Abstract
Study Objectives:
To determine efficacy and mechanisms of cognitive behavioral therapy for insomnia (CBT-I) and chronic obstructive pulmonary disease (COPD) education (COPD-ED) on clinical outcomes in adults with concurrent COPD and insomnia.
Methods:
We conducted a 2 × 2 factorial study to test the impact of CBT-I and COPD-ED delivered alone or in combination on severity of insomnia and fatigue, sleep, and dyspnea. Participants were randomized to 1 of 4 groups—group 1: CBT-I + attention control (AC; health videos, n = 27); group 2: COPD-ED + AC, n = 28; group 3: CBT-I + COPD-ED, n = 27; and group 4, AC only, n = 27. Participants received six 75-minute weekly sessions. Dependent variables included insomnia severity, sleep by actigraphy, fatigue, and dyspnea measured at baseline, immediately postintervention, and at 3 months postintervention. Presumed mediators of intervention effects included beliefs and attitudes about sleep, self-efficacy for sleep and COPD, and emotional function.
Results:
COPD patients (percent predicted forced expiratory volume in 1 second [FEV1pp] 67% ± 24% [mean ± standard deviation]), aged 65 ± 8 years, with insomnia participated in the study. Insomnia and sleep improved more in patients who received CBT-I than in those who did not, an effect that was sustained at 3 months postintervention and mediated by beliefs and attitudes about sleep. CBT-I was associated with clinically important improvements in fatigue and dyspnea. When CBT-I and COPD-ED were concurrently administered, effects on insomnia, fatigue, and dyspnea were attenuated.
Conclusions:
CBT-I produced significant and sustained decreases in insomnia improved sleep and clinically important improvement in fatigue, and dyspnea. The combination of CBT-I and COPD-ED reduced CBT-I’s effectiveness. Further research is needed to understand the mechanisms associated with effects of insomnia therapy on multiple symptoms in COPD.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: A Behavioral Therapy for Insomnia Co-existing with COPD; URL: https://clinicaltrials.gov/ct2/show/NCT01973647; Identifier: NCT01973647.
Citation:
Kapella M, Steffen A, Prasad B, et al. Therapy for insomnia with chronic obstructive pulmonary disease: a randomized trial of components. J Clin Sleep Med. 2022;18(12):2763–2774.
Keywords: cognitive behavioral therapy, sleep, insomnia, fatigue, dyspnea, chronic obstructive pulmonary disease
BRIEF SUMMARY
Current Knowledge/Study Rationale: Insomnia and fatigue are common complaints among patients with chronic obstructive pulmonary disease (COPD), but little is known about effects of nonpharmacologic insomnia therapy on COPD-related outcomes. The purpose of this study was to determine efficacy and mechanisms of cognitive behavioral therapy for insomnia (CBT-I) and COPD education (COPD-ED) on insomnia, fatigue, sleep, and dyspnea in adults with concurrent COPD and insomnia.
Study Impact: Insomnia and sleep improved more in patients who received CBT-I than in those who did not, an effect that was sustained 3 months post-intervention and mediated by the change in beliefs and attitudes about sleep. CBT-I was also associated with clinically important improvements in fatigue and dyspnea. Interestingly, COPD-ED reduced the effectiveness of CBT-I. Further research is needed to understand mechanisms associated with long-term effects of insomnia therapy on multiple symptoms in COPD.
INTRODUCTION
Insomnia and fatigue are commonly reported by patients with chronic obstructive pulmonary disease (COPD) and negatively impact health status,1 quality of life,2 and clinical outcomes including mortality.3 A growing body of evidence supports the effectiveness of nonpharmacologic therapy for insomnia in patients with coexisting medical illnesses, including patients with fibromyalgia,4 cancer,5 and cancer survivors.6 The effectiveness of nonpharmacologic therapy for insomnia in patients with COPD remains unclear. In an earlier pilot study7 we reported that cognitive behavioral therapy for insomnia (CBT-I) may minimize the severity of insomnia and fatigue in patients with COPD and concurrent insomnia. Moreover, we reported that COPD education (COPD-ED) could improve mood.7 Whether combining CBT-I and COPD-ED has an additive effect on ameliorating insomnia and fatigue in these patients and mechanisms associated with outcomes remains to be determined.
The study had 2 aims. Aim 1 was designed to determine the efficacy of administering CBT-I and COPD-ED alone and in combination on the severity of insomnia (primary outcome) and sleep, fatigue, and dyspnea (secondary outcomes). Aim 2 was designed to identify mechanistic contributors to pre/post-intervention change in insomnia and fatigue. Specifically, we hypothesized that the combination of CBT-I and COPD-ED is superior to each intervention on its own in decreasing insomnia and fatigue post-intervention and these effects are sustained for at least 3 months.
METHODS
Design
We conducted a 2 × 2 factorial study to test the impact of CBT-I and COPD-ED delivered alone or in combination on the severity of insomnia and fatigue as well as dyspnea in patients with concurrent insomnia and COPD. Details of the study were described previously.8 Study participants were randomly assigned to 1 of 4 groups consisting of combinations of CBT-I and COPD-ED (each separately, both, and neither) and attention control (AC).
The sample size calculation made when the study was designed was based on main effect differences at the end of the intervention.7 This called for 166 participants as to retain 140 (35 per group) participants in the study. These figures would result in 80% power to detect a moderate effect size (Cohen’s d = 0.47) for each treatment component (alpha = 0.05). However, we terminated recruitment before reaching our goal due to logistical challenges and time constraints. With the final sample of 109 (27–28 per group), the minimum detectable effect size increased by 15% to 0.54 for the main effects for treatment components assuming 80% power and alpha = 0.05. To maintain the 0.47 minimum detectable effect size at 80% power would require a larger type I error rate (alpha = 0.113).
Participants
The study took place from June 2014 to July 2019 and was approved by the Institutional Review Board at the University of Illinois at Chicago (UIC), Edward Hines Jr Veterans Hospital (Hines), and Jesse Brown Veterans Hospital. Participants were recruited through letters of invitation, newspaper advertisements, and word of mouth.
Inclusion criteria included the following: (1) COPD defined according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria,10 (2) age ≥ 45 years, (3) clinically stable at the time of enrollment, and (4) insomnia (Insomnia Severity Index [ISI] score ≥ 8).11,12 Participants were assessed for severity of COPD using spirometry (VMAX Encore 22; Viasys Healthcare, Inc, Yorba Linda, CA).13 Individuals who met initial eligibility criteria underwent a 1-night polysomnography (PSG) at the UIC Sleep Science Center (n = 31). When laboratory PSG was not available (n = 78), patients underwent an overnight sleep study at home (Alice NightOne with Sleepware G3 integrated Somnolyzer scoring; Philips Respironics, Murrysville, PA).
Exclusion criteria included the following: (1) restrictive lung disease or asthma; (2) pulse oximetry (SpO2) < 90% at rest or < 85% at night for > 5 minutes; (3) obstructive sleep apnea (apnea-hypopnea index > 15 events/h), periodic limb movements with arousals (> 10/h), positive Restless Legs Syndrome Rating Scale (> 30),14 history of narcolepsy; (4) current hypnotic use; (5) acute respiratory infection within the previous 2 months; (6) unstable chronic illness or alcohol or drug abuse; (7) major psychiatric disease or a Hospital Anxiety and Depression Scale (HADS) depression score of > 1115; and (8) actively participating in a pulmonary rehabilitation program.
