Abstract
Personal chemical exposure assessment is necessary to determine the frequency and magnitude of individual chemical exposures, especially since chemicals present in everyday environments may lead to adverse health outcomes. In the last decade, silicone wristbands have emerged as a new chemical exposure assessment tool and have since been utilized for assessing personal exposure to a wide range of chemicals in a variety of populations. Silicone wristbands can be powerful tools for quantifying personal exposure to chemical mixtures in a single sample, associating exposure with health outcomes, and potentially overcoming some of the challenges associated with quantifying the chemical exposome. However, as their popularity grows, it is crucial that they are used in the appropriate context and within the limits of the technology. This review serves as a guide for researchers interested in utilizing silicone wristbands as a personal exposure assessment tool. Along with briefly discussing the passive sampling theory behind silicone wristbands, this review performs an in-depth comparison of wrist-bands to other common exposure assessment tools, including biomarkers of exposure measured in biospecimens, and evaluates their utility in exposure assessments and epidemiological studies. Finally, this review includes recommendations for utilizing silicone wristbands to evaluate personal chemical exposure and provides suggestions on what research is needed to recognize silicone wristbands as a premier chemical exposure assessment tool.
Keywords: Human exposure, Personal exposure assessment, Passive sampling, Silicone wristbands, Exposome
1. Introduction
1.1. Environmental chemical exposures
Humans are exposed to a plethora of natural and manmade chemicals in their daily environment, some of which can have unintended consequences on health. Despite knowing generally that certain chemicals could affect human health and the environment, researchers often lack sufficient information about the frequency and magnitude of these exposures to adequately study links between chemical toxicants and health outcomes, and how they could affect health as a mixture. This forms a primary focus of exposure science and environmental epidemiology studies. In 2014, O’Connell et al. introduced the silicone wristband technology as a passive sampler and exposure assessment tool. Commercial silicone wristbands were modified to serve as personal samplers worn by individuals on their wrists, then returned to the laboratory for analysis of a wide array of consumer product and industrial chemicals (i.e., volatile and semi-volatile organic compounds, VOCs and SVOCs). Wristbands are inexpensive and noninvasive and have the potential to revolutionize how researchers can characterize personal chemical exposure. More simply, they may provide insights on the mixtures of chemical toxicants people are exposed to.
Knowledge of chemical exposure has been limited by our ability to measure exogenous chemicals quantitatively and especially mixtures of chemicals simultaneously. To assess human exposure to environmental pollutants, researchers rely on measurements in both biological and environmental matrices and often focus on quantifying individual chemicals or chemical classes. Exposure biomarkers are often measured in biological samples (e.g., urine, serum, milk, hair, saliva) in the form of parent compounds or metabolites and are regarded as representative measures of internal dose. However, such biomarkers are vulnerable to multiple sources of variability both between and within individuals, and, for urinary metabolites in particular, often represent a snapshot of exposure rather than long-term exposure (Aylward et al., 2014). Environmental matrices (e.g., air, dust) are analyzed for predominantly parent chemicals and describe potential external exposure via inhalation, inadvertent dust ingestion, and hand-to-mouth contact. Positive correlations with exposure biomarkers have indicated that these matrices contribute to internal dose, demonstrating that diet is not the only relevant pathway by which people are exposed to chemicals. Prior to the introduction of the silicone wristband, personal sampling techniques (e.g., wearable active air samplers, hand wipes, house dust) have been more prominently used to provide insights on individualized exposures to chemicals, but these methodologies may require trained personnel and can be cumbersome and time-intensive, particularly with air sampling.
Silicone wristbands provide a powerful tool for quantifying personal exposure to chemical mixtures in a single sample and are highly useful for exposure assessment. However, as their popularity grows, it is crucial that they are used in exposure assessment in the appropriate context (i. e., exposure pathway of interest) and within the limits of the technology. Previous reviews on the use of silicone wristband technology have focused on the incorporation of sensors such as wristbands into exposomics methods (Doherty et al., 2021) and provided a brief overview of their general use in exposure assessment, focusing on polycyclic aromatic hydrocarbons (Hamzai et al., 2021). Building on previous work, the overarching objective here was to review the literature to-date with regard to the background theory and current use of silicone wristbands for chemical exposure assessment of VOCs and SVOCs. Here, silicone wristbands were evaluated in the context of existing tools for individual exposure measurements, including alternative silicone configurations and wristband samplers, with a highlight on their associations with exposure biomarkers. Links between exposure measured by wristbands and health outcomes were explored for the purpose of informing their potential use in future epidemiological studies. Further, the strengths and limitations of using wristbands into exposure studies were examined, and recommendations for best practices were considered for future researchers who seek to use this novel technology.
2. Methods
A literature review was conducted to compile relevant articles using Web of Science and PubMed. We utilized the search term “silicone wristband” as our primary search term in both search engines. Web of Science yielded 70 results in total, with 57 articles being included as relevant articles for this review. A PubMed search resulted in 58 articles, 49 of which were relevant to our work here. Articles were removed if they were meeting abstracts, published comments/corrections, review articles, or news articles. Articles were excluded if they pertained to “flexible electronics” athletic activity, if wristbands were used for oxygen treatment, or if the sampling tool was the Fresh Air wristband, which is a different technology than the one discussed here. An additional search was conducted in Google Scholar for any scientific articles citing O’Connell et al., 2014, which was the first research article published utilizing silicone wristbands for exposure assessment. This yielded 14 additional articles for inclusion, resulting in 63 total publications, which will be discussed in this review (Tables 1, 2, and 4). An additional criterion for the inclusion of research articles was that the article was accepted and in press as of December 2021. Additional searches using terms such as “silicone passive sampler” were attempted, but these captured papers that had a broader scope and were not relevant to wristbands capturing personal exposure.
Table 1.
Summary of studies utilizing silicone wristbands to assess personal exposure, excluding studies that additionally evaluated for haelth biomarkers.
| Reference | Sampling Year |
Chemicals of Concern |
Targeted Population | Number of Samples |
Sampling Length |
Units of Measurement |
Location |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Aerts et al., 2018 | 2016 | Pesticide Residues | NA | n = 30 participants, n = 30 participant residences, n = 4 participant workplace, n=8 forest | 5 days | ng/g WB | Belgium |
| Alkon et al., 2021 | 2017–19 | Pesticides | Preschool age children | n = 125 | 30- weekday hours | ng/g WB | Northern California, USA |
| Arcury et al., 2021 | 2018–19 | Pesticides | 8-year-old LatinX children | n = 60 urban non-farmworker families, n = 75 rural farmworker families | 7 days | Number of chemical detections/WB | North Carolina, USA |
| Baum et al., 2020 | 2017 | PAHs | Structural firefighters | n = 72 | 24 hours | ng/g WB | Southern Florida, USA |
| Bergmann et al., 2017 | 2014 | Pesticides and SVOCs | NA | n = 68 | 1 month | ng/g WB | Alto Mayo, Peru |
| Bullock et al., 2020 | 2016 | VOCs and SVOCs | Beehives | n = 10 apiaries across 3 time points, and three locations within each apiary | 24 hours | Number of chemical detections/ WB | southeastern Pennsylvania, USA |
| Caban-Martinez et al., 2020 | 2019 | PAHs | Structural firefighters | n = 15 | 24 hours | ng/g WB | Dominican Republic |
| Craig et al., 2019 | 2016–17 | Phthalates, phthalate alternatives, OPEs | Nail Salon Workers (females, >18yo) | n = 9 wristbands and pinned lapels each | 1 work day | ng/g WB | Boston, Massachusetts, USA |
| Dixon et al., 2018 | 2013–15 | PAHs | Pregnant women | n = 22 | 48 hours | ng/WB | New York, New York, USA |
| Hendryx et al., 2020 | 2017–18 | PAHs | Appalachian coal mining communities with surface coal mining | n= 101 | 7 days | ng/g WB | Rural communities in the Central Appalachian portion of southern West Virginia, eastern Kentucky, and western Virginia |
| Hoffman et al., 2021 | 2018–19 | OPEs | North Carolina adults | n= 10 (1 wristband per day) | 5 days | ng/g WB | North Carolina, USA |
| Kile et al., 2016 | 2012–13 | PBDEs, OPEs, and HFRs | Preschool children (3–5yo) | n = 72 | 7 days | ng/g WB/day | Lane and Benton Counties, Oregon, USA |
| Levasseur et al., 2021 | 2014–16 | Environmental phenols | Children aged 3–6 | n = 76 | 7 days | ng/g WB | central North Carolina, USA |
| Dixon et al., 2019 | 2014–17 | VOCs and SVOCs | NA | n = 262 worn by 246 volunteers | Varied | Number of chemical detections/WB | Locations within Africa, North America, and South America |
| Doherty et al., 2020 | 2017–18 | VOCs and SVOCs | Pregnant women | n = 255 at 12 gestational weeks, n = 20 at 24 gestational weeks | 7 days | ng/g WB | New Hampshire, USA |
| Donald et al., 2016 | 2014 | Pesticides | Farmworkers | n = 35 for two time periods | 5 days | ng/g WB | Diender, Senegal |
| Gibson et al., 2016 | -- | OPEs | Mother and Child (3–6 yo) pairs | n = 32 pairs for two consecutive periods | 7 days | ng/g WB | New York, New York, USA |
| Hammel et al., 2016 | 2015 | OPEs | NA | n = 40 | 5 days | ng/WB | Durham, North Carolina, USA |
| Hammel et al., 2018 | 2016 | HFRs | NA | n = 30 | 7 days | ng/g WB | Durham, North Carolina, USA |
| Hammel et al., 2020 | 2015–16 | OPEs & phthalates | Children (3–6 yo) | n = 77 | 7 days | ng/g WB | North Carolina, USA |
| Harley et al., 2019 | 2016 | Pesticides | Latina adolescent girls in a farm working community | n = 97 | 7 days | ng/g WB/day | Salinas Valley, California, USA |
| Manzano et al., 2019 | 2016–17 | Targeted and untargeted SVOC analysis | Individuals in areas with different geographical/socioeconomic characteristics | n = 27; two time points (winter & summer) | 5 days | ng/g WB | Santiago, Chile |
| Mendoza-Sanchez et al., 2021 | 2015–16 | PAHs | Pregnant women | n = 17 for three consecutive periods | 24 hours | ng/WB | McAllen, Texas, USA |
| Nguyen et al., 2020 | 2017 | PBDEs & HFRs | e-waste recycling facility workers | n = 45 workers; n = 45 silicone brooches, n = 28 wristbands, and n = 9 armbands | 1 work day | ng/dm2 WB/h | Québec, Canada |
| O’Connell et al., 2014 | 2012 | PAHs | Roofers using hot asphalt | Worksite 1 (n = 3), worksite 2 (n = 5). Single, stacked and lapel/brooch wristbands deployed to every participant | 8hrs and workweek (32–39 hrs) | ng/WB | Willamette Valley, Oregon, USA |
| Paula Corrêa et al., 2021 | 2016–17 | VOCs & SVOCs | NA | n = 2 | 6 hours | ng/g WB | Brazilian cities |
| Paulik et al., 2018 | 2014 | PAHs | Individuals in close proximity to natural gas extraction sites | n= 19 | 3 weeks | ng/g WB | Carroll County, Ohio, USA |
| Poutasse et al., 2020 | 2018–19 | PAHs, VOCs & SVOCs | Structural Firefighters | nlow volume = 29; nhigh volume = 27 for on duty and off duty | 30 on-duty and off duty days | pmol/g tag | Kansas City, Kansas/Missouri, USA |
| Poutasse et al., 2022 | 2018–19 | Potential Endocrine Disruptors | Structural Firefighters | nlow volume= 29; nhigh volume = 27 for on duty and off duty | 30 on-duty and off duty days | nmol/tag | Kansas City, Kansas/Missouri, USA |
| Quintana et al., 2019 | 2017 | Nicotine | Children (4–14 yo) who lived with/without smoking adults | n= 31 children | 2 and 7 days | ng/WB | San Diego, California, USA |
| Santiago et al., 2021 | 2018 | PAHs | Fishermen | n= 17 | 24 work hrs | ng/g WB | Mississippi & Florida, USA |
| Swanson et al., 2018 | 2015–16 | Pesticides | Northern leopard frogs (Lithobates pipiens) | n = 72 | 2 weeks | ng/WB | northern Iowa, USA |
| Travis et al., 2020 | 2018 | PCBs, pesticides, PBDE, OPEs, HFR | Elementary school children | n = 24 | 7 days | ng/g WB | Montevideo, Uruguay |
| Quintana et al., 2021 | 2017–18 | Nicotine, cotinine, and tobacco-specific nitrosamines | Children (4–14 yo) who lived with/without smoking adults | n= 53 children, two wristbands were worn at the same time for 7 days, an additional wristband was worn the last two days | 2 and 7 days | ng/g WB | San Diego, California, USA |
| Reche et al., 2020 | 2019 | PAHs and personal care products | Volunteers in close proximity to professional athletes | n = 5 | 4.4 days | ng/WB | Yokohama, Kanagawa, Japan |
| Reddam et al., 2020 | 2019 | OPEs | University of California, Riverside students who commute | n = 89 | 5 days | ng/g WB | Riverside, California, USA |
| Rohlman et al., 2019 | 2015 | PAHs | Individuals with moderate to severe asthma | n= 10 | 7 days | ng/WB | Eugene, Oregon, USA |
| Rohlman et al., 2019 | 2016–17 | PAHs | Native American Communities | Spring (n = 10), Winter (n=22) | 7 days | ng/g WB | Swinomish Indian Tribal Community, Washington, USA |
| Roodt et al., 2018 | NA | Skin VOCs and SVOCs | NA | Subject 1 (6 wristbands, and 6 anklet samples), subject 2 (2 wristbands during 2 separate sample collections). | 4 hours | Normalized mean peak area | Pretoria, South Africa |
| Wang et al., 2019 | 2018 | PAHs, PBDE, HFRs, and OPEs | NA | n= 10 | 72 hours | ng/WB | Bloomington, Indiana, USA |
| Wang et al., 2020 | 2018–19 | PAHs, PBDEs, HFRs, and OPEs | NA | France (n = 40) and Italy (n = 31) | 5 days | ng/g WB | Turin, Italy and France |
| Wang et al., 2020 | 2018 | PBDEs, HFRs, OPEs, and dechlorane plus (DPs) | e-waste recycling facility workers | n= 15 | 24 hours | ng/WB/h | Dhaka, Bangladesh |
| Wise et al., 2020 | 2018 | VOCs & SVOCs | Human dog pairs | n = 30 human and dog pairs | 5 days | ng/g WB | Wake County, North Carolina, and New Jersey, USA |
| Wise et al., 2021 | 2018 | Pesticides | Human dog pairs | n = 30 human and dog pairs | 5 days | ng/g WB | Wake County, North Carolina, and New Jersey, USA |
| Wooding et al., 2020 | NA | Skin VOCs and SVOCs | NA | n = 20 (replicates of three for each wristband & anklet sampler) sampled on 5 consecutive days | 1 hr | Normalized mean peak area | Pretoria, South Africa |
| Xie et al., 2021 | 2018–19 | OPEs | Mother-child pairs | n = 47 mother- child pairs | 2 weeks | ng/WB | Guangzhou, South China |
| Young et al., 2021 | 2019 | PCBs, PBDEs, HFRs, OPEs, phthalates & phthalate alternatives, pesticides, & PAHs | Adult workers in office buildings | n = 251 from 36 office buildings | Monday-Thursday during work hrs. | ng/g WB | USA, UK, China, & India |
Table 2.
