Abstract
Study Objectives:
Trauma associated sleep disorder is a proposed parasomnia that develops after trauma with clinical features of trauma related nightmares, disruptive nocturnal behaviors, and autonomic disturbances. The purpose of this case series is to better characterize the clinical and video-polysomnographic features of patients meeting clinical criteria for this proposed parasomnia.
Methods:
Semistructured clinical interview and detailed video-polysomnography review of 40 patients. Movements and vocalizations in rapid eye movement sleep were quantified according to the rapid eye movement sleep behavior disorder severity scale.
Results:
Patients (n = 40, 32 males) were service members and veterans with a median age of 38.9 years (range 24–57 years) who reported trauma related nightmares and disruptive nocturnal behaviors at home. On video-polysomnography, 28 (71.8%) patients had disruptive nocturnal behaviors in rapid eye movement sleep consisting of limb, head, and axial movements; vocalizations were present in 8 (20%). On the rapid eye movement sleep behavior disorder severity scale, most (n = 28, 71.8%) had a low rating but those with greater severity (n = 11, 28.2%) had a higher prevalence of posttraumatic stress disorder (P = .013) and markedly less N3 sleep (P = .002). The cohort had a high rate of insomnia (n = 35, 87.5%) and obstructive sleep apnea (n = 19, 47.5%). Most patients were treated with prazosin (n = 29, 72.5%) with concomitant behavioral health interventions (n = 25, 64.1%); 15 (51.7%) patients receiving prazosin reported improved symptomatology.
Conclusions:
Disruptive nocturnal behaviors can be captured on video-polysomnography during rapid eye movement sleep, although they may be less pronounced than what patients report in their habitual sleeping environment. Clinical and video-polysomnographic correlations are invaluable in assessing patients with trauma associated sleep disorder to document objective abnormalities. This case series provides a further basis for establishing trauma associated sleep disorder as a unique parasomnia.
Citation:
Brock MS, Matsangas P, Creamer JL, et al. Clinical and polysomnographic features of trauma associated sleep disorder. J Clin Sleep Med. 2022;18(12):2775–2784.
Keywords: trauma associated sleep disorder, trauma related nightmares, disruptive nocturnal behaviors, nightmares, parasomnia, military, veterans, combat, dream enactment behavior
BRIEF SUMMARY
Current Knowledge/Study Rationale: Disruptive nocturnal behaviors are frequently reported by patients suffering with posttraumatic nightmares. This constellation of nocturnal symptoms is rarely captured in the sleep lab and lacks an established diagnosis.
Study Impact: Disruptive nocturnal behaviors including movements and vocalizations are frequently present on video-polysomnography during rapid eye movement sleep in trauma survivors reporting nightmares and these behaviors in their habitual sleeping environment. This case series provides additional evidence for establishing trauma associated sleep disorder as a unique parasomnia.
INTRODUCTION
Trauma associated sleep disorder (TSD) is a recently proposed parasomnia that develops after a traumatic experience.1 The first reports of this proposed parasomnia were in young active duty military personnel2 who presented with trauma related nightmares (TRN), disruptive nocturnal behaviors (DNB) to include dream enactment behavior (DEB), and autonomic hyperactivity primarily after combat-related trauma. More recently, there have been reports in older veterans with likely TSD,3–5 providing a basis that this is not a transient phenomenon, but likely a chronic sleep disorder. While the veterans were older than previous reports in military personnel, they were in their fifth decade, which would be relatively early onset for idiopathic rapid eye movement (REM) sleep behavior disorder (RBD).6
The currently proposed diagnostic criteria for TSD are primarily clinical in nature and rely on symptoms that are self-reported or provided by a bed partner (Table 1).7 The onset of symptoms after trauma helps to distinguish TSD from RBD, noting RBD is not reported to develop after traumatic exposure.6 Additionally, while DNB are present in both TSD and RBD, RBD lacks the self-reported symptoms and video-polysomnography (vPSG) documentation of autonomic hyperactivity (ie tachycardia, tachypnea, diaphoresis) that patients with TSD report.8 The other disorder that has been suggested may encompass TSD symptomatology is posttraumatic stress disorder (PTSD).9 It is well established that the primary nocturnal manifestation of PTSD is nightmares10; however, when nightmares are associated with DNB to include dream enactment, this transcends the established diagnostic criteria for nightmare disorder and merits consideration for another diagnosis.8,11
Table 1.
Diagnostic criteria for trauma associated sleep disorder.
Criteria A-E must be met:
|
Notes
|
EEG = electroencephalogram; PSG = polysomnogram; TSD = trauma associated sleep disorder.
There are limited reports of the objective findings of DNB to include DEB and autonomic hyperactivity occurring during polysomnography (PSG).12–14 In older reports, DNB and DEB were primarily reported in REM sleep along with elevated electromyography tone in REM sleep, but TRN were also documented in non-REM (NREM). In our initial report of TSD, we described a young soldier with vocalizations, tachycardia, tachypnea, and movements captured in REM sleep.2 The other 3 patients did not have these findings, although 1 had an elevated “any” electromyography activity index. A subsequent report by Feemster et al4 described a combat veteran with nightmares and DEB that mirrored his combat exposure. During this patient’s PSG, REM sleep without atonia (RSWA) was present, but detailed analysis found his RSWA was consistent with a medication effect (ie, selective serotonin reuptake inhibitors). In a series of 394 veterans (mean age 54.4 years) evaluated for parasomnias, Elliot et al3 reported 37% had DEB and 9% had DEB and RSWA, which were classified as RBD. In the RBD group, 9 of the 34 patients met proposed criteria for TSD but lacked abnormal movements or objective autonomic findings on PSG.
Symptoms consistent with TSD are not uncommon in military personnel or veterans with combat exposure.3 However, capture of DNB in the laboratory setting has been rare to date.15 In this case series we describe the clinical and vPSG characteristics of a cohort of 40 active duty military personnel and veterans with self-reported TRN, DNB, and autonomic hyperactivity who underwent a comprehensive sleep medicine evaluation at our sleep disorders center. The primary purpose of this case series was to better characterize the DNB that occur during vPSG in patients suspected of having TSD.