Procedures
Participants were screened by phone followed by a screening visit at the UIC Center for Sleep and Health Research. All participants provided written, informed consent to participate. Excluding the screening, participants had a total of 9 laboratory visits (1 pretest visit, 6 intervention visits, 1 post-test visit, and one 3-month follow-up visit [in laboratory or home]).
Eligible participants were scheduled for the baseline (pretest) visit and they were then randomly assigned to 1 of the 4 treatment groups. The treatment groups were randomly ordered in blocks of 4 and sequentially assigned through REDCap17,18 according to stratum. We had 4 strata defined by sex and COPD severity, managed separately by site. Participants were randomized by crossing the 2 factors CBT-I vs AC and COPD-ED vs AC. Thus, patients in group 1 received CBT-I + AC; patients in group 2 received COPD-ED + AC; those in group 3 received CBT-I + COPD-ED; and those in group 4 received AC + AC. All participants received 6 treatment sessions lasting approximately 75 minutes each. Nonblinded investigators generated allocation by REDCap and assigned participants to interventions. Investigators administering spirometry and scoring sleep studies and actigraphy were blinded to group assignment.
Participants attended the intervention visits at the UIC Center for Sleep and Health Research or at the Physical Performance Laboratory of Edward Hines Jr VA Hospital. For the 3-month follow-up, participants either went to the UIC Center for Sleep and Health Research or Physical Performance Laboratory or were mailed an actigraph and questionnaires. We tracked attendance and used strategies to reduce attrition such as communication between investigators and participants, minimizing study participant burden using REDCap and monetary incentives. Tests were performed in the same order for each participant. Selection bias was minimized by use of the randomized design. We documented dropout, adverse events, and missing data details.
Intervention components
The CBT-I protocol was based on the modified7 Perlis et al19 insomnia treatment protocol. The CBT-I protocol was administered by a behavioral sleep medicine specialist (C.T., J.L.) or a mental health registered nurse (G.K.) trained in behavioral sleep medicine. All sessions were conducted in individual format. We originally intended to conduct group sessions but found transportation issues interfered with participants arriving at scheduled appointment times. CBT-I sessions were guided by a study manual and session guidelines and included a review of the sleep diary and actigraphy data, a didactic/interactive presentation, and discussion. Topics of the didactic/interactive presentation included insomnia information, techniques and medications used to manage insomnia, and prevention of insomnia relapse. COPD-ED sessions, conducted by the same investigators who administered CBT-I, included nonsleep topics related to COPD such as how the lungs work, managing COPD and complications, medications, and breathing techniques. Participants attended sessions with AC when not assigned to receive a component. AC was composed of general health education videos and discussion. AC participants received the same dose (75 minutes) of in-person interaction as the intervention participants, but no COPD or insomnia elements of treatment were included in AC.
Fidelity
Therapists (C.T., J.L., and G.K.) were trained in CBT-I, COPD-ED, and AC topics by experts (Principles and Practice of CBT-I Course at the University of Pennsylvania) and practiced the interventions under supervision (M.K., C.T.). Therapists used a standardized form for guidance during CBT-I, COPD-ED, and AC sessions. To assure high-quality, live sessions were periodically reviewed. CBT-I sessions 2, 4, and 6 were reviewed. Over 95% of study sessions were conducted by 2 research specialists (G.K. and C.T.) over the course of the study.
Outcome measures
Dependent variables included insomnia (primary outcome) and fatigue, sleep, and dyspnea (secondary outcomes), measured 3 times: at baseline (pretest), immediately post-intervention (post-test), and at 3 months (follow-up).
We quantified severity of insomnia using the ISI. ISI scores range from 0 to 28, with higher scores indicating worse insomnia. Scores of 8 to 14 indicate subthreshold insomnia, 15 to 21 indicate moderate insomnia, and scores of 22–28 indicate severe insomnia.11 A 6-point reduction represents a clinically meaningful minimal improvement in insomnia.20 A reduction > 7 or 8 points is considered a moderate improvement and a reduction > 8 points is considered a marked improvement.11
We measured fatigue and dyspnea using the Chronic Respiratory Disease Questionnaire (CRQ) fatigue (CRQF) and dyspnea (CRQD).19,20 The CRQF and the CRQD consist of 4 and 5 items, respectively. Items are scored on a 7-point Likert scale. Lower scores indicate more fatigue or dyspnea. CRQF and CRQD are valid and reliable tools to quantify severity of fatigue and dyspnea in patients with COPD.21 The minimal clinically significant change in the CRQ score is a change of 0.5 points, and a moderate change is a CRQ change of 1 point.22
Sleep architecture was indirectly quantified using actigraphy (Actiwatch-2®; Minimitter; Philips Respironics, Murrysville, PA).23,24 Variables examined included wake after sleep onset (WASO) and sleep efficiency (SE). A WASO reduction of 20 minutes or a 5% increase in SE are considered clinically significant.25 All participants wore the actigraph during the 6-week intervention period and the report was reviewed at each session with those who received CBT-I. We found from our pilot work that participants enjoyed seeing their objective data and discussing it along with their sleep diary data during sessions.
Patients randomized to CBT-I were instructed to complete the Consensus Sleep Diary26 daily while wearing the actigraph. Diary data were discussed during the weekly CBT-I sessions and used for editing and scoring the actigraphy data. Patients not randomized to CBT-I were instructed to complete the Consensus Sleep Diary for 1 week at baseline, post-treatment, and at the 3-month follow-up. Reliability and validity in measuring self-reported sleep were reported.27,28
Mediator measures
The Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-30)29 was used to measure sleep-related beliefs in the participants. The scale consists of 30 items in 5 themes: misattributions or amplification of the consequences of insomnia, diminished perception of control and predictability of sleep, unrealistic sleep expectations, misconceptions about the causes of insomnia, and faulty beliefs about sleep-promoting practices. The response scale included a 0–10 Likert scale labeled “strongly disagree” to “strongly agree.” The DBAS score is an average of item scores. Adequate reliability and validity were demonstrated in older adults with and without sleep problems. The DBAS is sensitive to changes in sleep-related beliefs in older adults with insomnia.30 A DBAS score above 3.5 identifies clinically significant levels of unhelpful beliefs related to sleep.31
We measured self-efficacy for sleep using the Self-Efficacy Scale (SES) for sleep,32 a 9-item questionnaire including a 1–5 Likert scale. Respondents indicated their confidence (“not confident at all” to “very confident”) in falling asleep, staying asleep, and obtaining refreshing sleep. Reliability, validity, and test-retest reliability of the SES have been demonstrated in older adults.33
The COPD Self-efficacy Scale34 (COPDSE) was used to measure self-efficacy for COPD. The COPDSE is a 34-item questionnaire with 5 subscales that includes a 5-point Likert scale. Respondents indicated their confidence in managing breathing (dyspnea) during intense emotions, physical exertion, adverse weather or environmental conditions, and risk factors. Responses range from 34 to 170 (“very confident” to “not at all confident”) items scoring 1 to 5, with 1 representing higher self-efficacy. Reliability and validity of the COPDSE have been demonstrated in people with COPD.35
We measured the self-reported emotional arousal experienced in the previous 2 weeks using the 7 item CRQ Emotional Function domain (CRQEF).19,20 The questionnaire includes items on anxious and depressed mood and satisfaction with personal life.
