Abstract
Study Objectives:
Evaluate per-patient diagnostic performance of a wireless dual-sensor system (ANNE sleep) compared with reference standard polysomnography (PSG) for the diagnosis of moderate and severe obstructive sleep apnea (OSA) with a minimum prespecified threshold of 80% for both sensitivity and specificity.
Methods:
A multicenter clinical trial was conducted to evaluate ANNE sleep vs PSG to diagnose moderate and severe OSA in individuals 22 years or older. For each testing approach, apnea-hypopnea index (AHI) was manually scored and averaged by 3 registered sleep technologists blinded to the other system. Average variations > 15% were adjudicated by a sleep medicine physician.
Results:
In a total of n = 225 participants (mean age 53 years, range 22–88 years), PSG diagnosed 30% (n = 68) of participants with moderate or severe OSA (AHI ≥ 15 events/h) compared to 29% (n = 65) diagnosed by ANNE sleep (P = .55). The sensitivity and specificity for ANNE sleep were 90% (95% confidence interval: 80–96%) and 98% (95% confidence interval: 94–99%), respectively. Strong correlation was shown in terms of final AHI (r = .93), with an average AHI bias of 0.5 (95% limits of agreement: –12.8 to 11.8). The majority of users noted comfort with using the ANNE sleep in the home setting. No adverse events were noted.
Conclusions:
Using PSG as the gold standard, ANNE sleep demonstrated high sensitivity and specificity for the diagnosis of moderate or severe OSA.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Comparative Study of the ANNE™ One System to Diagnose Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT04643782; Identifier: NCT04643782.
Citation:
Davies C, Lee JY, Walter J et al. A single-arm, open-label, multicenter, and comparative study of the ANNE sleep system vs polysomnography to diagnose obstructive sleep apnea. J Clin Sleep Med. 2022;18(12):2703–2712.
Keywords: obstructive sleep apnea, polysomnography, home sleep apnea testing, diagnostic testing, wireless sensors, flexible electronics, patient preferences
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is widely underdiagnosed and access to gold-standard diagnostic testing (polysomnography) is limited, expensive, and uncomfortable.
Study Impact: We performed a multicenter clinical trial to compare the diagnostic performance of a flexible wireless dual-sensor system to diagnose moderate and severe OSA compared with in-laboratory polysomnography in adults aged 22 years or older. Compared with polysomnography, ANNE sleep demonstrated 90% sensitivity, 98% specificity, and 95% accuracy for moderate and severe OSA determined by the apnea-hypopnea index.
INTRODUCTION
Of the estimated 24.4 million Americans who have obstructive sleep apnea (OSA), approximately 90% of cases remain undiagnosed.1–3 Untreated OSA contributes to a 2-fold increase in health care expenditures given its associated risks for hypertension, myocardial infarction, stroke, metabolic disorders, and motor vehicle accidents.4–7 Efforts to increase the accessibility and efficiency of diagnosis are fundamental to improved identification of patients with OSA.
Currently, polysomnography (PSG) performed at an accredited sleep center is the gold standard for OSA diagnosis. Although the number of these facilities is rising, geographic availability varies considerably, resulting in unequal access.8 The ongoing coronavirus disease 2019 (COVID-19) pandemic has also changed the landscape of sleep diagnostics.9 In an effort to mitigate risk of exposure for both patients and staff, sleep laboratories have reduced their capacity. Furthermore, a single night of PSG may be subject to a “first night effect” in which the unfamiliar environment and equipment reduce the quantity and quality of sleep, thereby contributing to the night-to-night variability in sleep-disordered breathing.10,11 Thus, there is a growing need for home-based options to minimize COVID-19 exposure, reduce cost and time to diagnosis, while accommodating patient preferences.
As an alternative to PSG, a home sleep apnea test (HSAT) is used for patients with a high pretest probability of OSA. While HSATs do not include electroencephalography, electrooculography, or electromyography sensors, all of which are required to define wake and sleep stages, the American Academy of Sleep Medicine (AASM) endorses HSAT use in selected, medically uncomplicated patients.12 Studies comparing the diagnosis of OSA in the home compared with an accredited sleep laboratory demonstrated minimal differences in subsequent outcomes or treatment adherence when used in the appropriate patient population.13,14 Although HSATs offer a home-based alternative to PSG, there are still limitations of these systems. Type 3 and type 4 HSATs are often associated with underestimation of disease, given the use of total recording time, rather than total sleep time, of a study to determine the apnea-hypopnea index (AHI), leading to an estimated false-negative rate of 13–20%, with particularly poor discrimination of mild to moderate disease.15,16 Additionally, home studies have higher failure rates compared with PSG, lack real-time feedback of test adequacy, and have their own “first night” effect.17–19 A wrist-mounted device (WatchPAT; Itamar Medical Ltd, Caesarea, Israel) worn overnight at home has increasingly been studied as another technology to perform a sleep test in the home.20 The WatchPAT system is intended for single use and subsequent disposal. Some studies have demonstrated that WatchPAT may overestimate respiratory disturbances, given potential difficulties in distinguishing respiratory arousals from spontaneous arousals or periodic limb movements.21 Additional concerns include high cost and dependency on automated scoring algorithms that make the raw signal difficult to interpret by physicians.21
Given these limitations, there is a need for well-validated, home-based diagnostic systems for OSA with greater usability, comfort, affordability, and comparable accuracy to PSG. Furthermore, a system capable of real-time assessment of study adequacy paired with the capacity to reuse and recharge for multiple nights of testing would be valuable. Recent advances in soft, flexible electronics have enabled a wide range of biomedical applications including intensive care unit–grade monitoring in the home.22–24 The primary objective of this study was to validate a new flexible wireless dual sensor system (ANNE Sleep; Sibel Health, Niles Illinois) mounted on the chest and finger for the diagnosis of moderate and severe OSA compared with the reference standard, PSG, in the laboratory setting.