Summary of studies utilizing silicone wristbands to assess personal exposure in conjunction with evaluation for health biomarkers.
| Reference | Sampling Year |
of Concern | Targeted Population |
Number of Samples |
Study Length |
Units of Measurement |
Location | Health Endpoint |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Andersen et al., 2021 | 2018 | PAHs & OPEs | Air Force ground crew personnel | N = 79 | Working hours for 3 days | ng/g WB/day | Danish Air Force bases | Lung function, systemic inflammation, and genetic damage |
| Doherty et al., 2021 | 2017–19 | VOCs and SVOCs | Pregnant women | n= 177 | 7 days | ng/g WB | New Hampshire, USA | Plasma metabolome |
| Hardos et al., 2020 | -- | OPEs | Aircraft maintenance workers | n = 76, 2 WBs per person | 1 work day | ng/WB | Moody Air Force Base Georgia, Hill Air Force Base Utah and Davis-Monthan Air Force Base Arizona USA | Cholinesterase inhibition |
| Kassotis et al., 2020 | 2017–19 | HFRs, OPEs, pesticides, and phthalates | Papillary Thyroid Cancer Patients | n=72; case control study n=36 cancer and non -cancer patients | 7 days | ng/g WB | central North Carolina, USA | Thyroid Receptor antagonism |
| Lipscomb et al., 2017 | 2012–13 | PBDEs, OPEs, and HFRs | Preschool children age 3–5 | n = 72 | 7 days | ng/g WB/day | Lane and Benton counties, Oregon, USA | Social Behaviors |
| Poutasse et al., 2019 | 2017–28 | PBDEs, OPEs, and HFRs | Cats age 7 or older | n = 78; case control study n=39 ‘ hyperthyroid and non hyperthyroid cats | 7 days | pmol/g pet tag | New York, New York & Corvallis, Oregon USA | Hyperthyroidism |
| Vamell et al., 2021 | 2018 | Pesticides | Latina Farmworkers age 13–20 | n = 44 | 24 hours | Number of chemical detections/WB | North Carolina, USA | Menstrual cycle patterns |
| Vidi et al., 2021 | -- | Pesticides | Latinx children age 7–9 from farm working families | n= 10 | 7 days | ng/g WB | Benson, North Carolina, USA | DNA damage in hair follicles |
| Wang et al., 2020 | 2017–18 | PBDEs, OPEs, and HFRs | NA | n= 101 | 7 days | ng/g WB | Rural Central Appalachia | Thyroid function |
Table 4.
Publications related to the theory and quantification of chemicals absorbed to SPSDs in chronological order.
| Ref. | Publication Goal | Study Results |
|---|---|---|
|
| ||
| O’Connell et al., 2014 | Establish silicone wristbands as an effective passive sampling tool. | • Established laboratory practices concerning cleaning, infusion, and extraction of SPSD. • Measured 49 chemicals within SPSD extracts with KoW ranging from −0.07 to 9.49. • SPSDs were used in an occupational setting for the first time and demonstrated spatial sensitivity. |
| Anderson et al., 2017 | Establishing wristband practices and user guidelines | • Without proper conditioning analytical sensitivity is decreased, and the instrument will require a high degree of maintenance • Wristbands are stable for transport in air-tight containers at ambient air temperatures for 1–2 weeks. • Wristbands are stable for storage at 4°C for SVOCs and VOCs for up to 3 months, stable for longer if stored at −20°C. • Wristbands are suitable for sampling chemical classes with a log KOA range of 2.1 – 13.7. |
| Bergmann et al., 2018 | Development of a quantitative screen for 1550 chemicals using gas chromatography mass (GC-MS) spectrometry for use with analysis of silicone wristbands. | • Created a targeted analysis for a large number of chemicals ranging in phys-chem properties using a predictive model and Automated Mass Spectral Deconvolution and Identification System. • Analytical method improves efficient environmental monitoring paired with passive samplers such as silicone wristbands. |
| Romanak et al., 2019 | Development of a GC-MS method capable of screening 77 SVOCs from four chemical categories (PBDEs, HFRs, OPEs and PAHs) for use with SPSDs. | • Created a targeted analysis method for use with silicone wristbands that minimizes chromatographic interferences such as siloxanes and lipids. • Method was applied to wristbands worn by 10 individuals over a 7-day period, and found the method was capable of capturing personal exposure to various levels of target analytes. |
| Donald et al., 2019 | Co-deployment of LDPE and silicone wristbands to determine chemical flux above turf fields, and silicone-air portioning coefficients. | •Thermal extraction is a viable extraction method for silicone passive samplers. • Partitioning coefficients were derived for use in future studies. • Turf pore air concentrations measured by LDPE and air concentrations measure by SPSD were correlated. |
| Tromp et al., 2019 | Assess the variability of chemical uptake and uptake capacity into silicone sheets, silicone wristbands, and PUF. | • Derived a relationship for estimating sampling rates for passive samplers. • Differences amongst samplers in chemical uptake rates for gas-phase chemicals were not found. • Silicone-air partitioning coefficients were determined for 98 chemicals. |
| Travis et al., 2021 | Optimize a workflow for un-targeted analysis of silicone wristbands, provide confidence levels to features observed using high resolution mass spectroscopy – electron ionization, and evaluate different sample preparation techniques to maximize detections using orbitrap MS. | • Cleanup of complex matrices is necessary to produce accurate and reproducible results. • A workflow for non-targeted analysis was optimized and includes data acquisition, peak picking, feature filtering, and finally feature identification. |
| O’Connell et al.,2021 | Utilize measurements of silicone uptake and a chemicals boiling point to create predictive models for silicone-air partitioning coefficients. | • Data from silicone samplers were translated into an equivalent air concentration, that can be compared to regulatory air concentrations. |
For the purposes of this review, silicone wristbands were defined as passive sampling devices (PSDs), composed solely of polydimethylsiloxane (PDMS), either in the form of a wristband or an alternative configuration, worn on an individual or organismal unit for the duration of the sampling period. As such, other sampling devices worn on the wrist, including various configurations mounted on bracelets, are not included within the scope of this review.
3. Silicone passive sampling background
3.1. Silicone passive samplers
PSDs, including silicone wristbands, are composed of hydrophobic polymers that sequester organic molecules from the environment. Organic chemicals in the environmental media (e.g., sediment, air, soil, and water) are sequestered into PSDs via diffusion (Huckins et al., 2006). Because chemicals continually accumulate in passive sampling devices over time, the sensitivity of analytical detection is increased with time. This is also why passive sampling devices do not represent an episodic concentration, but rather a time-weighted average. Silicone wristbands are a type of PSD. Silicone wristbands used for personal exposure sampling have been primarily bought commercially, but have also been created from PDMS sheets and tubing.
3.2. Chemical uptake
Uptake into PSDs is unique to each chemical based on its physical-chemical properties, environmental concentrations, environmental conditions, and exposure time. Physical-chemical properties play a role in how compounds distribute in their environments, which can be predicted using chemical partitioning coefficients (i.e., how chemical molecules partitions between two phases (e.g., water, air, natural solids, or organisms)). To determine chemical partitioning in a laboratory setting, the organic solvent, n-octanol, is used as a surrogate to evaluate potential partitioning across cell membranes and into organisms. The octanol-air partition coefficient, log Koa, is most commonly used to estimate the rate at which chemicals will sequester into passive sampling devices (Huckins et al., 2006; Anderson et al., 2017; Donald et al., 2019; Shoeib and Harner, 2002; Shoeib and Harner, 2002; Harner and Shoeib, 2002; Okeme et al., 2018; Okeme et al., 2018). More specifically, it provides insight into the relative partitioning between air and organic fractions, such as soils, vegetation and lipid membranes (and thus into organisms). Silicone wristbands have previously sampled chemicals with log Koa ranging from 3.3 to 16. This includes smaller, more volatile compounds such as solvents (e.g., toluene) to higher molecular weight compounds such as flame retardants or plasticizers (Bergmann et al., 2018). This wide range enables the silicone wristband to function as a broad, nonspecific organic chemical sampler.
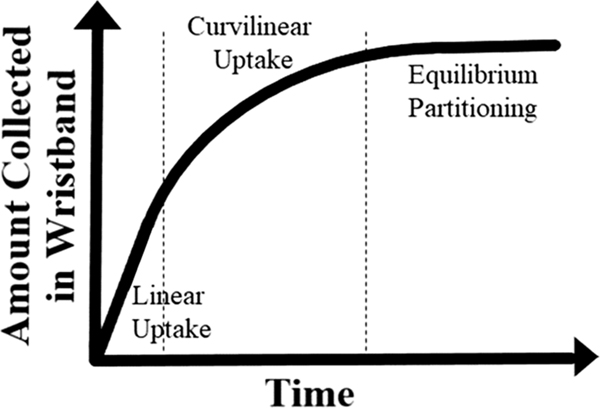
The rate of uptake of chemicals into PSDs is not consistent over time, and changes as a chemical approaches equilibrium between the environment and the PSD. This occurs in phases referred to as linear, curvilinear, and equilibrium (Fig. 1) (Huckins et al., 2006; O’Connell et al., 2021).Assuming that when a PSD is first deployed there are no chemicals in it, chemical uptake into the passive sampling device would first be in the linear phase. The linear phase is described as the period of time where uptake is occurring into the PSD at a constant rate, and there is greater potential for molecules to move into the PSD. The rate of uptake begins to slow as individual compounds near equilibrium, which is described as the curvilinear phase. At the curvilinear phase some amount of a compound has already partitioned into the PSD, and the potential for compounds to move inside the PSD decreases until thermodynamic equilibrium is reached. When in equilibrium, the potential for a compound to move between the PSD and the environmental media it’s sampling is equal, but depending on the character of the media and chemical physical properties of the chemical in question, the concentrations in the PSD and the environment it’s sampling may not be equal. For example, a compound that is hydrophobic is more likely to have a higher concentration in octanol than water when the potential across those two phases are equal. The same principle applies with uptake into a wristband; a hydrophobic compound will have a greater concentration in the wristband than the environmental media at equilibrium.
Fig. 1.
Theoretical chemical uptake curve for silicone wristbands over time. Every chemical will have an uptake curve for its physical-chemical properties.
The amount of chemical collected by the PSD depends on both the concentration in the environmental media and deployment time (Górecki and Namiésnik, 2002). The concentration obtained from the passive sampling device is a time weighted average. However, this is only true when certain conditions are met. First, the PSD must act as a “zero sink”, meaning once chemicals are trapped within the PSD they are not released back into the environmental media. Second, the chemical uptake rate of compounds into the PSD, must remain within the linear uptake phase (Huckins et al., 2006; Górecki and Namiésnik, 2002). The compounds frequently targeted when utilizing silicone wristbands tend to have a higher affinity for the PSD and once bound are not readily lost (desorbed) to the environment and the wristband thus acts as a “zero sink” (Booij and Smedes, 2010; Booij et al., 2016). However, since silicone wristbands are often utilized on individuals who are moving, in extreme cases one chemical could move between the phases of uptake many times during the duration of the deployment while a participant is migrating between microenvironments. Additionally, if equilibrium is reached the concentration gathered by the wristband is no longer a time weighted average. Calculating environmental concentrations using passive sampling devices often assumes that the compound is in the linear uptake phase (Huckins et al., 2006), and thus doing such calculations could be complicated if samplers are allowed to reach equilibrium. Currently more research is needed on the uptake and possible depuration (i.e. molecules moving out of the wristband into the environment) kinetics of silicone wristbands as well as the importance deployment duration/final uptake phase on reported concentrations.
3.3. Biomimetric properties
Much like how chemicals sequester into living organisms, passive sampling devices do not sequester all chemicals in the environment. The portion of chemicals that the PSDs captures is an estimate for the portion of chemicals in the environment that can transport across cellular membranes (Booij et al., 2016; Forsberg et al., 2014; Paulik et al., 2016; Minick et al., 2019). When evaluating environmental exposure, it is important to keep in mind that not all chemicals in the environment will be sequestered into living organisms. If the goal for a study is to examine the relationship between chemical exposures and health effects, then researchers should primarily be concerned with the fraction of chemicals in the environment that could enter an organism. Since silicone PSDs mainly reflect that fraction, they are ideal for chemical exposure and health assessment studies.
4. Measuring chemical exposure with personal silicone samplers
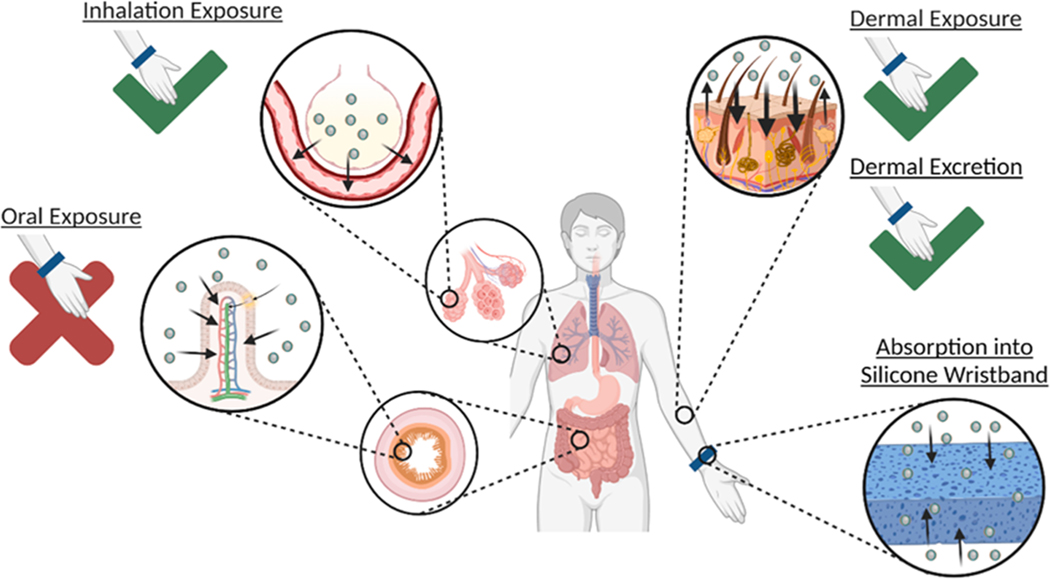
Silicone wristbands, like all PSDs, sample the chemicals in the environment surrounding them. When wristbands are worn on the wrist with dermal contact, these include vapor phase compounds as well as compounds located on the skin (Wang et al., 2019; O’Connell et al., 2014). Silicone wristbands are thought to account for inhalation exposure since they capture a portion of the volatile and semi-volatile chemicals that can be inhaled. Additionally, dermal contact with the wristband enables capture of a portion of the chemicals located on the skin. Compounds located on the skin can be due to direct contact with environmental contaminants (Wang et al., 2019), excretion of chemicals and their metabolites dermally (Bergmann et al., 2018; Quintana et al., 2021), and compounds associated with the skin microbiome (Roodt et al., 2018; Wooding et al., 2020). In summary, silicone wristbands capture a combination of inhalation exposure, dermal exposure, and dermal excretion (Fig. 2).