Statistical analyses
Descriptive statistics and plots were used to screen the data prior to our main analyses. Baseline adjustment for covariates (eg, age, COPD severity [pulmonary function test], site, and sex) were incorporated into the main effect analyses to reduce error variance and improve statistical power.36 All tests were 2-sided and an error rate of α < 0.05 was considered statistically significant. The test of each specific aim is described below, and all statistical analysis used an intent-to-treat approach, with all cases retained regardless of attrition or missing data utilizing all available data with a full information maximum likelihood approach. Data analyses were performed using SAS version 9.4 statistical software (SAS Institute, Cary, NC), IBM SPSS Statistics for Windows, version 27 (IBM Corporation, Armonk, NY), and Stata Statistical Software 15.1 (StataCorp 2017; StataCorp LLC, College Station, TX).
Test of specific hypotheses
We used separate covariance pattern models regressing outcomes on treatment component indicators (effect coded –1, +1), time (categorical), and their interactions as fixed effects. An unstructured residual covariance pattern was used to account for correlations due to repeated measurements. The null hypothesis was rejected when a significant treatment × time interaction was observed. For example, a significant CBT-I × time effect in the hypothesized direction meant that participants receiving CBT-I improved more than those who did not, averaged across the other conditions at the specified time point. Models run with and without a priori covariates yielded the same statistical conclusions for efficacy findings, and unadjusted models are presented. Potential moderators suggested by our previous work include sex, severity of baseline insomnia, baseline fatigue, and COPD and were tested as interactions in the context of the Aim 1 models. Significant moderation suggests characteristics associated with better response to the intervention.
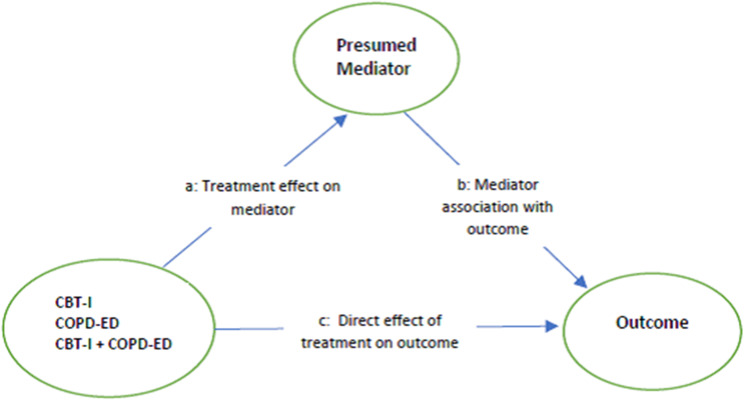
We hypothesized that CBT-I and COPD-ED components impact insomnia and fatigue through complementary mechanisms. We used path analysis to test the conceptual model illustrated in Figure 2.37 The models tested if each treatment component was related to change in the hypothesized mediator post-treatment, and whether that change is associated with improvement in the outcome post-treatment. The “a” paths represent the direct effects of each treatment component on the mediator, also known as the action theory part of a mediation model, and indicate that treatment impacted the mediator (ie, difference in change between those who received the component compared with those who did not). The “b” path is the effect of the mediator on the outcome, representing the conceptual theory aspect of mediation, and indicates the relationship between mediator and outcome. Significant conceptual theory (b paths) supports that the mediator is an appropriate target to improve the outcome. The “c’” paths are the direct effects of the treatment component on the outcome, showing what aspect of a treatment effect is not explained by the mediator. A significant indirect effect (a × b) provides the strongest support for mediation. A total effect is the sum of the indirect (a × b) and direct paths (c) and is useful to determine what proportion of an effect is due to the mediated effect. These path models used dummy-coded indicators for treatment variables and their interaction, included baseline measures of mediators and outcomes as covariates, and used full information maximum likelihood estimation to incorporate all available data.
Figure 2. Mediation model.
CBT-I = cognitive behavioral therapy for insomnia, COPD-ED = chronic obstructive pulmonary disease education.
RESULTS
Baseline characteristics
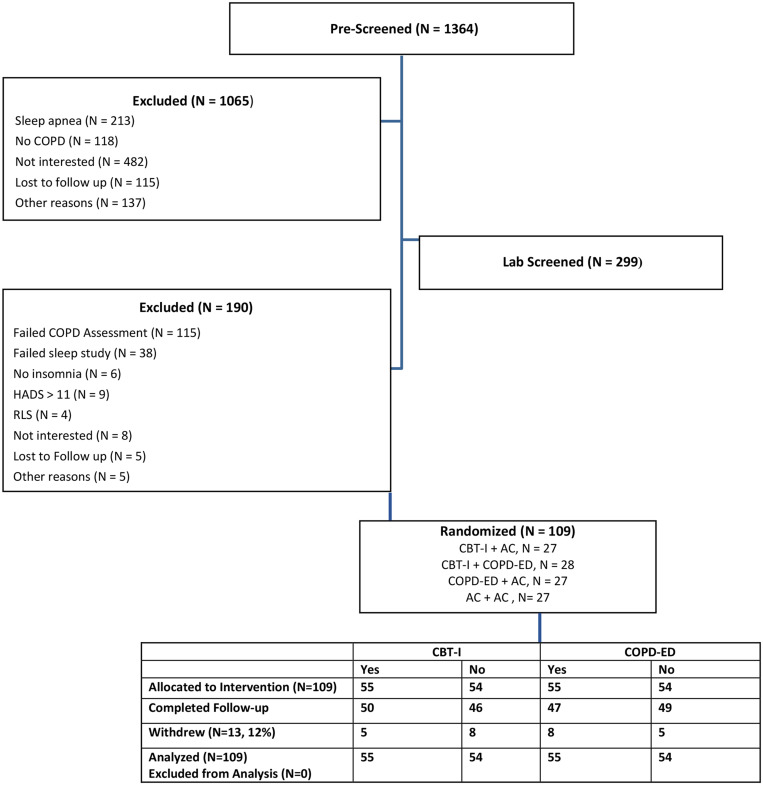
A total of 109 patients (percent predicted forced expiratory volume in 1 second [FEV1%] 67% ± 24% [mean ± SD], age 65 ± 8 years) were enrolled in the study (Figure 1). Demographic characteristics were balanced among groups (Table 1). In contrast, at baseline, patients randomized to COPD-ED had more severe insomnia (ISI = 17) than the other patients (ISI = 14.8). Thirty-six percent of participants were women and 73% were Black. As per the study design, all participants had COPD40; GOLD stage 1 (n = 30), GOLD stage 2 (n = 53), GOLD stage 3 (n = 17), GOLD stage 4 (n = 9). Descriptive statistics for measures and baseline outcome scores are summarized in Table 2 and Table 3. Participants reported moderate levels of insomnia and fatigue, more than 1 hour awake during the night, and low SE at baseline.
Figure 1. CONSORT participant flow.
AC = attention control, CBT-I = cognitive behavioral therapy for insomnia, CONSORT = Consolidated Standards of Reporting Trials, COPD = chronic obstructive pulmonary disease, COPD-ED = COPD education, HADS = Hospital Anxiety and Depression Scale, RLS = Restless Legs Syndrome Questionnaire.
Table 1.