METHODS
Patients who presented to an academic military sleep disorders center from December 2015 to June 2019 for evaluation of sleep disturbances consisting of TRN (ie, replicative or nonreplicative nightmare of a traumatic experience), DNB (vocalizations or abnormal movements reported by the patient or bed partner), and symptoms of autonomic hyperarousal (ie, tachycardia, tachypnea, or diaphoresis reported after awakening from a TRN and/or DNB) developing after a traumatic event were included. All patients underwent a semistructured clinical interview and physical examination. In patients who had a bed partner and agreed, the onset of and nature of the patient’s nightmares and DNB were further elucidated. Approval for this research was obtained from the Wilford Hall Ambulatory Surgical Center institutional review board.
Clinical measures
As part of the evaluation, patients completed several clinical instruments. The Epworth Sleepiness Scale was used to determine their daytime sleepiness.16 Participants rate the likelihood that they will doze or fall asleep on a 4-point scale (0 = would never doze to 3 = high chance of dozing) across 8 situations, with a score ≥ 10 consistent with excessive daytime sleepiness. The Insomnia Severity Index is a 7-item self-report measure that was used to evaluate for insomnia. The items of the Insomnia Severity Index are summed to produce a total score (range 0–28), with a total score ≥ 15 consistent with clinically significant insomnia.17 The Pittsburgh Sleep Quality Index (PSQI) was used to determine sleep quality during the previous month.18 The PSQI consists of open-ended items (eg, during the past month, when have you usually gone to bed at night?) and 14 Likert items rated on a 4-point scale. A global sleep quality index is derived from 7 components to include self-reported sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. A score of ≥ 5 is consistent with poor quality sleep. The PSQI-Addendum (PSQI-A) was used to evaluate for TRN and DNB.19 The PSQI-A was specifically used to quantify how often during the previous month patients had memories or nightmares of a traumatic experience, episodes of terror or screaming during sleep without fully awakening, and episodes of “acting out” their dreams such as kicking, punching, running, or screaming. To characterize the patient’s nightmare content and DNB, a semistructured interview was performed. The interview included determining if the nightmare was combat or noncombat related and whether specific content from the nightmare could be recalled. Regarding DNB, the presence or absence of DNB was ascertained; if DNB were present, the patient’s movements were characterized as well as if any injuries occurred to the patient or their bed partner. Patients were also queried about symptoms of autonomic hyperactivity (ie, night sweats, rapid breathing, racing heart) related to their DNB and nightmares. As previously noted, when allowed by the patient, these questions were discussed with their bed partner.
Video-polysomnography
As part of their evaluation, all patients had a level 1 attended in-lab diagnostic vPSG performed in accordance with American Academy of Sleep Medicine (AASM) standards within an AASM accredited lab (Sleepware G3 version 3.9.0; Philips Respironics, Murrysville, PA). Six patients had a second PSG performed on positive airway pressure (PAP) therapy that was used for review because the presence of moderate-severe sleep-disordered breathing on their initial diagnostic vPSG made interpretation of movements in sleep difficult. Studies were scored utilizing the 2012 AASM scoring guidelines.
All patients had a detailed vPSG review performed (M.B. or V.M.) to evaluate for the presence or absence of abnormal movements in sleep to include vocalizations and DNB. While the entirety of the PSG was reviewed, all patients had their movements and vocalizations in REM sleep quantified according to the REM sleep behavior disorder severity scale (RBDSS), a reliable instrument with scores ranging from 0 (no movements or vocalization) to 4 (major body movements and vocalization) to evaluate motor events in REM.20 Patients additionally had their periods of REM sleep evaluated for evidence of autonomic nervous system activation. PSG epochs were analyzed for ≥ 10 beats per minute increase in heart rate occurring during a REM epoch when movements and/or vocalizations were present and without an otherwise apparent etiology (ie, not associated with apnea, hypopnea, or limb movements suggestive of periodic limb movement disorder).
Sleep and comorbid diagnoses
Patients were evaluated for insomnia and obstructive sleep apnea (OSA) in our sleep disorders center in accordance with International Classification of Sleep Disorders third edition criteria.8 For the diagnosis of insomnia, patients were required to have difficulty falling asleep, staying asleep, or awakening too early for at least 3 nights per week for 3 months with associated daytime impairment. Furthermore, their insomnia symptoms could not be better explained by a co-occurring sleep, medical, or psychiatric disorder. OSA was diagnosed in patients who had an apnea-hypopnea index (AHI) ≥ 5 events/h and associated symptoms and/or associated medical or mood disorder. The co-occurring illnesses of PTSD, anxiety, depression, and traumatic brain injury were ascertained if these diagnoses were present in the patient’s medical record.
Statistical analysis
First, all variables underwent descriptive analysis. Next, patients were classified in 2 groups based on their RBDSS rating, the group with low ratings (0, 1, 2) and the group with high ratings (3, 4). The 2 groups were compared in terms of their characteristics.
Statistical analysis was conducted with JMP Pro 15 software package (SAS Institute; Cary, NC). Data normality was assessed with the Shapiro-Wilk test. Normally distributed continuous data were presented as mean ± standard deviation, whereas nonnormally distributed data were presented as median (interquartile range). Categorical variables were presented as percentages with the number of patients in parentheses.
Pairwise comparisons between continuous variables were based on t-test and Wilcoxon rank sum test, whereas comparisons between proportions were based on Fisher’s exact test. An α level of .05 was used to determine statistical significance. Accounting for family-wise error, post hoc statistical significance was assessed by the Benjamini–Hochberg false discovery rate controlling procedure with q = 0.20 (Benjamini & Hochberg, 1995).
