Abstract
Study Objectives:
A high body mass index (BMI) is a risk factor for obstructive sleep apnea. However, to our knowledge there is no reported equation that quantifies the relationship between weight, as measured by BMI, and apnea severity, as assessed by the apnea-hypopnea index (AHI). Our objective was to find a mathematical relationship between BMI and AHI.
Methods:
We prospectively recruited 434 veterans from our polysomnography laboratory. Veterans already undergoing a sleep study were approached, and those who consented were enrolled. The veterans who enrolled in our study also participated in their scheduled sleep study. This study was approved by our institutional review board.
Results:
We found a simple mathematical relationship between BMI and AHI: for every 1-point drop in BMI (corresponding to 5–8 pounds, depending on a person’s height), AHI decreases by 6.2%. And limiting BMI to 25–40 kg/m2 (which includes about 80% of the BMIs), then AHI drops by 7.1%. Simply put as a rule of thumb: For every 7-pounds drop in weight, expect a 7% drop in AHI.
Conclusions:
To our knowledge, this is the first simple mathematical equation that associates the severity of weight with the severity of apnea in veterans. This equation can be a practical rule of thumb that can be implemented in clinics to predict the amount of weight a patient needs to lose to decrease their apnea, which might help motivate patients to lose weight.
Citation:
Fattal D, Hester S, Wendt L. Body weight and obstructive sleep apnea: a mathematical relationship between body mass index and apnea-hypopnea index in veterans. J Clin Sleep Med. 2022;18(12):2723–2729.
Keywords: obstructive sleep apnea, polysomnography, body mass index, BMI, apnea-hypopnea index, AHI, mathematics of sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is a known association between body weight and the presence of obstructive sleep apnea as well as the severity of apnea. The rationale of this study is to find a mathematical relationship between body weight and severity of apnea. We found a simple, proportional, mathematical formula between body weight, as measured by body mass index, and sleep apnea severity, as measured by apnea-hypopnea index.
Study Impact: We found the following: for every 7-pound drop in weight, expect a 7% drop in apnea-hypopnea index. We hope our formula plays a role in motivating patients to improve their weight and apnea risk by providing them with a practical guidance of how much they need to lose.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder whose prevalence and severity increase with body weight. OSA is characterized by episodes of apnea (cessation of breathing), hypopnea (reduction in ventilation), or both, and is quantified by the apnea-hypopnea index (AHI).1–3 Weight is one of the chief risk factors for OSA, and high body mass index (BMI) coexists with abnormal AHI.4–6 Overweight and obese persons are at risk of having OSA.7 Population studies throughout the United States, Europe, Asia, and Australia have consistently shown a graded increase in the prevalence of OSA as BMI, or other measures of body habitus, increases in adults.7 For example, 26% of overweight men or women have mild apnea, and about 20% of men and 10% of women have moderate to severe apnea. Among obese individuals, 22% of men and 34% of women have mild apnea, and about 62% of men and 20% of women have moderate to severe apnea.5 Finally, about 60–90% of adult patients with OSA have a BMI of 25 kg/m2 or more.6
OSA has multiple negative consequences on health and is a high-impact public health issue.6,7 There is a close relationship between OSA and metabolic syndrome–related diseases—namely, hypertension, type 2 diabetes mellitus, and hyperlipidemia.6 For example, OSA has a bidirectional relationship with diabetes mellitus and shares common risk factors, including obesity.8 An abnormal AHI is associated with an increase in all causes of mortality, cardiovascular events, stroke, diabetes mellitus, and depression.9 Stelmach-Mardas et al10 found that reducing weight not only improved apnea but also improved cholesterol, triglycerides, fasting insulin, and blood pressure levels. Therefore, helping overweight or obese individuals who are apneic understand the relationship between weight and apnea could help them better manage these ailments. Finding a numerical relationship between weight and apnea is 1 step toward helping patients understand their condition and taking steps to improve it.
METHODS
Protocol registration
This study was approved by the institutional review board at Iowa City VA Medical Center (IRB approval # 202101393). Recruitment of participants took place between May 2021 and December 2021.
Participants
Every veteran who participated in a sleep test in our polysomnography laboratory was approached by one of our sleep technicians to determine their interest in participating in this research study. Our inclusion criterion was adult veterans (age ≥ 18 years). We did not enroll anyone 90 years old or above. No other exclusionary criteria were set. This study was done during the coronavirus disease 2019 (COVID-19) pandemic; thus, we adhered to Veterans Affairs (VA) guidelines on personal protective equipment for staff and veterans.