Demographic and clinical characteristics at baseline.
| CBT-I | COPD-ED | ||||
|---|---|---|---|---|---|
| Characteristics | All Participants (n = 109) | Yes (n = 55) | No (n = 54) | Yes (n = 55) | No (n = 54) |
| Age, y | 65 ± 8 | 64 ± 8 | 65 ± 9 | 65 ± 8 | 64 ± 9 |
| Women, % | 36% | 36% | 35% | 36% | 36% |
| FEV1, % predicted | 66.8 ± 23.6 | 67.2 ± 23.7 | 66.4 ± 23.8 | 67.3 ± 24.8 | 66.2 ± 22.5 |
| FEV1/FVC | 55.0 ± 11.5 | 55.5 ± 12.0 | 54.5 ± 11.1 | 55.1 ± 11.6 | 55.0 ± 11.6 |
| BMI, kg/m2 | 27.2 ± 6.1 | 27.6 ± 6.3 | 26.8 ± 5.9 | 27.2 ± 7.1 | 27.2 ± 5.0 |
| Race, % | |||||
| Black | 73% | 80% | 67% | 74% | 73% |
| White | 23% | 18% | 28% | 20% | 23% |
| Unknown or other | 4% | 2% | 5% | 6% | 4% |
| Education, % | |||||
| > High school | 66% | 75% | 80% | 80% | 74% |
| ≤ High school | 34% | 25% | 20% | 20% | 26% |
| Smoking, pack-years | 31.8 ± 26.3 | 30.0 ± 21.6 | 33.5 ± 30.4 | 32.5 ± 28.8 | 31.1 ± 24.0 |
| Apnea-hypopnea index, events/h | 5.1 ± 3.4 | 5.5 ± 3.3 | 4.7 ± 3.4 | 5.3 ± 3.4 | 5.0 ± 3.4 |
| Baseline SaO2, % | 93.4 ± 2.7 | 93.1 ± 2.6 | 93.6 ± 2.8 | 93.2 ± 2.9 | 93.5 ± 2.5 |
| Inhaled beta-agonist, % | 82.41% | 80% | 83% | 84% | 82% |
| Oral beta-agonists, % | 100% | 100% | 98% | 100% | 99% |
| Inhaled glucocorticoid, % | 51.85% | 56% | 46% | 53% | 52% |
| Inhaled anticholinergic, % | 49.07% | 45% | 60% | 45% | 49% |
Data are presented as mean ± SD unless otherwise indicated. BMI = body mass index, CBT-I = cognitive behavioral therapy for insomnia, COPD-ED = chronic obstructive pulmonary disease education, FEV1 = forced expiratory volume in 1 second, FVC = forced vital capacity, SaO2 = arterial oxygen saturation, SD = standard deviation.
Table 2.
Descriptive statistics for outcomes and presumed mechanisms at baseline.
| Mean ± SD | Range | Potential Range | Cronbach’s Alpha | |
|---|---|---|---|---|
| Insomnia Severity (ISI) | 15.9 ± 4.4 | 6–27 | 0–28 | 0.80 |
| Wake after Sleep Onset (minutes) (WASO) | 72.1 ± 32.9 | 17–170 | N/A | N/A |
| Sleep Efficiency (%) (SE) | 74.2 ± 10.3 | 39–90 | 0–100 | N/A |
| CRQ Fatigue Level (CRQF, lower score = more fatigue) | 3.7 ± 1.1 | 1–6 | 1–7 | 0.85 |
| CRQ Dyspnea level (CRQD, lower score = worse dyspnea) | 4.4 ± 1.4 | 1–7 | 1–7 | 0.86 |
| CRQ Emotional Function (CRQEF, lower score = worse emotional function) | 4.4 ± 1.2 | 1.0–6.7 | 1–7 | 0.89 |
| Dysfunctional Beliefs and Attitudes about Sleep (DBAS, lower score = less dysfunctional beliefs about sleep) | 4.4 ± 1.5 | 1.2–7.9 | 0–10 | 0.92 |
| Self-Efficacy for Sleep (SES, lower score = less confident about sleep) | 23.4 ± 6.7 | 9–45 | 9–45 | 0.79 |
| Self-Efficacy for COPD (COPDSE, lower score = more confident in managing breathing) | 101.7 ± 25.1 | 39–163 | 34–170 | 0.96 |
Difference for COPD-ED (yes, no), Insomnia Severity, P = .0078. COPD = chronic obstructive pulmonary disease, COPD-ED = COPD education, CRQ = Chronic Respiratory Disease Questionnaire, ISI = Insomnia Severity Index, N/A = not applicable, SD = standard deviation.
Table 3.
Baseline scores and change for four outcomes by treatment components: COPD-ED and CBT-I.
| Insomnia Severity Index | CRQ Fatigue | WASO | Sleep Efficiency | |||||
|---|---|---|---|---|---|---|---|---|
| Y | N | Y | N | Y | N | Y | N | |
| COPD-ED | ||||||||
| Baseline | 17.0 | 14.8 | 3.6 | 3.9 | 74.5 | 68.8 | 73.8 | 74.9 |
| Change at post-test | −4.8 | −3.3 | 0.5 | 0.4 | −7.2 | −2.4 | 1.9 | 2.7 |
| Change at follow-up | −5.1 | −4.9 | 0.6 | 0.6 | −6.2 | −0.8 | 3.3 | 1.3 |
| CBT-I | ||||||||
| Baseline | 16.0 | 15.8 | 3.8 | 3.7 | 76.8 | 66.5 | 72.7 | 76.0 |
| Change at post-test | −5.8 | −2.2 | 0.6 | 0.4 | −13.1 | 3.5 | 4.5 | 0.1 |
| Change at follow-up | −6.8 | −3.3 | 0.7 | 0.4 | −13.3 | 6.2 | 4.5 | 0.1 |
Sleep efficiency (actigraphy), n = 101. Bold = P < .05. Numbers are model-based least-square means adjusted for treatment components, time, and their interaction. Participants who received CBT-I are compared with those who did not receive it and participants who received COPD-ED are compared with those who did not receive it. We observed a baseline difference where those receiving COPD-ED had more severe insomnia (17) than those who did not (14.8), despite randomization. There were no other statistically significant differences between those who received COPD-ED and those who did not for all outcomes at baseline, and the change observed at post-test and follow-up. CBT-I = cognitive behavioral therapy for insomnia, COPD-ED = chronic obstructive pulmonary disease education, CRQ, Chronic Respiratory Disease Questionnaire, N = no, WASO = wake after sleep onset (actigraphy), Y = yes.
Primary outcome
In Table 3 we summarize the main effects of the interventions on clinical outcomes. This analysis focuses on a between-group comparison of difference in change (DIC) from baseline to a follow-up time point. Severity of insomnia decreased more from baseline to immediately post-treatment (6 weeks) among patients who received CBT-I compared with those who did not receive CBT-I (DIC = –5.8 to –2.2 = –3.6 points). The decrease in insomnia associated with administration of CBT-I was sustained at the 3-month follow-up visit (DIC = –3.5). This CBT-I group’s mean decrease in insomnia at 3 months was clinically meaningful, almost to the moderate level (–6.8). The corresponding DIC for COPD-ED compared with no COPD-ED was not statistically significant: –1.5 at 6 weeks and –0.2 at follow-up weeks.
Full-model results for the Aim 1 analyses are summarized in Table 4. At follow-up, a 3-way interaction was significant and plotted to aid interpretation (Figure 3a). The largest decrease in ISI occurred in patients randomized to CBT-I (+ AC). Compared with CBT-I (+ AC), CBT-I + COPD-ED had an attenuated effect on ISI. While the model identified baseline differences in ISI for these subgroups, we obtained the same results in an alternative model adjusting for baseline ISI and other a priori covariates.
Table 4.