RESULTS
Patients were predominantly men (n = 32, 80.0%) between 24 and 57 years of age (38.9 ± 7.35). All patients were active duty military or veterans at the time of their evaluation, and all but 1 (n = 39, 97.5%) had deployment experience. Four major branches of service were represented, with the majority from the US Army (n = 20) and US Air Force (n = 16), followed by the US Navy (n = 3) and US Marine Corps (n = 1). Overall, the cohort was characterized by a high rate of insomnia (n = 35, 87.5%) and OSA (n = 19, 47.5%; 10 patients had mild OSA (AHI 5–15 events/h), 8 had moderate OSA (AHI 15–30 events/h), 1 had severe OSA (AHI > 30 events/h)). The demographic characteristics and PSG variables are shown in Table 1. Within our cohort, the following co-occurring illnesses were present: PTSD (n = 26, 65.0%), anxiety disorder (n = 25, 62.5%), depression (n = 20, 50.0%), and traumatic brain injury (n = 13, 32.5%). Sleep aids (sedative hypnotics and/or sedating antidepressants such as trazodone) were used by 25 (64.1%) of the patients and antidepressants (selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants) by 24 (60.0%) patients. Regarding the potential confounders of antidepressant use and OSA that may contribute to movements during REM sleep or RSWA, of the 24 patients taking antidepressants, almost half (n = 11, 27.5% of the cohort) had a diagnosis of OSA, whereas 13 (32.5%) did not have OSA. Eight (20%) patients had OSA and were not taking any antidepressants and 8 (20%) patients did not have OSA or antidepressant use.
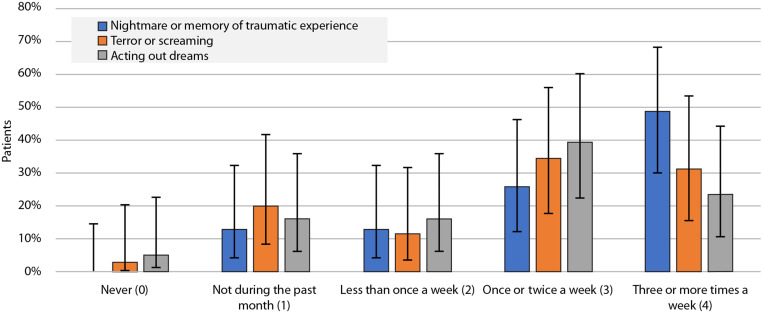
Regarding their nocturnal sleep disturbances during the previous month as evaluated by questions from the PSQI-A, 85% of the patients reported having trouble sleeping due to a bad dream once/twice per week or more often. In the same timeframe, ie, during the previous month, ∼74% of the patients reported having had memories or nightmares of a traumatic experience, ∼66% endorsed episodes of terror or screaming during sleep without fully awakening, and ∼63% reported episodes of “acting out” their dreams such as kicking, punching, running, or screaming. These results are shown in Figure 1. A diagnosis of OSA was not associated with ratings in severity of having bad dreams (Fisher’s exact test, P = .778), nightmares or memories of a traumatic experience (Fisher’s exact test, P = .899), terror or screaming ratings (Fisher’s exact test, P = .134), or acting out dreams (Fisher’s exact test, P = .738).
Figure 1. Responses to the Pittsburgh Sleep Quality Index-Addendum (PSQI-A) questions.
Patient responses to the PSQI-A questions “During the past month, how often … (can be based on report of roommate or bed partner): …have you had memories or nightmares of a traumatic experience/…have you had episodes of terror or screaming during sleep without fully awakening/…have you had episodes of ‘acting out’ your dreams such as kicking, punching, running, or screaming.” Vertical bars denote the standard error.
Overall, PSG characteristics (Table 2) aggregated for the cohort were notable for reduced total sleep time (345 ± 47.0 minutes), decreased sleep efficiency (87.9% [15.3%]), increased wake after sleep onset (33.0 minutes [58.4]), an increased arousal index (18.8 events/h [16.5]), slightly reduced REM sleep (19.6% [7.63]), and normal AHI (4.70 events/h [12.7]), and periodic limb movement index (1.30 events/h [14.7]). Ten patients (25%) had a periodic limb movement index > 15 events/h. No patients had vocalizations, somnambulism, or abnormal body movements during NREM sleep. The majority of patients (n = 33, 82.5%) met criteria for RSWA; of those with RSWA, 28 (71.8%) had DNB present on vPSG review ranging from limb and head movements to axial movements. Vocalizations were present in 8 (20%) patients during REM sleep. An increase in heart rate of ≥ 10 beats per minute during an epoch of REM when movements and/or vocalizations were present and without an otherwise apparent etiology was present in half (n = 20) of the patients.
Table 2.
Sample demographic characteristics and polysomnographic variables.
