Abstract
Study Objectives:
To describe the outcomes of central sleep apnea requiring home supplemental oxygen therapy in otherwise healthy term infants.
Methods:
All children < 1 year of age undergoing polysomnography between 2015 and 2020 at the Queensland Children’s Hospital were retrospectively studied. Children with gestational age < 37 weeks, underlying syndrome, cleft palate, those with obstructive apnea-hypopnea index > 50% of total apnea-hypopnea index, or with underlying cardiac or pulmonary parenchymal pathology were excluded. Polysomnography parameters were extracted for periods both on and off supplemental oxygenation.
Results:
Fifty-two (mean [standard deviation] age at polysomnography 32.6 [34.7] days; 21 females) term infants were included. There was a statistically significant improvement in apnea-hypopnea index on supplemental oxygen (mean [standard deviation] in room air 50.2 [36.3] vs 11.6 [9], P < .001 on supplemental oxygen), in both rapid eye movement and nonrapid eye movement sleep, as well as in mean oxygen saturations (96.6% in room air to 98.9% on oxygen; P < .001). There was no statistically significant change in transcutaneous carbon dioxide levels or sleep duration. Oxygenation was prescribed for a median (interquartile range) age of 197 (127) days.
Conclusions:
Central sleep apnea in term infants who are otherwise healthy generally has a good prognosis, with oxygen therapy prescribed for around 6 months. Oxygen therapy was associated with improved saturations and decrease in apnea-hypopnea index when assessed with polysomnography.
Citation:
Hayashi A, Suresh S, Kevat A, Robinson J, Kapur N. Central sleep apnea in otherwise healthy term infants. J Clin Sleep Med. 2022; 18(12):2813–2817.
Keywords: central sleep apnea, oxygen therapy, term infants, periodic breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Central apneas requiring intervention are considered uncommon in term, otherwise healthy infants. Previous studies have mainly described apnea in infants with known risk factors including prematurity and children with complex syndromes such as Prader Willi Syndrome. We endeavored to fill in the knowledge gap present within the field of central apnea in term infants by describing the effect and duration of oxygen therapy.
Study Impact: This study describes the polysomnography outcomes of improved oxygen saturation parameters and decreased apnea-hypopnea index with supplemental oxygenation along with the good prognosis of central sleep apnea in our cohort of term, otherwise healthy infants. This information is highly useful to guide clinicians and counsel parents of infants with central sleep apnea.
INTRODUCTION
Central sleep apnea (CSA) is defined as lack of respiratory effort during cessations of airflow, resulting in insufficient or absent ventilation and compromised gas exchange.1 CSA is diagnosed through PSG, with an apnea event defined as occurring when there is a reduction in airflow of at least 90% with decreased/absent respiratory effort, lasting at least 20 seconds or more than 2 baseline respiratory cycles, associated with an at least 3% reduction in oxygen saturation and/or an arousal and/or an episode of bradycardia in infants.2 In comparison, central hypopnea is reported if there is reduction in airflow of 30–90% with concurrent reduced inspiratory effort lasting for at least 2 breaths associated with at least 3% desaturation and/or arousal.2,3 A central apnea-hypopnea index (CAHI) ≤ 1 event/h is widely considered as normal, whereas CAHI ≥ 5 events/h is currently proposed as a pathological threshold.4,5