METHODS
Pivotal trial: ANNE sleep vs PSG
We performed a multicenter clinical trial to evaluate the accuracy, sensitivity, and specificity of the ANNE sleep system as a diagnostic tool for moderate and severe OSA in adults compared with PSG. Individuals at least 22 years old with either suspected OSA (based on history or physical examination), or prescribed either PSG or HSAT study by a health care provider, were eligible. Consenting individuals provided demographics and medical history and completed a sleep survey to ensure eligibility. Individuals were excluded if they had a medical condition posing a health risk related to trial participation or interfering with trial completion, such as oxygen dependence, respiratory muscle weakness secondary to neuromuscular disease, awake or sleep-related hypoventilation, chronic opioid use, dementia, severe insomnia, inability to follow instructions, severe skin abnormalities, implanted pacemakers or defibrillators, stroke, or pregnancy.
Enrolled eligible participants completed PSG supervised by a respiratory sleep technologist for 1 night at an AASM-certified sleep center. Concurrent with PSG, ANNE sleep sensors were applied by a study coordinator. Data were collected by both systems simultaneously. Participants completed a sleep diary and a usability survey postprocedure. The study was approved by Northwestern University and the Carle Foundation Hospital Institutional Review Boards (IRB ID: STU00213322 and IRB ID: 20NCI3196) and registered at ClinicalTrials.gov (NCT04643782). All participants provided written consent prior to participation.
Both PSG and ANNE sleep outputs were manually scored by 3 blinded registered sleep technologists. Scoring of PSG data followed the guidelines in The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (version 2.6) using a 4% oxygen desaturation criteria for hypopneas.25 The respiratory sleep technologists were provided the ANNE sleep scoring manual for guidelines to score the ANNE sleep data. Sleep-disordered breathing events were identified through collective evaluation of the multiple channel outputs for chest wall movement, peripheral arterial tonometry (PAT) for an attenuated signal, arterial oxygen saturation (SpO2), heart rate, and snoring. ANNE sleep’s sleep-wake classifier is based on an artificial intelligence–enabled classifier that combines recurrent and convolutional neural networks. If the inter–respiratory sleep technologist mean variation for either PSG or ANNE-determined AHI exceeded 15%, a board-certified sleep medicine physician blinded to the experimental condition provided the final determination of AHI after review of the raw data per AASM and ANNE sleep scoring manual guidelines.
Equipment
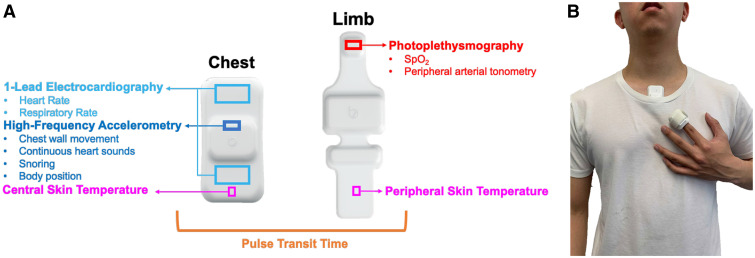
The novel system used in both the pivotal and pilot studies (ANNE One; Sibel Health) is pictured in Figure 1; it consists of 2 flexible wireless sensors, 1 placed at the suprasternal notch (ANNE chest) and the second wrapped around the index finger (ANNE limb). Both sensors are Food and Drug Administration–cleared as part of a continuous physiological monitoring system for patients 18 years or above to aid in clinical decision making. The ANNE chest unit contains a single-lead electrocardiogram sensor, high-frequency 3-axis accelerometer, and temperature sensor, and is capable of continuous measurement of heart rate, respiratory rate, chest wall movement, body position, and skin temperature. Direct mechanical coupling of the 3-axis accelerometer to the skin at the suprasternal notch with a bio-compatible, single-use adhesive accesses this anatomical location of high information density. This enables continuous stethoscope-like detection of snoring, respiration, and seismocardiography.22 The ANNE limb unit, coupled to the index finger with a latex-free, biocompatible adhesive, has a photo-plethysmograph to measure SpO2, PAT, and temperature. The accuracy and performance of the system have been published previously for core vital signs.24 ANNE sleep’s accuracy in quantifying total sleep time was validated against PSG records via 30-second epochs. The mean absolute percentage error for total sleep time was 17% with a mean difference of 12.9 minutes.
Figure 1. Experimental system.
The components applied to the body for the experimental system (ANNE sleep) are shown (A). They consist of 2 soft, flexible, wireless sensors that couple to the suprasternal notch and the index finger. The onboard sensors include 1-lead ECG, 3-axis high-frequency accelerometry, thermography, and transmissive mode photoplethysmography. Collectively, these onboard sensors generate measurements for heart rate, respiratory rate, chest wall movement, continuous heart sounds, snoring, body position, SpO2, skin temperature at both a central and peripheral location, and peripheral arterial tonometry. The sensors are software-linked, enabling time synchronization to derive pulse arrival time and pulse transit time. The sensors are mounted (B) on the suprasternal notch and finger with single-use consumables. ECG = electrocardiography, SpO2 = arterial oxygen saturation.