We prospectively enrolled 434 veterans (age range 24–88 years, 6% women and 94% men) with any sleep complaint who presented at our polysomnography laboratory to undergo a sleep test. Although we did not keep records of those who did not participate, we estimate that we enrolled approximately 95% of the total number of veterans who underwent a sleep test at our laboratory during our research study period.
Polysomnography
Each veteran participated in an overnight sleep test that was referred by their provider. All of the sleep tests were performed in accordance with American Academy of Sleep Medicine (AASM) criteria and included measuring the following parameters: electroencephalographic (EEG) activity (EEG leads were at locations F3-A1, C3-A1, O1-A1 on the scalp), electro-oculographic activity, submental electromyographic activity, lower limb movement, electrocardiographic activity, chest and abdominal movements, airflow, and oxygen saturation.
Sleep was recorded between the times when the lights were switched off (after calibration was complete, approximately 9:00–10:00 pm) and when the lights were switched back on (normally between 05:30 and 06:00 am). As per our protocol, no medications were stopped prior to the study. The sleep tests were scored and interpreted in accordance with standard criteria,1 as we routinely do in our sleep laboratory, and this was independent of our research study.
Apnea and hypopnea were defined as per standard criteria.1 An apnea requires both of the following criteria to be met: (1) a drop in the peak breathing excursion by ≥ 90% of pre-event baseline, detected using a sensor, and (2) duration of the ≥ 90% drop in the sensor signal is ≥ 10 seconds. A hypopnea requires both of the following criteria to be met: (1) peak respiratory excursion drops by ≥ 30% of pre-event baseline, detected using a sensor, and (2) duration of the ≥ 30% drop in signal excursion is ≥ 10 seconds.
At our VA hospital, we use ≥ 4% oxygen desaturation from the pre-event baseline as an additional criterion to identify hypopneas (from criteria by the Centers for Medicare and Medicaid Services11).
We used the AASM criteria to diagnose OSA using the following AHI values: normal = <5, mild OSA = 5–15 (we use 5–14), moderate OSA = 15–30, and severe OSA > 30 events/h (AASM website2).
Statistical analysis
To evaluate the association between BMI and AHI, we used a Spearman correlation, along with confidence intervals (CIs) obtained using a Fisher’s z-transformation. We obtained a coefficient for BMI by log-transforming AHI (our outcome variable) and subsequently fitting a linear regression model. P values < .05 were statistically significant.
To accomplish the target sample size needed for enough power, an iterative analysis was performed periodically once we started to gather data. At approximately 5 months into our study, we did interim analysis and then repeated the iterative analysis approximately 1 month later. We discovered that, even if we collected data from 1000 participants, the results were not likely to change significantly. This is when we terminated the study.
RESULTS
Participants enrolled
We recruited 434 veterans. We did not keep records of those who did not enroll, but from the total number of sleep studies we typically perform each month, we estimate that we enrolled 95% of eligible patients.
Only 8 patients did not have plausible values for certain measurements. We counted these values as missing, and the analyses were adjusted for these missing data. For example, the sleep efficiency for 1 participant was reported as 543%, so their sleep efficiency measurement was removed from the data, but the rest of their data remained in the study. A total of 5% of the data were missing.
Six percent of our sample was female, consistent with the veteran population, which is reported as 9% by the US Census Bureau.12
BMI and AHI
Of the 434 veterans involved in our study, 279 (64.4%) had OSA (AHI ≥ 5 events/h). Forty-nine participants (11.3%) had normal weight, while 385 (88.7%) of our veterans were overweight or obese. We compared BMI in veterans with normal vs abnormal AHI and found a P value of < .001 (Table 1). We also compared BMI with mild, moderate, and severe AHI and found an overall P value of < .001 (Table 2). Finally, we compared age and AHI and obtained an overall P value of < .001 (Table 1 and Table 2).
Table 1.
BMI and presence or absence of apnea.
| No Apnea: AHI < 5 events/h | Apnea: AHI ≥ 5 events/h | P | |
|---|---|---|---|
| Age, mean (SD), y | 52.4 (16.6) (n = 154) | 62.6 (13.6) (n = 279) | <.001 |
| BMI, mean (SD), kg/m2 | 30.2 (5.6) (n = 152) | 33.5 (7.1) (n = 276) | <.001 |
AHI = apnea-hypopnea index, BMI = body mass index, SD = standard deviation.
