive fraction (11 kBq/100 μL/mouse) to a 5 week old male ICR mice (Japan SLC Inc., Shizuoka, Japan). Groups of five mice were used for the experiments. Organs of interest were removed and weighed, and the radioactivity counts were determined with an auto-well γ counter at 1 and 3 h postinjection.
Statistical Analyses
Data are expressed as the means ± SD where appropriate. The results were statistically analyzed using Tukey’s test for the one-way ANOVA.
Acknowledgments
This work was supported in part by a grant from Global and Prominent Research, Chiba University. We appreciate Dr. Tmoki Yoneda for his technical assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c06814.
Synthesis of DOTAGAMBu, DO3AMBu, and p-NO2-Bn-DO3A; biodistribution of 67Ga-labeled TOC analogues; RP-HPLC chromatograms of 67Ga complexes, nonradioactive complexes, and ligands of DOTAGAMBu, DO3AMBu, p-NO2-Bn-DO3A, DOTA, DOTA-Bn-TOC, and DOTATOC; RP-TLC radiochromatograms of 67Ga-labeled model chelators and TOC analogues; MS spectra of isomer A and isomer B; and comparison of in vitro stability (PDF)
Crystallographic information of isomer B (CIF)
Crystallographic information of Ga-p-NO2-Bn-DO3A (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Clarke E. T.; Martell A. E. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12-, 13- and 14-membered tetraazamacrocycles. Inorg. Chim. Acta 1991, 190, 37–46. 10.1016/s0020-1693(00)80229-7. [DOI] [Google Scholar]
- Kumar K.; Chang C. A.; Francesconi L.; Dischino D.; Malley M.; Gougoutas J.; Tweedle M. F. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III) Macrocyclic Poly(amino carboxylates). Inorg. Chem. 1994, 33, 3567–3575. 10.1021/ic00094a021. [DOI] [Google Scholar]
- Tóth Ė.; Brücher E. Stability constants of the lanthanide (III)-1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetate complexes. Inorg. Chim. Acta 1994, 221, 165–167. 10.1016/0020-1693(94)03964-X. [DOI] [Google Scholar]
- Deal K. A.; Davis I. A.; Mirzadeh S.; Kennel S. J.; Brechbiel M. W. Improved in vivo stability of actinium-225 macrocyclic complexes. J. Med. Chem. 1999, 42, 2988–2992. 10.1021/jm990141f. [DOI] [PubMed] [Google Scholar]
- Price E. W.; Orvig C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. 10.1039/c3cs60304k. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S.; Kuji I.; Hamacher K. A.; Konishi S.; Kostakoglu L.; Kothari P. A.; Milowski M. I.; Nanus D. M.; Bander N. H.; Goldsmith S. J. Pharmacokinetics and biodistribution of 111In-and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu?. J. Nucl. Med. 2005, 46, 634–641. [PubMed] [Google Scholar]
- Schottelius M.; Wester H.-J. Molecular imaging targeting peptide receptors. Methods 2009, 48, 161–177. 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Pandya D. N.; Hantgan R.; Budzevich M. M.; Kock N. D.; Morse D. L.; Batista I.; Mintz A.; Li K. C.; Wadas T. J. Preliminary Therapy Evaluation of 225Ac-DOTA-c(RGDyK) Demonstrates that Cerenkov Radiation Derived from 225Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization. Theranostics 2016, 6, 698. 10.7150/thno.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadvar H.; Chen X.; Cai W.; Mahmood U. Radiotheranostics in cancer diagnosis and management. Radiology 2018, 286, 388–400. 10.1148/radiol.2017170346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani M.; André J. P.; Maecke H. R. 68Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 2008, 3, 53–63. 