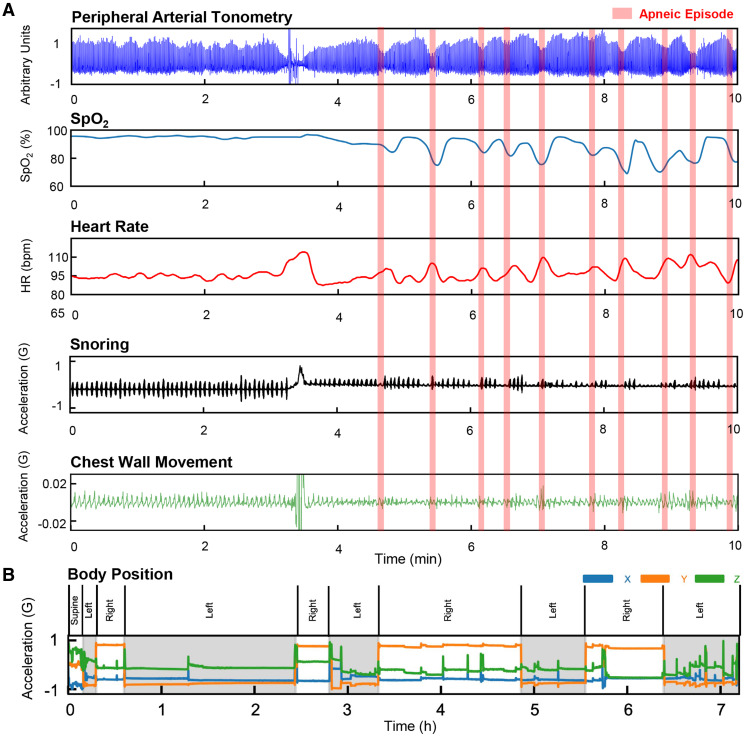
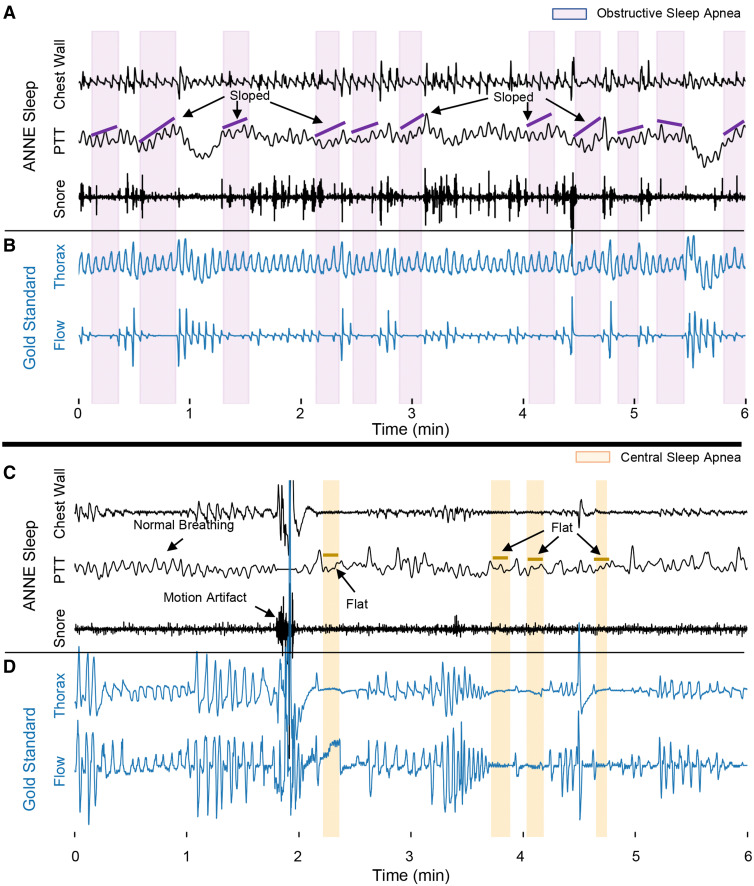
Figure 2 demonstrates data outputs from the system. Both sensors record data on board and can stream and store all output channels of physiological data to a mobile device via encrypted Bluetooth. Data are then automatically transmitted to a secure cloud for analysis. Furthermore, the sensors are software-linked and time-synchronized to produce continuous measurements of pulse transit time (PTT) as a novel index. PTT represents a time interval that corresponds to a pulse wave traveling from the aortic valve to the finger, providing a reliable measurement of continuous blood pressure.26 Previously, PTT has been further established as a reliable method to differentiate obstructive and central sleep respiratory events, and respiratory effort.27,28 Systematic reviews have concluded the utility of PTT as an important digital biomarker of OSA in both children and adults without the need for a nasal cannula or thermistor—PTT has been described previously with a sensitivity of 93% and specificity of 96% for differentiating obstructive and central respiratory events against standard techniques.27,29–31 We illustrate the utility of PTT derived from the ANNE sleep system to identify both central sleep apnea and OSA events compared with flow and respiratory inductance plethysmography outputs from gold-standard systems. In cases where obstructive events occur, the PTT signal is more variable with a sloped appearance. In cases where central apnea events occur, the PTT signal is flat (Figure 3).
Figure 2. Outputs of the experimental system.
The outputs of the ANNE sleep system are shown derived from a suite of onboard sensors. (A) The outputs derived from the ANNE limb sensor for PAT and SpO2 and ANNE chest sensor for heart rate, snoring, and chest wall movement. During apneic events, there is clear illustration of PAT attenuation, heart rate increases, SpO2 drops, snoring changes, and chest wall movement changes. (B) The system accurately determines body position changes based on the ANNE chest sensor. bpm = beats per minute, HR = heart rate, PAT = peripheral arterial tonometry, SpO2 = arterial oxygen saturation.
Figure 3. PTT derived from experimental system.
(A–D) PTT represents a time interval that corresponds to a pulse wave traveling from the aortic valve to the finger, which can be used to distinguish central and obstructive sleep apnea events. In the case of obstructive events, PTT varies with a sloped appearance given the movement of the chest. In the case of central events, PTT remains relatively flat given the lack of movement of the chest. PTT = pulse transit time.