Fig. 2.
Routes of chemical exposure include inhalation exposure, dermal exposure, and oral exposure. The wristband is able to capture a representative fraction of both inhalation exposure and dermal exposure. The wristband is also able to capture dermal excretion.
4.1. What silicone wristbands have been used to measure
As shown in Table 1 and Table 2 silicone wristbands have been deployed for a range of sampling lengths and silicone wristband extracts have been reported in a variety of measurements. The sampling length ranged from four hours to 30 days. The most frequently used sampling lengths were seven days (38% of studies) and five days (16% of studies). The ideal length of sampling time will depend on target analytes’ physical chemical properties and uptake into the wristband. Other considerations that need to be made are whether the researcher wants to investigate concentrations that are at equilibrium or in the linear uptake phase. These are also important considerations for how wristband extract concentrations should be reported. Current units of measurement included in the literature include mass target analyte/wristband, mass target analyte/mass of wristband, and mass target analyte/mass of wristband/sampling length.
Most research utilizing silicone wristbands have focused on measuring environmental VOCs SVOCs, which comprise of chemicals from several different chemical classes. Chemical categories that have been measured using silicone wristbands include polycyclic aromatic hydrocarbons (PAHs), oxygenated PAHs (OPAHs), polychlorinated biphenyls (PCBs), phthalates and non-phthalate plasticizers, dioxins and furans, environmental phenols, pharmaceuticals (i.e., nicotine), pesticides, and flame retardants. Flame retardants can include polybrominated diphenyl ethers (PBDEs), halogenated flame retardants (HFRs), and organophosphate esters (OPEs). Many chemical categories can also fall under the pesticide umbrella: organochlorines, organophosphates, neonicotinoids, pyrethroids, amides, pyrazoles, and more. While not a single chemical category, multiple papers have focused on exposure to endocrine disruptors (Poutasse et al., 2022; Varnell et al., 2021; Dixon et al., 2019).Two studies have utilized silicone wristbands to measure VOCs and SVOCs that originate from the human cells and skin microflora (Roodt et al., 2018; Wooding et al., 2020). There have been a few analytical methods explicitly developed for silicone wristbands. These include a quantitative screen for 1530 targeted organic compounds (Bergmann et al., 2018), a targeted analysis for 77 compounds including PBDEs, HFRs, OPEs, and PAHs (Romanak et al., 2019), and lastly, a non-targeted analysis using high-resolution mass spectroscopy - electron ionization (Travis et al., 2021).
Silicone wristbands can be easily integrated as exposure assessment tools for various unique study populations and have hence been utilized in diverse communities across several continents. For instance, wristbands were first used occupationally to assess exposure in roofers using hot asphalt (O’Connell et al., 2014) but have since expanded to sample various populations where occupational exposure to environmental contaminants is likely. These include firefighters, agricultural workers, e-waste recycling facility workers, fishermen, aircraft maintenance workers, air force personnel, and office workers. Using wristbands in occupational settings provides unique opportunities to easily characterize potential acute exposure over a short time period, such as a single workday, without overburdening workers with multiple sampling devices (e.g., active air sampling packs).
In a similar vein, wristbands have been used to evaluate exposure to those who may be exposed to environmental contaminants due to proximity, such as families of agricultural workers, individuals living in coal mining communities, individuals living near natural gas drilling pads, individuals who commute on heavily trafficked roads, and individuals living near a natural-technological disaster (Oluyomi et al., 2021). Lastly, wristbands have been used to sample populations more highly susceptible to adverse health outcomes such as children, pregnant women, and individuals with preexisting health conditions. Outside of the use of silicone wristbands as personal human passive sampling devices, they have also been used as stationary air samplers and to evaluate chemicals exposure in animals. This includes bees and frogs, as well as felines and canines as sentinel species. For a complete list of personal exposure studies, including target chemicals and populations, see Table 1 and Table 2 for studies that included health biomarkers.
5. Comparison to other exposure assessment tools
5.1. Biological samples
Often touted as the gold standard for quantifying human exposure, measurements of chemical biomarkers in biological matrices, such as urine and serum, have been used extensively to evaluate exposures to SVOCs (Aylward et al., 2014). These exposure biomarkers are useful for evaluating internal dose of a compound and verifying that an external exposure resulted in the compound entering the human body. Biomarkers allow for the analysis of chemical concentrations and trends over time in population-wide examinations [e.g., United States National Health and Nutrition Examination Survey (NHANES) (Choi et al., 2015), Canadian Health Measures Survey (CHMS) (Choi et al., 2015), Human Biomonitoring for EU (HBM4EU) (Apel et al., 2020), Korea National Survey for Environmental Pollutants in the Human Body (KorSEP) (Choi et al., 2015), China National Human Biomonitoring (CNHB) (Cao et al., 2021), Japan Environment and Children’s Study (JECS) (Kawamoto et al., 2014). They also serve as an integrative measure for all pathways of exposure by which individuals may encounter an environmental chemical.
However, identifying exposure biomarkers with adequate sensitivity and specificity for effective epidemiological studies can be difficult (Mayeux, 2004). Further, evaluating differences in biomarkers based on factors contributing to inter- and intra-individual variability (e.g., metabolism rates, chemical toxicokinectics, timing of exposure, timing of sample, body mass index) can lead to limitations in how these levels can be interpreted across populations (Aylward et al., 2014; Mayeux, 2004; Koch et al., 2014). Variation in persistence of chemicals in the body can lead to exposure misclassification, particularly for compounds with short half-lives and when spot samples are used to indicate individual exposures. Spot urine samples only represent a snapshot of what that individual is exposed to at a single time point, and repeated sampling would be required to characterize long-term exposure. Certain chemicals or their metabolites in blood could have longer half-lives, which could reflect more historical exposure, but would have limited utility for determining when a person was exposed. Additionally, while biomarkers indicate the presence of xenobiotics in the body and may be correlated with external exposure measures, they may not represent a mass balance for total exposure (e.g., currently measured metabolites of di(2-ethylhexyl) terephthalate (DEHTP) only account for 16% of total ingested dose) (Lessmann et al., 2016). Further, not every chemical measured in the external environment has a known measurable exposure biomarker. For instance, the CDC measures about seven OPAHs that are relevant biomarkers in urine to PAH exposure; however, with increasing molecular weight of a PAH, less is excreted via urine, and not every PAH measured in an external matrix (e.g., wristband, dust, hand wipe) has a corresponding urinary biomarker (Aylward et al., 2014). It is also extremely difficult and expensive to identify appropriate biomarkers, particularly for chemicals that are rapidly metabolized, and chemicals that have multiple metabolites (e.g., certain pesticides (Sudakin, 2006; Buratti et al., 2007).
Collection of biological samples can also be invasive (e.g., blood sampling), thereby making biospecimen collection challenging, especially among children. Once collected, the biospecimens themselves typically need to be frozen or kept on ice to maintain sample’s integrity and biomarkers, whether parent compounds or metabolites, as they tend to be sensitive to temperature changes. Sample volumes also tend to be small, and sample analyses are expensive, leading to limited numbers of targeted analytes available for evaluation (Forsberg et al., 2011). In addition, this limitation reduces the ability for researchers to evaluate exposure to mixtures across classes of compounds, which may be necessary for evaluating health outcomes.
Silicone wristbands overcome these challenges and do not have these same limitations (Fig. 2). They are easily worn on the wrist and therefore are minimally invasive. They are stable for transport in cold and hot temperatures with minimal chemical loss, and they can by analyzed for hundreds of chemicals (Anderson et al., 2017; O’Connell et al., 2014; Dixon et al., 2020). In validating the use of silicone wristbands as a measure of personal exposure, it has been important to determine if wristband chemical concentrations correspond to internal dose measurements (i.e., exposure biomarkers). To date, wristband concentrations of SVOCs have been compared primarily to corresponding biomarkers in urine, with two papers including serum measurements (Table 3).
Table 3.
Comparison of silicone wristbands as passive sampling devices to other sampling approaches.
| Other personal exposure sampling approaches |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref. | Target Compounds |
Urine | Plasma | Hand wipes |
Dust | Active air sampling |
Passive air sampling (PUFs) |
Alternative Silicone Device |
Key Conclusions |
|
| |||||||||
| Anderson et al, 2021 | PAHs & OPEs | X | X | • The correlation between PAH levels measured in different matrixes was weak. • Levels of PAHs & OPEs measured in silicone wristbands and urinary metabolites were not consistent throughout sampling. • Urine samples were taken as spot samples before sampling with wristbands. |
|||||
| Craig et al., 2019 | SVOCs (phthalates, phthalate alternatives, organophosphate esters) | X | lapel | • For DEHP urine metabolites were more highly correlated to lapel than wristbands (non-significant). • For DEHTP urinary metabolites were moderately correlated with lapels and wristbands. • Spot urine samples were taken before and after the wristband sampling event and the differences between the two urine samples metabolites were correlated to lapels and wristbands. |
|||||
| Dixon et al., 2018 | PAHs | X | PUFs-filters | • Two comparisons between PAHs found on PUFs-filters correlated significantly with urinary metabolites. • Six comparisons between PAHs found on wristbands correlated significantly with urinary metabolites. • Spot urine samples were collected at the end of the wristband sampling period. |
|||||
| Gibson et al., 2019 | Organophosphate ester flame retardants | X | • Three flame retardants (TPHP, TDCIPP, and TCIPP) measured in wristbands were associated with urinary metabolites. • Spot urine samples were taken at baseline and after one and two weeks of sampling. Wristband concentrations during week one were correlated to the week one urine samples & wristband concentrations during week two were correlated to the week two urine samples. |
||||||
| Hammel et al., 2016 | Organophosphate flame retardants | X | X | • Levels of TDCIPP and TCIPP on wristbands were more significantly correlated with urinary metabolites then levels of TDCIPP and TCIPP on hand wipes. • There was also significant correlation between TDCIPP and TCIPP measured using wristbands and handwipes. • Urine samples utilized for comparison were pooled from the first morning void collected on three separate days during the five-day sampling scheme. • Hand wipes were collected at the end of the wristband sampling period. |
|||||
| Hammel et al., 2018 | Brominated flame retardants | X | • Wristband levels for four polybrominated diphenyl ether were significantly correlated with serum biomarkers. • Serum samples were collected at the start of the wristband sampling period. |
||||||
| Mendoza-Sanchez et al., 2021 | PAHs | Filter and XAD sorbent | Wristbands worn on active sampling backpack | • Concentrations of the PAHs varied across sampling matrix. • PAH profiles in filters were composed of small amounts of many PAHS, but XADs and WBs were predominated by only a few PAHs. |
|||||
| Hammel et al., 2020 | Organophosphate esters & phthalates | X | X | X | •Six OPEs and four phthalates were significantly associated with corresponding urinary metabolites. • There was some significant correlation between OPEs measured in wristbands and hand wipes, but dust wristbands were not correlated to dust. • Phthalate concentrations found in dust was not significantly associated with hand wipes, dust, or urinary metabolites. • Three spot urine samples were collected during the wristband sampling period and pooled. • Hand wipes and home dust samples were collected at the start of the wristband sampling period. |
||||
| Hendryx et al., 2020 | PAHs | Stationary indoor & outdoor | • Three significant correlations were found between PAH concentrations in wristbands and outdoor air. Correlations between indoor air and wristbands were not included. | ||||||
| Hoffman et al., 2021 | Organophosphate esters |
X | •OPE uptake in wristbands occurred linearly during the study period. • TDCIPP and TCIPP wristband and spot urine samples were moderately to strongly correlated to internal dose, while DPHP wristband and spot urine samples were not correlated. • Spot urine samples and a 24hr pooled urine sample were collected for each sampling day. |
||||||
| Levasseur et al., 2021 | Environmental phenols | X | X | X | •Ethyl, methyl, and propylparaben levels observed in hand wipes, dust and on wristbands were significantly correlated to their associated urinary metabolites. •Correlations to urinary biomarkers were generally larger for wristbands than correlations for dust, and correlations were similar to or greater than hand wipe correlations. •Three spot urine samples were collected during the wristband sampling period and pooled. •Hand wipes and home dust samples were collected at the start of the wristband sampling period. |
||||
| Wang et al., 2020 | PBDEs, HFRs, OPEs, & dechlorane plus (DPs) | •Silicone wristbands and cotton t-shirts were both used to assess exposure. • Concentrations of 5 compounds in t-shirts were significantly correlated to WBs. • Wristbands accumulated approximately 7 times the mass than the cotton t-shirts. • Utilized a silicone “sandwich where there is direct contact of silicone with a textile to calculate partitioning of chemicals from textile to silicone. |
|||||||
| Nguyen et al., 2020 | Flame retardants | X | X | OVS cartridges | Brooch and armband | •Stronger correlations were found between active air samplers and brooches, compared to wristbands and armbands. • BDE-209 concentrations in brooches and wristbands were moderately correlated with levels in blood plasma. • OPEs in brooches and wristbands were not correlated with urine or plasma biomarkers. • Spot urine samples were collected at the end of the wristband sampling period and plasma samples were collected the following day. |
|||
| O’Connell et al., 2014 | PAHs | Lapel and stacked wristbands | • There was no statistical difference between wristband configurations and most discrepancies could be explained by the silicone being covered by protective clothing. | ||||||
| Quintana et al., 2019 | Nicotine | X | 2-days vs 7-day wristband | •Nicotine detected in wristbands for both two and seven days were highly correlated with the urinary biomarker cotinine. • Concentrations found in the 2-day and 7- day wristbands were significantly correlated. • Spot urine samples were collected at the end of the sampling period. |
|||||
| Quintana et al., 2021 | Nicotine, cotinine, and tobacco-specific nitrosamines | X | X | 2-days vs 7-day wristband | • Concentrations found in the 2-day and 7- day wristbands were significantly correlated. • The wristbands had significantly higher detection frequencies for nicotine and cotinine than the passive air sampler. • Concentrations found in the 2-day and 7-day wristbands were significantly correlated to urinary concentrations collected on day 7. • Spot urine samples were collected at the end of the sampling period. |
||||
| Wang et al., 2019 | PAHs, PBDE, nBFRs, and OPEs | X | X | OVS cartridges | Brooches | • Concentrations for nBFRs and OPEs in OVS cartridges were positively associated with wristbands and brooches. • Concentrations for PAHs, PBDE, nBFRs, and OPEs in wristbands were positively associated with hand wipes and brooches, but more so for hand wipes. • Significant correlations between measures on dog tags and wristbands were observed. |
|||
| Wise et al.,2020 | SVOCs | X | Sentinel animal dog tags | • Correlations with respective urinary metabolites were stronger in dog tags compared to that in wristbands for several OPEs. • Three urine samples were collected over a five-day sampling period and pooled for both owners and pets. |
|||||
| Wise et al., 2021 | Pesticides | X | Sentinel animal dog tags | • There were significant and positive correlations between silicone samplers and urinary metabolites in both species for DEET and permethrin. • Three urine samples were collected over a five-day sampling period and pooled for both owners and pets. |
|||||
| Xie et al., 2021 | OPEs | X | X | •More significant correlations existed between house dust and wristbands for children then their mothers. • There was a significant correlation between mother and child urinary DPHP and BDCIPP concentrations. • Wristband concentrations of TDCIPP were more significantly correlated with urinary metabolites than house dust. • Pooled urine from two collections during the sampling period were utilized for comparison. |
|||||
5.1.1. Urine
The majority of studies comparing wristbands with chemical measurements in biospecimens have focused on organophoshate esters, and although many of them have observed statistically significant and positive correlations, relationships for specific compounds have varied between studies. Overall, these studies suggest that in a general population, wristbands are effective at predicting internal dose for OPEs, especially TDCIPP, among mother-child pairs (Gibson et al., 2019; Xie et al., 2021), adults (Hammel et al., 2016; Hoffman et al., 2021), and pet dogs (Wise et al., 2020), and TCIPP (Gibson et al., 2019; Hammel et al., 2016; Hoffman et al., 2021). When utilized in occupational settings, these same trends did not hold (Craig et al., 2019; Nguyen et al., 2020). Some studies reported correlations to other OPEs, including TPHP (Gibson et al., 2019; Hammel et al., 2016; Wise et al., 2020) and TMPP (Xie et al., 2021), and a phthalate replacement, DEHTP (Craig et al., 2019). OPE correlation to wristbands could be highly dependent on the sources (e.g., home furnishings, building materials, personal care products) and pathways (i.e., dermal absorption versus diet) by which these individuals may be exposed to OPEs. As previously mentioned, urine samples represent a snapshot of exposure and therefore timing of when that sample was taken, whether pooled or spot urine samples were utilized, whether the urinary metabolites were creatinine corrected, when and how the wristband was worn, and whether the chemical of concern reached equilibrium in the wristband may impact correlation. This may be why variable results have been observed for associations between OPEs and urinary biomarkers among certain study populations.