CSA is a recognized problem in preterm infants and in term infants with underlying disease or disorders,6,7 although central apneas requiring intervention are considered uncommon in term infants with no underlying medical condition and in this group, its pathophysiology remains poorly understood.8 Ventilatory control begins to develop early in gestation and this continues prenatally to prepare the baby to be “ready” to breathe at birth.9 While term infants are expected to have a robust respiratory control, recent evidence indicates that some may still be vulnerable to centrally disordered breathing during sleep, especially in the first 6 months of life.8,9 This stability of the respiratory control system has been quantified using the engineering concept of loop gain, where a controller (the central respiratory centers in the brain) and a plant (the lungs) operate in a negative feedback system to regulate the level of oxygen and carbon dioxide in the blood.10 It has been hypothesized that some term infants may still have residual instability of respiratory control, resulting in “overshooting” (high loop gain) of ventilation increase as a response to triggers such as hypoxia and arousal. This leads to excessive decrease in partial pressure of carbon dioxide (paCO2), below apnea threshold, causing apnea while waiting for paCO2 to increase.11 Infants also have limited lung reserves, making desaturation events quicker and more frequent during an apneic episode. This hypoxia continues the cycle of central apnea.1,11
Apnea, bradycardia, and oxygen desaturation events in term infants are known to cause significant effects on length of hospital stay, hospital cost, and quality of life for the family.12 Treatment for central apnea in children includes pharmacological therapies, supplemental oxygenation, and noninvasive or invasive ventilation.4,11 Supplemental oxygen has been shown to abolish periodic breathing and reduce central apnea numbers and has the potential of breaking the cycle of central apnea in children by preventing the reactive desaturation.3,11 In clinical practice, the rationale for initiating supplemental oxygen can be to reduce cyclical periods of hypoxia and to reduce the risks repetitive hypoxia could bring, including those of poor feeding/weight gain as well as possible adverse neurodevelopmental effects. However, there exists a lack of general consensus around when this therapy should be initiated. Similar to preterm infants with chronic neonatal lung disease,13 term infants have also benefited from the home oxygen therapy to assist early discharge and improve family’s quality of life.1,14–16
The aim of our study was to describe the clinical features, investigational parameters, and outcomes of neurodevelopmentally normal term infants with PSG-proven CSA prescribed supplemental oxygen.
METHODS
Study design
Through the PSG database of the Respiratory & Sleep Unit at Queensland Children’s Hospital from January 2015 to December 2020, a list of all children < 1 year of age who had formal PSG with results available was retrospectively obtained. Exclusion criteria then applied to this list were:
Gestation age < 37 weeks
Infants with complex syndromes, abnormal neuroimaging, significant developmental impairment, and/or cleft palate
Infants with predominant obstructive sleep-disordered breathing on PSG defined as obstructive apnea-hypopnea index > 50% of the total apnea-hypopnea index
Underlying cardiac or pulmonary parenchymal pathology
Not prescribed home oxygen therapy
PSG only available at the end of supplemental oxygen therapy
The PSG parameters extracted were sleep duration (minutes), rapid eye movement (REM) CAHI, nonrapid eye movement (NREM) CAHI, total CAHI, REM apnea-hypopnea index, NREM apnea-hypopnea index, total apnea-hypopnea index, time saturations < 90%, mean saturation, mean transcutaneous carbon dioxide (TcCO2), and percent time TcCO2 > 50 mm Hg. For each child who trialed supplemental oxygen during the course of the PSG, the abovementioned parameters were extracted for periods both with and without supplemental oxygen therapy. If more than one flow rate of oxygen was used, the oxygen flow rate used for the greatest duration was the only one included.
Statistical analyses
Results were tabulated and expressed as mean with standard deviation. SPSS software was used for analysis. Paired t-tests were used to compare results with and without supplemental oxygen for infants studied.
Ethics approval
The Children’s Health Queensland Human research ethics committee approved the study as a quality improvement project (EX/22/QCHQ/85215).