Statistical methods
The primary endpoints of the pivotal study were sensitivity and specificity of ANNE sleep to diagnose moderate and severe OSA compared with PSG with a prespecified goal of at least 80% for both sensitivity and specificity. An AHI between 15 to 30 events/h was defined as moderate OSA and an AHI greater or equal to 30 events/h was designated as severe disease. Sensitivity was defined as the proportion of participants with moderate or severe OSA by PSG correctly identified by ANNE sleep. Specificity was defined as the proportion of participants without moderate or severe OSA by PSG with similarly negative testing by ANNE sleep. Secondary endpoints included ANNE sleep accuracy and positive predictive values (PPVs) and negative predictive values (NPVs) for moderate and severe OSA. Accuracy was defined as the proportion of true results. PPV was defined as the proportion of participants accurately identified as having moderate or severe OSA of the total number of positive screening tests. NPV was defined as the proportion of participants correctly screening negative among the total number of negative tests.
We determined point estimates and 95% confidence intervals (CIs) for sensitivity, specificity, accuracy, area under the curve (AUC) of the receiver operating characteristic (ROC) curve, PPV, and NPV of ANNE sleep to diagnose moderate and severe sleep apnea compared with PSG. We also calculated the diagnostic characteristics for AHI cutoffs of 5, 15, and 30 events/h. Bland-Altman plots and linear regressions for AHI were generated to evaluate bias between mean differences and to estimate a 95% interval of differences between ANNE sleep and PSG.32 It was determined, a priori, that a minimum sample size of 181 participants would be required to achieve 80% power to detect (1) sensitivity at least as large as 0.80, based on a target significance level of .05 with a 1-sided binomial test, and (2) specificity at least as large as 0.80, based on a target significance level of .05 with a 1-sided binomial test after accounting for an estimated 10% missingness. Statistical programming and analyses were performed independently by HealthCore, using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 287 individuals were recruited from 4 clinical sites in Illinois between May 11, 2021, and November 17, 2021. A total of n = 225 individuals were included in the final analysis after accounting for patient cancellations and withdrawals (Figure S1 (21.4KB, pdf) in the supplemental material). The mean age of participants was 53 years (standard deviation [SD] 14 years) and 56% were women. The mean body mass index (BMI) of participants was 31.2 kg/m2, and 83% were considered overweight or obese by BMI. Nearly one-third of patients (n = 73) reported a history of hypertension and 12.5% (n = 28) had a history of diabetes (Table 1).
Table 1.
Participant demographics (n = 225).
| Values | |
|---|---|
| Age, mean (SD), y | 52.7 (14) |
| Sex, n (%) | |
| Male | 98 (43.6) |
| Female | 127 (56.4) |
| Race, n (%) | |
| American Indian or Alaska Native | 1 (0.4) |
| Asian | 20 (8.9) |
| Native Hawaiian or other Pacific Islander | 1 (0.4) |
| Black or African American | 26 (11.6) |
| White | 164 (72.9) |
| More than 1 race | 10 (4.4) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 19 (8.4) |
| Highest education level completed, n (%) | |
| Some high school | 1 (0.4) |
| High school graduate (or equivalent) | 21 (9.3) |
| Some college or technical school | 50 (22.2) |
| College graduate | 152 (67.6) |
| Current employment status, n (%) | |
| Employed | 146 (64.9) |
| Unemployed | 26 (11.6) |
| Retired | 48 (21.3) |
| Retired due to disability | 4 (1.8) |
| BMI, mean (SD), kg/m2 | 31.2 (7.1) |
| BMI categories,* n (%) | |
| Underweight | 4 (1.8) |
| Normal | 34 (15.1) |
| Overweight | 73 (32.4) |
| Obese | 113 (50.2) |
| Medical history, n (%) | |
| Diabetes | 28 (12.4) |
| Hypertension | 73 (32.4) |
| Atrial fibrillation | 5 (1.7) |
| Other cardiopulmonary disease | 6 (2.1) |
| Cardiopulmonary disease requiring hospitalization | 6 (2.1) |
| OSA disease severity,† n (%) | |
| Normal | 91 (40.4) |
| Mild | 66 (29.3) |
| Moderate | 37 (16.4) |
| Severe | 31 (13.8) |
*BMI categories: underweight = BMI < 18.5 kg/m2, normal weight = BMI 18.5 to < 25 kg/m2, overweight = BMI 25 to < 30 kg/m2, and obese = BMI ≥ 30 kg/m2. †OSA disease severity: normal = AHI < 5 events/h, mild = AHI 5 to < 15 events/h, moderate = AHI 15 to < 30 events/h, and severe = AHI ≥ 30 events/h. AHI = apnea-hypopnea index, BMI = body mass index, OSA = obstructive sleep apnea, SD = standard deviation.
Overall, the mean PSG-derived AHI was 13.4 (SD 16.7) events/h and mean ANNE sleep–derived AHI was 12.9 (SD 15.1) events/h. Forty percent of the cohort had a normal AHI based on PSG (n = 91), compared to 37% (n = 83) by ANNE sleep; 30% of the cohort had mild OSA by PSG-derived AHI (n = 66), compared to 34% (n = 77) by ANNE sleep; 16% of the cohort had moderate OSA by PSG (n = 37), compared to 19% by ANNE sleep (n = 43). Last, 14% of the cohort had severe OSA by PSG (n = 31) compared to 10% (n = 22) by ANNE sleep (Table 2). PSG diagnosed 30% of the cohort (n = 68) with moderate or severe OSA based on an AHI ≥ 15 events/h compared to 29% (n = 65) identified by ANNE sleep (P = .55). Variation in scoring exceeding 15% by respiratory sleep technologists required adjudication by a sleep medicine physician in 42% of PSG studies (n = 94) and in 8% of ANNE sleep studies (n = 19).
Table 2.