Table 2.
BMI and apnea severity.
| Normal: AHI < 5 events/h | Mild: AHI 5–14 events/h | Moderate: AHI 15–30 events/h | Severe: AHI > 30 events/h | P (Overall Test) | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 52.4 (16.6) (n = 154) | 61.6 (14.4) (n = 134) | 64.4 (12.2) (n = 81) | 62.6 (13.6) (n = 64) | <.001 |
| BMI, mean (SD), kg/m2 | 30.2 (5.6) (n = 152) | 32.8 (6.8) (n = 132) | 32.9 (7.6) (n = 80) | 35.6 (6.8) (n = 64) | <.001 |
AHI = apnea-hypopnea index, BMI = body mass index, SD = standard deviation.
Higher BMI is associated with higher AHI
Veterans with a higher BMI tended to have more severe apnea—that is, higher AHI (Table 3). We did not have enough women in each BMI and AHI category to stratify our data by sex. Of our 23 female veterans, 12 had a normal AHI, 5 had mild OSA, 2 had moderate OSA, and 4 had severe OSA.
Table 3.
BMI severity and OSA prevalence and severity.
| Normal: AHI < 5 events/h | Mild: AHI 5–14 events/h | Moderate: AHI 15–30 events/h | Severe: AHI > 30 events/h | |
|---|---|---|---|---|
| Normal weight: BMI < 25 kg/m2, n (%) | 28 (58.3%) | 10 (20.8%) | 8 (16.7%) | 2 (4.2%) |
| Overweight: BMI 25–30 kg/m2, n (%) | 56 (40.6%) | 43 (31.2%) | 27 (19.6%) | 12 (8.7%) |
| Obese: BMI > 30 kg/m2, n (%) | 70 (28.3%) | 81 (32.8%) | 46 (18.6%) | 50 (20.2%) |
AHI = apnea-hypopnea index, BMI = body mass index.
OSA and sex
The difference in AHI between female and male veterans is presented in Table 4. Males are more likely to have OSA. The P value for the Wilcoxon rank-sum test used for this comparison is .0054.
Table 4.
OSA in female vs male veterans.
| Female | Male | P | |
|---|---|---|---|
| Median (IQR) AHI, events/h | 2.2 (0.6, 12.2) (n = 27) | 9.2 (3.0, 20.8) (n = 407) | .0054 |
IQR = interquartile range, OSA = obstructive sleep apnea.
Mathematical relationship between BMI and AHI
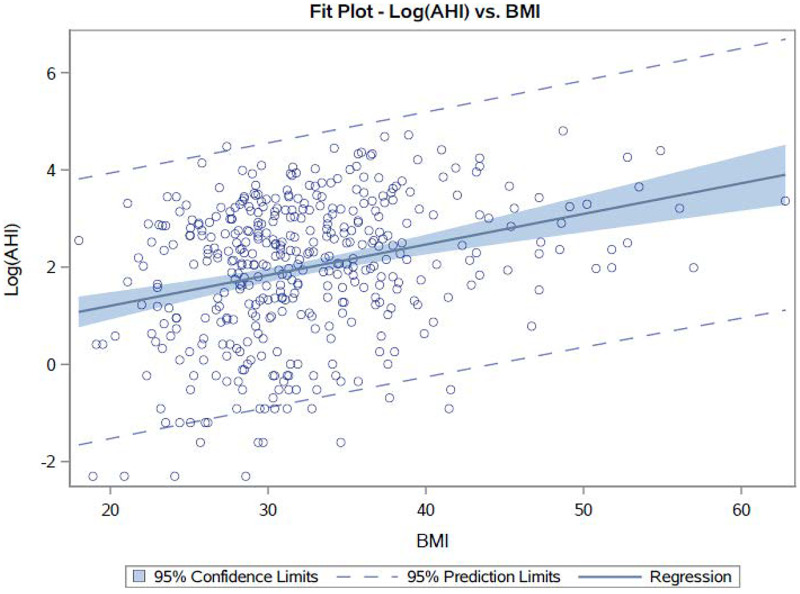
Of our total 434 veterans, we had data on both BMI and AHI in 428 of them. Using a Spearman correlation, we compared BMI with OSA severity using the AHI and found that BMI correlates with AHI (P < .01, n = 428; Figure 1). The Spearman’s rank correlation coefficient was 0.272, with a 95% CI of 0.181–0.357. Using a linear regression model in which the AHI variable was log-transformed, we were able to find a more interpretable relationship: for every 1-point drop in BMI, AHI is expected to drop by 6.2% (95% CI: 4.2–7.9%).