It has been hypothesized that the wristbands may be better predictors of internal dose among chemicals whose urinary metabolites tend to have lower intraclass correlation coefficients (ICCs), or increased variability in spot urine samples over time. For instance, wristbands and spot urinary metabolites for parent OPEs, TDCIPP and TCIPP, were statistically correlated to 24-hr urine collections, whereas a similar trend was not observed for TPHP (Hoffman et al., 2021). Both of the metabolites of TDCIPP and TCIPP have fairly high ICCs (Bastiaensen et al., 2021) compared to other metabolites of similar half-lives but much lower ICCs (DPHP included), providing an explanation why correlations between wristband OPEs and their metabolites have been observed more frequently than those with phthalates and PAHs. While one study reported weak correlations between PAHs in wristbands and spot urine samples in air force workers (Andersen et al., 2021), another reported statistically significant correlations between six PAHs detected in the wristband and urinary metabolites in pregnant women (Dixon et al., 2018). Similarly, positive and statistically significant, but relatively low to moderate, relationships were observed among children to environmental phenols (specifically BPA, triclosan, and three parabens) and 4 phthalates and phthalate replacements (Hammel et al., 2020; Levasseur et al., 2021). Lastly, strong positive correlations between wristband nicotine and urinary cotinine have been observed among children who wore wristbands for both 2 and 7 days (Quintana et al., 2021; Quintana et al., 2019).
5.1.2. Serum
Wristband associations have only been compared to serum measurements in two published studies and specifically for PBDEs. In both studies, wristbands concentrations were positively correlated with serum measurements for a range of PBDEs in a general adult population (Hammel et al., 2018) and BDE-209 in occupationally exposed e-waste workers (Nguyen et al., 2020). While the first study in the general adult population demonstrated associations for PBDEs with longer half-lives in the body (t½ = 1.6–6.5 years), notably BDE-209 has an estimated half-life of roughly 15 days (Thuresson et al., 2006). More studies are necessary to evaluate the utility of wristbands for other compounds with serum exposure biomarkers; however, these studies suggest the wristbands can be useful for predicting exposure to chemicals with longer half-lives in the body, at least when inhalation and dermal absorption are the dominant exposure pathways.
5.2. Environmental samples
5.2.1. Dust
Indoor dust has been used to evaluate individual exposures to environmental chemicals over the last several decades, and dust measurements have been used in epidemiology studies. Because people spend a majority of time indoors, particularly their home, dust measurements are thought to represent potential exposure in the home environment. More recent studies have quantified flame retardants, plasticizers, pesticides, and other consumer product chemicals in house dust, suggesting links with human exposure via inhalation, inadvertent dust ingestion, or dermal contact with dust (Mitro et al., 2016; Johnson et al., 2010; Stapleton et al., 2008; Hou et al., 2021; Jones-Otazo et al., 2005; Whitehead et al., 2011; Rudel et al., 2003; Lucattini et al., 2018). House dust collection is often performed using a vacuum, whether sampling occurs by collecting dust out of individual homes’ vacuum cleaner containers or research personnel visit homes to vacuum the homes in a standardized procedure. Dust is also a reasonably convenient matrix since there is typically a large enough amount available for multiple analyses to be conducted similar to wristbands. SVOCs tend to be relatively stable over time in the dust matrix, having low temporal variability over time (Kim et al., 2021; Al-Omran and Harrad, 2018). In addition, the National Institute of Standards and Technology (NIST) has several indoor dust Standard Reference Materials (SRMs) available and certified for several classes of compounds to support QA/QC needs.
However, there are a series of limitations when trying to link house dust to human exposure. Oftentimes, a single room of the home is sampled out of convenience, such as the main living area, which limits the ability of researchers to comment on the totality of human exposure from dust in the home. Differences may occur by particle size for evaluating concentrations of specific analytes and by location of sampling within a single room or home (i.e., floor versus elevated surface), which could lead to difficulties in comparing dust across studies (Al-Omran and Harrad, 2018; Al-Omran and Harrad, 2016; Kajiwara and Takigami, 2016). House dust measurements are limited to evaluating a single microenvironment (i.e., home), and occasionally a single room in the home. Thus, the extrapolation of a chemical concentration in house dust to total human exposure could be less insightful than utilizing silicone wristbands, which sample all microenvironments that a participant occupies.
Studies comparing silicone wristbands with house dust have been fairly rare, including only 3 to-date (Table 2). In terms of versatility, wristbands are comparable to dust in their availability of sample mass to support targeted analyses of a wide range of chemical compounds or non-targeted analyses. In two studies on children’s exposure to OPEs, phthalates, and environmental phenols, wristbands performed better than indoor dust for predicting urinary metabolites, with the exception of two higher molecular weight phthalate compounds, specifically dibutyl phthalate (DBP) and di(2-ethylhexyl) terephthalate (DEHTP) (Hammel et al., 2020; Levasseur et al., 2021). Alternatively, a recent study examining OPEs among mother-child pairs observed that the statistically significant correlations between wristband or dust and corresponding urinary metabolites were more compound-specific and differed in pattern between adults and children (Xie et al., 2021).
5.2.2. Hand wipes
Hand wipes have been used to evaluate individual exposure to SVOCs in both children and adults. When the entire surface area of the hands is sampled, hand wipes are hypothesized to capture hand-to-mouth behaviors and dermal contact and absorption. In terms of sample collection, hand wipes are fairly simple and inexpensive to collect, typically requiring only a pre-cleaned wipe and isopropanol. Because they have been shown to be significantly associated with biomarkers of exposure in several studies across multiple classes of compounds (i.e., OPEs, phthalates, PBDEs, PCBs, phenols, some pesticides), hand wipes are a standard tool for examining individual exposure to common environmental exposures (Levasseur et al., 2021; Hou et al., 2021; Gong et al., 2015; Hoffman et al., 2014; Hoffman et al., 2015; Stapleton et al., 2012; Hines et al., 2018; Frederiksen et al., 2020; Oerlemans et al., 2021).
However, a significant limitation in using hand wipes as an exposure tool is that hand washing behaviors may modify the relationship between the wipes and biomarkers of exposure (Gibson et al., 2019). It is challenging to control for how recently an individual washed their hands prior to wipe collection. Additionally, for the purpose of consistency and especially among children, they often require research personnel to wipe the individual’s hands, and the technique used for wiping the skin surface could vary from person to person. Hand wipes are also limited in their ability to capture the totality of an individual’s exposure, as they may only capture exposure from the person’s most recent environment and not every microenvironment a person may occupy throughout the day. Few studies have examined the intra-individual variability over time for chemicals measured on hand wipes.
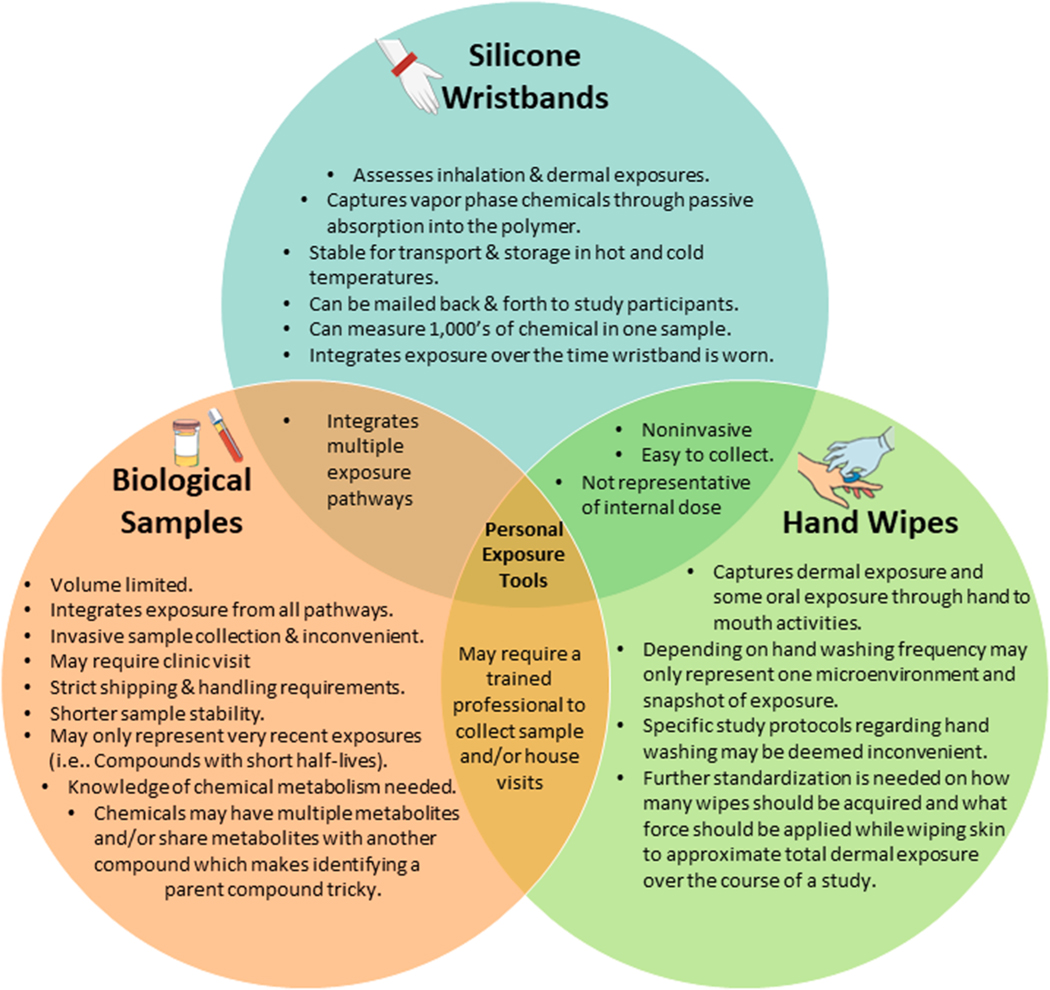
As with house dust, few studies have evaluated hand wipes compared to wristbands for effectiveness in exposure assessment. When hand wipes and wristbands were evaluated for OPE parent compounds and their corresponding urinary metabolites, wristband correlations with urinary metabolites were generally similar or higher than for hand wipes (Hammel et al., 2016). Among children wearing wristbands, similar conclusions were drawn for OPEs, phthalates, and environmental phenols; additionally, SVOC concentrations and corresponding urinary metabolites were greater or roughly similar in magnitude than hand wipe correlations (Hammel et al., 2020; Levasseur et al., 2021). Concentrations for PAHs, BFRs (PBDEs and NBFRs), and OPEs in wristbands were correlated and exhibited similar composition patterns to hand wipe measurement when hand wipes were taken prior to every handwashing event and pooled for the sampling period (Wang et al., 2019). All four of these studies demonstrate that concentrations of SVOCs on hand wipes and wristbands tend to be significantly and positively correlated, which indicates an overlap in the sampled exposure pathways for SVOCs (i.e., dermal absorption). These studies also provide evidence that for silicone personal sampling devices on the wrist, the dermal exposure pathway is a significant exposure route for these chemicals (Fig. 3).
Fig. 3.
Comparison of key features of silicone wristbands and other personal exposure assessment tools including biological samples and hand wipes.
5.2.3. Active air sampling
Active air samplers have previously been used to quantify environmental contaminants in a person’s breathing space to estimate inhalation exposure (Bohlin et al., 2007). Personal forms of active samplers are often placed into backpacks so that the sampler travels with a participant (Dixon et al., 2018; Bohlin et al., 2007). These devices include a battery pack and pump that continuously pulls air through the sampler at a known flow rate. The pump draws air through a filter where particle-associated chemicals are captured before reaching a sorbent. Sorbents used in comparison to silicone wristbands include a polyurethane foam (PUF) cartridge (Dixon et al., 2018) and XAD-2 cartridges (Mendoza-Sanchez et al., 2021). Wristbands have also been compared to OSHA versatile sampler (OVS) cartridges which contained both PUF and XAD-2 components (Wang et al., 2019; Nguyen et al., 2020). OVS cartridges combine the sorbent and filter in one tube; a filter to trap aerosols and a two-section sorbent bed to adsorb vapor. Since all of the studies that have used active air sampling concurrently with the silicone wristbands have used different sorbents, it is impossible to directly compare the studies to each other. Additionally, multiple studies placed silicone passive samplers’ locations other than being worn on a wrist such as on an active air sampling backpack (Mendoza-Sanchez et al., 2021) and as a brooch worn on the chest (Nguyen et al., 2020), thereby only comparing the ability of the silicone wristband to act as an air sampler. The differences in sorbent used could explain differences in the reported correlation between worn silicone wristbands and active air samplers.
Several studies have evaluated how uptake of PAHs into silicone wristbands compare to active air samplers and have had varied results. One study reported moderate to strong correlations between wristband and active air sampler concentrations for a majority of the PAHs evaluated (Dixon et al., 2018). In contrast, other studies reported no statistically significant correlations between wristband and active air sampler concentrations of PAHs (Wang et al., 2019; Mendoza-Sanchez et al., 2021).
Interestingly, a study that reported no correlations between PAH concentrations in wristbands and active air samplers reported statistically significant correlations for different chemical classes, including HFRs and OPEs (Wang et al., 2019). Another factor to consider when examining the differences between studies is the physical-chemical properties of the compounds being measured. The chemical properties will play a role in partitioning both into the wristband and into the sorbent in active air samplers. This could explain why (Mendoza-Sanchez et al., 2021) reported a lack of correlation between wristband PAH concentrations and active air samplers (Mendoza-Sanchez et al., 2021), but (Nguyen et al., 2020) reported many statistically significant correlations for concentrations of flame retardants found in the silicone and active air sampler (Nguyen et al., 2020), even though in both studies’ personal silicone samplers had no dermal contact and were thus only capturing inhalation exposure.