Subjects categorized by OSA severity by PSG and ANNE sleep.
| OSA Severity* | PSG-Derived AHI | ANNE Sleep–Derived AHI |
|---|---|---|
| Normal | 91 (40.0) | 83 (37.0) |
| Mild | 66 (30.0) | 77 (34.0) |
| Moderate | 37 (16.0) | 43 (19.0) |
| Severe | 31 (14.0) | 22 (10.0) |
Values are presented as n (%). *OSA disease severity: normal = AHI < 5 events/h, mild = AHI 5 to < 15 events/h, moderate = AHI 15 to < 30 events/h, and severe = AHI ≥ 30 events/h. AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, PSG = polysomnography.
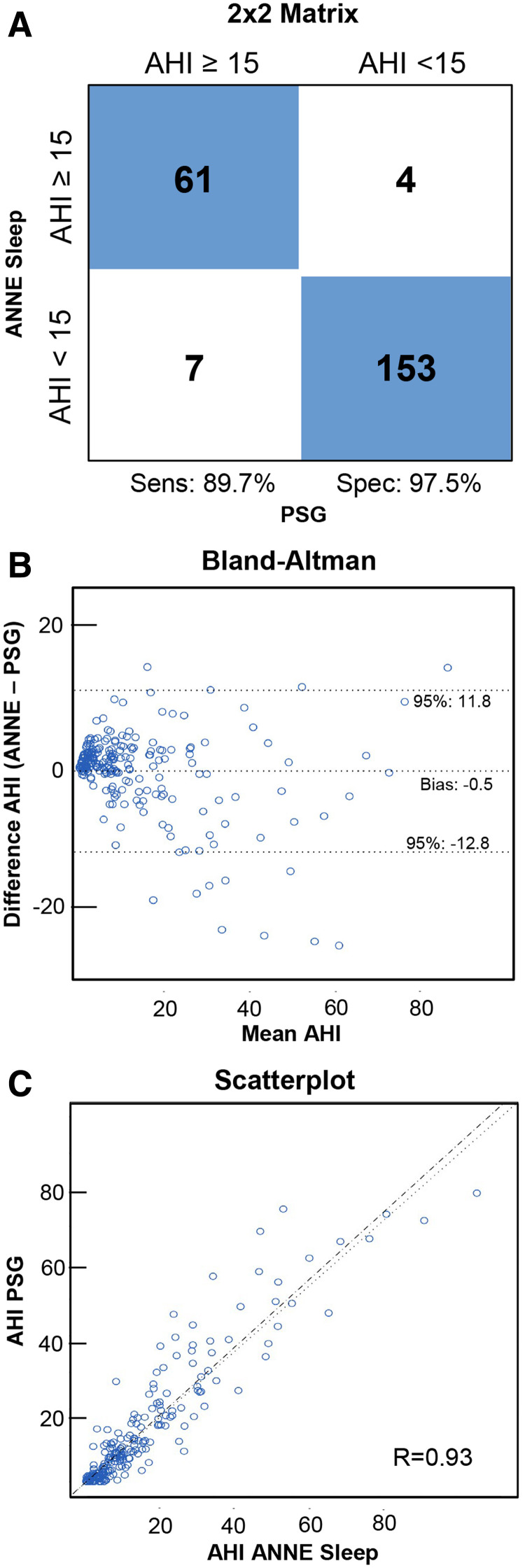
The sensitivity and specificity for ANNE sleep in the diagnosis of moderate to severe OSA were 89.7% (95% CI: 79.9–95.8%) and 97.5% (95% CI: 93.6–99.3%), respectively (Figure 4). The overall accuracy between ANNE sleep and PSG was 95.1% (95% CI: 91.4–97.5%). The PPV and NPV were 93.8% (95% CI: 85.0–98.3%) and 95.6% (95% CI: 91.2–98.2%), respectively. The overall bias was –0.5 events/h (95% limits of agreement: –12.8 to 11.8). AHI determined by PSG and ANNE sleep showed a correlation of r = .93 (95% CI: 0.91 to 0.94). Furthermore, in post hoc analyses we determined the diagnostic performance of ANNE sleep vs PSG at cutoffs of 5, 10, and 15 events/h for AHI (Table 3).
Figure 4. AHI scored by experimental system compared with PSG.
(A) A 2 × 2 matrix for the diagnosis of moderate to severe OSA for ANNE sleep and PSG. (B) Bland-Altman plot for AHI between ANNE sleep and PSG. (C) The scatterplot illustrates high linear agreement between AHI derived from ANNE sleep compared with AHI derived from PSG. AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, PSG = polysomnography, Sens = sensitivity, Spec = specificity.
Table 3.
Diagnostic performance of ANNE sleep vs PSG at AHI thresholds.
| AHI Cutoff (events/h) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Accuracy | PPV (95% CI) | NPV (95% CI) | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|
| 5 | 0.96 (0.92–0.99) | 0.86 (0.77–0.92) | 0.91 (0.87– 0.95) | 0.92 | 0.91 (0.85–0.95) | 0.94 (0.87–0.98) | 6.7 | 0.04 |
| 15 | 0.90 (0.8–0.96) | 0.97 (0.94–0.99) | 0.94 (0.90–0.97) | 0.95 | 0.94 (0.85–0.98) | 0.96 (0.91–0.98) | 35.2 | 0.11 |
| 30 | 0.65 (0.45–0.81) | 0.99 (0.96–0.99) | 0.82 (0.73–0.90) | 0.94 | 0.91 (0.71–0.99) | 0.95 (0.91–0.97) | 62.6 | 0.36 |
AHI = apnea-hypopnea index, AUC = area under the curve, CI = confidence interval, LR = likelihood ratio, NPV = negative predictive value, PPV = positive predictive value, PSG = polysomnography.
Overall, 93% of participants agreed or strongly agreed that ANNE sleep sensors would be easy to use at home and reported they could see themselves using the ANNE sensors at home for monitoring sleep. A subanalysis of user preferences for older adults in this study (≥65 years; n = 43) reported similarly high comfort levels using the ANNE sensors and expressed potential interest in future home use. There were no serious adverse events during the study.