RESULTS
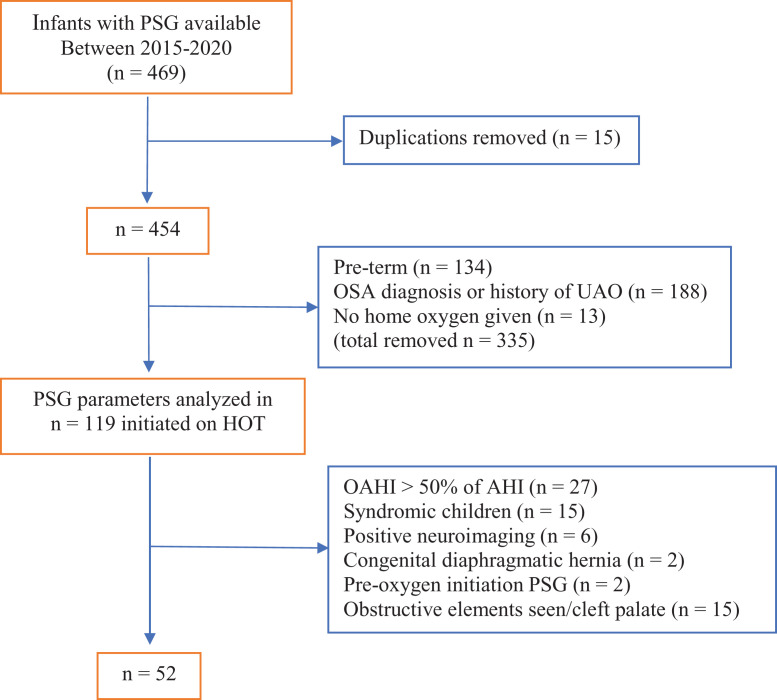
Over the 6-year period, 469 infants had a formal diagnostic PSG through the Queensland Children’s Hospital. Of these, 52 [mean (standard deviation) age at PSG 32.6 (34.7) days; 21 females] term neurologically normal nonsyndromic infants met inclusion criteria and were further analyzed (Figure 1). The median (interquartile range) gestation of the cohort was 38 + 6 [271.25 days] (11.5 days) with neuroimaging being normal in all 47 infants (magnetic resonance imaging in 40 infants; ultrasound head in 30 infants) for whom such investigations were conducted. Thirty children also had echocardiography performed with structurally normal hearts found in all. Metabolic screens were normal in 8 infants (urine screen in 4 infants, serological screen in 4 infants). The mean (standard deviation) apnea-hypopnea index for the cohort without supplemental oxygen at PSG was 50.2 (36.3) events/h, which showed significant improvement on supplemental oxygen [mean (standard deviation): 11.6(9), P < .001] (Table 1). Most PSG parameters showed statistically significant improvement on addition of supplemental oxygen, except TcCO2-related parameters, which were worse on supplemental oxygen, although this change did not reach statistical significance. The frequency of central events inversely correlated with the age at the diagnostic PSG (Figure 2).
Figure 1. Study cohort flow diagram.
Positive neuroimaging includes presence of hypoxic ischemic encephalopathy, Arnold-Chiari malformation, and developmental hypoplasia of corpus callosum. History of UAO includes presence of Pierre-Robin Sequence and laryngomalacia. AHI = apnea-hypopnea index, HOT = home oxygen therapy, OAHI = obstructive apnea-hypopnea index, OSA = obstructive sleep apnea, PSG = polysomnography, UAO = upper airway obstruction.
Table 1.
Polysomnography parameters without and with supplemental oxygen.
| Without Supplemental Oxygen, mean (SD) | On Supplemental Oxygen, mean (SD) | P* | |
|---|---|---|---|
| Sleep duration (minutes) | 143.0 (115.1) | 189.5 (106.1) | 0.09 |
| AHI, events/h | 50.2 (36.3) | 11.6 (9) | <0.001 |
| REM AHI, events/h | 71.0 (52.5) | 17.5 (13.8) | <0.001 |
| NREM AHI, events/h | 24.1 (26.4) | 3.6 (3.6) | <0.001 |
| CAHI, events/h | 47.1 (33.2) | 9.9 (8) | <0.001 |
| % Time saturation < 90% | 4.4 (11.9) | 0.2 (0.4) | 0.015 |
| Mean saturation | 96.6 (2.2) | 98.9 (0.8) | <0.001 |
| Mean TcCO2 | 43.8 (5.2) | 45.1 (6.3) | 0.08 |
| % time TcCO2 > 50 mm | 12.3 (9.3) | 21.2 (35.3) | 0.08 |
*Paired t-test. AHI = apnea-hypopnea index, CAHI = central apnea-hypopnea index, NREM AHI = non-rapid eye movement apnea-hypopnea index, REM AHI = rapid eye movement apnea-hypopnea index, SD = standard deviation, TcCO2 = transcutaneous carbon dioxide.
Figure 2. Correlation between apnea indices measured in room air and age.
AHI = apnea-hypopnea index, CAHI = central apnea-hypopnea index, PSG = polysomnography, RA = room air.