Figure 1. Plot of BMI (x axis) vs AHI (y axis) in all participants.
AHI = apnea-hypopnea index, BMI = body mass index.
For example, if a person with an AHI of 50.0 events/h loses weight, reducing their BMI by 1 point (5–8 pounds, depending on their height) will decrease their AHI to 46.9 events/h.
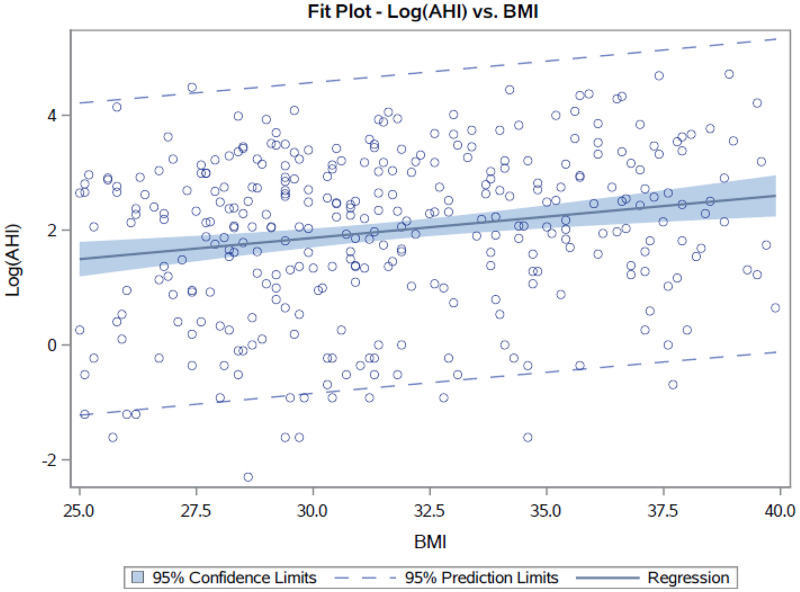
The majority of our veterans (339/434, 78.1%) had a BMI between 25–40 kg/m2, so we additionally analyzed the data using only this group of veterans to evaluate the sensitivity of our findings to outliers. Using only BMI values ranging from 25–40 kg/m2, we observed a similar mathematical equation—namely, for a BMI between 25 and 40 kg/m2, a 1-point decrease in BMI corresponds with a 7.1% decrease in AHI (95% CI: 3.4–10.7%). Our Spearman’s rank correlation coefficient is 0.187 with a 95% CI of 0.081–0.287 (P = .0005; Figure 2).
Figure 2. Plot of BMI (x axis) vs AHI (y axis) in participants with a BMI in the range of 25–40 kg/m2.
AHI = apnea-hypopnea index, BMI = body mass index.
The summary of our findings can be simplified by the following rule of thumb: for every 7-pound drop in weight, expect a 7% drop in AHI.
DISCUSSION
The main finding of our study is that in 428 of the 434 veterans who participated in our study, we found a mathematical formula linking a change in weight, as measured by BMI, proportionally to a change in OSA severity, as measured by AHI. A 1-point drop in BMI corresponds to a 6–7% drop in AHI. A 1-point drop in BMI is equivalent to 5–8 pounds, depending on the height of the person (National Institutes of Health website13).