Evidence suggests that some correlation exists between active air samplers and worn silicone wristbands but that other sampling methods that capture dermal exposure (i.e., hand wipes) (Wang et al., 2019) or exposure from multiple sources such as biological samples more strongly correlate with concentrations found on the silicone wristband (Dixon et al., 2018). This is likely due to the ability of silicone wristbands to sequester chemicals that are representative of dermal and inhalation exposure, whereas the active air sampler sorbent only captures inhalation vapors. For example, (Dixon et al., 2018) found three times more correlations that were positive and statistically significant between PAH metabolite pairs in wristbands and urine samples than between PUF-filters and urine samples (Dixon et al., 2018).
5.2.4. Passive air sampling & other passive sampling devices
During passive air sampling, the amount of a chemical taken up in a sorbent from the air without the help of a pump is quantified and converted into an air concentration (Wania and Shunthirasingham, 2020). The same principles discussed for silicone wristband uptake apply, except that since passive air samplers are only exposed to air, if the uptake rates into the passive samplers are known, post-deployment concentrations can be back-calculated to an air concentration. Similar to active air samplers, there are many different forms of passive air samplers. Passive air samplers have been used in studies that utilized silicone wristbands to assess the wristbands’ practicality and use for specific target compounds (Quintana et al., 2021) and assess where a chemical found in a participant wristband was coming from (e.g., indoor air vs. outdoor air) (Hendryx et al., 2020). Further, unworn silicone wristbands have been deployed as stationary passive air sampling devices, however this reviews focus was on personal silicone samplers, and unworn wristbands were thus excluded.
5.2.5. Alternative wristband configurations
The most common form of alternative wristband configuration (i.e., the wristband is not worn on the wrist) is a silicone piece worn similar to a brooch or a lapel on the chest (Wang et al., 2019; O’Connell et al., 2014; Craig et al., 2019; Nguyen et al., 2020). These configurations limit the ability of the silicone to capture dermal exposure since there is no dermal contact with the sampler. Wearing a wristband as a brooch/lapel and stacking the wristbands on a wrist (O’Connell et al., 2014) have been compared to the use of a single wristband worn on a wrist. Results are study-dependent, and both sampling period length and chemical properties of target analytes could have played a role in the results. Studies have reported differences in both chemical detections and concentrations for silicone brooches/lapels and silicone samplers worn on the wrist (Craig et al., 2019; Mendoza-Sanchez et al., 2021; Nguyen et al., 2020) signifying that while wristbands capture dermal exposure, silicone lapels/brooches do not. Air flow (e.g., wrist movement) and possibly surface contact could also contribute to these differences in reported concentration. Other studies have reported a correlation between the configurations (Wang et al., 2019; O’Connell et al., 2014). See Table 1 for specific study details and findings.
Alternative configurations that have not been directly compared to the standard sampler (i.e., a wristband worn on a wrist) include wristbands worn as air samplers on participants with no dermal contact aside from brooches/lapels (Aerts et al., 2018; Bullock et al., 2020; Mendoza-Sanchez et al., 2021; Swanson et al., 2018; Andersen et al., 2021), silicone military-style dog tags worn around the neck (Poutasse et al., 2022; Poutasse et al., 2020), and pet tags (Wise et al., 2020; Wise et al., 2021; Poutasse et al., 2019). Two studies have utilized PDMS tubing to create silicone wristbands and anklets, but these studies were not concerned with environmental chemical exposure (Roodt et al., 2018; Wooding et al., 2020). A recent study included a silicone watch band mounted with a silicone piece encased with a filter to assess phthalate exposure, but it did not provide any comparison to silicone wristbands (Hong et al., 2021).
5.2.6. Fresh air wristbands
First introduced by (Lin et al., 2020), the Fresh Air wristband consists of a triethanolamine-coated foam pad and a clean PDMS sorbent bar enclosed in a polytetrafluoroethylene (PTFE) chamber and mounted on a bracelet. The two separate samplers are intended to capture exposure to NO2 (foam pad) and a combination of VOCs and SVOCs (sorbent bar) (Lin et al., 2020). They have been used in several separate sampling campaigns, such as older adult exposures to chemicals mixtures in China (Doherty et al., 2021; Guo et al., 2021; Koelmel et al., 2021). Additional work with the Fresh Air wristbands has compared participant exposure profiles based on the sampler placement (i.e., chest, wrist, and shoe) and season of sampling (Koelmel et al., 2021). It is mentioned here due to its similar configuration to the silicone wristband (i.e., a sampler worn on the wrist). However, the Fresh Air wristband utilizes a different technology by using a PDMS bar housed within a PTFE chamber. Therefore, it is not included in the scope of this review.
6. Use of personal silicone samplers to assess health risks
6.1. Utility of wristbands in health studies
Several studies have begun to examine chemical concentrations from wristbands in association with adverse health effects (Table 2). Most of these studies focused on the association between flame retardants and thyroid function (Kassotis et al., 2020; Poutasse et al., 2019; Wang et al., 2020) and a later downstream target of thyroid function, neurocognitive functioning (Kile et al., 2016; Lipscomb et al., 2017). Using silicone wristbands as personal sampling devices, there were positive correlations between: concentrations of several phthalates, OPEs, and brominated flame retardants and thyroid receptor β antagonism (Kassotis et al., 2020); tris(1,3-dichloro-2-isopropyl) phosphate (TDCIPP) concentrations and feline hyperthyroidism (Poutasse et al., 2019), ΣOPFR concentration with less responsible behavior and more externalizing behavior problems in preschool-age children, and ΣBDE concentration with being less assertive (Lipscomb et al., 2017). Lastly, significant positive and negative associations existed between several PBDEs, and NFRs concentration and free thyroxine (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) (Wang et al., 2020). Two other studies focused on occupational exposure of aircraft workers, which included evaluating the association between OPE exposure with cholinesterase inhibition (Hardos et al., 2020), and evaluating OPE as well as PAH exposure on lung function, systemic inflammation, and genetic damage (Andersen et al., 2021); but in both instances significant associations were not seen.
Other chemical categories evaluated in wristbands for their association with health effects included pesticides, which focused on Hispanic farmworkers and their families (Varnell et al., 2021; Vidi et al., 2017). Several studies have utilized silicone wristbands to look at Hispanic farmworkers and farmworker families’ exposure to pesticides (Varnell et al., 2021; Arcury et al., 2021; Harley et al., 2019; Vidi et al., 2017), but only two to-date have also looked at a health endpoint such as menstrual cycle irregularities (Varnell et al., 2021) or biomarkers such as DNA damage in hair follicles (Vidi et al., 2017). While Varnell et al., 2021 reported that there were no significant associations between pesticide exposure and menstrual cycle irregularities, over 90% of the study population was exposed to pesticides to some degree and more than half had menstrual cycle irregularities (Varnell et al., 2021). (Vidi et al., 2017) characterized children’s para-occupational pesticide exposures in association with DNA damage in hair follicles and found that an increased number of pesticide detections was significantly associated with DNA damage in the papilla region of the hairs, indicative of DNA damage to epithelial cells (Vidi et al., 2017). Lastly, one study has investigated the influence of a large range of SVOCs on the plasma metabolomics during pregnancy (Doherty et al., 2021). While this study found that the combined chemical mixture of chemical exposures was not significantly related to metabolic pathway enrichment, specific chemicals including N,N-diethyl-m-toluamide, tonalide, galaxolide, and benzyl salicylate were associated with disturbances in metabolic pathways and concentrations of free amino acids (Doherty et al., 2021).
Although the studies that have utilized silicone wristbands to assess health risks differ in both target chemicals and endpoints, collectively, they highlight the usefulness of silicone wristbands as an exposure assessment tool. Multiple studies demonstrated the usefulness on using silicone wristbands to assess exposure both in rural setting (Varnell et al., 2021; Vidi et al., 2017) and in children (Lipscomb et al., 2017; Vidi et al., 2017). Additionally, silicone wristbands have been successful exposure assessment tools when deployed only in occupational settings (Varnell et al., 2021; Andersen et al., 2021; Hardos et al., 2020) and have demonstrated the relationship between occupational exposure to environmental contaminants and adverse health outcomes (Varnell et al., 2021). If silicone wristbands are deployed only while an individual is in an occupational setting, occupational exposure can be separated from total exposure. Furthermore, silicone wristbands mainly capture dermal and inhalation exposure and not ingestion, which we would not expect from occupational exposure. Lastly, silicone wristbands are easy to use and are amenable for use in large scale population studies evaluating health outcomes.
Special considerations may need to be made when comparing studies examining the effect of exogenous chemicals on human health. Differences in study design, populations, and analytical measurements for exogenous and endogenous substrates can all play a role in discrepancies between studies. Bias can occur when comparing studies that use different sampling devices. Future studies should consider how differences in exposure scenarios and study populations will impact their results and if they are interested in current bioavailable environmental exposure or body burden of a chemical.
6.2. Sentinel animal species
Silicone passive sampling devices have been used to assess exposure to numerous animals including frogs (Swanson et al., 2018; Yaw et al., 2017), bee hives (Bullock et al., 2020), horses (Rivera, 2020), cats (Poutasse et al., 2019), and dogs (Wise et al., 2020; Wise et al., 2021). This opens up a door to utilize silicone passive samplers to assess chemical exposure and health outcomes in sentinel animals. Sentinel animals are used to evaluate various hazards within an ecosystem and the possible health effects on the populations. Sentinels may react to environmental toxicants before major problems occur in the environment and may be more vulnerable than humans to some types of environmental contaminants (Reif, 2011; Scotch et al., 2009). A focus on sentinel species makes it practical to predict toxicological risks associated with the environment-animal-human interactions (Wise et al., 2020). Specifically, evaluating the chemical exposure and health impacts of companion animals such as cats (Poutasse et al., 2019) and dogs (Wise et al., 2020; Wise et al., 2021) can better inform the etiology of human diseases. Two studies evaluated human chemical exposure in comparison to their pet dogs and statistically significant correlations between measures on dog tags and wristbands worn by owners were observed (Wise et al., 2020; Wise et al., 2021).
7. Silicone wristband strengths and weaknesses
7.1. Benefits of using silicone wristbands for exposure assessment
Wristbands can provide a noninvasive and inexpensive method of quantitatively measuring personal exposure in large populations. Compared to a biological sample, collection does not require a trained professional, which reduces cost and undue stress for participants. Additionally, purchasing a silicone wristband for research purposes may be cheaper than vials and collection containers necessary for biological samples or pumps necessary for active air sampling. The cost of sample analysis is likely similar regardless of the exposure assessment tool used (i.e., biological samples, hand wipes, dust, active air samplers, and wristbands).
As more research is conducted to evaluate chemicals on the wristbands with their corresponding biomarkers of exposure in paired samples, and therefore validate that the wristbands are reflective of meaningful exposure, we can reduce the reliance on biomarkers. Biological samples (e.g., serum) for biomarker analysis can be challenging to collect and invasive, particularly among small children. The wristbands can support large-scale biomonitoring studies to inform population-level exposure assessments and epidemiological studies because of their ease of collection and transport. Given the stability of SVOCs on wristbands in transport (up to 30 °C for a month) and in storage ( −20 °C for up to 6 months), (Anderson et al., 2017) pre-cleaned wristbands can to be mailed to and from participants nationally and/or globally for personal exposure analysis, as has been done in previous studies (Dixon et al., 2019; Doherty et al., 2020; Young et al., 2021). This ease of collection allows for increased flexibility, especially when compared to collection of biospecimens, which typically requires a clinic visit (for blood) and need to be placed on ice or frozen soon after collection to maintain the stability of the sample.
Further, wristbands remove the need for research personnel to be present for collection, such as for the collection of house dust, hand wipes, or biological samples (e.g., serum), reducing the time and resources required to conduct the studies. For these reasons, silicone wristbands are ideal tools in disaster scenarios where normal researcher and participant contact is not possible (Rohlman et al., 2022). This includes scenarios such as the SARS-COV-2 pandemic, natural disasters such as hurricanes, earthquakes, or wildfires, and any other crises that would render the use of traditional sampling approaches unfeasible.
The wristbands also hold the potential for advances in exposure assessment of mixtures. The silicone material binds chemicals across a wide range of physicochemical properties, which allows for an integrated measure of exposure in a single sample (Bergmann et al., 2018; O’Connell et al., 2014). As epidemiologists and toxicologists begin evaluating exposure in the context of mixtures, and deemphasize studies based on single chemicals, the wristbands may provide insights on relative levels of chemicals to which individuals may be exposed across a specified period of time. Currently, research indicates that wristbands are an average measure over a defined time period (O’Connell et al., 2021; O’Connell et al., 2014), which is a vast improvement over some exposure biomarkers measured in urine and serum. This is particularly the case with spot urine samples, which are typically cross-sectional measures of exposure since many compounds with urinary metabolite biomarkers have short half-lives in the body. Oftentimes, due to a lack of resources to collect and analyze more samples, spot urine samples are analyzed and assumed to be representative of total exposure and internal dose, based on the assumption that exposure to these chemicals is constant and chronic. As such, an abnormally high exposure event or the time of day for sampling (i.e., morning void versus daytime spot sample) could easily lead to exposure misclassification. A spot urine sample would provide insights on a smaller window of time compared to wristbands, and a more frequent and intensive urine sampling would presumably be better matched with the concentrations measured by wristbands. In addition, wristbands could support prospective studies to understand changes in exposure over time (e.g., wearing multiple wristbands across pregnancy (Doherty et al., 2020), which is more difficult to conduct using biospecimens. The concentrations of SVOCs on the wristbands tend to be high and often larger in magnitude than hand wipes, thereby allowing for increased analytical sensitivity for evaluating personal exposure to consumer product chemicals (Hammel et al., 2016; Hammel et al., 2020; Levasseur et al., 2021). The range of analytical sensitivity is large in targeted analyses for a segment of a wristband, allowing for nanogram range concentrations to be measured and accounting for much larger concentrations captured by a whole wristband. The absorptivity of compounds to the wristbands themselves and concentrations when present allow for other pieces of each wristband sample to be reserved for future analyses (e.g., non-targeted work, cell assays) (Travis et al., 2021; Manzano et al., 2019; Kassotis et al., 2020). Further, this could lead to the development of individual quantitative exposure profiles, which could be extremely helpful in defining the personal exposome and providing greater insight for the evaluation of health outcomes.
7.2. Best practices & recommendations
The exact methodology for chemical extraction and analysis vary to match a project’s data quality objectives individually and the laboratory performing the analysis. Similarities are based around the basic passive sampling principle that unless in a contained environment, they are always sampling. With this in mind, the following recommendations for best practices should be considered when using wristbands, some of which are standard protocols for any kind of sampling device.
Wristbands should be cleaned or conditioned pre-deployment then stored in airtight containers both before and after a participant has worn the wristband for the sampling period.
-
Quality control samples should be deployed for every step of the wristband deployment journey, including appropriate laboratory and field blanks (i.e., predeployment cleaning blank, travel blank, extraction blank, etc.).
Predeployment cleaning blank is a blank wristband that was cleaned alongside other wristbands but remains in the laboratory until analysis.
Travel blank is a wristband which has been cleaned then proceeds through all transport procedures, but is not deployed, and then is analyzed.
Extraction blank is a blank sample that is processed alongside wristband samples to account for any potential contamination during extraction procedures.