| Entire sample (n = 40) | |
|---|---|
| Demographics | |
| Age in years, M ± SD | 38.9 ± 7.35 |
| Males, % (No.) | 80.0% (32) |
| BMI in kg/m2, M ± SD | 29.0 ± 3.58 |
| Previously deployed, % (No.) | 97.5% (39) |
| Medication usage: % (No.) | |
| Sleep aid | 64.1% (25) |
| Antidepressant | 60.0% (24) |
| Prazosin | 72.5% (29) |
| Self-reported measures | |
| Sleep duration in hours, M ± SD | 4.60 ± 1.26 |
| ESS, M ± SD | 12.1 ± 5.11 |
| Excessive daytime sleepiness, % (No.) | 67.5% (27) |
| ISI, MD (IQR) | 22.5 (7.50) |
| PSQI, M ± SD | 14.7 ± 4.05 |
| Poor sleepers, % (No.) | 100% (39 – one missing value) |
| Primary sleep diagnoses: % (No.) | |
| Insomnia (ISI score ≥ 15) | 87.5% (35) |
| OSA (AHI ≥ 5) | 47.5% (19) |
| Associated illnesses: % (No.) | |
| Anxiety | 62.5% (25) |
| Depression | 50.0% (20) |
| PTSD | 65.0% (26) |
| TBI | 32.5% (13) |
| PSG variables | |
| TST (min), M ± SD | 345 ± 47.0 |
| SE (%), MD (IQR) | 87.9 (15.3) |
| SOL (min), MD (IQR) | 12.6 (20.3) |
| WASO (min), MD (IQR) | 33.0 (58.4) |
| REM latency (min), MD (IQR) | 104 (74.6) |
| N1 (%), MD (IQR) | 8.90 (8.00) |
| N2 (%), M ± SD | 55.4 ± 11.8 |
| N3 (%), MD (IQR) | 11.6 (14.7) |
| REM (%), MD (IQR) | 19.6 (7.63) |
| AHI (events/h), MD (IQR) | 4.70 (12.7) |
| Arousal index (events/h), MD (IQR) | 18.8 (16.5) |
| Lowest oxygen desaturation (%), MD (IQR) | 89.0 (6.00) |
| PLMS Index, MD (IQR) | 1.30 (14.7) |
AHI = apnea-hypopnea index, BMI = body mass index, desaturation = minimum recorded oximetry value during sleep, ESS = Epworth Sleepiness Scale, ISI = Insomnia Severity Index, M ± SD = mean ± standard deviation, MD (IQR) = median (interquartile range), OSA = obstructive sleep apnea, PLMS = periodic limb movements of sleep, PSG = polysomnogram, PSQI = Pittsburgh Sleep Quality Index, PTSD = posttraumatic stress disorder, REM = rapid eye movement, SE = sleep efficiency, SOL = sleep onset latency, TBI = traumatic brain injury, TST = total sleep time, WASO = wakefulness after sleep onset.
Next, we classified patients in 2 groups based on their RBDSS rating, the group with low scores (0, 1, 2) and the group with high scores (3, 4). Comparisons between groups were based on 39 patients because 1 patient could not be classified because video was unavailable for this PSG. Two patients with vocalizations were in the low RBDSS scores group. Compared to the low scores group (n = 28, 71.8%), the group with high RBDSS scores (n = 11, 28.2%) had a higher prevalence of PTSD (P = .013) and significantly less N3 sleep (P = .002). Also, the patients in the group with high RBDSS scores spent more time in N1 sleep stage (P = .059, effect size r = .302). In terms of medication use, the 2 groups were equivalent in the use of sleep aids and antidepressants (Fisher’s exact test; all P > .471). Detailed results are shown in Table 3. An epoch of sleep from a patient with an RBDSS score of 4 is presented in Figure 2.
Table 3.
Demographic characteristics and polysomnographic variables by RBDSS group.
| Patients with DNB Ratings 0 + 1+2 (n = 28) | Patients with DNB Ratings 3 + 4 (n = 11) | P a | Effect Size | |
|---|---|---|---|---|
| Demographics | ||||
| Age in years, M ± SD | 37.8 ± 7.39 | 40.8 ± 6.78 | 0.240b | 0.415h |
| Males, % (No.) | 82.1% (23) | 72.7% (8) | 0.663d | 0.89 (0.59–1.32)f |
| BMI in kg/m2, M ± SD | 29.1 ± 3.19 | 28.3 ± 4.40 | 0.560b | 0.225h |
| Medication usage: % (No.) | ||||
| Sleep aid | 60.7% (17) | 70.0% (7) | 0.715d | 1.15 (0.70–1.91)f |
| Antidepressant | 53.6% (15) | 72.7% (8) | 0.471d | 1.36 (0.82–2.24)f |
| Prazosin | 71.4% (20) | 72.7% (8) | 0.999d | 1.02 (0.66–1.57)f |
| Self-reported measures | ||||
| PSQI, M ± SD | 14.3 ± 3.87 | 15.5 ± 4.53 | 0.453b | 0.295h |
| ESS, M ± SD | 11.8 ± 5.40 | 13.2 ± 4.38 | 0.423b | 0.556h |
| ISI, MD (IQR) | 20.0 (8.00) | 23.0 (6.00) | 0.150c | 0.231g |
| Primary sleep diagnoses: % (No.) | ||||
| Insomnia (ISI score ≥ 15) | 82.1% (23) | 100% (11) | 0.296d | 1.22 (1.02–1.45)f |
| OSA (AHI ≥ 5 events/h) | 53.6% (15) | 27.3% (3) | 0.171d | 0.51 (0.18–1.42)f |
| Associated illnesses: % (No.) | ||||
| Anxiety | 53.6% (15) | 81.8% (9) | 0.150d | 1.53 (0.98–2.38)f |
| Depression | 42.9% (12) | 72.7% (8) | 0.155d | 1.70 (0.97–2.97)f |
| PTSD | 50.0% (13) | 100% (9) | 0.003d,e | 2.00 (1.38–2.90)f |
| TBI | 28.6% (8) | 36.4% (4) | 0.709d | 1.27 (0.48–3.38)f |
| PSG variables | ||||
| TST (min), M ± SD | 351 ± 43.6 | 333 ± 56.1 | 0.343b | 0.389h |
| SE (%), MD (IQR) | 89.3 (13.5) | 79.4 (18.2) | 0.138c | 0.237g |
| SOL (min), MD (IQR) | 6.86 (18.5) | 13.4 ± 26.6 | 0.224c | 0.192g |
| WASO (min), MD (IQR) | 31.3 (46.3) | 75.5 (94.7) | 0.130c | 0.242g |
| REM latency (min), MD (IQR) | 104 (55.8) | 82.0 (78.5) | 0.674c | 0.067g |
| N1 (%), MD (IQR) | 7.50 (5.98) | 13.0 (18.8) | 0.059c | 0.302g |
| N2 (%), M ± SD | 55.2 ± 12.2 | 54.8 ± 15.4 | 0.931b | 0.033h |
| N3 (%), MD (IQR) | 13.8 (16.2) | 1.00 (11.4) | 0.002c,e | 0.489g |
| REM (%), MD (IQR) | 19.5 (6.80) | 21.0 (17.3) | 0.374c | 0.142g |
| AHI (events/h), MD (IQR) | 5.20 (11.3) | 3.10 (21.9) | 0.325c | 0.157g |
| Arousal index (events/h), MD (IQR) | 17.1 (13.5) | 25.0 (19.8) | 0.206c | 0.202g |
| Lowest oxygen desaturation (%), MD (IQR) | 89.5 (5.00) | 89.0 (19.8) | 0.950c | 0.010g |
| PLMS Index, MD (IQR) | 1.90 (25.7) | 1.20 (3.80) | 0.450c | 0.124g |
aUnadjusted P. bComparisons based on t-test for groups with unequal sample sizes. cComparisons based on Wilcoxon Rank Sum test. dComparisons based on Fisher’s exact test. eStatistically significant according to post hoc analysis with the Benjamini–Hochberg false discovery rate controlling procedure. fRelative risk (95% confidence interval) for patients with DNB ratings of 3 and 4. gEffect size r. hEffect size Hedges g. AHI = apnea-hypopnea index, BMI = body mass index, desaturation = minimum recorded oximetry value during sleep, ESS = Epworth Sleepiness Scale, ISI = Insomnia Severity Index, M ± SD = mean ± standard deviation, MD (IQR) = median (interquartile range), OSA = obstructive sleep apnea, PLMS = periodic limb movements of sleep, PSG = polysomnogram, PSQI = Pittsburgh Sleep Quality Index, PTSD = posttraumatic stress disorder, REM = rapid eye movement, SE = sleep efficiency, SOL = sleep onset latency, TBI = traumatic brain injury, TST = total sleep time, WASO = wakefulness after sleep onset.