We reviewed the BMI vs AHI relationship reported in the literature (Table 5). In a study of 260 patients, Kuna et al14 found a relationship between weight and AHI: for every 1 kg of weight loss, AHI dropped by 0.54–0.68 points. Therefore, if a hypothetical 250-pound, 6-ft tall person (BMI of 33.9 kg/m2) with an AHI of 30 events/h loses approximately 8 pounds (3.6 kg), the equivalent of a 1-point drop in BMI, they will reduce their AHI by 1.9–2.4 events/h. This is similar to our formula—in this case, we would predict that AHI drops by 2 points. Furthermore, Kline et al15 found that, in 114 adults, for those who lost at least 5% of their weight, their AHI was reduced by 2.1 ± 0.9 points (adjusted mean ± SE) more than for those who lost less than 5% of weight (P < .05). The authors did not provide an equation linking BMI to AHI. Therefore, we will indirectly compare their result to our result, and estimate using our hypothetical person. If a 250-pound, 6-ft tall person with a BMI of 33.9 kg/m2 loses 6% of their weight, their BMI would decrease to 31.9 kg/m2 (a 2-point drop in BMI), and their AHI would drop by 2–3 points. If the AHI were 30 events/h, then our formula would predict that AHI drops by 4 points. If we use an AHI of 15 events/h (similar to the average of 15.18 events/h found in our study), then Kline et al’s calculation would again be similar to our formula.
Table 5.
BMI vs AHI in literature.
| Study/First Author, Year | Number of Articles and Article Type | Sample Size | Decrease in Weight | Decrease in AHI |
|---|---|---|---|---|
| Iowa City VA (current study) | 1 | 428 | 1 BMI | 6–7% |
| Stelmach-Mardas,10 2021 (this review includes Kuna’s study) | 10, Surgical intervention | 1,070 | 1 BMI | 2.83 |
| Kuna,14 2021 | 1, 10-Year follow-up | 134 | 1 kg | 0.54 and 0.68 in each intervention group |
| Lins-Filho,20 2021 (includes Kline’s study) | 12, Exercise intervention | 596 | 0.55 BMI | 8.06 |
| Kline,15 2018 | 1, Nonsurgical intervention | 114 | 5% Weight | 2.1 ± 0.9 |
| Quintas-Neves,19 2016 (includes all Greenburg articles) | 22, Surgical intervention | 1,669 | Average 30.5% | 65% |
| Greenburg,18 2009 | 12, Surgical intervention | 342 | 17.9 BMI (95% CI: 55.3–37.7) | 38.2 (95% CI: 31.9–44.4) |
| Ashrafian,16 2015 | 19, Surgical intervention | 525 | 14 BMI | 29 |
| Ashrafian,16 2015 | 20, Nonsurgical intervention | 825 | 3.1 BMI | 11 |
| Romero-Corral,4 2010 | n/a, Review | n/a | 15 BMI | 36 |
| Tuomilehto,17 2009 | 1, Nonsurgical intervention | 72 | Weight loss: 10.7 ± 6.5 kg; BMI: 3.5 ± 2.1 | 4 |
AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, VA = Veterans Affairs, n/a = not applicable.
In a review of 20 studies on nonsurgical weight loss, Ashrafian et al16 found that, overall, when there was a 3-point decrease in BMI there was an 11-point reduction in AHI. This study was averaged from several studies. Using the same example above, our hypothetical person’s BMI would drop by 6.3 points (which is within a similar order of magnitude to ours). The authors also reviewed 19 surgical studies and found higher weight loss with surgical interventions than with nonsurgical interventions.16 Romero-Corral et al’s review4 found that bariatric surgery (regardless of the type of intervention) resulted in an average reduction of 15 points in BMI and 36 points in the AHI. Again, this is a review of data from multiple studies, and we estimate that their result suggests that every 1-point reduction in BMI translated to a reduction of 2.3 points in AHI, similar to our results. Finally, Stelmach-Mardas et al10 reviewed 10 articles and found that a 1-point reduction in BMI corresponded to an approximate 1.4- to 7-point drop in AHI. Although this is a wide range, our result falls within this range.
Other literature review studies could not be directly compared with ours and are listed here. In Tuomilehto et al,17 72 patients with mild apnea underwent a low diet program and, on average, their BMI dropped by 3.5 points, and this corresponded to a “markedly lower” adjusted odds ratio for having mild OSA. Greenburg et al18 reviewed 12 bariatric surgery studies (total of 342 patients) and found that the pooled mean BMI reduction was approximately 18 points and the AHI was reduced by approximately 38 points. Quintas-Neves et al19 reviewed 22 surgical intervention articles and found a significant reduction in BMI (13–43%) and AHI (28–93%). Peppard et al21 found that, in 690 randomly selected employed Wisconsin residents, a 10% increase in weight predicted an approximate 32% increase in AHI and a 10% weight loss predicted a 26% reduction in AHI (Table 5; summary of weight measurements and OSA in the literature).