Deployment of a silicone wristband has lasted anywhere between four hours and 30 days. However, most studies to date have typically used a deployment period that lasts seven days to capture both weekday and weekend exposure (Table 1), after which the wristbands are returned via the airtight container for extraction and analysis.
While wristbands are stable at room temperature and therefore safe for transport for (up to seven days for some VOCs and up to 30 days for SVOCs), it is generally recommended to store wristbands at −20° C until extraction to minimize chemical loss (Anderson et al., 2017). Chemical stability has been evaluated with little degradation in storage at −20 °C for up to 6 months among SVOCs and up to 3 months for VOCs (Anderson et al., 2017).
Wristbands have been used to evaluate some VOC exposures; however, researchers should keep in mind that more volatile compounds will likely desorb more quickly from the silicone and thus may not represent a completely accurate representation of personal exposures to VOCs.
Although further research is needed to specifically investigate what the wristbands measurements represent, current studies suggest that wristbands capture a combination of dermal and inhalation exposures, as well as dermal excretion for some compounds (Roodt et al., 2018; Wooding et al., 2020) such as caffeine (Dixon et al., 2020). As such, the wristbands should not be directly used to investigate exposures from drinking water or dietary sources.
Silicone wristbands sample continuosly once deployed and therefore can capture exposure from all environments that a person is in. If the goal of a study is to capture typical chemical exposure of a participant then wristbands should be worn at all times including activities such gardening painting, sleeping etc. If the study aims to only capture occupational exposure than wristbands should only be worn while in an occupational setting.
Since wristbands come in a variety of sizes, it is recommended to report the exposure amount as mass target analyte/mass of wristband. At this time, it is not recommended to report exposure in mass target analyte/mass of wristband/sampling length since this assumes all chemicals are in the linear uptake phase. However, deployment time should be carefully noted in the study design.
7.3. Gaps in research & future directions
7.3.1. Gaps pertaining to sample deployment
7.3.1.1. Wristband location.
There is evidence location of a personal passive sampling device on a person’s body plays a role in what chemicals are captured (Koelmel et al., 2021). There are a few possible explanations for this. The first being that some silicone PSD placements have direct dermal contact, while others do not (Wang et al., 2019; O’Connell et al., 2014). The second is that specific chemical weights, intermolecular forces, and environmental conditions contribute to speed of diffusion and chemical gradation. Passive sampling locations closer to the ground will likely capture less volatile and higher molecular weight compounds, whereas more elevated PSD placements, such as on the chest in the case of brooches, will likely capture more volatile compounds. For wristbands specifically, this could have further implications when comparing individuals with notable height differences (e.g., children versus adults), and for assessing exposures of sentinel species and needs to be explored further. There is also limited evidence on the impact of obstructive items such as clothing (i.e., if the wristband is worn under or comes in contact with clothing), or in the case of sentinel species hair/fur, on the wristband’s ability to uptake vapor phase compounds.
7.3.1.2. Does personal bathing behavior make a difference.
In most wristband studies, participants are asked to wear the wristbands through showering and bathing. However, to date, there are still no studies that address how showering, bathing, or handwashing impact wristband concentrations of SVOCs. It is hypothesized that bathing practices affect the whole skin surface, including the worn wristband, so any removal of SVOCs from the skin surface could be washed off in a similar fashion to the wristband. However, this may depend on the chemical-physical properties of the compounds of concern, since SVOCs already absorbed into the wristband may not readily partition into water while bathing or handwashing. In addition, VOCs that individuals may be exposed to during bathing from soaps and cleaning products may also be captured in wristbands. Preliminary results evaluating wristbands worn over 5 days, with one removed each day, suggest that uptake for OPEs on the wristbands is generally linear (Hoffman et al., 2021), which indicates that accumulation may be consistent over this study period, despite any potential washing. There is limited knowledge regarding the effects of single sporadic or non-daily activities which could increase exposure substantially (e.g., gardening, painting, woodworking), and whether wristbands should be worn during these cases when trying to capture average exposure. Additional information regarding how the amount of sweating and the use of personal care products like lotion affects wristband concentrations and mediates exposure would be insightful as well.
7.3.2. Interpretation and usefulness of exposure assessments using Wristbands.
7.3.2.1. Translating silicone wristband extract concentrations to a meaningful exposure measure (Not necessarily internal dose).
Models and equations used to estimate environmental concentrations from PSDs are common, but are dependent on sampler type and target chemical, and may rely on a number of assumptions such as first-order uptake, an ideal sampling matrix, and/or standard environmental conditions, and calibration with an active sampler (Huckins et al., 2006; Harner and Shoeib, 2002; Booij et al., 2016). As discussed in Section 3.2, chemical uptake into PSDs is dependent upon a chemical’s physical-chemical properties and thus similar extract concentrations may represent varying environmental concentrations. Not only does this make it difficult for researcher to compare data across studies, it makes it challenging to compare the relative abundance of different chemicals captured in wristband extracts since chemicals have different affinities and uptake rates into the silicone that have not been normalized in reported extract concentrations. Since silicone wristbands capture exposure from multiple routes, attempting to estimate concentrations that could be compared to a level used in risk assessment (i.e. dermal or air concentration) is often not included in current research. Instead, reported concentrations often represent concentrations present in wristbands on a mass per mass basis.
Recent research has determined silicone air partitioning constants (Ksa) and sampling rates (ke and/or Rs) for several chemical categories so that an air concentration can be calculated from silicone passive samplers, including wristbands, used as air samplers. The experiments have included the use of a silicone passive sampler in addition to an active air sampler (Okeme et al., 2018; Okeme et al., 2018; Mendoza-Sanchez et al., 2021; Nguyen et al., 2020; Okeme et al., 2016; Vorkamp et al., 2016), another form of a passive air sampler (Donald et al., 2019), or through controlled experimental procedures (Tromp et al., 2019; O’Connell et al., 2021). One of the challenges in calculating Ksa; Rs, or ke for silicone is that it has traditionally involved other partitioning estimates that do not exist for every chemical (O’Connell et al., 2021). Most recently (O’Connell et al., 2021) utilized boiling points to aid in modeling Ksa and ke; and then further refined the Ksa model utilizing preexisting data from Tromp et al., 2019 (O’Connell et al., 2021; Tromp et al., 2019). The end result was a predictive model that could be utilized for most target analytes (Koa: 2.4–9.8, boiling points: ∼80–400 °C) (O’Connell et al., 2021). These models, aid in the conversion of extract concentrations from silicone wristbands as air samplers into an air “equivalent” concentration, which represent air estimates assuming 25°C and 50% humidity.
In summary, silicone wristbands are limited in their ability to derive an internal dose from the extract concentration measurement due to the complexity of the sampling environment, and the inability of the wristband to capture oral exposure. Additionally, sampling rates and affinity to silicone under environmental conditions are unique to each target chemical. For these reasons, care should be taken when comparing extract concentrations between studies, and exact concentrations of different chemicals within the same study interpreted with caution. More advances have been made where silicone passive samplers have been used solely as air samplers. In these instances, modeled partitioning coefficients and sampling rates exist so that an air equivalent concentration may be calculated. Future work may include advances in estimating dermal concentrations or generating models that help predict the primary route of exposure for a given chemical (air, dermal, mixed, or other), since physiochemical characteristics may provide much insight into likely exposure pathways.
7.3.2.2. Using wristband data to effectively study the exposome.
Tools are available to efficiently assess personal environmental exposure, but capturing enough data to facilitate comparisons between personal chemical exposures and health outcomes still poses challenges. The silicone wristband itself is able to capture a vast amount of information. Therefore, future studies should consider spending ample time on study design to formulate how researchers intend to utilize the wristband data and what participant data is necessary to mechanistically link an exposure to a health outcome. As an example, Rohlman et al. (2019) developed an Exposure, Location, and (lung) Function (ELF) tool to concurrently collect daily individualized chemical exposure (silicone wristbands), location (cell phone), and respiratory health outcomes (spirometer and questionnaires). An ELF phone app collected questionnaire data about personal behavior, potential exposure sources, and respiratory health symptoms. Volunteers also used a handheld, Bluetooth-linked spirometer to assess lung function throughout the study (Rohlman et al., 2019). This study highlights methods that could be developed to collect personalized data needed to compare personal chemical exposure and evaluate health outcomes while controlling for covariates and considering potential effect modifiers.
8. Conclusion
It can be difficult to assess what exposure assessment tool is most appropriate for certain research goals and selection of an exposure assessment tool is not a “one size fits all” endeavor. Since the first wristband paper was published by O’Connell et al. in 2014, wristbands have been deployed for exposure assessment of a wide range of chemicals across numerous circumstances. In a growing number of studies, wristbands have been shown to be positively and significantly correlated with exposure biomarkers in addition to being equivalent or better than contemporary exposure assessment tools (Dixon et al., 2018; Levasseur et al., 2021; Quintana et al., 2019; Xie et al., 2021; Hammel et al., 2016; Hammel et al., 2018; Hammel et al., 2020). Silicone wristbands have been demonstrated to be an effective sampling strategy for personal exposure studies as they capture both inhalation and dermal exposure. Correlation with metabolites in biological samples further adds evidence that wristbands can be used in exposure assessment, including epidemiology studies interested in health outcomes.
The usefulness of the silicone wristband as a personal passive sampling device stems from its ability to provide an integrated exposure measure for hundreds of environmental contaminants with minimal participant burden. Utilizing silicone wristbands to assess chemical exposures has the potential to reshape how scientists think of the chemical exposome and opens up many research possibilities. These could include evaluation of mixtures exposure, screening for population-level exposure, examination of exposures prospectively (i.e., throughout a population over time and with a single person across life stages), and linking chemical exposures to other components of the exposome (e.g., diet, lifestyle) to examine health in a more holistic manner through exposome-wide association studies. The exposome is the measure of all exogenous and endogenous exposures over the course of a lifetime from conception onwards (Wild et al., 2013; DeBord et al., 2016). As compared to traditional research/sampling approaches that are limited in their ability to address multiple chemical and non-chemical stressors, silicone wristbands integration into exposome-wide association studies can provide a new approach for conceptualizing the roles and relationships of multiple chemical and non-chemical exposures in the etiology and progression of diseases (Juarez and Matthews-Juarez, 2018; Juarez et al., 2014; Stingone et al., 2017; Juarez et al., 2020; Kim and Hong, 2017).
Acknowledgments
Fig. 2. Created with BioRender.com.
Funding
SCH was supported by FFIKA, Focused Research Effort on Chemicals in the Working Environment, a grant from the Danish Government. HMS was supported by a grant from the National Institute of Health (U2C-ES030851). SMS and KAA were both supported by multiple grants from the National Institute of Environmental Health Sciences of the National Institute of Health (P42-ES016465, P30-ES000210, P30-ES030287, R21-ES029460, T32-ES0007060) and one grant provided by the Federal Emergency Management Agency through the Assistance to Firefighters Grant Program (EMW-2016-FP-000754).
Abbreviations:
- VOC
volatile organic compound
- SVOC
semi-volatile organic compounds
- PAH
polycyclic aromatic hydrocarbon
- OPAH
oxygenated PAH
- PCB
polychlorinated biphenyl
- PBDE
polybrominated diphenyl ether
- HFR
halogenated flame retardant
- OPE
organophosphate ester
- PSD
passive sampling device
- PDMS
polydimethylsiloxane
- ICC
intraclass correlation coefficient
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SCH, HMS, SMS- none. KAA discloses a financial interest in MyExposome, Inc., which markets products related to the research being reported. The terms of this arrangement have been reviewed and approved by Oregon State University in accordance with its policy on research conflicts of interest.