The median level for supplemental oxygen was 250 mL/min with duration of supplemental oxygenation (n = 47; 4 infants excluded since they were still on supplemental oxygen at the time of data collection and 1 infant was lost to follow-up) being median (interquartile range) 197 (127) days. Need for ongoing supplemental oxygen requirement was assessed based on repeat PSG or overnight oximetry performed every 8–12 weeks. The frequency of assessment and modality used was on individual sleep physician’s discretion.
DISCUSSION
Central sleep-disordered breathing in term infants, who are otherwise healthy, remains poorly understood and its outcomes have been infrequently reported in the literature to date.17 Using data from 52 term infants with PSG-proven CSA severe enough to warrant supplemental oxygenation, we report excellent overall outcomes with successful cessation of oxygen therapy in almost all cases within 1 year of use. Investigations available, including neuroimaging, cardiac imaging, and metabolic screen, were all normal and did not contribute to change in management. Oxygen therapy greatly reduced the frequency of central events and improved oxygen saturation parameters on PSG, without a significant difference emerging in TcCO2 level-related parameters.
The prevalence of CSA in children is unclear, with the reported incidence ranging from 1 in 1,000 term infants7 to affecting up to 5% of healthy children.4 Although CSA can be considered a physiological, idiopathic, or pathological condition, pediatric reports of those requiring treatment mainly focus on children with underlying medical conditions.4 There is still a paucity of data on the clinical consequences and best treatment course for CSA in otherwise healthy term infants,6 with most data being extrapolated from studies on preterm infants and infants with underlying morbidity.
Our study found that central events occurred much more frequently in REM compared to NREM sleep. This REM-related preponderance of central sleep-disordered breathing has not been commonly reported, although in 49 infants, McNamara and Sullivan18 reported the incidence of apneic events to be more than double in REM compared to NREM (NREM total apnea index 9 events/h, REM total apnea index 20 events/h, P <.005). We hypothesize that REM-related generalized muscle hypotonia and diaphragm-dependent breathing due to inhibition of intercostal and accessory muscles may be contributing to this phenomenon.19 Furthermore, hypoxic and hypercapnic ventilatory drives are also deceased in REM compared to NREM sleep, and this may further exaggerate the events.19 This becomes especially relevant during infancy, when a significantly higher proportion of sleep time (up to a third of total sleep time) is spent in REM.18,20
Polysomnography remains the “gold standard” in diagnosing apnea in children by measuring air flow, respiratory effort, and oxygen saturation as well as heart rate and electroencephalographically captured neural activity to detect respiratory events during sleep and differentiate central from obstructive events.11,19 It also can assess the severity of sleep-disordered breathing and detect associated hypoventilation,11 which may guide further investigations such as cardiac and neuroimaging, thyroid function testing, metabolic investigations, and genetic tests.1,4,21
Our results are consistent with previously reported data on the role of supplemental oxygen in abolishing periodic breathing and reducing central apnea numbers. Urquhart et al11 demonstrated reduction in frequency of central apnea when in 0.25 L/min compared to in air via a split-night sleep study of a child with idiopathic central sleep disorder. Home oxygen therapy has been increasingly used over the past 45 years,22,23 and this has benefited both patients and the hospital system by facilitating earlier hospital discharge,7,15,16,24 reducing costs for the medical system and improving hospital service efficiency.22,25,26 Pharmacological treatment involves use of methylxanthines, caffeine and theophyllines,11,27 and is most known for treatment in premature infants, despite some use in older children.3 Methylxanthine compounds are nonselective antagonists of adenosine receptors that are known to reduce apnea by increasing minute ventilation, CO2 sensitivity, and neural respiratory drive while also reducing hypoxic depression of breathing. It has added benefit of improving diaphragmatic contraction and respiratory muscle function.28 It has been found to reduce apneic episodes and need for ventilation in the first week of life11 and effective as early as within 2 to 7 days of commencing treatment.28 Caffeine is most preferred due to its longer half-life of 100 hours compared to 30 hours of theophylline, wider therapeutic range, and better side effect profile.27,28 Cases of apnea associated with hypoventilation may require ventilation therapy such as continuous positive airway pressure or bilevel ventilatory support,3,11 although it is rarely needed in term-born infants without a significant contributing underlying condition. Our cohort of such infants who were treated with oxygen therapy were followed up and found to have an overall excellent prognosis; the majority were treated with an average of 250 mL/min oxygen and required treatment for median 6.5 months. Our study demonstrates that infants who have higher CSA reflect the immature end of the spectrum of respiratory controller maturity, and, with supplemental oxygen, there is gradual improvement and attain normal breathing pattern without any detrimental consequences.