DISCUSSION
This validation study demonstrated a high level of per-patient diagnostic agreement between ANNE sleep and PSG for moderate and severe OSA. The ANNE sleep system achieved a sensitivity of 89.7% and specificity of 97.5% with gold-standard in-laboratory PSG, meeting our prespecified threshold of at least 80% for sensitivity and specificity. The cohort used in this validation study was appropriately representative of a high-risk cohort of patients undergoing diagnostic testing for OSA; overall, 60% of the cohort had some level of dysfunctional breathing, while approximately 30% had moderate to severe disease.25 Additionally, comorbidities of obesity, hypertension, and diabetes were highly prevalent.
Although the study was primarily designed to characterize performance of ANNE sleep to diagnose moderate to severe OSA, we demonstrated strong discriminatory agreement of ANNE sleep at all clinically relevant thresholds of disease severity with an AUC of at least 0.8 at each AHI cutpoint of 5, 15, and 30 events/h.33 ANNE sleep underestimated the PSG AHI minimally by a mean of 0.5 events/h and, although it performed well for the a priori diagnostic designation of moderate and severe apnea, it was less accurate at the higher end of apneic events, differentiating moderate from severe disease. Arguably, differentiation of moderate from severe disease has fewer clinical implications, given the AASM recommendation to treat all patients with moderate disease (AHI 15 events/h) even in the absence of symptoms according to the AASM guidelines.25 Furthermore, maintaining a high PPV is fundamental to preventing overdiagnosis and therefore increasing accessibility and reducing cost. A post hoc analysis of variable AHI cutoffs demonstrated that a threshold of 15 or more events/h maximized both the sensitivity and specificity of ANNE sleep and the AUC of 0.94. Furthermore, we demonstrated less interrater variability of ANNE sleep when compared with the interrater variability of PSG. Exploration of the clinical applicability of fully automated ANNE sleep scoring is under way.
In light of the global disease burden of OSA, affecting an estimated 24% of men and 9% of women, most of whom are undiagnosed, innovation to improve access to efficient, accurate, high-quality diagnostic testing is of utmost importance.34 The COVID-19 pandemic has accelerated the interest in wearable devices and home-based diagnostic tools. While PSG does offer high accuracy and low failure rates as a supervised study, it remains prohibitively labor-intensive, inconvenient, costly, and uncomfortable, potentially leading to decreased total sleep time, lower sleep efficiency, and reduction in rapid eye movement sleep that may compromise its diagnostic value.35 HSAT systems address some of the limitations of PSG, given their home-based use and lower costs. However, a negative HSAT in the setting of high clinical suspicion still requires confirmatory PSG evaluation.36 Randomized clinical trial–based cost-effectiveness analyses comparing HSAT and PSG generally favor home-based screening, although the margin of benefit narrows when considering the lower accuracy of HSAT, higher technical failures, and requirements for confirmatory testing.18,37,38
It is important to note that there are multiple HSAT systems commercially available. Traditional type 3 HSAT systems (eg, Philip’s Alice NightOne, Murrysville, PA) include a large base unit strapped to the chest with multiple cable connections that allow for a single night use before being returned for refurbishment. Alternatively, WatchPAT (Itamar Medical Ltd, Caesarea, Israel) is a single-use disposable wrist-bound system that first used PAT to assess apnea events.17,20 In addition, SomnaPatch (Somnarus Inc, Mountain View, CA) was recently Food and Drug Administration–cleared as a single-use system with a nasal cannula and a forehead mounted pulse oximeter.37 The ANNE Sleep system, also FDA-cleared as a diagnostic platform for sleep-related breathing disorders in 2022, offers several potential advantages over these existing solutions. The unique soft mechanics and low-profile nature of the ANNE sleep system allow for mechanical deformation with natural body movement and lower skin contact stress, enabling high-fidelity monitoring and comfort.24 Thus, the ANNE sleep system allows for more natural sleeping positions and automatically determines body position over a sleep night via the chest sensor (Figure 2A). This may offer a more realistic assessment of AHI and reduce the first-night effects observed with both PSG and traditional type 3 HSAT systems.39 Furthermore, the ANNE sensors are fully rechargeable and reusable by the users themselves, allowing for multiple testing nights without the need to dispose of the system or reset it. Currently, the WatchPAT system is a single-use disposable device. This is a relevant advantage as multiple nights of home testing may be beneficial to increase diagnostic performance and reduce the impact of night-to-night variability of AHI; in a previous study of 47,423 adults, the average nightly variation of HSAT-derived AHI was 5.5 events/h, leading to a change in classification of severity of disease (mild, moderate, or severe) in one-third of the sample.39 Furthermore, ANNE sleep’s ability to link to ubiquitous mobile devices offers near-immediate data transfer to a secure cloud for analysis after each night, which may further reduce the need for confirmatory PSGs. Finally, the ANNE sleep system offers continuous electrocardiogram measurements unlike other HSAT systems and WatchPAT, and derives total sleep time and core body position from the chest sensor, mitigating limb movement artifacts.
There are several important limitations to acknowledge. First, our scoring criteria do not differentiate between apnea and hypopnea events. Although apneas and hypopneas are delineated clearly in AASM scoring guidelines, there is little empirical evidence of the clinical significance of differentiating these events.40 Several studies have shown that differentiating apneas and hypopneas results in limited clinical differences in treatment outcomes, or imaging findings.41–44 In addition, 1 study showed no differences in clinical comorbidities for patients with higher apnea indices compared with hypopnea indices—in fact, scoring apneas and hypopneas together, as done here with the experimental system, may increase interrater reliability and save resources in technician and physician time.40 While the system has the potential to distinguish central vs obstructive apnea events via PTT, the population addressed in this study was selected for a high pretest probability for obstructive apnea. Finally, the ANNE sleep sensor system was deployed under optimal conditions in this study—applied by a trained study coordinator in a sleep laboratory. Future efforts are ongoing to further validate the ANNE sleep system’s performance in the home setting by users themselves and the system’s ability to distinguish central from obstructive events.