Figure 2. PSG epoch depicting disruptive nocturnal behaviors (DNB) in patient with trauma related nightmares.
A 30-second epoch of REM sleep in a 40-year-old woman who presented with nightmares related to combat and nightly DNB. She reported nocturnal sweating, tachycardia, and hyperventilation and her spouse substantiated her DNB to include jolting movements, sitting up in bed and kicking, along with vocalizations. The epoch is unambiguous REM sleep and there is increased electromyography tone during the mid and latter half of the epoch corresponding with semipurposeful thoracic movements and moaning. REM = rapid eye movement.
The nightmare content reported was mostly related to combat (n = 35, 87.5%), although 5 patients reported noncombat themes. Of those with combat-related nightmares, 21 (60.0%) relayed specific details of the nightmare content, whereas 12 (34.3%) described general combat themes without specific detail. Two (5.7%) patients declined to describe their nightmares but acknowledged they were related to combat. Two primary themes were present in the patient’s nightmares that consisted of being exposed to rocket attacks or gunfire or seeing human remains to include body parts, dead comrades or combatants, or mutilated or burnt bodies.
DNB were reported to occur at home in all 40 (100%) patients. The DNB were primarily descriptions reported to the patient by their bed partner. These DNB included punching, kicking, thrashing, and jumping or running out of bed. In 28 (70%) patients, the descriptions of the DNB were related to the trauma unfolding in their dream, including performing cardiopulmonary resuscitation, running out of bed screaming, and jumping from a supine position to a kneeling posture “ready to pounce”. Vocalizations ranging from mumbling to screaming obscenities and more specific commands such as “get down” were reported by 16 (40%) patients. Injuries to either the patient or the bed partner occurred in 9 (22.5%) cases. The majority of the injuries were minor and reported as punching or kicking the bed partner; however, 2 patients had lacerations and 1 had multiple injuries from running into a wall. Illustrative TRN content and associated DNB from six patients are presented in Table 4.
For treatment, 29 patients (72.5%) were prescribed prazosin after their PSG for their TSD symptoms with doses ranging from 1 mg to 8 mg depending on efficacy and tolerance. Of these, 15 (51.7%) reported reduction in nightmare and DNB frequency and/or nightmare and DNB severity, 3 (10.3%) reported no improvement before discontinuing therapy, 4 (13.8%) discontinued therapy due to side effects, and 8 (27.6%) had an unknown response as they could not be reached for follow up. Notably, 25 (62.5%) patients were concomitantly receiving behavioral health therapy, including cognitive behavioral therapy for insomnia (CBT-I) and imagery rehearsal therapy for nightmares.
DISCUSSION
Video-polysomnographic features of TSD
Disruptive nocturnal behaviors
In this case series of 40 patients who presented with TRN and DNB we report findings of their comprehensive sleep evaluation. On detailed vPSG review, 8 patients had vocalizations and 28 had abnormal movements present during REM sleep with no abnormal movements or vocalizations noted in NREM sleep. Of those with abnormal movements, 11 had an RBDSS score of 3 or 4, which consisted of either proximal muscle movement (ie, punching or kicking) or axial movements (eg, thrashing) with or without a vocalization. While there have been previous reports of TRN in NREM sleep,13,14,21 these studies did not conduct video review of DNB and either did not evaluate sleep-disordered breathing or used nonstandard thresholds (ie, AHI > 10 events/h). Our findings suggest that TSD is likely a REM-related as opposed to NREM-related parasomnia and possibly a REM sleep arousal disorder.
Another important finding from this study is that DNB occurs relatively frequently in patients with TSD. Most prior studies, to include 2 recent reports, have not found DNB in patients with TRN and PTSD22 or likely TSD,3,4 consistent with the common perception that DNB in the lab are rare.10,22 There are a number of potential reasons for the high rate of DNB in our patients compared to previous studies, which include detailed vPSG review performed as part of this study; that movements occurring in the lab may be more subtle than what is described in the habitual sleeping environment; and treating underlying OSA with PAP therapy during a second PSG, if indicated, aids in excluding movements secondary to sleep-disordered breathing. Less pronounced movements, which were present in the majority of patients, included limb and head jerks with or without vocalizations, which is similar to what has also been reported in patients with RBD.23 As opposed to the proximal or axial movements with vocalizations, which are unequivocal DNB, these less pronounced movements in REM sleep, which are abnormal, may not necessarily have been viewed as DNB in previous studies.