Models of 4 outcomes regressed on treatment components, time, and their interaction.
| Insomnia Severity | Fatigue Level | WASO | SE | Dyspnea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | |
| Intercept | 15.89 | <.0001 | 3.72 | <.0001 | 71.64 | <.0001 | 74.37 | <.0001 | 4.42 | <.0001 |
| COPD-ED | 1.11 | .01 | −0.14 | .17 | 2.88 | .38 | −0.53 | .61 | 0.02 | .87 |
| CBT-I | 0.13 | .75 | 0.05 | .65 | 5.18 | .12 | −1.65 | .11 | −0.26 | .07 |
| COPD-ED × CBT-I | −0.84 | .04 | 0.08 | .42 | 2.55 | .44 | −0.30 | .77 | −0.11 | .41 |
| Baseline | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — |
| Post-test | −4.03 | <.0001 | 0.45 | <.0001 | −4.84 | .06 | 2.31 | <.0001 | 0.38 | <.0001 |
| Follow-up | −5.05 | <.0001 | 0.59 | <.0001 | −3.54 | .20 | 2.29 | <.0001 | 0.44 | .0004 |
| COPD-ED × baseline | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — |
| COPD-ED × post-test | −0.74 | .12 | 0.03 | .72 | −2.41 | .34 | −0.44 | .54 | 0.09 | .31 |
| COPD-ED × follow-up | −0.10 | .83 | −0.03 | .73 | −2.69 | .32 | 0.97 | .17 | −0.11 | .34 |
| CBT-I × baseline | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — |
| CBT-I × post-test | −1.80 | <.001 | 0.10 | .27 | −8.30 | <.001 | 2.23 | <.001 | 0.11 | .21 |
| CBT-I × follow-up | −1.77 | <.001 | 0.16 | .09 | −9.78 | <.001 | 2.21 | <.001 | 0.21 | .09 |
| COPD-ED × CBT-I × baseline | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — | 0.00 | — |
| COPD-ED × CBT-I × post-test | 0.91 | .06 | −0.14 | .12 | −1.40 | .58 | −0.14 | .84 | −0.15 | .08 |
| COPD-ED × CBT-I × follow-up | 1.56 | <.001 | −0.21 | .02 | 3.80 | .16 | −1.84 | .01 | −0.13 | .26 |
Components (COPD-ED and CBT-I) were effect coded (−1, 1). Bold = P < .05. AC = attention control, CBT-I = cognitive behavioral therapy for insomnia, COPD-ED, chronic obstructive pulmonary disease education, SE = actigraphy sleep efficiency, WASO = actigraphy wake after sleep onset.
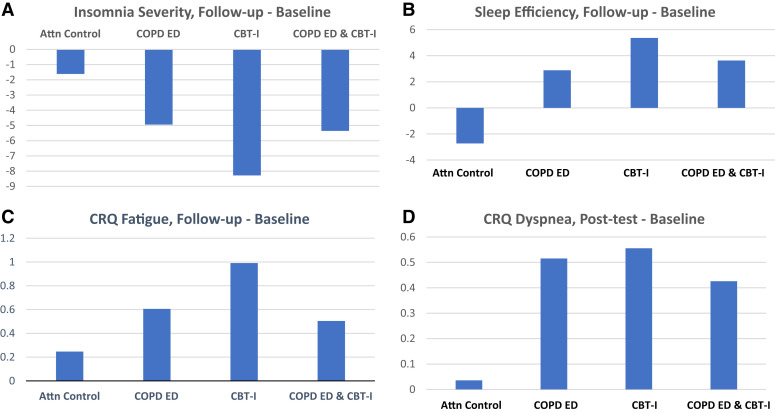
Figure 3. Changes in insomnia severity (A), sleep (B), fatigue (C), and dyspnea (D).
Higher CRQ scores indicate less symptom levels. Attn = attention, CBT-I = cognitive behavioral therapy for insomnia, COPD ED = chronic obstructive pulmonary disease education, CRQ = Chronic Respiratory Disease Questionnaire.
Secondary outcomes
Actigraphy WASO and SE results showed findings like ISI. CBT-I was associated with a significant DIC compared with those who did not receive CBT-I at post-treatment and 3-month follow-up. SE increased in a similar pattern (Table 3). On average, improvements in WASO and SE did not reach the clinically significant level. At follow-up, the interaction effect was evident for SE (Figure 3b).
Fatigue decreased post-treatment and continued to be less than pretreatment at the 3-month follow-up; however, the DIC was not significant for main effect comparisons of CBT-I and COPD-ED (Table 3 and Table 4). At the 3-month follow-up, there was an interaction between CBT-I and COPD-ED like that observed for insomnia and SE, suggesting that the combination of CBT-I and COPD-ED was less effective than CBT-I alone (Table 4 and Figure 3c). There was a clinically important improvement in fatigue and dyspnea for those randomized to either CBT-I or COPD-ED (Table 3 and Figure 3d).
Moderators
We assessed whether sex, initial ISI, and fatigue severity and COPD severity modulated the effects of CBT-I and COPD-ED in the differed subgroups. CBT-I was more effective among participants with greater fatigue at baseline [b = 1.08 (0.48), P = .028], an effect that was also evident at 3 months [b = 1.02 (0.48), P = .037]. In contrast, initial ISI and COPD severity had no impact on the effects of CBT-I. No moderator had any impact on the effects of COPD-ED.
Mediation
To identify potential mechanistic contributors to pre/post-intervention change in insomnia and fatigue we examined the hypothesized mediators including COPDSE, SES, DBAS, and CRQEF.8 Model results are presented in Table 5.
Table 5.
Mediation models.