References
- Aerts R, Joly L, Szternfeld P, Tsilikas K, De Cremer K, Castelain P, Aerts JM, Van Orshoven J, Somers B, Hendrickx M, Andjelkovic M, Van Nieuwenhuyse A, 2018. Silicone Wristband Passive Samplers Yield Highly Individualized Pesticide Residue Exposure Profiles. Environ. Sci. Technol. 52 (1), 298–307. [DOI] [PubMed] [Google Scholar]
- Alkon A, Gunier RB, Hazard K, Castorina R, Hoffman PD, Scott RP, Anderson KA, Bradman A, 2021. Preschool-Age Children’s Pesticide Exposures in Child Care Centers and at Home in Northern California. J. Pediatric Health Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Omran LS, Harrad S, 2016. Distribution pattern of legacy and “novel” brominated flame retardants in different particle size fractions of indoor dust in Birmingham, United Kingdom. Chemosphere 157, 124–131. [DOI] [PubMed] [Google Scholar]
- Al-Omran LS, Harrad S, 2018. Within-room and within-home spatial and temporal variability in concentrations of legacy and “novel” brominated flame retardants in indoor dust. Chemosphere 193, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Andersen MHG, Saber AT, Frederiksen M, Clausen PA, Sejbaek CS, Hemmingsen CH, Ebbehøj NE, Catalán J, Aimonen K, Koivisto J, Loft S, Møller P, Vogel U, 2021. Occupational exposure and markers of genetic damage, systemic inflammation and lung function: a Danish cross-sectional study among air force personnel. Sci. Rep. 11 (1), 17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Points GL, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG, Hoffman PD, Herbstman JB, O’Connell SG, 2017. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Exp. Sci. Environ. Epidemiol. 27 (6), 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel P, Rousselle C, Lange R, Sissoko F, Kolossa-Gehring M, Ougier E, 2020. Human biomonitoring initiative (HBM4EU) - Strategy to derive human biomonitoring guidance values (HBM-GVs) for health risk assessment. Int. J. Hyg. Environ. Health 230, 113622. [DOI] [PubMed] [Google Scholar]
- Arcury TA, Chen H, Quandt SA, Talton JW, Anderson KA, Scott RP, Jensen A, Laurienti PJ, 2021. Pesticide exposure among Latinx children: Comparison of children in rural, farmworker and urban, non-farmworker communities. Sci. Total Environ. 763, 144233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward LL, Hays SM, Smolders R, Koch HM, Cocker J, Jones K, Warren N, Levy L, Bevan R, 2014. Sources of Variability in Biomarker Concentrations. J. Toxicol. Environ. Health, Part B 17 (1), 45–61. [DOI] [PubMed] [Google Scholar]
- Bastiaensen M, Gys C, Malarvannan G, Fotache M, Bombeke J, Ait Bamai Y, Araki A, Covaci A, 2021. Short-term temporal variability of urinary biomarkers of organophosphate flame retardants and plasticizers. Environ. Int. 146, 106147. [DOI] [PubMed] [Google Scholar]
- Baum JLR, Bakali U, Killawala C, Santiago KM, Dikici E, Kobetz EN, Solle NS, Deo S, Bachas L, Daunert S, 2020. Evaluation of silicone-based wristbands as passive sampling systems using PAHs as an exposure proxy for carcinogen monitoring in firefighters: Evidence from the firefighter cancer initiative. Ecotoxicol. Environ. Saf. 205, 111100. [DOI] [PubMed] [Google Scholar]
- Bergmann AJ, North PE, Vasquez L, Bello H, Ruiz MDG, Anderson KA, 2017. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Exp. Sci. Environ. Epidemiol. 27 (6), 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann AJ, Points GL, Scott RP, Wilson G, Anderson KA, 2018. Development of quantitative screen for 1550 chemicals with GC-MS. Anal. Bioanal. Chem. 410 (13), 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin P, Jones KC, Strandberg B, 2007. Occupational and indoor air exposure to persistent organic pollutants: A review of passive sampling techniques and needs. J. Environ. Monit. 9 (6), 501–509. [DOI] [PubMed] [Google Scholar]
- Booij K, Robinson CD, Burgess RM, Mayer P, Roberts CA, Ahrens L, Allan IJ, Brant J, Jones L, Kraus UR, Larsen MM, Lepom P, Petersen J, Pröfrock D, Roose P, Schäfer S, Smedes F, Tixier C, Vorkamp K, Whitehouse P, 2016. Passive Sampling in Regulatory Chemical Monitoring of Nonpolar Organic Compounds in the Aquatic Environment. Environ. Sci. Technol. 50 (1), 3–17. [DOI] [PubMed] [Google Scholar]
- Booij K, Smedes F, 2010. An improved method for estimating in situ sampling rates of nonpolar passive samplers. Environ. Sci. Technol. 44 (17), 6789–6794. [DOI] [PubMed] [Google Scholar]
- Bullock EJ, Schafsnitz AM, Wang CH, Broadrup RL, Macherone A, Mayack C, White HK, 2020. Silicone Wristbands as Passive Samplers in Honey Bee Hives. Vet Sci 7 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti FM, Leoni C, Testai E, 2007. The Human Metabolism of Organophosphorothionate Pesticides: Consequences for Toxicological Risk Assessment. J. für Verbraucherschutz und Lebensmittelsicherheit 2 (1), 37–44. [Google Scholar]
- Caban-Martinez AJ, Louzado-Feliciano P, Santiago KM, Baum J, Schaefer Solle N, Rivera G, Miric M, Perez-Then E, Kobetz-Kerman EN, Daunert S, 2020. Objective Measurement of Carcinogens Among Dominican Republic Firefighters Using Silicone-Based Wristbands. J. Occup. Environ. Med. 62 (11), e611–e615. [DOI] [PubMed] [Google Scholar]
- Cao Z, Lin S, Zhao F, Lv Y, Qu Y, Hu X, Yu S, Song S, Lu Y, Yan H, 2021. Cohort profile: China National Human Biomonitoring (CNHBM)—A nationally representative, prospective cohort in Chinese population. Environ. Int. 146, 106252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Morck TA, Joas A, Knudsen E, 2015. Major national human biomonitoring programs in chemical exposure assessment. Environ. Sci. 2, 782–802. [Google Scholar]
- Craig JA, Ceballos DM, Fruh V, Petropoulos ZE, Allen JG, Calafa AM, Ospina M, Stapleton HM, Hammel S, Gray R, Webster TF, 2019. Exposure of Nail Salon Workers to Phthalates, Di(2-ethylhexyl) Terephthalate, and Organophosphate Esters: A Pilot Study. Environ. Sci. Technol. 53 (24), 14630–14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Corrêa M, Germano Marciano A, Silveira Barreto Carvalho V, Bernardo de Souza PM, da Silveira Carvalho Ripper J, Roy D, Breton L, De Vecchi R, 2021. Exposome extrinsic factors in the tropics: The need for skin protection beyond solar UV radiation. Sci. Total Environ. 782, 146921. [Google Scholar]
- DeBord DG, Carreon T, Lentz TJ, Middendorf PJ, Hoover MD, Schulte PA, 2016. Use of the “Exposome” in the Practice of Epidemiology: A Primer on -Omic Technologies. Am. J. Epidemiol. 184 (4), 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Poutasse, Carolyn M., Anderson, Kim A., 2020. Silicone wristbands and wearables to assess chemical exposures. In: Phillips Kirk A., LeeAnn Racz DPY. (Eds.), Total Exposure Health, first ed. Boca Raton: CRC Press, pp. 139–160. [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA, 2018. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem. 410 (13), 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Armstrong G, Barton M, Bergmann AJ, Bondy M, Halbleib ML, Hamilton W, Haynes E, Herbstmanw J, Hoffman P, Jepson P, Kile ML, Kincl L, Laurienti PJ, North P, Pauilk LB, Petrosino J, Points GL, Poutasse CM, Rohlman D, Scott RP, Smith B, Tidwell LG, Walker C, Waters KM, Anderson KA, 2019. Discovery of common chemical exposures across three continents using silicone wristbands. Royal Soc. Open Sci. 6 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Pearce JL, Anderson KA, Karagas MR, Romano ME, 2020. Assessment of Multipollutant Exposures During Pregnancy Using Silicone Wristbands. Front. Public Health 8, 547239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, McRitchie SL, Pathmasiri WW, Stewart DA, Kirchner D, Anderson KA, Gui J, Madan JC, Hoen AG, Sumner SJ, Karagas MR, Romano ME, 2021. Chemical exposures assessed via silicone wristbands and endogenous plasma metabolomics during pregnancy. J. Exposure Sci. Environ. Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Koelmel JP, Lin EZ, Romano ME, Godri Pollitt KJ, 2021. Use of Exposomic Methods Incorporating Sensors in Environmental Epidemiology. Curr. Environ. Health Rep. 8 (1), 34–41. [DOI] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, Anderson KA, 2016. Silicone wristbands detect individuals’ pesticide exposures in West Africa. R. Soc. Open Sci. 3 (8), 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald CE, Scott RP, Wilson G, Hoffman PD, Anderson KA, 2019. Artificial turf: chemical flux and development of silicone wristband partitioning coefficients. Air Quality Atmos. Health 12 (5), 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg ND, Rodriguez-Proteau R, Ma L, Morré J, Christensen JM, Maier CS,Jenkins JJ, Anderson KA, 2011. Organophosphorus pesticide degradation product in vitro metabolic stability and time-course uptake and elimination in rats following oral and intravenous dosing. Xenobiotica 41 (5), 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg ND, Smith BW, Sower GJ, Anderson KA, 2014. Predicting Polycyclic Aromatic Hydrocarbon Concentrations in Resident Aquatic Organisms Using Passive Samplers and Partial Least-Squares Calibration. Environ. Sci. Technol. 48 (11), 6291–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Andersen HV, Haug LS, Thomsen C, Broadwell SL, Egsmose EL, Kolarik B, Gunnarsen L, Knudsen LE, 2020. PCB in serum and hand wipes from exposed residents living in contaminated high-rise apartment buildings and a reference group. Int. J. Hyg. Environ. Health 224, 113430. [DOI] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Anderson KA, Herbstman JB, 2019. Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere 219, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA, Stapleton HM, Calero L, Holmes D, Burke K, Martinez R, Cortes B, Nematollahi A, Evans D, Herbstman JB, 2019. Flame retardant exposure assessment: findings from a behavioral intervention study. J. Eposure Sci. Environ. Epidemiol. 29 (1), 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Weschler CJ, Liu L, Shen H, Huang L, Sundell J, Zhang Y, 2015. Phthalate metabolites in urine samples from Beijing children and correlations with phthalate levels in their handwipes. Indoor Air 25 (6), 572–581. [DOI] [PubMed] [Google Scholar]
- Górecki T, Namiésnik J, 2002. Passive sampling. TrAC, Trends Anal. Chem. 21 (4), 276–291. [Google Scholar]
- Guo P, Lin EZ, Koelmel JP, Ding E, Gao Y, Deng F, Dong H, Liu Y, Cha YE, Fang J, Shi X, Tang S, Godri Pollitt KJ, 2021. Exploring personal chemical exposures in China with wearable air pollutant monitors: A repeated-measure study in healthy older adults in Jinan, China. Environ. Int. 156. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 50 (8), 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Phillips AL, Hoffman K, Stapleton HM, 2018. Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol. 52 (20), 11875–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Phillips AL, Levasseur JL, Lorenzo AM, Webster TF, Stapleton HM, 2020. Comparing the Use of Silicone Wristbands, Hand Wipes, And Dust to Evaluate Children’s Exposure to Flame Retardants and Plasticizers. Environ. Sci. Technol. 54 (7), 4484–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzai L, Lopez Galvez N, Hoh E, Dodder NG, Matt GE, Quintana PJ, 2021. A systematic review of the use of silicone wristbands for environmental exposure assessment, with a focus on polycyclic aromatic hydrocarbons (PAHs). J. Exposure Sci. Environ. Epidemiol. [DOI] [PubMed] [Google Scholar]
- Hardos JE, Rubenstein M, Pfahler S, Sleight T, 2020. Cholinesterase Inhibition and Exposure to Organophosphate Esters in Aircraft Maintenance Workers. Aerosp. Med. Hum. Perform. 91 (9), 710–714. [DOI] [PubMed] [Google Scholar]
- Harley KG, Parra KL, Camacho J, Bradman A, Nolan JES, Lessard C, Anderson KA, Poutasse CM, Scott RP, Lazaro G, Cardoso E, Gallardo D, Gunier RB, 2019. Determinants of pesticide concentrations in silicone wristbands worn by Latina adolescent girls in a California farmworker community: The COSECHA youth participatory action study. Sci. Total Environ. 652, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harner T., Shoeib M., 2002. Measurements of Octanol Air Partition Coefficients (KOA) for Polybrominated Diphenyl Ethers (PBDEs): Predicting Partitioning in the Environment. J. Chem. Eng. Data 47 (2), 228–232. [Google Scholar]
- Hendryx M, Wang S, Romanak KA, Salamova A, Venier M, 2020. Personal exposure to polycyclic aromatic hydrocarbons in Appalachian mining communities. Environ. Pollut. 257, 113501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines CJ, Christianson AL, Jackson MV, Ye X, Pretty JR, Arnold JE, Calafat AM, 2018. An Evaluation of the Relationship among Urine, Air, and Hand Measures of Exposure to Bisphenol A (BPA) in US Manufacturing Workers. Ann. Work Exposures Health 62 (7), 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM, 2014. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster® 550. Environ. Health Perspect. 122 (9), 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM, 2015. Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ. Health Perspect. 123 (2), 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K, Levasseur JL, Zhang S, Hay D, Herkert NJ, Stapleton HM, 2021. Monitoring Human Exposure to Organophosphate Esters: Comparing Silicone Wristbands with Spot Urine Samples as Predictors of Internal Dose. Environ. Sci. Technol. Lett. 8 (9), 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Chen C-Y, Wu C-C, Bao L-J, Zeng EY, 2021. A Novel Personal Passive Sampler for Collecting Gaseous Phthalates. Environ. Sci. Technol. 55 (23), 15961–15968. [DOI] [PubMed] [Google Scholar]
- Hou MM, Shi YL, Na GS, Cai YQ, 2021. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure. Environ. Int. 146. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Booij K, 2006. Monitors of Organic Chemicals in the z. Springer Science & Business Media. [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD, 2010. Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environ. Sci. Technol. 44 (14), 5627–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B, 2005. Is House Dust the Missing Exposure Pathway for PBDEs? An Analysis of the Urban Fate and Human Exposure to PBDEs. Environ. Sci. Technol. 39 (14), 5121–5130. [DOI] [PubMed] [Google Scholar]
- Juarez P, Matthews-Juarez P, Hood D, Im W, Levine R, Kilbourne B, Langston M, Al-Hamdan M, Crosson W, Estes M, Estes S, Agboto V, Robinson P, Wilson S, Lichtveld M, 2014. The Public Health Exposome: A Population-Based, Exposure Science Approach to Health Disparities Research. Int. J. Environ. Res. Public Health 11 (12), 12866–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez PD, Matthews-Juarez P, 2018. Applying an Exposome-Wide (ExWAS) Approach to Cancer Research. Front. Oncol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez PD, Hood DB, Song MA, Ramesh A, 2020. Use of an Exposome Approach to Understand the Effects of Exposures From the Natural, Built, and Social Environments on Cardio-Vascular Disease Onset, Progression, and Outcomes. Front. Public Health 8, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara N, Takigami H, 2016. Particle size distribution of brominated flame retardants in house dust from Japan. Emerg. Contaminants 2 (2), 109–117. [Google Scholar]
- Kassotis CD, Herkert NJ, Hammel SC, Hoffman K, Xia QY, Kullman SW, Sosa JA, Stapleton HM, 2020. Thyroid Receptor Antagonism of Chemicals Extracted from Personal Silicone Wristbands within a Papillary Thyroid Cancer Pilot Study. Environ. Sci. Technol. 54 (23), 15296–15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, Yamagata Z, Kayama F, Kishi R, Ohya Y, 2014. Rationale and study design of the Japan environment and children’s study (JECS). BMC Publ. Health 14 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA, 2016. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res. 147, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Hong YC, 2017. The exposome and the future of epidemiology: a vision and prospect. Environ Health Toxicol 32, e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Shin H-M, Wong L, Young TM, Bennett DH, 2021. Temporal variability of indoor dust concentrations of semivolatile organic compounds. Indoor Air 31 (3), 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, Jones K, Warren N, Levy L, Bevan R, 2014. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: Personal care product ingredients. Toxicol. Lett. 231 (2), 261–269. [DOI] [PubMed] [Google Scholar]