Reduced N3 sleep
The most notable PSG finding in our cohort was the markedly decreased percentage of N3 sleep in patients with TSD and high RBDSS scores. While medications and depression can impact sleep architecture, there was no significant difference in these factors between the low and high RBDSS scores groups. Decreased N3 or slow wave sleep is a recognized sleep architectural abnormality in patients with PTSD and the reduced N3 correlates with increased severity of PTSD24 as determined by validated scales. As the amount of N3 in the patients with TSD and high RBDSS scores was substantially lower than what is reported in patients with PTSD alone, this finding be may a polysomnographic marker to suggest the presence of this proposed parasomnia, specifically when TRN and DNB are present as opposed to nightmares alone.
Autonomic nervous system activation
Symptoms consistent with autonomic hyperactivity were reported by all patients. PSG findings of increased heart rate and/or tachypnea associated with observed disruptive nocturnal behaviors support the presence of REM-arousal behaviors with autonomic nervous system activation. In REM sleep, half of the patients had an increase in heart rate of ≥ 10 beats per minute associated with movements and/or vocalizations and without an otherwise apparent etiology. One patient had unequivocal PSG findings of tachycardia and tachypnea during REM sleep associated with a TRN as shown in Figure 3. Pronounced objective findings of autonomic hyperactivity are uncommon, noting 1 patient in the current cohort and 1 patient in our initial case series3 had this degree of both tachycardia and tachypnea (see figure 1 in Mysliwiec et al2). Our findings differ from that reported by Elliot et al3 who did not find any patients with objective autonomic hyperactivity in their large veteran cohort. A potential reason they did not find autonomic hyperactivity is the high rate of OSA in their cohort and absence of treatment for this disorder, whereby patients may have had tachycardia and/or tachypnea due to respiratory events. As epochs of sleep with pronounced autonomic hyperactivity are uncommon, future research utilizing more sensitive measures for determining the presence of autonomic hyperactivity are required, as this is a distinguishing feature of TSD compared to RBD and may support the classification of TSD as a distinct REM sleep arousal disorder.8
Figure 3. PSG capture of a trauma related nightmare.
The figure depicts 2 PSG epochs in a 24-year-old marine who presented for evaluation of trauma related nightmares (TRN). Upon clinical evaluation he reported a distinct onset of TRN after a deployment to Africa, where fellow marines were ambushed in a bazaar 5 minutes before he arrived. His nocturnal symptoms included sleeping sitting up in an attempt to lessen the nocturnal dyspnea associated with his nightly TRN. The findings of unexplained tachycardia and tachypnea in REM sleep were captured during his PSG. PSG = polysomnogram; REM = rapid eye movement.
Clinical features of TSD
Co-occurring medical and psychiatric conditions
The cohort of primarily young to middle-aged men with combat exposure was defined by a high rate of co-occurring illnesses, including PTSD, traumatic brain injury, as well as anxiety and depression. Unsurprisingly, the majority of patients reported taking an antidepressant. Antidepressants such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are associated with an increase in RSWA; however, these medications are not associated with an increased rate of RBD.25 Furthermore, antidepressant use did not differ between the low and high RBDSS patient groups. In our cohort, we did not include any patients who reported nightmares and DNB which developed after starting antidepressant therapy. This likely precludes a medication induced onset of TSD symptoms in patients in our cohort but does not exclude that antidepressants may have augmented symptoms, especially movements in REM sleep.
Co-occurring sleep disorders
Overall, our TSD cohort had disturbed sleep and nearly all of the patients also had insomnia, with self-reported sleep duration of 4.6 hours and reduced objective total sleep time of 345 minutes. Sleep apnea was diagnosed in 47.5%, with the majority having mild disease. While sleep-disordered breathing can be associated with DNB,26 we excluded movements in REM sleep from analysis if they were associated with a respiratory event. In the patients with confounding OSA, 6 required a second vPSG on PAP therapy to evaluate for the presence of abnormal movements in REM sleep. Furthermore, a second vPSG with positive airway pressure therapy in those with OSA aided in our evaluation of DNB. It is likely that comorbid sleep disorders, and most notably OSA, contributed to prior reports of TRN in NREM sleep.27 Earlier studies that reported TRN could occur in NREM sleep did not evaluate for OSA13,14; however, Phelps et al21 evaluated OSA and noted that the majority of TRN were associated with respiratory and/or leg movements. While we do not suggest that comorbid sleep disorders should be part of the diagnostic criteria for TSD, comorbid sleep disorders more than likely contributed to the persistence and/or worsening of this disorder, and treating sleep-disordered breathing aided in our evaluation of DNB. Regarding OSA, PAP therapy has been reported to decrease nightmares28,29 as well as DNB in patients with RBD.6 Similarly, CBT-I in patients with PTSD and insomnia can improve not only insomnia symptoms, but nightmares and DNB.30 In our cohort, all patients with OSA were prescribed PAP and 25 (62.5%) patients received either CBT-I and/or imagery rehearsal therapy for their TRN. It is likely that the combination of addressing these comorbid disorders along with pharmacologic therapy contributed to their self-reported improved symptoms.