Our study had many limitations. First, oxygen therapy initiation was not standardized and was at the treating physician’s discretion. Secondly, in the absence of standardized criteria for cessation of supplemental therapy, again this was performed at the treating physician’s discretion, with clinical progress and downloadable oximetry tests generally used to guide this in a nonstandardized fashion. Finally, it remains debatable whether these children required any intervention and if clinical outcomes would have been any different if oxygen therapy was not initiated. Despite these limitations, to our knowledge this is the first study to report on the outcomes of PSG-proven CSA in otherwise healthy term-born infants prescribed oxygen therapy for the condition.
In conclusion, the present study portrays the presence of a severe CSA phenotype in otherwise healthy term infants that improves with age, has good clinical prognosis, and may be treated with home supplemental oxygen therapy. This outcome-based descriptive study will address the current knowledge gap regarding this group of children and highlights the need for future prospective studies examining the role of oxygen supplementation and other treatment modalities in infants with CSA.
ABBREVIATIONS
- CAHI
central apnea-hypopnea index
- CSA
central sleep apnea
- ECG
electrocardiogram
- NREM
non-rapid eye movement
- PSG
polysomnography
- REM
rapid eye movement
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
REFERENCES
- 1. Eckert DJ , Jordan AS , Merchia P , Malhotra A . Central sleep apnea: pathophysiology and treatment . Chest. 2007. ; 131 ( 2 ): 595 – 607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berry RB , Budhiraja R , Gottlieb DJ , et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Urquhart D . Investigation and management of childhood sleep apnoea . Hippokratia. 2013. ; 17 ( 3 ): 196 – 202 . [PMC free article] [PubMed] [Google Scholar]
- 4. McLaren AT , Bin-Hasan S , Narang I . Diagnosis, management and pathophysiology of central sleep apnea in children . Paediatr Respir Rev. 2019. ; 30 : 49 – 57 . [DOI] [PubMed] [Google Scholar]
- 5. Harman K , Weichard AJ , Davey MJ , Horne RSC , Nixon GM , Edwards BA . Assessing ventilatory control stability in children with and without an elevated central apnoea index . Respirology. 2020. ; 25 ( 2 ): 214 – 220 . [DOI] [PubMed] [Google Scholar]
- 6. Ghirardo S , Amaddeo A , Griffon L , Khirani S , Fauroux B . Central apnea and periodic breathing in children with underlying conditions . J Sleep Res. 2021. ; 30 ( 6 ): e13388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levin JC , Jang J , Rhein LM . Apnea in the otherwise healthy, term newborn: National Prevalence and Utilization during the birth Hospitalization . J Pediatr. 2017. ; 181 : 67 – 73.e1 . [DOI] [PubMed] [Google Scholar]
- 8. Patrinos ME , Martin RJ . Apnea in the term infant . Semin Fetal Neonatal Med. 2017. ; 22 ( 4 ): 240 – 244 . [DOI] [PubMed] [Google Scholar]
- 9. Carroll JL , Agarwal A . Development of ventilatory control in infants . Paediatr Respir Rev. 2010. ; 11 ( 4 ): 199 – 207 . [DOI] [PubMed] [Google Scholar]
- 10. Siriwardhana LS , Nixon GM , Horne RSC , Edwards BA . Journey towards a personalised medicine approach for OSA: can a similar approach to adult OSA be applied to paediatric OSA? Paediatr Respir Rev. 2020. ; 36 : 128 – 135 . [DOI] [PubMed] [Google Scholar]
- 11. Urquhart D , Hill E , Morley A . Sleep disordered breathing in children . Paediatr Child Health. 2017. ; 27 ( 7 ): 328 – 336 . [Google Scholar]