CONCLUSIONS
Given the large burden of undiagnosed OSA and the ongoing COVID-19 pandemic, which has further driven the demand for virtual care, there remains a continued clinical need for more diagnostic platforms suitable for the home setting. Herein, we show high accuracy and strong positive user feedback for ANNE sleep compared with PSG for OSA. The advantages of the ANNE sleep system include reusability of both sensors with a simple sanitization wipe, enabling a potential lower cost per night. In addition, future opportunities include assessment of improved diagnostic performance with multiple sleep nights with minimal patient discomfort.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Northwestern Memorial Hospital, Carle Foundation Hospital, Central DuPage Hospital, and Lake Forest Hospital. This study was funded by Elevance Health (S.B., L.K.). J.Y.L., H.C., and S.X. also recognize support from the National Institute of Health’s National Heart, Lung, and Blood Institute (1R43HL151549-01). J.Y.L., D.K., J.P., H.C., and D.S.R. are all employees of Sibel Health, the developer of the ANNE One system. The spouse of J.R.W. has stock ownership in Sibel Health and royalties as an inventor. J.Y.L., D.K., J.P., and S.X. report stock ownership in Sibel Health, and royalties as inventors related to the patents associated with the technology. The other authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- HSAT
home sleep apnea test
- NPV
negative predictive value
- OSA
obstructive sleep apnea
- PAT
peripheral arterial tonometry
- PPV
positive predictive value
- PSG
polysomnography
- PTT
pulse transit time
- SD
standard deviation
REFERENCES
- 1. Suen C , Wong J , Ryan CM , et al . Prevalence of undiagnosed obstructive sleep apnea among patients hospitalized for cardiovascular disease and associated in-hospital outcomes: a scoping review . J Clin Med. 2020. ; 9 ( 4 ): E989 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simpson L , Hillman DR , Cooper MN , et al . High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls . Sleep Breath. 2013. ; 17 ( 3 ): 967 – 973 . [DOI] [PubMed] [Google Scholar]
- 3. Finkel KJ , Searleman AC , Tymkew H , et al . Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center . Sleep Med. 2009. ; 10 ( 7 ): 753 – 758 . [DOI] [PubMed] [Google Scholar]
- 4. Tarasiuk A , Reuveni H . The economic impact of obstructive sleep apnea . Curr Opin Pulm Med. 2013. ; 19 ( 6 ): 639 – 644 . [DOI] [PubMed] [Google Scholar]
- 5. Gozal D , Kheirandish-Gozal L . Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more . Am J Respir Crit Care Med. 2008. ; 177 ( 4 ): 369 – 375 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw JE , Punjabi NM , Wilding JP , Alberti KG , Zimmet PZ ; International Diabetes Federation Taskforce on Epidemiology and Prevention . Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention . Diabetes Res Clin Pract. 2008. ; 81 ( 1 ): 2 – 12 . [DOI] [PubMed] [Google Scholar]
- 7. Tregear S , Reston J , Schoelles K , Phillips B . Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis . J Clin Sleep Med. 2009. ; 5 ( 6 ): 573 – 581 . [PMC free article] [PubMed] [Google Scholar]
- 8. Tachibana N , Ayas NT , White DP . A quantitative assessment of sleep laboratory activity in the United States . J Clin Sleep Med. 2005. ; 1 ( 1 ): 23 – 26 . [PubMed] [Google Scholar]
- 9. Patel SR , Donovan LM . The COVID-19 pandemic presents an opportunity to reassess the value of polysomnography . Am J Respir Crit Care Med. 2020. ; 202 ( 3 ): 309 – 310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curcio G , Ferrara M , Piergianni A , Fratello F , De Gennaro L . Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography . Clin Neurophysiol. 2004. ; 115 ( 5 ): 1178 – 1188 . [DOI] [PubMed] [Google Scholar]
- 11. Agnew HW Jr , Webb WB , Williams RL . The first night effect: an EEG study of sleep . Psychophysiology. 1966. ; 2 ( 3 ): 263 – 266 . [DOI] [PubMed] [Google Scholar]
- 12. Rosen IM , Kirsch DB , Chervin RD , et al. ; American Academy of Sleep Medicine Board of Directors . Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement . J Clin Sleep Med. 2017. ; 13 ( 10 ): 1205 – 1207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skomro RP , Gjevre J , Reid J , et al . Outcomes of home-based diagnosis and treatment of obstructive sleep apnea . Chest. 2010. ; 138 ( 2 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 14. Rosen CL , Auckley D , Benca R , et al . A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study . Sleep. 2012. ; 35 ( 6 ): 757 – 767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Shayeb M , Topfer LA , Stafinski T , Pawluk L , Menon D . Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis . CMAJ. 2014. ; 186 ( 1 ): E25 – 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg R , Hirshkowitz M , Rapoport DM , Kryger M . The role of home sleep testing for evaluation of patients with excessive daytime sleepiness: focus on obstructive sleep apnea and narcolepsy . Sleep Med . 2019. ; 56 : 80 – 89 . [DOI] [PubMed] [Google Scholar]
- 17. Collop NA , Anderson WM , Boehlecke B , et al. Portable Monitoring Task Force of the American Academy of Sleep Medicine . Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients . J Clin Sleep Med. 2007. ; 3 ( 7 ): 737 – 747 . [PMC free article] [PubMed] [Google Scholar]
- 18. He RK , Kapur VK . Home- vs laboratory-based management of OSA: an economic review . Curr Sleep Med Rep. 2016. ; 2 ( 2 ): 107 – 113 . [Google Scholar]