| Model | Treatment Component | (Action Theory) | (Conceptual Theory) | Total Effect (a × b + c) | (Mediation) |
|---|---|---|---|---|---|
| Path a | Path b | Indirect Effect | |||
| Outcome-> | COPD Self-Efficacy | Insomnia Severity | Insomnia Severity | ||
| 1. COPD Self-Efficacy and Insomnia Severity | COPD-ED | −6.2 (4.4) | 0.08 (0.03)* | −1.8 (1.3) | −0.49 (0.39) |
| CBT-I | −2.7 (4.2) | −4.7 (1.2)* | −0.22 (0.35) | ||
| COPD-ED × CBT-I | 5.9 (6.1) | 2.6 (1.7) | 0.47 (0.52) | ||
| Outcome-> | Sleep Self-Efficacy | Insomnia Severity | Insomnia Severity | ||
| 2. Sleep Self-Efficacy and Insomnia Severity | COPD-ED | 0.97 (1.8) | −0.47 (0.06)* | −1.5 (1.3) | −0.46 (0.84) |
| CBT-I | 2.2 (1.7) | −4.5 (1.2)* | −1.0 (0.82) | ||
| COPD-ED × CBT-I | −0.31 (2.4) | 2.1 (1.8) | 0.14 (1.1) | ||
| Outcome-> | Beliefs and Attitudes about Sleep | Insomnia Severity | Insomnia Severity | ||
| 3. Beliefs and Attitudes about Sleep and Insomnia Severity | COPD-ED | −0.75 (0.35)* | 1.2 (0.35)* | −2.0 (1.3) | −0.90 (0.49) |
| CBT-I | −0.88 (0.33)* | −4.8 (1.2)* | −1.05 (0.50)* | ||
| COPD-ED × CBT-I | 0.64 (0.48) | 2.7 (1.8) | 0.77 (0.62) | ||
| Outcome-> | CRQ Emotional Function | Insomnia Severity | Insomnia Severity | ||
| 4. CRQ Emotional Function and Insomnia Severity | COPD-ED | 0.37 (0.21) | −1.9 (0.57)* | −1.8 (1.3) | −0.72 (0.46) |
| CBT-I | 0.45 (0.21)* | −5.0 (1.2)* | −0.87 (0.47) | ||
| COPD-ED × CBT-I | −0.56 (0.29) | 2.5 (1.8) | 1.1 (0.65) | ||
| Outcome-> | COPD Self-Efficacy | Fatigue | Fatigue | ||
| 5. COPD Self-Efficacy and CRQ Fatigue | COPD-ED | −6.2 (4.4) | −0.01 (0.001)* | 0.16 (0.23) | 0.07 (0.06) |
| CBT-I | −2.7 (4.2) | 0.44 (0.22)* | 0.03 (0.05) | ||
| COPD-ED × CBT-I | 5.9 (6.1) | −0.43 (0.31) | −0.07 (0.08) | ||
| Outcome-> | Sleep Self-Efficacy | Fatigue | Fatigue | ||
| 6. Sleep Self-Efficacy and CRQ Fatigue | COPD-ED | 0.97 (1.8) | 0.03 (0.01)* | 0.18 (0.23) | 0.03 (0.05) |
| CBT-I | 2.2 (1.7) | 0.46 (0.22)* | 0.07 (0.06) | ||
| COPD-ED × CBT-I | −0.31 (2.4) | −0.42 (0.31) | −0.01 (0.07) | ||
| Outcome-> | Beliefs and Attitudes about Sleep | Fatigue | Fatigue | ||
| 7. Belief and Attitudes about Sleep and CRQ Fatigue | COPD-ED | −0.75 (0.35)* | −0.09 (0.06) | 0.29 (0.22) | 0.07 (0.06) |
| CBT-I | −0.88 (0.33)* | 0.51 (0.21)* | 0.08 (0.06) | ||
| COPD-ED × CBT-I | 0.64 (0.48) | −0.58 (0.31) | −0.06 (0.06) | ||
| Outcome-> | CRQ Emotional Function | Fatigue | Fatigue | ||
| 8. CRQ Emotional Function and CRQ Fatigue | COPD-ED | 0.37 (0.21) | 0.44 (0.10)* | 0.18 (0.23) | 0.16 (0.10) |
| CBT-I | 0.45 (0.21)* | 0.49 (0.22)* | 0.20 (0.10) | ||
| COPD-ED × CBT-I | −0.56 (0.29) | −0.46 (0.31) | −0.25 (0.14) |
All models are adjusted for baseline measures of the mediator and outcome. Cell values are parameter estimate (standard error). *P < .05. Italic: P < .10. -> = effects on variables in columns to the right, CBT-I = cognitive behavioral therapy for insomnia, COPD-ED = chronic obstructive pulmonary disease education, CRQ = Chronic Respiratory Disease Questionnaire.
Models 1–4 present the mediated effects on insomnia severity and 5–8 are the mediated effects on fatigue. Generally, the effects of CBT-I were greater on SES, DBAS, and CRQEF; the effect of COPD-ED was greater for COPDSE; and the combined components resulted in attenuated effects. The treatment component effects on mediators (“a” paths) show significant effects for both COPD-ED and CBT-I on DBAS with (nonsignificant) attenuation when components are given together. CBT-I has a significant effect on CRQEF, while COPD-ED and the attenuation from the combined components is marginally significant (P < .10). The mediator effect paths (“b” paths) were highly significant for nearly every mediator predicting both insomnia severity and fatigue. The 1 exception was that DBAS was not related to fatigue. A significant indirect mediation effect was observed for the impact of CBT-I on ISI through DBAS, accounting for 22% of CBT-I’s total effect on insomnia severity.
DISCUSSION
This is the largest study to date designed to examine the efficacy of CBT-I and COPD-ED on insomnia and fatigue in patients with concurrent COPD and insomnia. The study has 3 major findings. First, contrary to our hypothesis, the combination of CBT-I and COPD-ED is not superior to each intervention on its own in decreasing insomnia and fatigue. Second, CBT-I produces significant and sustained decreases in insomnia and clinical improvements in fatigue and dyspnea. Finally, changes in behavior and attitudes toward sleep associated with CBT-I are potential mechanisms through which this nonpharmacological intervention improves insomnia.
Although the benefits of CBT-I have been documented in other patient populations, its benefits have been investigated in only our pilot study and one other study conducted by Rybarczyk and colleagues38 with a sample of 17 participants with COPD. Our results augment theirs showing that, in addition to self-report measures of insomnia and sleep, objectively measured sleep improved in patients after CBT-I compared with patients treated with a COPD-specific active intervention. Our results also add COPD-related evidence to findings of studies that tested CBT-I against active and contact control conditions and found sustained, positive effects on outcomes in other chronic medical or psychiatric conditions.39,40 We found superiority of CBT-I over the COPD-ED in patients with COPD at post-treatment and that the combination of both treatment components may actually reduce long-term effectiveness. Additionally, our findings add new evidence that changes in sleep-related beliefs (DBAS) mediate the decrease in insomnia in patients with COPD who receive CBT-I and evidence suggesting a clinically important improvement in dyspnea for those who received either CBT-I or COPD-ED.
Consistent with our pilot work, we found positive treatment-related effects of the CBT-I intervention on severity of insomnia, WASO, and SE.7 However, the magnitude of improvement was less in the current study than in our previous pilot study. A possible explanation for the difference is that, unlike the pilot study, the factorial study design controlled for attention and allowed for examination of interactions among the components on the outcomes. Another reason could be that the current study included more women than the pilot study, which may have affected the results. Women with COPD likely experience concurrent symptoms differently than men. Investigators have reported differences between men and women in responses to CBT-I that could be attributed to physiological, psychological, and situational dissimilarities.41,42 Additional research is needed to further understand sex-related effects of interventions for insomnia on persons with chronic conditions.
Findings of this study add to those of Parsons and colleagues43 who found a significant effect of CBT-I on insomnia severity through DBAS in their meta-analysis of mediators of change. We found that, in addition to sleep-related beliefs (DBAS), self-efficacy for sleep (SES), self-efficacy for COPD (COPDSE), and self-reported emotional arousal experienced in the previous 2 weeks (CRQEF) were highly significant predicting both insomnia severity and fatigue. This finding supports the possibility that these constructs relate to the outcomes. Future investigations will need to address the effects of enhancing self-efficacy and addressing anxiety and depressed mood on insomnia and fatigue. Parsons et al43 also examined arousal-related mediators and found a partial mediation effect of arousal, while ours did not. These differences could be related to differences in sample size that could have limited our ability to detect fatigue-related significance. In addition, to quantify the emotional arousal experienced in the previous 2 weeks we used the CRQEF. This is an instrument that includes both anxiety and depression questions to measure arousal, whereas the studies in the meta-analysis of Parsons et al43 used primarily anxiety/worry/rumination measures.
Our results augment the understanding of how treatments affect fatigue levels in COPD. Although fatigue levels marginally decreased only from baseline to follow-up in patients who received CBT-I, the decrease was clinically important. In addition, results revealed that patients receiving CBT-I + COPD-ED had an attenuated effect, suggesting the combination of both treatment components reduces CBT-I’s effectiveness on fatigue and refuting our hypothesis that the combination of CBT-I and COPD-ED is superior to each intervention on its own. It is possible that combining CBT-I and COPD-ED limits the focus on each intervention, lessening effects on outcomes. Also, participants continued to have poor sleep that could have contributed to continued fatigue. Results suggest that fatigue was most improved long term for the CBT-I–only group. This could be related to a lasting effect of CBT-I on mediators of the change in insomnia, including beliefs and attitudes about sleep, self-efficacy, and emotional function, which were also associated with fatigue levels. Our findings are in line with a meta-analysis of 47 studies of insomnia therapy by Ballesio et al44 who found that CBT-I administered individually has a role in symptom management, but not specifically on fatigue. Our results are also consistent with others who found that fatigue decreased from pretest to post-test CBT-I in adults with other chronic medical conditions.44 The decrease in fatigue did not reach statistical significance in our study of patients with COPD, which could be attributed to our sample size, as well as differences in study design, measures, and participant characteristics, such as other condition-specific symptoms.