The BMIs in our population of veterans are consistent with those reported in the literature. In our population, 90.4% were overweight or obese, similar to the 88% reported in the literature.22 In our apneic participants, the mean BMI was 33.5 kg/m2, and in controls, the mean BMI was 30.3 kg/m2. This is consistent with what Myers et al3 found in their review, in which individuals with OSA had a mean BMI of 31.4 kg/m2, and those without had a mean BMI of 28.3 kg/m2. Similarly, Camacho et al23 found in their review of non-Asian (as opposed to Asian) studies that the means for BMI in apneics and controls were 40.6 and 30.9 kg/m2, respectively.
Finally, our age and sex results were also consistent with the literature.5,24 We found apnea to be more prevalent with age, as in prior literature5 and that apnea is more common in men vs women, similar to prior literature.3–5
Pathophysiology of OSA in relation to weight
The etiology of OSA is complex, but obesity is one of its chief causes.6 Population studies of weight loss provide support for a causal relationship between AHI and weight.7 From such studies, we know that weight loss can improve OSA. A meaningful improvement in OSA can be achieved when weight is reduced by 7–11%.25 On the other side, we also know that an increase in weight increases the risk of having apnea. For example, a BMI increase of 5.3 leads to 4 times greater risk of developing OSA.26
It is postulated that weight gain worsens apnea because fat deposition, such as around the upper airways, can predispose individuals to a narrower lumen and increased the collapsibility of the upper airways.4 In fact, obese patients with OSA have 42% more fat in their neck.19 Furthermore, fat deposition around the chest reduces chest compliance and reduces resting lung volume.4,6 Such reductions in lung volume reduce tracheal traction, which increases pharyngeal collapsibility.6 Another mechanism that may explain the predisposition to OSA in people who are obese is that adipocytes produce hormones such as leptin.19 Leptin is an adipocyte-derived hormone regulating energy homeostasis and body weight.27 Leptin concentration is increased in patients with OSA,27 which may lead to reduced neuromuscular control of the pharyngeal lumen.28 A leptin receptor gene polymorphism was studied in OSA and it was found that individuals who are carriers of the arginine (Arg) allele were more obese and developed OSA as compared with carriers of homozygotic glutamine (Gln/Gln) individuals.27
Although obesity is the most significant predisposing factor for OSA,19 it is not the only factor, and improvement in OSA with weight loss is not linked entirely to the loss of fat per se.20 Other factors, such as genetic variation, also play a role in OSA. In fact, Patel et al29 suggest that obesity only explains about 40% of the genetic variance in sleep apnea. In addition, factors other than weight play a role because the same amount of weight gain increases AHI more in males than in females30 and in younger individuals compared with in older individuals.26 Furthermore, from prevalence data of OSA in individuals with normal weight and in those who are overweight or obese, it is estimated that, in adults with moderate-to-severe OSA, only 58% have OSA attributable to excess weight.31 Our results support this concept that weight is not the only factor in OSA since 42% of our normal-weight veterans had OSA. Finally, one can see from Figure 1 and Figure 2 that BMI vs AHI data are not heavily correlated, further confirming that weight is not the only factor contributing to OSA.
Strengths and limitations
The strengths of our study are that it is a prospective study that could be conducted efficiently and safely during the time of COVID-19. We had a high participation rate; we recruited a large number of participants in a short amount of time. To our knowledge, this is the only study that looks at the relationship between weight and OSA in veterans.
The limitations of our study are that we did not collect self-reported sleep symptoms, so we cannot differentiate between apneic individuals who have symptoms of OSA vs those who are asymptomatic. Since this was done during COVID-19, we did not want to prolong the testing time and we believe that our study results were not related to the sleep symptoms. We assume all veterans had sleep-related symptoms, because they were referred to our polysomnography lab. Further, we did not collect data on comorbid illnesses, such as hypertension or diabetes mellitus, or on central apnea, which we estimate that the latter was present in only a few of our veterans. Central apnea is another factor that contributes to apnea independent of weight; future studies can separate it from OSA in weight-management evaluations. Medications were reviewed and are listed on each veteran’s sleep report; and although we focused on current weight, we did not include in our analysis whether or not a veteran’s weight is related to a medication side effect. Finally, we used AASM and Centers for Medicare and Medicaid Services criteria. It would be interesting to repeat such a study with AASM criteria only, although with our large sample and the indirect similarity of our results to the literature, we predict that a similar rule of thumb is likely to emerge.