- 19. Pietzsch JB , Garner A , Cipriano LE , Linehan JH . An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea . Sleep. 2011. ; 34 ( 6 ): 695 – 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zou D , Grote L , Peker Y , Lindblad U , Hedner J . Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography . Sleep. 2006. ; 29 ( 3 ): 367 – 374 . [DOI] [PubMed] [Google Scholar]
- 21. Yuceege M , Firat H , Demir A , Ardic S . Reliability of the Watch-PAT 200 in detecting sleep apnea in highway bus drivers . J Clin Sleep Med. 2013. ; 9 ( 4 ): 339 – 344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee K , Ni X , Lee JY , et al . Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch . Nat Biomed Eng. 2020. ; 4 ( 2 ): 148 – 158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu S , Jayaraman A , Rogers JA . Skin sensors are the future of health care . Nature. 2019. ; 571 ( 7765 ): 319 – 321 . [DOI] [PubMed] [Google Scholar]
- 24. Chung HU , Rwei AY , Hourlier-Fargette A , et al . Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units . Nat Med. 2020. ; 26 ( 3 ): 418 – 429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapur VK , Auckley DH , Chowdhuri S , et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y , Choi J , Hourlier-Fargette A , et al . Relation between blood pressure and pulse wave velocity for human arteries . Proc Natl Acad Sci USA. 2018. ; 115 ( 44 ): 11144 – 11149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Argod J , Pépin JL , Lévy P . Differentiating obstructive and central sleep respiratory events through pulse transit time . Am J Respir Crit Care Med. 1998. ; 158 ( 6 ): 1778 – 1783 . [DOI] [PubMed] [Google Scholar]
- 28. Argod J , Pépin JL , Smith RP , Lévy P . Comparison of esophageal pressure with pulse transit time as a measure of respiratory effort for scoring obstructive nonapneic respiratory events . Am J Respir Crit Care Med. 2000. ; 162 ( 1 ): 87 – 93 . [DOI] [PubMed] [Google Scholar]
- 29. Pépin JL , Tamisier R , Borel JC , Baguet JP , Lévy P . A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure . Curr Opin Pulm Med. 2009. ; 15 ( 6 ): 550 – 558 . [DOI] [PubMed] [Google Scholar]
- 30. Katz ES , Lutz J , Black C , Marcus CL . Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing . Pediatr Res. 2003. ; 53 ( 4 ): 580 – 588 . [DOI] [PubMed] [Google Scholar]
- 31. Pèpin JL , Delavie N , Pin I , et al . Pulse transit time improves detection of sleep respiratory events and microarousals in children . Chest. 2005. ; 127 ( 3 ): 722 – 730 . [DOI] [PubMed] [Google Scholar]
- 32. Giavarina D . Understanding Bland Altman analysis . Biochem Med (Zagreb). 2015. ; 25 ( 2 ): 141 – 151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandrekar JN . Receiver operating characteristic curve in diagnostic test assessment . J Thorac Oncol. 2010. ; 5 ( 9 ): 1315 – 1316 . [DOI] [PubMed] [Google Scholar]
- 34. Young T , Peppard PE , Gottlieb DJ . Epidemiology of obstructive sleep apnea: a population health perspective . Am J Respir Crit Care Med. 2002. ; 165 ( 9 ): 1217 – 1239 . [DOI] [PubMed] [Google Scholar]
- 35. Byun JH , Kim KT , Moon HJ , Motamedi GK , Cho YW . The first night effect during polysomnography, and patients’ estimates of sleep quality . Psychiatry Res. 2019. ; 274 : 27 – 29 . [DOI] [PubMed] [Google Scholar]
- 36. Gottlieb DJ , Punjabi NM . Diagnosis and management of obstructive sleep apnea: a review . JAMA. 2020. ; 323 ( 14 ): 1389 – 1400 . [DOI] [PubMed] [Google Scholar]
- 37. Kim RD , Kapur VK , Redline-Bruch J , et al . An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea . Sleep. 2015. ; 38 ( 7 ): 1027 – 1037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Safadi A , Etzioni T , Fliss D , Pillar G , Shapira C . The effect of the transition to home monitoring for the diagnosis of OSAS on test availability, waiting time, patients’ satisfaction, and outcome in a large health provider system . Sleep Disord. 2014. ; 2014 : 418246 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dzierzewski JM , Dautovich ND , Rybarczyk B , Taylor SA . Night-to-night fluctuations in sleep apnea severity: diagnostic and treatment implications . J Clin Sleep Med. 2020. ; 16 ( 4 ): 539 – 544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spector AR , Loriaux D , Farjat AE . The clinical significance of apneas versus hypopneas: is there really a difference? Cureus. 2019. ; 11 ( 4 ): e4560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gould GA , Whyte KF , Rhind GB , et al . The sleep hypopnea syndrome . Am Rev Respir Dis. 1988. ; 137 ( 4 ): 895 – 898 . [DOI] [PubMed] [Google Scholar]
- 42. Sutherland K , Takaya H , Qian J , Petocz P , Ng AT , Cistulli PA . Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea . J Clin Sleep Med. 2015. ; 11 ( 8 ): 861 – 868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Özer T , Selçuk A , Yılmaz Z , et al . The role of upper airway morphology in apnea versus hypopnea predominant obstructive sleep apnea patients: an exploratory study . Br J Radiol. 2018. ; 91 ( 1087 ): 20170322 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang AL , Cohen AP , Benke JR , Stierer KD , Stanley J , Ishman SL . Obstructive sleep apnea resolution in hypopnea-versus apnea-predominant children after adenotonsillectomy . Otolaryngol Head Neck Surg. 2016. ; 155 ( 4 ): 670 – 675 . [DOI] [PubMed] [Google Scholar]