Therapeutic considerations
The primary pharmacologic treatment received by patients was prazosin in doses ranging from 1 mg to 8 mg. While the majority of patients had improvement in their TSD symptoms, the ability to determine that the patients responded to this pharmacologic treatment alone vs some combination of their overall sleep management is limited as they received PAP therapy, CBT-I, or imagery rehearsal therapy as clinically indicated. While there has been a change in the clinical practice guidelines regarding the use of prazosin for the treatment of nightmare disorder,31 primarily based on the randomized clinical trial that showed no change in overall PTSD outcomes,32 this medication remains our primary therapy. It is our contention that PSG could identify patients with DNB and/or autonomic hyperactivity that may better respond to antiadrenergic therapy.5,33
Consideration of TSD as a distinct parasomnia
As a recently proposed parasomnia, there is some controversy about whether TSD is a distinct disorder given its overlap of features with RBD and PTSD.9,34 While these conditions share some commonalities, they are likely different in pathophysiology and underlying mechanisms.35 Compared to RBD, TSD patients are younger, have an inciting traumatic event, and a unique sleep phenotype as it relates dream mentation, DNB, and PSG findings. As opposed to the RSWA which characterizes RBD, patients with TSD appear to have distinct periods of movement in REM sleep that are more than likely associated with their TRNs. Another finding that distinguishes TSD is that these movements can have associated autonomic hyperactivity that is not seen with RBD. In terms of pathophysiology, RBD is an insidious disorder associated with neurodegeneration and α-synucleinopathies such as Parkinson’s disease. The current neurobiological hypothesis of TSD includes alterations in the central fear network structures including hyperactivity in the amgydala, locus ceruleus, and peri-locus ceruleus and increased neuroendocrine activity resulting in an overdrive phenomenon causing loss of both REM atonia and sympathetic suppression.1 Furthermore, RBD is typically treated with benzodiazepines or melatonin while TSD responds to prazosin and possibly behavioral health interventions and in our experience is relatively unresponsive to benzodiazepines.2
PTSD also occurs following trauma exposure but unlike TSD, the nightmares in PTSD do not include DNB, which is a defining feature of TSD. Also, TSD is likely a REM-related parasomnia with purely nocturnal symptoms, whereas PTSD is a behavioral health disorder with both nocturnal and diurnal symptoms. Lastly, although TSD may be comorbid with PTSD, this is not always the case, as with 35% of our cohort. Large-scale longitudinal studies are needed to assess long-term consequences of TSD that can further distinguish it from RBD and PTSD and determine if TSD is potentially associated with neurodegenerative disorders or adverse long-term health outcomes.
Study limitations
The primary limitation of this study is that all of the patients were from 1 academic military sleep facility, which limits the generalizability. More research is needed in patients who have trauma not related to combat. Another potential limitation is that we utilized the RBDSS to assess movements and vocalizations in our patient population. This metric was designed to quantify symptom severity in patients with RBD rather than TSD. Additionally, detailed RSWA analysis was not performed. We suspect that RSWA is present during the patient’s nightmare and therefore RSWA is focal and lesser in degree than in idiopathic RBD, but this contention remains unproven.11 Another study limitation is that we did not consistently assess for the self-report of nightmares during the PSG as performed by Phelps et al.21 Future research should focus on the many unknowns facing TSD including differences in TSD caused by combat vs noncombat trauma, sex differences in TSD symptomatology, and the development of additional therapies for TSD.
Summary
In summary, trauma associated sleep disorder has distinct clinical manifestations that can be present on vPSG. When present, the objective nocturnal findings provide an important context for understanding the frequent self-reported DNB in trauma survivors. Patients with TSD frequently have multiple comorbid sleep and behavioral medicine disorders, which makes diagnosis and treatment challenging. Further studies in military, veteran, and civilian populations are required to address this proposed novel parasomnia.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CBT-I
cognitive behavioral therapy for insomnia
- DEB
dream enactment behavior
- DNB
disruptive nocturnal behaviors
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- PSQI-A
Pittsburgh Sleep Quality Index-Addendum
- PTSD
posttraumatic stress disorder
- RBD
rapid eye movement sleep behavior disorder
- RBDSS
rapid eye movement sleep behavior disorder severity scale
- REM
rapid eye movement
- RSWA
rapid eye movement sleep without atonia
- TRN
trauma related nightmare
- TSD
trauma associated sleep disorder
- vPSG
video-polysomnography
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Wilford Hall Ambulatory Surgical Center, JBSA Lackland, TX. The views expressed are those of the presenters and do not reflect the official views or policy of the Department of Defense or its Components. There is no financial support to disclose. Dr. Mysliwiec works for NOCTEM Health and has served as a consultant for CPAP Medical, Bluegrass Oxygen, Sleep Care Inc., Ebb Therapeutics, Nightware and Jazz Pharmaceuticals. He has also received an honoraria from Springer Healthcare. The other authors report no conflicts of interest.
REFERENCES
- 1. Mysliwiec V , Brock MS , Creamer JL , O’Reilly BM , Germain A , Roth BJ . Trauma associated sleep disorder: a parasomnia induced by trauma . Sleep Med Rev. 2018. ; 37 : 94 – 104 . [DOI] [PubMed] [Google Scholar]