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- Rösch F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Wei L.; Miao Y.; Gallazzi F.; Quinn T. P.; Welch M. J.; Va̅vere A. L.; Lewis J. S. Gallium-68-labeled DOTA-rhenium-cyclized α-melanocyte-stimulating hormone analog for imaging of malignant melanoma. Nucl. Med. Biol. 2007, 34, 945–953. 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M.; Hisada H.; Temma T.; Shimizu Y.; Kimura H.; Ono M.; Nakamoto Y.; Togashi K.; Saji H. Gallium-68-labeled anti-HER2 single-chain Fv fragment: development and in vivo monitoring of HER2 expression. Mol. Imag. Biol. 2015, 17, 102–110. 10.1007/s11307-014-0769-5. [DOI] [PubMed] [Google Scholar]
- Srirajaskanthan R.; Kayani I.; Quigley A. M.; Soh J.; Caplin M. E.; Bomanji J. The Role of 68Ga-DOTATATE PET in Patients with Neuroendocrine Tumors and Negative or Equivocal Findings on 111In-DTPA-Octreotide Scintigraphy. J. Nucl. Med. 2010, 51, 875–882. 10.2967/jnumed.109.066134. [DOI] [PubMed] [Google Scholar]
- Afshar-Oromieh A.; Sattler L. P.; Mier W.; Hadaschik B. A.; Debus J.; Holland-Letz T.; Kopka K.; Haberkorn U. The Clinical Impact of Additional Late PET/CT Imaging with 68Ga-PSMA-11 (HBED-CC) in the Diagnosis of Prostate Cancer. J. Nucl. Med. 2017, 58, 750–755. 10.2967/jnumed.116.183483. [DOI] [PubMed] [Google Scholar]
- Weineisen M.; Schottelius M.; Simecek J.; Baum R. P.; Yildiz A.; Beykan S.; Kulkarni H. R.; Lassmann M.; Klette I.; Eiber M.; Schwaiger M.; Wester H.-J. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176. 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- Nock B. A.; Kaloudi A.; Lymperis E.; Giarika A.; Kulkarni H. R.; Klette I.; Singh A.; Krenning E. P.; de Jong M.; Maina T.; Baum R. P. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: preclinical and first clinical results. J. Nucl. Med. 2017, 58, 75–80. 10.2967/jnumed.116.178889. [DOI] [PubMed] [Google Scholar]
- Li D.; Minnix M.; Allen R.; Bading J.; Chea J.; Wong P.; Bowles N.; Poku E.; Shively J. E. Preclinical PET Imaging of NTSR-1-Positive Tumors with 64Cu-and 68Ga-DOTA-Neurotensin Analogs and Therapy with an 225Ac-DOTA-Neurotensin Analog. Cancer Biother. Rad. 2020, 36, 651. 10.1089/cbr.2020.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni H. R.; Singh A.; Langbein T.; Schuchardt C.; Mueller D.; Zhang J.; Lehmann C.; Baum R. P. Theranostics of prostate cancer: from molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. Br. J. Radiol. 2018, 91, 20180308. 10.1259/bjr.20180308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decristoforo C.; Hernandez Gonzalez I. H.; Carlsen J.; Rupprich M.; Huisman M.; Virgolini I.; Wester H.-J.; Haubner R. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imag. 2008, 35, 1507–1515. 10.1007/s00259-008-0757-6. [DOI] [PubMed] [Google Scholar]
- Blom E.; Långström B.; Velikyan I. 68Ga-Labeling of Biotin Analogues and their Characterization. Bioconjugate Chem. 2009, 20, 1146–1151. 10.1021/bc800538s. [DOI] [PubMed] [Google Scholar]
- Sephton R. G.; Hodgson G. S.; De Abrew S.; Harris A. W. Ga-67 and Fe-59 distributions in mice. J. Nucl. Med. 1978, 19, 930–935. [PubMed] [Google Scholar]
- Bernstein L. R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–82. [PubMed] [Google Scholar]
