Abstract
Using a pulmonary model of infection, we demonstrated previously that A/Sn and B10.A mice are, respectively, resistant and susceptible to Paracoccidioides brasiliensis infection. Employing the same experimental model, we examined herein the role of CD8+ T cells in the course of paracoccidioidomycosis. Treatment with anti-CD8 monoclonal antibodies caused a selective depletion of pulmonary and splenic CD8+ T cells in both mouse strains. The number of pulmonary CD4+ T cells and immunoglobulin-positive cells was independent of the number of CD8+ T cells. In susceptible mice, the loss of CD8+ T cells by in vivo treatment with anti-CD8 monoclonal antibodies impaired the clearance of yeasts from the lungs and increased the fungal dissemination to the liver and spleen. The same treatment in resistant mice increased fungal dissemination to extrapulmonary tissues but did not alter the pulmonary fungal load. Furthermore, CD8+ T-cell depletion did not modify delayed-type hypersensitivity reactions of A/Sn mice but increased these reactions in B10.A mice. The production of P. brasiliensis-specific antibodies by resistant and susceptible mice depleted of CD8+ T cells was similar to that of mice given control antibody. Histopathologically, depletion of CD8+ T cells did not disorganize the focal granulomatous lesions developed by both mouse strains. These results indicate that CD8+ T cells are necessary for optimal clearance of the fungus from tissues of mice infected with P. brasiliensis and demonstrate more prominent protective activity by those cells in the immune responses mounted by susceptible animals.
Paracoccidioidomycosis (PCM), caused by Paracoccidioides brasiliensis, a thermally dimorphic fungus, is the major systemic mycosis caused by a frank pathogen in Latin America (4, 35). The infection is usually acquired by the respiratory route, probably by inhalation of airborne propagules (25, 35). Most infected subjects develop an asymptomatic pulmonary infection, which indicates that they are resistant hosts. However, some individuals are susceptible to this fungal agent and develop overt PCM. The disease presents a wide gamut of clinical and pathological manifestations, ranging from benign and localized forms to severe disseminated disease (4, 27). In most cases, the disease involves the lungs primarily and then disseminates to other organs and systems.
Experimental (14, 36) and clinical (10, 30, 31, 33) investigations have indicated the relevance of humoral and/or cellular immune responses in the pathogenesis and evolution of PCM. Specific cell-mediated immune responses seem to play an important role in resistance to P. brasiliensis. Patients with systemic PCM tend to show depressed cellular immune responses compared to those with localized disease (31, 33); also, the most severe forms of infection are associated with high levels of specific antibodies (reviewed in reference 10).
Using a murine model of intraperitoneally (i.p.) induced PCM, we observed significant differences in susceptibility among inbred strains: A/Sn mice were found to be the most resistant, while B10.A animals were the most susceptible, to P. brasiliensis infection (8, 9, 36). More recently, using the intratracheal (i.t.) route of infection, we developed a pulmonary PCM model with the same inbred mouse strains and verified that A/Sn and B10.A mice maintain the same resistance patterns as those observed with the i.p. route of infection (12). These studies demonstrated that A/Sn mice develop a chronic benign pulmonary-restricted PCM associated with low mortality rates, the presence of positive and persistent delayed-type hypersensitivity (DTH) reactions, and production of high levels of specific antibodies in which immunoglobulin G2a (IgG2a) and IgG3 isotypes are higher than those observed in susceptible mice. In contrast, B10.A mice develop a progressive disseminated disease resulting in high mortality rates, discrete DTH reactions, and production of an IgG2b isotype at levels higher than those observed in the resistant strain.
Studies using athymic BALB/c mice (nu/nu) and their heterozygous counterparts (nu/+), which are phenotypically normal, revealed that susceptibility to P. brasiliensis infection is exacerbated in athymic animals (6). This demonstrates that the integrity of the cellular immune response is fundamental to the establishment of resistance mechanisms to P. brasiliensis infection. However, the contributions of the different components of the T-cell response are unclear.
Various studies have shown that the role of CD8+ T cells in the immune response may be protective (15, 19, 32), suppressive (34), or just innocuous (1), depending both on the infecting organism and on the genetic characteristics of the host. To our knowledge, the role of CD8+ T cells in resistance against P. brasiliensis pulmonary infection has never been investigated. Thus, we have undertaken a series of studies of CD8+ T-cell-depleted A/Sn and B10.A mice, investigating their responses to i.t. infection. In particular, we have characterized the T- and B-cell subpopulations in the spleen and lung of infected and CD8+ T-cell-depleted animals and investigated the progression of pulmonary and extrapulmonary infections, the specific DTH reactions, the specific humoral responses, and the histopathology of pulmonary lesions at weeks 4 and 8 postinfection. The data obtained demonstrate that, irrespective of the mouse strain, CD8+ T cells are involved in clearance of fungal cells and in control of dissemination to extrapulmonary tissues. These cells also seem to play a role in suppressing DTH reactions in susceptible mice but show a negligible effect on the pattern of pulmonary lesions, as well as the production of specific antibody, by both resistant and susceptible mice.