- 12. Montenegro B , Freiberger C , Veit L , Amberson M , Mukhopadhyay S , Rhein L . An occurrence of apnea, bradycardia, and desaturation events resulting in a delay of discharge in late preterm and full term infants . Pediatr Neonatol. 2018. ; 59 ( 6 ): 630 – 631 . [DOI] [PubMed] [Google Scholar]
- 13. Kapur N , Nixon G , Robinson P , et al . Respiratory management of infants with chronic neonatal lung disease beyond the NICU: a position statement from the Thoracic Society of Australia and New Zealand . Respirology. 2020. ; 25 ( 8 ): 880 – 888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balfour-Lynn IM , Field DJ , Gringras P , et al. Paediatric Section of the Home Oxygen Guideline Development Group of the BTS Standards of Care Committee . BTS guidelines for home oxygen in children . Thorax. 2009. ; 64 ( Suppl 2 ): ii1 – ii26 . [DOI] [PubMed] [Google Scholar]
- 15. Wong MD , Chung H , Chawla J . Using continuous overnight pulse oximetry to guide home oxygen therapy in chronic neonatal lung disease . J Paediatr Child Health. 2020. ; 56 ( 2 ): 309 – 316 . [DOI] [PubMed] [Google Scholar]
- 16. Serginson JG , Yang IA , Armstrong JG , et al . Variability in the rate of prescription and cost of domiciliary oxygen therapy in Australia . Med J Aust. 2009. ; 191 ( 10 ): 549 – 553 . [DOI] [PubMed] [Google Scholar]
- 17. Xiao L , Sunkonkit K , Chiang J , Narang I . Unexplained significant central sleep apnea in infants: clinical presentation and outcomes . Sleep Breath. 2022. . [DOI] [PubMed] [Google Scholar]
- 18. McNamara F , Sullivan CE . Sleep-disordered breathing and its effects on sleep in infants . Sleep. 1996. ; 19 ( 1 ): 4 – 12 . [DOI] [PubMed] [Google Scholar]
- 19. Davey MJ . Investigation of sleep disorders . J Paediatr Child Health. 2005. ; 41 ( 1–2 ): 16 – 20 . [DOI] [PubMed] [Google Scholar]
- 20. Kato I , Franco P , Groswasser J , Kelmanson I , Togari H , Kahn A . Frequency of obstructive and mixed sleep apneas in 1,023 infants . Sleep. 2000. ; 23 ( 4 ): 487 – 492 . [PubMed] [Google Scholar]
- 21. Amimoto Y , Okada K , Nakano H , Sasaki A , Hayasaka K , Odajima H . A case of congenital central hypoventilation syndrome with a novel mutation of the PHOX2B gene presenting as central sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 3 ): 327 – 329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cherian S , Morris I , Evans J , Kotecha S . Oxygen therapy in preterm infants . Paediatr Respir Rev. 2014. ; 15 ( 2 ): 135 – 141 . [DOI] [PubMed] [Google Scholar]
- 23. MacLean JE , Fitzgerald DA . A rational approach to home oxygen use in infants and children . Paediatr Respir Rev. 2006. ; 7 ( 3 ): 215 – 222 . [DOI] [PubMed] [Google Scholar]
- 24. Ejiawoko A , Lee HC , Lu T , Lagatta J . Home oxygen use for preterm infants with bronchopulmonary dysplasia in California . J Pediatr. 2019. ; 210 : 55 – 62.e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pirr S , Peter C . Home oxygen therapy after hospital discharge . Semin Fetal Neonatal Med. 2020. ; 25 ( 2 ): 101082 . [DOI] [PubMed] [Google Scholar]
- 26. Hayes D Jr , Wilson KC , Krivchenia K , et al . Home oxygen therapy for children. An official American Thoracic Society clinical practice guideline . Am J Respir Crit Care Med. 2019. ; 199 ( 3 ): e5 – e23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahlfeld SK . Apnea . In: Kliegman RM , St Geme J , Blum N , et al ., eds. Nelson Textbook of Pediatrics. 21st ed. Philadelphia, PA: : Elsevier; ; 2020. : 3990 – 3991 . [Google Scholar]
- 28. Zhao J , Gonzalez F , Mu D . Apnea of prematurity: from cause to treatment . Eur J Pediatr. 2011. ; 170 ( 9 ): 1097 – 1105 . [DOI] [PMC free article] [PubMed] [Google Scholar]