We reason that our finding that the success of insomnia treatment may be related to decreases in a clinically important symptom of COPD, dyspnea, merits further investigation. Considering that dyspnea is the most common and distressing symptom experienced by patients with COPD, it is noteworthy that CBT-I and COPD-ED treatments were associated with a clinically important decrease in dyspnea measured with the CRQD. A possible explanation for the decrease in dyspnea in the CBT-I and COPD-ED groups is that patients with COPD and insomnia may have altered their perception of dyspnea. Previous studies found differences between patients with COPD and healthy controls in the perception and processing of respiratory sensations in the prefrontal cortex, an area of the brain associated with sleep.45–47 Klumpp et al48 reported that, in anxious or depressed patients, worse sleep measured by the Pittsburgh Sleep Quality Index and actigraphy corresponded with less activation in the dorsal anterior cingulate cortex, an area of the brain commonly associated with dyspnea and pain.49 Our findings draw attention to a need for further research on how interventions to improve sleep and minimize fatigue may also affect other symptoms associated with COPD.
Strengths and limitations
This study has several strengths. We enrolled participants with concurrent COPD and insomnia that allowed us to examine intervention components in persons with both conditions. Characteristics of the sample, comprising 36% women and over 70% Black participants, increased the likelihood that findings can be generalized to a variety of people with COPD. We used an efficient factorial study design that allowed us to test the 2 factors alone and combined, as well as to examine additional variables of interest. Our therapists were trained in CBT-I, COPD-ED, and AC and conducted all sessions, limiting therapist bias on effects.
The following limitations must be considered. Over 75% of participants had mild or moderate COPD. Whether patients with severe or very severe COPD would respond to CBT-I and COPD-ED similar to patients with mild or moderate COPD remains to be determined. We also had a lack of power, which may have reduced the ability to detect a true effect in secondary outcomes. In addition, patients randomized to COPD-ED + AC had worse (but moderate) insomnia (ISI 17.0) than patients not randomized to COPD-ED + AC (ISI 14.8) at baseline, which could have affected our results. In addition, as more than half of the participants underwent a screening sleep study at home (home sleep apnea test), it is possible that some with periodic limb movements or those whose apnea-hypopnea index may have been underestimated by a home sleep apnea test may have been included in the study. Finally, the dropout rate was greater among patients with severe COPD and those randomized to COPD-ED + AC than in patients randomized to the other 3 groups. The most common reason given for dropout was lack of interest followed by lack of time. Insomnia therapy conducted remotely could make it much easier for patients to complete the therapy sessions.
The 3 main conclusions of this study are as follows. First, contrary to our hypothesis, the combination of CBT-I and COPD-ED is not superior to each intervention on its own in decreasing insomnia and fatigue in patients with concurrent COPD and insomnia. Second, in these patients, CBT-I produces significant and sustained decreases in insomnia and clinical improvements in fatigue and dyspnea. Finally, changes in behavior and attitudes toward sleep associated with CBT-I are potential mechanism through which this nonpharmacological intervention improves insomnia.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was supported by the National Institute of Nursing Research of the National Institutes of Health under award number R01NR013937. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Study data were collected and managed using REDCap electronic data capture tools hosted at UIC. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources.
ABBREVIATIONS
- AC
attention control
- CBT-I
cognitive behavioral therapy for insomnia
- COPD
chronic obstructive pulmonary disease
- COPD-ED
COPD education
- COPDSE
self-efficacy for COPD
- CRQ
Chronic Respiratory Disease Questionnaire
- CRQD
Chronic Respiratory Disease Questionnaire Dyspnea
- CRQEF
Chronic Respiratory Disease Questionnaire Emotional Function
- CRQF
Chronic Respiratory Disease Questionnaire Fatigue
- DBAS
Dysfunctional Beliefs and Attitudes about Sleep Questionnaire
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- ISI
Insomnia Severity Index
- SE
sleep efficiency
- SES
Self-Efficacy Scale
- UIC
University of Illinois at Chicago
- WASO
wake after sleep onset
REFERENCES
- 1. Ban WH , Joo H , Lim JU , Kang HH , Moon HS , Lee SH . The relationship between sleep disturbance and health status in patients with COPD . Int J Chron Obstruct Pulmon Dis. 2018. ; 13 : 2049 – 2055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Budhiraja R , Parthasarathy S , Budhiraja P , Habib MP , Wendel C , Quan SF . Insomnia in patients with COPD . Sleep. 2012. ; 35 ( 3 ): 369 – 375 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Omachi TA , Blanc PD , Claman DM , et al . Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes . Sleep Med. 2012. ; 13 ( 5 ): 476 – 483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martínez MP , Miró E , Sánchez AI , et al . Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial . J Behav Med. 2014. ; 37 ( 4 ): 683 – 697 . [DOI] [PubMed] [Google Scholar]
- 5. Fleming L , Randell K , Harvey CJ , Espie CA . Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. 2014. ; 23 ( 6 ): 679 – 684 . [DOI] [PubMed] [Google Scholar]
- 6. Heckler CE , Garland SN , Peoples AR , et al . Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial . Support Care Cancer. 2016. ; 24 ( 5 ): 2059 – 2066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kapella MC , Herdegen JJ , Perlis ML , et al . Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects . Int J Chron Obstruct Pulmon Dis. 2011. ; 6 : 625 – 635 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kapella MC , Herdegen JJ , Laghi F , Steffen AD , Carley DW . Efficacy and mechanisms of behavioral therapy components for insomnia coexisting with chronic obstructive pulmonary disease: study protocol for a randomized controlled trial . Trials. 2016. ; 17 ( 1 ): 258 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vestbo J , Hurd SS , Agustí AG , et al . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary . Am J Respir Crit Care Med. 2013. ; 187 ( 4 ): 347 – 365 . [DOI] [PubMed] [Google Scholar]
- 10. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bastien CH , Vallières A , Morin CM . Validation of the Insomnia Severity Index as an outcome measure for insomnia research . Sleep Med. 2001. ; 2 ( 4 ): 297 – 307 . [DOI] [PubMed] [Google Scholar]
- 12. Miller MR , Hankinson J , Brusasco V , et al. ATS/ERS Task Force . Standardisation of spirometry . Eur Respir J. 2005. ; 26 ( 2 ): 319 – 338 . [DOI] [PubMed] [Google Scholar]
- 13. Walters AS , LeBrocq C , Dhar A , et al. International Restless Legs Syndrome Study Group . Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome . Sleep Med. 2003. ; 4 ( 2 ): 121 – 132 . [DOI] [PubMed] [Google Scholar]