CONCLUSIONS
Weight is a major risk factor for OSA, and weight loss should be a cornerstone of treatment for OSA. We found a mathematical relationship between BMI and AHI that can simply be stated as a rule of thumb: for every 7-pound drop in weight, expect a 7% drop in AHI. Although this is not guaranteed, this equation serves as guidance to patients and clinicians and is a useful and practical way to explain to patients the role of weight gain or loss as it relates to their OSA. Weight management is multifaceted and needs a multidisciplinary approach for each patient.32 Our formula is a practical one and can be easily used in clinics. We hope our formula plays a role in motivating patients to improve their weight and apnea risk.
ACKNOWLEDGMENTS
Author contributions: D.F.: developed the study design and prepared the manuscript. L.W.: performed statistical analysis and contributed to the manuscript. S.H. collected all the data.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- OSA
obstructive sleep apnea
DISCLOSURE STATEMENT
All authors have read and approved the content of the manuscript. Work for this study was performed at Iowa City VA Health Care System. Research reported in this publication was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002537. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
REFERENCES
- 1. Berry RB , Brooks R , Gamaldo CE , et al. American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.2. Darien, IL: : American Academy of Sleep Medicine; ; 2015. . [Google Scholar]
- 2. American Academy of Sleep Medicine . https://aasm.org/resources/factsheets/sleepapnea.pdf . Accessed January 17, 2022.
- 3. Myers KA , Mrkobrada M , Simel DL . Does this patient have obstructive sleep apnea? The Rational Clinical Examination systematic review . JAMA. 2013. ; 310 ( 7 ): 731 – 741 . [DOI] [PubMed] [Google Scholar]
- 4. Romero-Corral A , Caples SM , Lopez-Jimenez F , Somers VK . Interactions between obesity and obstructive sleep apnea: implications for treatment . Chest. 2010. ; 137 ( 3 ): 711 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Senaratna CV , Perret JL , Lodge CJ , et al . Prevalence of obstructive sleep apnea in the general population: a systematic review . Sleep Med Rev. 2017. ; 34 : 70 – 81 . [DOI] [PubMed] [Google Scholar]
- 6. Ming X , Yang M , Chen X . Metabolic bariatric surgery as a treatment for obstructive sleep apnea hypopnea syndrome: review of the literature and potential mechanisms . Surg Obes Relat Dis. 2021. ; 17 ( 1 ): 215 – 220 . [DOI] [PubMed] [Google Scholar]
- 7. Young T , Peppard PE , Gottlieb DJ . Epidemiology of obstructive sleep apnea: a population health perspective . Am J Respir Crit Care Med. 2002a. ; 165 ( 9 ): 1217 – 1239 . [DOI] [PubMed] [Google Scholar]
- 8. Song SO , He K , Narla RR , Kang HG , Ryu HU , Boyko EJ . Metabolic consequences of obstructive sleep apnea especially pertaining to diabetes mellitus and insulin sensitivity . Diabetes Metab J. 2019. ; 43 ( 2 ): 144 – 155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kendzerska T , Mollayeva T , Gershon AS , Leung RS , Hawker G , Tomlinson G . Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review . Sleep Med Rev. 2014. ; 18 ( 1 ): 49 – 59 . [DOI] [PubMed] [Google Scholar]
- 10. Stelmach-Mardas M , Brajer-Luftmann B , Kuśnierczak M , Batura-Gabryel H , Piorunek T , Mardas M . Body mass index reduction and selected cardiometabolic risk factors in obstructive sleep apnea: meta-analysis . J Clin Med. 2021. ; 10 ( 7 ): 1485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Medicare and Medicaid Services . CPAP for Obstructive Sleep Apnea. https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/CPAP . Accessed January 17, 2022.
- 12. US Census Bureau . https://www.census.gov/newsroom/press-releases/2020/veterans-report.html . Accessed January 17, 2022.
- 13. National Institutes of Health . Aim for Healthy Weight. Key Recommendations - National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_tbl.htm . Accessed January 17, 2022.