- 2. Mysliwiec V , O’Reilly B , Polchinski J , Kwon HP , Germain A , Roth BJ . Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors . J Clin Sleep Med. 2014. ; 10 ( 10 ): 1143 – 1148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elliott JE , Opel RA , Pleshakov D , et al . Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in Veterans with and without comorbid traumatic brain injury . Sleep. 2020. ; 43 ( 3 ): zsz237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feemster JC , Smith KL , McCarter SJ , St Louis EK . Trauma associated sleep disorder: a posttraumatic stress/REM sleep behavior disorder mash-up? J Clin Sleep Med. 2019. ; 15 ( 2 ): 345 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brock MS , Mysliwiec V , Shirley S . Severe Sleep Disruption in PTSD: Trauma Associated Sleep Disorder . In: Khawaja S , ed. Comorbid Sleep and Psychiatric Disorders. Cham, ZG, Switzerland: : Springer; ; 2019. : 123 – 135 . [Google Scholar]
- 6. Fernández-Arcos A , Iranzo A , Serradell M , Gaig C , Santamaria J . The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients . Sleep. 2016. ; 39 ( 1 ): 121 – 132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mysliwiec V , Brock MS . Time to recognize trauma associated sleep disorder as a distinct parasomnia . Sleep. 2020. ; 43 ( 3 ): zsaa019 . [DOI] [PubMed] [Google Scholar]
- 8. American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd ed. Darien, IL: : American Academy of Sleep Medicine; ; 2014. . [Google Scholar]
- 9. Rachakonda TD , Balba NM , Lim MM . Trauma associated sleep disturbances: a distinct sleep disorder? Curr Sleep Med Rep. 2018. ; 4 ( 2 ): 143 – 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Germain A . Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013. ; 170 ( 4 ): 372 – 382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brock MS , Powell TA , Creamer JL , Moore BA , Mysliwiec V . Trauma associated sleep disorder: clinical developments 5 years after discovery . Curr Psychiatry Rep. 2019. ; 21 ( 9 ): 80 . [DOI] [PubMed] [Google Scholar]
- 12. Schlosberg A , Benjamin M . Sleep patterns in three acute combat fatigue cases . J Clin Psychiatry. 1978. ; 39 ( 6 ): 546 – 549 . [PubMed] [Google Scholar]
- 13. van der Kolk B , Blitz R , Burr W , Sherry S , Hartmann E . Nightmares and trauma: a comparison of nightmares after combat with lifelong nightmares in veterans . Am J Psychiatry. 1984. ; 141 ( 2 ): 187 – 190 . [DOI] [PubMed] [Google Scholar]
- 14. Hefez A , Metz L , Lavie P . Long-term effects of extreme situational stress on sleep and dreaming . Am J Psychiatry. 1987. ; 144 ( 3 ): 344 – 347 . [DOI] [PubMed] [Google Scholar]
- 15. Jones MB , Jeevan S , Wang J , et al . Clinical correlates of dream enactment behaviors in previously deployed OEF/OIF/OND veterans: an exploratory analysis . J Neuropsychiatry Clin Neurosci. 2020. ; 32 ( 2 ): 147 – 153 . [DOI] [PubMed] [Google Scholar]
- 16. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 17. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buysse DJ , Reynolds CF 3rd , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 19. Germain A , Hall M , Krakow B , Katherine Shear M , Buysse DJ . A brief sleep scale for Posttraumatic Stress Disorder: Pittsburgh Sleep Quality Index Addendum for PTSD . J Anxiety Disord. 2005. ; 19 ( 2 ): 233 – 244 . [DOI] [PubMed] [Google Scholar]
- 20. Sixel-Döring F , Schweitzer M , Mollenhauer B , Trenkwalder C . Intraindividual variability of REM sleep behavior disorder in Parkinson’s disease: a comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine . J Clin Sleep Med. 2011. ; 7 ( 1 ): 75 – 80 . [PMC free article] [PubMed] [Google Scholar]
- 21. Phelps AJ , Kanaan RAA , Worsnop C , Redston S , Ralph N , Forbes D . An ambulatory polysomnography study of the posttraumatic nightmares of posttraumatic stress disorder . Sleep. 2018. ; 41 ( 1 ): zsx188 . [DOI] [PubMed] [Google Scholar]
- 22. Woodward SH , Arsenault NJ , Murray C , Bliwise DL . Laboratory sleep correlates of nightmare complaint in PTSD inpatients . Biol Psychiatry. 2000. ; 48 ( 11 ): 1081 – 1087 . [DOI] [PubMed] [Google Scholar]
- 23. Högl B , Stefani A . REM sleep behavior disorder (RBD): update on diagnosis and treatment . Somnologie (Berl). 2017. 21 ( S1 , Suppl 1 ): 1 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y , Ren R , Sanford LD , et al . Sleep in posttraumatic stress disorder: a systematic review and meta-analysis of polysomnographic findings . Sleep Med Rev. 2019. ; 48 : 101210 . [DOI] [PubMed] [Google Scholar]
- 25. Lee K , Baron K , Soca R , Attarian H . The prevalence and characteristics of REM sleep without atonia (RSWA) in patients taking antidepressants . J Clin Sleep Med. 2016. ; 12 ( 3 ): 351 – 355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iranzo A , Santamaría J . Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder . Sleep. 2005. ; 28 ( 2 ): 203 – 206 . [DOI] [PubMed] [Google Scholar]
- 27. Hicks RA , Bautista J . Snoring and nightmares . Percept Mot Skills. 1993. ; 77 ( 2 ): 433 – 434 . [DOI] [PubMed] [Google Scholar]
- 28. Tamanna S , Parker JD , Lyons J , Ullah MI . The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) . J Clin Sleep Med. 2014. ; 10 ( 6 ): 631 – 636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krakow B , Lowry C , Germain A , et al . A retrospective study on improvements in nightmares and posttraumatic stress disorder following treatment for co-morbid sleep-disordered breathing . J Psychosom Res. 2000. ; 49 ( 5 ): 291 – 298 . [DOI] [PubMed] [Google Scholar]
- 30. Talbot LS , Maguen S , Metzler TJ , et al . Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial . Sleep. 2014. ; 37 ( 2 ): 327 – 341 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morgenthaler TI , Auerbach S , Casey KR , et al . Position paper for the treatment of nightmare disorder in adults: an American Academy of Sleep Medicine Position Paper . J Clin Sleep Med. 2018. ; 14 ( 6 ): 1041 – 1055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raskind MA , Peskind ER , Chow B , et al . Trial of prazosin for posttraumatic stress disorder in military veterans . N Engl J Med. 2018. ; 378 ( 6 ): 507 – 517 . [DOI] [PubMed] [Google Scholar]
- 33. Mysliwiec V , Brock MS . Prazosin for posttraumatic stress disorder . N Engl J Med. 2018. ; 378 ( 17 ): 1649 . [DOI] [PubMed] [Google Scholar]
- 34. Mellman TA . A time to recognize trauma related sleep disorder or a time to pause and consider? Sleep. 2020. ; 43 ( 6 ): zsaa069 . [DOI] [PubMed] [Google Scholar]
- 35. Barone DA . Dream enactment behavior—a real nightmare: a review of PTSD, RBD, and trauma associated sleep disorder . J Clin Sleep Med. 2020. ; 16 ( 11 ): 1943 – 1948 . [DOI] [PMC free article] [PubMed] [Google Scholar]