- Koelmel JP, Lin EZ, Nichols A, Guo P, Zhou Y, Godri Pollitt KJ, 2021. Head, Shoulders, Knees, and Toes: Placement of Wearable Passive Samplers Alters Exposure Profiles Observed. Environ. Sci. Technol. 55 (6), 3796–3806. [DOI] [PubMed] [Google Scholar]
- Koelmel JP, Lin EZ, Guo P, Zhou J, He J, Chen A, Gao Y, Deng F, Dong H, Liu Y, Cha YE, Fang J, Beecher C, Shi X, Tang S, Godri Pollitt KJ, 2021. Exploring the external exposome using wearable passive samplers - The China BAPE study. Environ. Pollut. 270. [DOI] [PubMed] [Google Scholar]
- Lessmann F, Schütze A, Weiss T, Langsch A, Otter R, Brüning T, Koch HM, 2016. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) terephthalate (DEHTP) in three male volunteers after oral dosage. Arch. Toxicol. 90 (7), 1659–1667. [DOI] [PubMed] [Google Scholar]
- Levasseur JL, Hammel SC, Hoffman K, Phillips AL, Zhang S, Ye XY, Calafat AM, Webster TF, Stapleton HM, 2021. Young children’s exposure to phenols in the home: Associations between house dust, hand wipes, silicone wristbands, and urinary biomarkers. Environ. Int. 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EZ, Esenther S, Mascelloni M, Irfan F, Godri Pollitt KJ, 2020. The Fresh Air Wristband: A Wearable Air Pollutant Sampler. Environ. Sci. Technol. Lett. 7 (5), 308–314. [Google Scholar]
- Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML, 2017. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattini L, Poma G, Covaci A, de Boer J, Lamoree MH, Leonards PEG, 2018. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere 201, 466–482. [DOI] [PubMed] [Google Scholar]
- Manzano CA, Dodder NG, Hoh E, Morales R, 2019. Patterns of Personal Exposure to Urban Pollutants Using Personal Passive Samplers and GC x GC/ToF-MS. Environ. Sci. Technol. 53 (2), 614–624. [DOI] [PubMed] [Google Scholar]
- Mayeux R, 2004. Biomarkers: Potential uses and limitations. NeuroRX 1 (2), 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Sanchez I, Uwak I, Myatt L, Van Cleve A, Pulczinski JC, Rychlik KA,Sweet S, Ramani T, Zietsman J, Zamora ML, Koehler K, Carrillo G, Johnson NM, 2021. Maternal exposure to polycyclic aromatic hydrocarbons in South Texas, evaluation of silicone wristbands as personal passive samplers. J. Exposure Sci. Environ. Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minick DJ, Paulik LB, Smith BW, Scott RP, Kile ML, Rohlman D, Anderson KA, 2019. A passive sampling model to predict PAHs in butter clams (Saxidomus giganteus), a traditional food source for Native American tribes of the Salish Sea Region. Mar. Pollut. Bull. 145, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, Zota AR, 2016. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of U.S. Studies. Environ. Sci. Technol. 50 (19), 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LV, Gravel S, Labrèche F, Bakhiyi B, Verner MA, Zayed J, Jantunen LM, Arrandale VH, Diamond ML, 2020. Can Silicone Passive Samplers be Used for Measuring Exposure of e-Waste Workers to Flame Retardants? Environ. Sci. Technol. 54 (23), 15277–15286. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Anderson KA, Epstein MI, 2021. Determining chemical air equivalency using silicone personal monitors. J. Exposure Sci. Environ. Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, McCartney MA, Paulik LB, Allan SE, Tidwell LG, Wilson G, Anderson KA, 2014. Improvements in pollutant monitoring: Optimizing silicone for co-deployment with polyethylene passive sampling devices. Environ. Pollut. 193, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA, 2014. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 48 (6), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans A, Figueiredo DM, Mol JGJ, Nijssen R, Anzion RBM, van Dael MFP, Duyzer J, Roeleveld N, Russel FGM, Vermeulen RCH, Scheepers PTJ, 2021. Personal exposure assessment of pesticides in residents: The association between hand wipes and urinary biomarkers. Environ. Res. 199, 111282. [DOI] [PubMed] [Google Scholar]
- Okeme JO, Saini A, Yang CQ, Zhu JP, Smedes F, Klanova J, Diamond ML, 2016. Calibration of polydimethylsiloxane and XAD-Pocket passive air samplers (PAS) for measuring gas- and particle-phase SVOCs. Atmos. Environ. 143, 202–208. [Google Scholar]
- Okeme JO, Nguyen LV, Lorenzo M, Dhal S, Pico Y, Arrandale VH, Diamond ML, 2018. Polydimethylsiloxane (silicone rubber) brooch as a personal passive air sampler for semi-volatile organic compounds. Chemosphere 208, 1002–1007. [DOI] [PubMed] [Google Scholar]
- Okeme JO, Yang C, Abdollahi A, Dhal S, Harris SA, Jantunen LM, Tsirlin D, Diamond ML, 2018. Passive air sampling of flame retardants and plasticizers in Canadian homes using PDMS, XAD-coated PDMS and PUF samplers. Environ. Pollut. 239, 109–117. [DOI] [PubMed] [Google Scholar]
- Oluyomi AO, Panthagani K, Sotelo J, Gu X, Armstrong G, Luo DN, Hoffman KL, Rohlman D, Tidwell L, Hamilton WJ, 2021. Houston hurricane Harvey health (Houston-3H) study: assessment of allergic symptoms and stress after hurricane Harvey flooding. Environ. Health 20 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB, Smith BW, Bergmann AJ, Sower GJ, Forsberg ND, Teeguarden JG, Anderson KA, 2016. Passive samplers accurately predict PAH levels in resident crayfish. Sci. Total Environ. 544, 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB, Hobbie KA, Rohlman D, Smith BW, Scott RP, Kincl L, Haynes EN, Anderson KA, 2018. Environmental and individual PAH exposures near rural natural gas extraction. Environ. Pollut. 241, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse CM, Herbstman JB, Peterson ME, Gordon J, Soboroff PH, Holmes D, Gonzalez D, Tidwell LG, Anderson KA, 2019. Silicone Pet Tags Associate Tris (1,3-dichloro-2-isopropyl) Phosphate Exposures with Feline Hyperthyroidism. Environ. Sci. Technol. 53 (15), 9203–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse CM, Poston WSC, Jahnke SA, Haddock CK, Tidwell LG, Hoffman PD, Anderson KA, 2020. Discovery of firefighter chemical exposures using military-style silicone dog tags. Environ. Int. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutasse CM, Haddock CK, Poston WSC, Jahnke SA, Tidwell LG, Bonner EM, Hoffman PD, Anderson KA, 2022. Firefighter exposures to potential endocrine disrupting chemicals measured by military-style silicone dog tags. Environ. Int. 158, 106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana PJE, Hoh E, Dodder NG, Matt GE, Zakarian JM, Anderson KA, Akins B, Chu L, Hovell MF, 2019. Nicotine levels in silicone wristband samplers worn by children exposed to secondhand smoke and electronic cigarette vapor are highly correlated with child’s urinary cotinine. J. Expo. Sci. Environ. Epidemiol. 29 (6), 733–741. [DOI] [PubMed] [Google Scholar]
- Quintana PJE, Lopez-Galvez N, Dodder NG, Hoh E, Matt GE, Zakarian JM, Vyas M, Chu L, Akins B, Padilla S, Anderson KA, Hovell MF, 2021. Nicotine, Cotinine, and Tobacco-Specific Nitrosamines Measured in Children’s Silicone Wristbands in Relation to Secondhand Smoke and E-cigarette Vapor Exposure. Nicotine Tob. Res. 23 (3), 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reche C, Viana M, van Drooge BL, Fernandez FJ, Escribano M, Castano-Vinyals G, Nieuwenhuijsen M, Adami PE, Bermon S, 2020. Athletes’ exposure to air pollution during World Athletics Relays: A pilot study. Sci. Total Environ. 717. [DOI] [PubMed] [Google Scholar]
- Reddam A, Tait G, Herkert N, Hammel SC, Stapleton HM, Volz DC, 2020. Longer commutes are associated with increased human exposure to tris(1,3-dichloro-2-propyl) phosphate. Environ. Int. 136, 105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif JS, 2011. Animal Sentinels for Environmental and Public Health. Public Health Rep. 126 (1_suppl), 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera B, 2020. In: Time-Integrated Exposures to Identify Chemical Profiles between Healthy and Dysphagic Foals, Society of Toxicology, New Frontiers in Dynamic Toxicology, Virtual, Virtual, 2020. [Google Scholar]
- Rohlman D, Dixon HM, Kincl L, Larkin A, Evoy R, Barton M, Phillips A, Peterson E, Scaffidi C, Herbstman JB, Waters KM, Anderson KA, 2019. Development of an environmental health tool linking chemical exposures, physical location and lung function. BMC Public Health 19 (1), 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman D, Donatuto J, Heidt M, Barton M, Campbell L, Anderson KA, Kile ML, 2019. A Case Study Describing a Community-Engaged Approach for Evaluating Polycyclic Aromatic Hydrocarbon Exposure in a Native American Community. Int. J. Environ. Res. Public Health 16 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman D, Samon S, Allan S, Barton M, Dixon H, Ghetu C, Tidwell L, Hoffman P, Oluyomi A, Symanski E, Bondy M, Anderson K, 2022. Designing Equitable, Transparent, Community-engaged Disaster Research. Citizen Science: Theory and Practice 7 (1), 22. 10.5334/cstp.443. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanak KA, Wang SR, Stubbings WA, Hendryx M, Venier M, Salamova A, 2019. Analysis of brominated and chlorinated flame retardants, organophosphate esters, and polycyclic aromatic hydrocarbons in silicone wristbands used as personal passive samplers. J. Chromatogr. A 1588, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodt AP., Naudé Y., Stoltz A., Rohwer E., 2018. Human skin volatiles: Passive sampling and GC × GC-ToFMS analysis as a tool to investigate the skin microbiome and interactions with anthropophilic mosquito disease vectors. J. Chromatogr. B 1097–1098, 83–93. [DOI] [PubMed] [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG, 2003. Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environ. Sci. Technol. 37 (20), 4543–4553. [DOI] [PubMed] [Google Scholar]
- Santiago KM, Louzado-Feliciano P, Baum J, Bakali U, Caban-Martinez AJ, 2021. Self-reported and objectively measured occupational exposures, health, and safety concerns among fishermen: A cross-sectional Fishing Industry Safety and Health (FISH) pilot study. Am. J. Ind. Med. 64 (1), 58–69. [DOI] [PubMed] [Google Scholar]
- Scotch M, Odofin L, Rabinowitz P, 2009. Linkages between animal and human health sentinel data. BMC Veterinary Res. 5 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeib M, Harner T, 2002. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 36 (19), 4142–4151. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, 2002. Using measured octanol-air partition coefficients to explain environmental partitioning of organochlorine pesticides. Environ. Toxicol. Chem.: Int. J. 21 (5), 984–990. [PubMed] [Google Scholar]
- Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF, 2008. Alternate and New Brominated Flame Retardants Detected in U.S. House Dust. Environ. Sci. Technol. 42 (18), 6910–6916. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjödin A, Webster TF, 2012. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 120 (7), 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone JA, Buck Louis GM, Nakayama SF, Vermeulen RC, Kwok RK, Cui Y, Balshaw DM, Teitelbaum SL, 2017. Toward Greater Implementation of the Exposome Research Paradigm within Environmental Epidemiology. Annu. Rev. Public Health 38, 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin DL, 2006. Pyrethroid Insecticides: Advances and Challenges in Biomonitoring. Clin. Toxicol. 44 (1), 31–37. [DOI] [PubMed] [Google Scholar]
- Swanson JE, Muths E, Pierce CL, Dinsmore SJ, Vandever MW, Hladik ML, Smalling KL, 2018. Exploring the amphibian exposome in an agricultural landscape using telemetry and passive sampling. Sci. Rep. 8 (1), 10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuresson K, Höglund P, Hagmar L, Sjödin A, Bergman A, Jakobsson K, 2006. Apparent half-lives of hepta-to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 114 (2), 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis SC, Aga DS, Queirolo EI, Olson JR, Daleiro M, Kordas K, 2020. Catching flame retardants and pesticides in silicone wristbands: Evidence of exposure to current and legacy pollutants in Uruguayan children. Sci. Total Environ. 740, 140136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis SC, Kordas K, Aga DS, 2021. Optimized workflow for unknown screening using gas chromatography high-resolution mass spectrometry expands identification of contaminants in silicone personal passive samplers. Rapid Commun. Mass Spectrom, e9048. [DOI] [PubMed] [Google Scholar]
- Tromp PC, Beeltje H, Okeme JO, Vermeulen R, Pronk A, Diamond ML, 2019. Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design. Chemosphere 227, 435–443. [DOI] [PubMed] [Google Scholar]
- Varnell RR, Arnold TJ, Quandt SA, Talton JW, Chen H, Miles CM, Daniel SS, Sandberg JC, Anderson KA, Arcury TA, 2021. Menstrual Cycle Patterns and Irregularities in Hired Latinx Child Farmworkers. J. Occup. Environ. Med. 63 (1), 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidi PA, Anderson KA, Chen HY, Anderson R, Salvador-Moreno N, Mora DC, Poutasse C, Laurienti PJ, Daniel SS, Arcury TA, 2017. Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat. Res.-Genetic Toxicol. Environ. Mutagen. 822, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkamp K, Odsbjerg L, Langeland M, Mayer P, 2016. Utilizing the partitioning properties of silicone for the passive sampling of polychlorinated biphenyls (PCBs) in indoor air. Chemosphere 160, 280–286. [DOI] [PubMed] [Google Scholar]
- Wang Y, Peris A, Rifat MR, Ahmed SI, Aich N, Nguyen LV, Urík J, Eljarrat E, Vrana B, Jantunen LM, Diamond ML, 2020. Measuring exposure of e-waste dismantlers in Dhaka Bangladesh to organophosphate esters and halogenated flame retardants using silicone wristbands and T-shirts. Sci. Total Environ. 720, 137480. [DOI] [PubMed] [Google Scholar]
- Wang SR, Romanak KA, Stubbings WA, Arrandale VH, Hendryx M, Diamond ML, Salamova A, Venier M, 2019. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SR, Romanak KA, Hendryx M, Salamova A, Venier M, 2020. Association between Thyroid Function and Exposures to Brominated and Organophosphate Flame Retardants in Rural Central Appalachia. Environ. Sci. Technol. 54 (1), 325–334. [DOI] [PubMed] [Google Scholar]
- Wang SR, Romanak KA, Tarallo S, Francavilla A, Viviani M, Vineis P, Rothwell JA, Mancini FR, Cordero F, Naccarati A, Severi G, Venier M, 2020. The use of silicone wristbands to evaluate personal exposure to semi-volatile organic chemicals (SVOCs) in France and Italy. Environ. Pollut. 267. [DOI] [PubMed] [Google Scholar]
- Wania F, Shunthirasingham C, 2020. Passive air sampling for semi-volatile organic chemicals. Environ. Sci. Processes Impacts 22 (10), 1925–2002. [DOI] [PubMed] [Google Scholar]
- Whitehead T, Metayer C, Buffler P, Rappaport SM, 2011. Estimating exposures to indoor contaminants using residential dust. J. Eposure Sci. Environ. Epidemiol. 21 (6), 549–564. [DOI] [PubMed] [Google Scholar]
- Wild CP, Scalbert A, Herceg Z, 2013. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environ. Mol. Mutagen. 54 (7), 480–499. [DOI] [PubMed] [Google Scholar]
- Wise CF, Hammel SC, Herkert N, Ma J, Motsinger-Reif A, Stapleton HM, Breen M, 2020. Comparative Exposure Assessment Using Silicone Passive Samplers Indicates That Domestic Dogs Are Sentinels To Support Human Health Research. Environ. Sci. Technol. 54 (12), 7409–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise CF, Hammel SC, Herkert NJ, Ospina M, Calafat AM, Breen M, Stapleton HM, 2021. Comparative Assessment of Pesticide Exposures in Domestic Dogs and Their Owners Using Silicone Passive Samplers and Biomonitoring. Environ. Sci. Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding M., Rohwer ER., Naudé Y, 2020. Chemical profiling of the human skin surface for malaria vector control via a non-invasive sorptive sampler with GC × GC-TOFMS. Anal. Bioanal. Chem. 412 (23), 5759–5777. [DOI] [PubMed] [Google Scholar]
- Xie Q, Guan Q, Li L, Pan X, Ho C-L, Liu X, Hou S, Chen D, 2021. Exposure of children and mothers to organophosphate esters: Prediction by house dust and silicone wristbands. Environ. Pollut. 282, 117011. [DOI] [PubMed] [Google Scholar]
- Yaw TJ, Swanson JE, Pierce CL, Muths E, Smalling KL, Vandever MW, Zaffarano BA, 2017. Placement of Intracoelomic Radiotransmitters and Silicone Passive Sampling Devices in Northern Leopard Frogs (Lithobates pipiens). J. Herpetol. Med. Surg. 27 (3–4), 111–115. [Google Scholar]
- Young AS, Herkert N, Stapleton HM, Cedeño Laurent JG, Jones ER, MacNaughton P, Coull BA, James-Todd T, Hauser R, Luna ML, Chung YS, Allen JG, 2021. Chemical contaminant exposures assessed using silicone wristbands among occupants in office buildings in the USA, UK, China, and India. Environ. Int. 156, 106727. [DOI] [PMC free article] [PubMed] [Google Scholar]