MATERIALS AND METHODS
Animals.
Unless otherwise stated, groups of 8 to 10 male mice (8 to 11 weeks old) from the susceptible (B10.A) and resistant (A/Sn) strains were used for each period of infection. All animals were bred at the University of São Paulo animal facilities and provided with acidified water and sterilized food and bedding.
Fungus.
P. brasiliensis Pb18, a highly virulent isolate (21), was used throughout this investigation. To ensure the maintenance of its virulence, the isolate was used after three animal passages (22). Pb18 yeast cells were then maintained by weekly subcultivation in semisolid Fava Netto's culture medium (16) at 35°C and used on day 7 after culture. The yeast cells were washed in phosphate-buffered saline (PBS) (pH 7.2) and adjusted to 20 × 106 cells/ml based on hemacytometer counts. Viability was determined with Janus green B vital dye (3) (Merck, Darmstadt, Germany) and was always higher than 80%.
P. brasiliensis infection.
Mice were anesthetized and submitted to i.t. P. brasiliensis infection, as previously described (12). Briefly, after i.p. anesthesia the animals were infected with 106 P. brasiliensis Pb18 yeast cells, contained in 50 μl of PBS, by surgical i.t. inoculation that allowed dispensing of the fungal cells directly into the lungs. The skin was then sutured and the mice were allowed to recover under a heat lamp.
In vivo depletion of CD8+ T cells.
H-35 hybridoma cells secreting rat IgG1 anti-Lyt-2 monoclonal antibody (MAb) (murine CD8) were used in this study. These cells were grown as ascites in pristane (Sigma Chemical Co., St. Louis, Mo.)-primed, sublethally irradiated (550 rads) BALB/c mice. The H-35 antibodies were purified from ascites as described elsewhere (26) and assessed for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Groups of B10.A and A/Sn mice were given 150 μg of H-35 MAb or normal rat IgG (controls) by the i.p. route at 1 day before infection with P. brasiliensis cells (day −1) and 100 μg of H-35 MAb or rat IgG weekly for 8 weeks thereafter. Infected, noninfected, and nondepleted mice were all used as controls.
Cell preparation.
Spleen and lung lymphocyte cell suspensions were prepared as previously described (19). Spleen cells were removed and homogenized in RPMI to obtain a single cell suspension. Erythrocytes were lysed before washing of cells for staining. Lung-infiltrating lymphocytes were obtained from individual mice after bronchoalveolar lavage by repeated injections of 1 ml of sterile PBS (final volume, 3 ml). Lungs were then removed, minced, and digested for 60 min in digestion buffer (RPMI, 10% fetal calf serum, antibiotics, and 1 mg of collagenase per ml [Boehringer Mannheim Biochemicals, Indianapolis, Ind.]). The cell suspension and undigested fragments were further dissociated by using thick-walled glass tubes fitted with Teflon pestles, washed, and pelleted, and erythrocytes were lysed. The lymphocytes were then isolated by standard Percoll (Pharmacia Biotech AB, Uppsala, Sweden) gradient centrifugation. Cells were recovered and counted, and viability was determined with trypan blue vital stain.
Cytofluorometry.
The efficiency of in vivo CD8+ T-cell depletion and the effect of i.t. infection on the profile of lymphocyte subpopulations were determined at 4 weeks postinfection by flow cytofluorometric analysis. Cells were obtained from the spleen and lung of mice inoculated with the MAb or normal rat IgG and i.t. infected with 106 P. brasiliensis yeast cells. Normal, saline-injected as well as only infected animals were used as controls. Cell suspensions of each animal were washed, adjusted to 5 × 106 viable cells/ml, and stained with phycoerythrin-conjugated anti-CD4, anti-CD8, or rat anti-mouse Ig MAb (Pharmingen, San Diego, Calif.). The stained cells were analyzed immediately by FACScan equipment using PC-Lysys software (Becton Dickinson, San Jose, Calif.) and gating on lymphocytes as judged from forward and side light scatter. Ten thousand lymphocytes were counted, and the data are expressed as the number of viable CD8+ T cells, CD4+ T cells, and Ig+ cells. The efficiency of the CD8+ T-cell depletion was assessed by calculating the decrease of this subpopulation relative to infected, normal IgG-treated animals.
Assay for organ CFU.
The numbers of viable microorganisms in the lungs, liver, and spleen from CD8+ T-cell-depleted and nondepleted mice were determined by counting the numbers of CFU. Eight to 10 animals from each mouse strain were sacrificed at 4 and 8 weeks postinfection, and enumerations of viable organisms in the three organs were done by counting the numbers of CFU, as previously described (37). Briefly, aliquots (100 μl) of the cellular suspensions of each organ were plated on brain heart infusion agar (Difco) supplemented with 4% (vol/vol) horse serum (Instituto Butantan, São Paulo, Brazil) and 5% Pb192 culture filtrate, the latter constituting a source of growth-promoting factor. Plates were incubated at 35°C, and colonies were counted daily until no increase in counts was observed. The numbers (log10) of viable P. brasiliensis colonies per gram of tissue are expressed as means ± standard errors.