- 14. Snaith RP . The Hospital Anxiety and Depression Scale . Health Qual Life Outcomes. 2003. ; 1 ( 1 ): 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PA , Taylor R , Thielke R , Payne J , Gonzalez N , Conde JG . Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform. 2009. ; 42 ( 2 ): 377 – 381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA , Taylor R , Minor BL , et al. REDCap Consortium . The REDCap Consortium: building an international community of software platform partners . J Biomed Inform. 2019. ; 95 : 103208 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perlis ML , Jungquist C , Smith MT , Posner D . Cognitive Behavioral Treatment of Insomnia. Springer, New York; , New York: ; 2005. . [Google Scholar]
- 18. Yang M , Morin CM , Schaefer K , Wallenstein GV . Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference . Curr Med Res Opin. 2009. ; 25 ( 10 ): 2487 – 2494 . [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH , Berman LB , Townsend M , Pugsley SO , Chambers LW . A measure of quality of life for clinical trials in chronic lung disease . Thorax. 1987. ; 42 ( 10 ): 773 – 778 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams JE , Singh SJ , Sewell L , Guyatt GH , Morgan MD . Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR) . Thorax. 2001. ; 56 ( 12 ): 954 – 959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wijkstra PJ , TenVergert EM , Van Altena R , et al . Reliability and validity of the Chronic Respiratory Questionnaire (CRQ) . Thorax. 1994. ; 49 ( 5 ): 465 – 467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jaeschke R , Singer J , Guyatt GH . Measurement of health status. Ascertaining the minimal clinically important difference . Control Clin Trials. 1989. ; 10 ( 4 ): 407 – 415 . [DOI] [PubMed] [Google Scholar]
- 23. Cole RJ , Kripke DF , Gruen W , Mullaney DJ , Gillin JC . Automatic sleep/wake identification from wrist activity . Sleep. 1992. ; 15 ( 5 ): 461 – 469 . [DOI] [PubMed] [Google Scholar]
- 24. Sadeh A , Hauri PJ , Kripke DF , Lavie P . The role of actigraphy in the evaluation of sleep disorders . Sleep. 1995. ; 18 ( 4 ): 288 – 302 . [DOI] [PubMed] [Google Scholar]
- 25. Sateia MJ , Buysse DJ , Krystal AD , Neubauer DN , Heald JL . Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline . J Clin Sleep Med. 2017. ; 13 ( 2 ): 307 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carney CE , Buysse DJ , Ancoli-Israel S , Edinger JD , Krystal AD , Lichstein KL , Morin CM . The consensus sleep diary: standardizing prospective sleep self-monitoring . Sleep . 2012. Feb 1; 35 ( 2 ): 287 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coates TJ , Killen JD , George J , Marchini E , Silverman S , Thoresen C . Estimating sleep parameters: a multitrait–multimethod analysis . J Consult Clin Psychol. 1982. ; 50 ( 3 ): 345 – 352 . [PubMed] [Google Scholar]
- 28. Guilleminault C , Clerk A , Black J , Labanowski M , Pelayo R , Claman D . Nondrug treatment trials in psychophysiologic insomnia . Arch Intern Med. 1995. ; 155 ( 8 ): 838 – 844 . [PubMed] [Google Scholar]
- 29. Morin CM . Insomnia: Psychological Assessment and Management. Guilford Press, New York; , New York: ; 1993. . [Google Scholar]
- 30. Morin CM , Stone J , Trinkle D , Mercer J , Remsberg S . Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints . Psychol Aging. 1993. ; 8 ( 3 ): 463 – 467 . [DOI] [PubMed] [Google Scholar]
- 31. Carney CE , Edinger JD , Morin CM , et al . Examining maladaptive beliefs about sleep across insomnia patient groups . J Psychosom Res. 2010. ; 68 ( 1 ): 57 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacks P . Psychology Practitioner. Pergamon Press; , Oxford, United Kingdom: ; 1987. . [Google Scholar]
- 33. Fichten CS , Libman E , Creti L , et al . Role of thoughts during nocturnal awake times in the insomnia experience of older adults . Cognit Ther Res. 2001. ; 25 ( 6 ): 665 – 692 . [Google Scholar]
- 34. Wigal JK , Creer TL , Kotses H . The COPD Self-Efficacy Scale . Chest. 1991. ; 99 ( 5 ): 1193 – 1196 . [DOI] [PubMed] [Google Scholar]
- 35. Bentsen SB , Wentzel-Larsen T , Henriksen AH , Rokne B , Wahl AK . Self-efficacy as a predictor of improvement in health status and overall quality of life in pulmonary rehabilitation—an exploratory study . Patient Educ Couns. 2010. ; 81 ( 1 ): 5 – 13 . [DOI] [PubMed] [Google Scholar]
- 36. Senn SS . Statistical Issues in Drug Development. John Wiley & Sons; , Hoboken, NJ: ; 2008. . [Google Scholar]
- 37. MacKinnon DP , MacKinnon D . Introduction to Statistical Mediation Analysis. 1 Pap/Cdr. Routledge Academic; 2008. .
- 38. Rybarczyk B , Stepanski E , Fogg L , Lopez M , Barry P , Davis A . A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults . J Consult Clin Psychol. 2005. ; 73 ( 6 ): 1164 – 1174 . [DOI] [PubMed] [Google Scholar]
- 39. Geiger-Brown JM , Rogers VE , Liu W , Ludeman EM , Downton KD , Diaz-Abad M . Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis . Sleep Med Rev. 2015. ; 23 : 54 – 67 . [DOI] [PubMed] [Google Scholar]
- 40. Garland SN , Xie SX , DuHamel K , et al . Acupuncture versus cognitive behavioral therapy for insomnia in cancer survivors: a randomized clinical trial . J Natl Cancer Inst. 2019. ; 111 ( 12 ): 1323 – 1331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lami MJ , Martínez MP , Sánchez AI , et al . Gender differences in patients with fibromyalgia undergoing cognitive-behavioral therapy for insomnia: preliminary data . Pain Pract. 2016. ; 16 ( 2 ): E23 – E34 . [DOI] [PubMed] [Google Scholar]
- 42. Nowakowski S , Meers JM . Cognitive behavioral therapy for insomnia and women’s health: sex as a biological variable . Sleep Med Clin. 2019. ; 14 ( 2 ): 185 – 197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parsons CE , Zachariae R , Landberger C , Young KS . How does cognitive behavioural therapy for insomnia work? A systematic review and meta-analysis of mediators of change . Clin Psychol Rev. 2021. ; 86 : 102027 . [DOI] [PubMed] [Google Scholar]
- 44. Ballesio A , Aquino MRJV , Feige B , et al . The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: a systematic review and network meta-analysis . Sleep Med Rev. 2018. ; 37 : 114 – 129 . [DOI] [PubMed] [Google Scholar]
- 45. Esser RW , Stoeckel MC , Kirsten A , et al . Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease . Front Physiol. 2017. ; 8 : 617 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herigstad M , Hayen A , Evans E , et al . Dyspnea-related cues engage the prefrontal cortex: evidence from functional brain imaging in COPD . Chest. 2015. ; 148 ( 4 ): 953 – 961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reijnders T , Troosters T , Janssens W , et al . Brain activations to dyspnea in patients with COPD . Front Physiol. 2020. ; 11 : 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klumpp H , Roberts J , Kapella MC , Kennedy AE , Kumar A , Phan KL . Subjective and objective sleep quality modulate emotion regulatory brain function in anxiety and depression . Depress Anxiety. 2017. ; 34 ( 7 ): 651 – 660 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. von Leupoldt A , Sommer T , Kegat S , et al . Dyspnea and pain share emotion-related brain network . Neuroimage. 2009. ; 48 ( 1 ): 200 – 206 . [DOI] [PubMed] [Google Scholar]