- Sugyo A.; Tsuji A. B.; Sudo H.; Nomura F.; Satoh H.; Koizumi M.; Kurosawa G.; Kurosawa Y.; Saga T. Uptake of 111In-labeled fully human monoclonal antibody TSP-A18 reflects transferrin receptor expression in normal organs and tissues of mice. Oncol. Rep. 2017, 37, 1529–1536. 10.3892/or.2017.5412. [DOI] [PubMed] [Google Scholar]
- Kubíček V. c.; Havlíčková J.; Kotek J.; Tircsó G.; Hermann P.; Tóth É.; Lukeš I. Gallium(III) Complexes of DOTA and DOTA–Monoamide: Kinetic and Thermodynamic Studies. Inorg. Chem. 2010, 49, 10960–10969. 10.1021/ic101378s. [DOI] [PubMed] [Google Scholar]
- Bernhard C.; Moreau M.; Lhenry D.; Goze C.; Boschetti F.; Rousselin Y.; Brunotte F.; Denat F. DOTAGA-Anhydride: A Valuable Building Block for the Preparation of DOTA-Like Chelating Agents. Chem.—Eur. J. 2012, 18, 7834–7841. 10.1002/chem.201200132. [DOI] [PubMed] [Google Scholar]
- Varasteh Z.; Mitran B.; Rosenström U.; Velikyan I.; Rosestedt M.; Lindeberg G.; Sörensen J.; Larhed M.; Tolmachev V.; Orlova A. The effect of macrocyclic chelators on the targeting properties of the 68 Ga-labeled gastrin releasing peptide receptor antagonist PEG 2-RM26. Nucl. Med. Biol. 2015, 42, 446–454. 10.1016/j.nucmedbio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Deshpande S.; Subramanian R.; McCall M.; DeNardo S. J.; DeNardo G.; Meares C. F. Metabolism of indium chelates attached to monoclonal antibody: minimal transchelation of indium from benzyl-EDTA chelate in vivo. J. Nucl. Med. 1990, 31, 218–24. [PubMed] [Google Scholar]
- Suzuki H.; Ichinohe K.; Araki M.; Muramatsu S.; Uehara T.; Arano Y. Synthesis and evaluation of a para-carboxylated benzyl-DOTA for labeling peptides and polypeptides. Nucl. Med. Biol. 2022, 114–115, 18–28. 10.1016/j.nucmedbio.2022.08.003. [DOI] [PubMed] [Google Scholar]
- Viola N. A.; Rarig R. S.; Ouellette W.; Doyle R. P. Synthesis, structure and thermal analysis of the gallium complex of 1,4,7,10-tetraazacyclo-dodecane-N,N′,N″,N‴-tetraacetic acid (DOTA). Polyhedron 2006, 25, 3457–3462. 10.1016/j.poly.2006.06.039. [DOI] [Google Scholar]
- Kilian K. 68Ga-DOTA and analogs: Current status and future perspectives. Rep. Pract. Oncol. Radiother. 2014, 19, S13–S21. 10.1016/j.rpor.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoli G.; Dolmella A.; Tisato F.; Porchia M.; Refosco F. Mononuclear six-coordinated Ga(III) complexes: A comprehensive survey. Coord. Chem. Rev. 2009, 253, 56–77. 10.1016/j.ccr.2007.12.001. [DOI] [Google Scholar]
- Notni J.; Pohle K.; Wester H.-J. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012, 2, 28. 10.1186/2191-219x-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-T.; Li Y.; Liu S. Synthesis and structural characterization of complexes of a DO3A-conjugated triphenylphosphonium cation with diagnostically important metal ions. Inorg. Chem. 2007, 46, 8988–8997. 10.1021/ic7010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knetsch P. A.; Petrik M.; Rangger C.; Seidel G.; Pietzsch H.-J.; Virgolini I.; Decristoforo C.; Haubner R. [68Ga]NS3-RGD and [68Ga] Oxo-DO3A-RGD for imaging αvβ3 integrin expression: synthesis, evaluation, and comparison. Nucl. Med. Biol. 2013, 40, 65–72. 10.1016/j.nucmedbio.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Lee Y. S.; Mou Z.; Opina A. C. L.; Vasalatiy O. Origin of the Isomer Stability of Polymethylated DOTA Chelates Complexed with Ln 3+ Ions. Eur. J. Inorg. Chem. 2021, 2021, 1428–1440. 10.1002/ejic.202100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.