- 14. Kuna ST , Reboussin DM , Strotmeyer ES , et al. Sleep AHEAD Research Subgroup of the Look AHEAD Research Group . Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD Study . Am J Respir Crit Care Med. 2021. ; 203 ( 2 ): 221 – 229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kline CE , Burke LE , Sereika SM , et al . Bidirectional relationships between weight change and sleep apnea in a behavioral weight loss intervention . Mayo Clin Proc. 2018. ; 93 ( 9 ): 1290 – 1298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashrafian H , Toma T , Rowland SP , et al . Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses . Obes Surg. 2015. ; 25 ( 7 ): 1239 – 1250 . [DOI] [PubMed] [Google Scholar]
- 17. Tuomilehto HP , Seppä JM , Partinen MM , et al. Kuopio Sleep Apnea Group . Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea . Am J Respir Crit Care Med. 2009. ; 179 ( 4 ): 320 – 327 . [DOI] [PubMed] [Google Scholar]
- 18. Greenburg DL , Lettieri CJ , Eliasson AH . Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis . Am J Med. 2009. ; 122 ( 6 ): 535 – 542 . [DOI] [PubMed] [Google Scholar]
- 19. Quintas-Neves M , Preto J , Drummond M . Assessment of bariatric surgery efficacy on obstructive sleep apnea (OSA) . Rev Port Pneumol. 2016. ; 22 ( 6 ): 331 – 336 . [DOI] [PubMed] [Google Scholar]
- 20. Lins-Filho O , Porto Aguiar JL , Vieira de Almeida JR , et al . Effect of exercise training on body composition in patients with obstructive sleep apnea: a systematic review and meta-analysis . Sleep Med. 2021. ; 87 : 105 – 113 . [DOI] [PubMed] [Google Scholar]
- 21. Peppard PE , Young T , Palta M , Dempsey J , Skatrud J . Longitudinal study of moderate weight change and sleep-disordered breathing . JAMA. 2000. ; 284 ( 23 ): 3015 – 3021 . [DOI] [PubMed] [Google Scholar]
- 22. Rose S , Pretto J , Paul C , Emmett B , Hensley M , Henskens F . Relationships between nutritional knowledge, obesity, and sleep disorder severity . J Sleep Res. 2016. ; 25 ( 3 ): 350 – 355 . [DOI] [PubMed] [Google Scholar]
- 23. Camacho M , Riaz M , Tahoori A , Certal V , Kushida CA . Mathematical equations to predict positive airway pressures for obstructive sleep apnea: a systematic review . Sleep Disord. 2015. ; 2015 : 293868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y , Wang Y . Obstructive sleep apnea-hypopnea syndrome as a novel potential risk for aging . Aging Dis. 2021. ; 12 ( 2 ): 586 – 596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garvey WT , Mechanick JI , Brett EM , et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines . American Association of Clinical Endocrinologist and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity . Endocr Pract. 2016. ; 22 ( Suppl 3 ): 1 – 203 . [DOI] [PubMed] [Google Scholar]
- 26. Young T , Shahar E , Nieto FJ , et al. Sleep Heart Health Study Research Group . Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study . Arch Intern Med. 2002b. ; 162 ( 8 ): 893 – 900 . [DOI] [PubMed] [Google Scholar]
- 27. Popko K , Gorska E , Wasik M , et al . Frequency of distribution of leptin gene polymorphism in obstructive sleep apnea patients . J Physiol Pharmacol. 2007. ; 58 ( Suppl 5, Pt 2 ): 551 – 561 . [PubMed] [Google Scholar]
- 28. Cowan DC , Livingston E . Obstructive sleep apnoea syndrome and weight loss: review . Sleep Disord. 2012. ; 2012 : 163296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel SR , Larkin EK , Redline S . Shared genetic basis for obstructive sleep apnea and adiposity measures . Int J Obes Lond. 2008. ; 32 ( 5 ): 795 – 800 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newman AB , Foster G , Givelber R , Nieto FJ , Redline S , Young T . Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study . Arch Intern Med. 2005. ; 165 ( 20 ): 2408 – 2413 . [DOI] [PubMed] [Google Scholar]
- 31. Young T , Peppard PE , Taheri S . Excess weight and sleep-disordered breathing . J Appl Physiol. (1985). 2005. ; 99 ( 4 ): 1592 – 1599 . [DOI] [PubMed] [Google Scholar]
- 32. Saunders KH , Igel LI , Tchang BG . Surgical and nonsurgical weight loss for patients with obstructive sleep apnea . Otolaryngol Clin North Am. 2020. ; 53 ( 3 ): 409 – 420 . [DOI] [PubMed] [Google Scholar]