DTH assay.
DTH reactions were always evaluated just before sacrifice of the animals used in the CFU assays by the footpad test, under previously determined conditions (17). Briefly, mice were inoculated with 25 μl of Fava Netto's antigen (16) and footpad thickness was measured with a caliper (Mitutoyo Corporation, Tokyo, Japan) immediately before and 24 h after antigen inoculation. The increase in thickness was calculated and expressed in millimeters. Noninfected mice submitted to the footpad test were used as controls.
Specific antibody levels.
Specific antibody levels (total Ig, IgM, IgG1, IgG2a, IgG3, and IgG2b) were measured by a previously described enzyme-linked immunosorbent assay (ELISA) (12) employing a cell-free antigen (11) prepared from a pool of different P. brasiliensis isolates (Pb339, Pb265, and Pb18). The average of the optical densities obtained with sera from control mice (PBS inoculated), diluted 1:20, was considered the cutoff for each respective isotype. Optical densities for each dilution of experimental serum were compared to control values. The titer for each sample was expressed as the reciprocal of the highest dilution which presented absorbance higher than the cutoff.
Histopathologic analysis.
Groups of five normal rat IgG-treated and CD8+ T-cell-depleted P. brasiliensis-infected A/Sn and B10.A mice were killed at week 4 after infection. Lungs were collected, fixed in 10% formalin, and embedded in paraffin. Five-micrometer sections were stained by the hematoxylin-eosin method. Pathologic changes were analyzed based on the number, size, morphology, and cell composition of granulomatous lesions, number of fungi, and intensity of inflammatory infiltrates.
Statistical analysis.
The results obtained in the groups of each mouse strain were analyzed by nonparametric two-way analysis of variance (Kruskal-Wallis method) followed by multiple comparisons according to Dunn's procedure (41). A P value of <0.05 was considered significant.
RESULTS
Phenotypic characterization of cells in the spleen and lungs of mice.
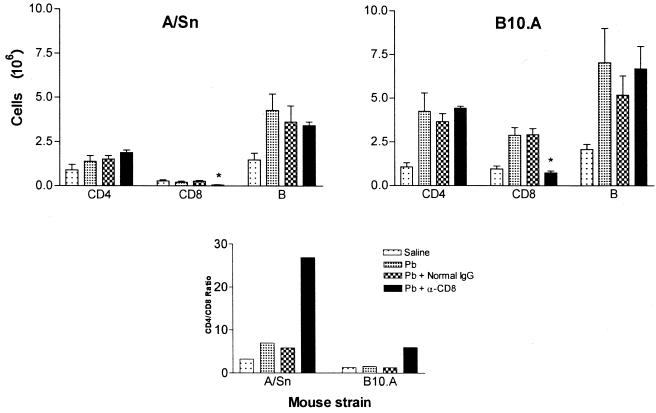
To evaluate the efficiency of anti-CD8 MAb in vivo administration as well as its specificity, the phenotypes of lymphocytes obtained from the lungs and spleen of infected and control mice were determined by flow cytometric analysis. Four groups (n = 4 to 6) of A/Sn and B10.A mice were used: saline-injected normal mice, P. brasiliensis-infected mice, and infected mice treated with either normal rat IgG or anti-CD8+ T cell MAb. At week 4 of the experiment, splenic lymphocytes were analyzed to monitor T-cell depletion and revealed a significant decrease of CD8+ T lymphocytes in the anti-CD8-treated group (71.5%, P = 0.004 [for A/Sn mice]; 67.1%, P = 0.005 [for B10.A mice]). This fact is reflected in the increased CD4/CD8 ratios detected in the MAb-treated groups (Fig. 1). Flow cytometric analysis of lymphocytes found in the lungs of B10.A mice by week 4 of infection (Table 1) demonstrated an increased number of CD4+ T cells, CD8+ T cells, and Ig+ cells in the P. brasiliensis-infected and normal IgG-treated groups. The equivalent groups of A/Sn mice presented increased numbers of CD4+ T cells and Ig+ cells but not of CD8+ T lymphocytes. These data indicate that both CD4+ and CD8+ T cells are involved in the pulmonary immune response developed by susceptible animals, while in the resistant strain the CD4+ T cells constitute the major subpopulation involved in the local inflammatory response. Thus, a twofold increase in the CD4/CD8 ratio was detected in A/Sn mice but not in B10.A animals. Treatment with anti-CD8 MAb led to a specific decrease in the CD8+ T-cell subset in both mouse strains (62.8%, P = 0.05 [for A/Sn mice]; 77.7%, P = 0.035 [or B10.A mice]) when compared with the normal IgG-treated group. An important observation was that Ig+ and CD4+ T-cell influx still occurred in mice depleted of CD8+ cells. Analysis of bronchoalveolar lavage cells gave results similar to those obtained with lung-infiltrating lymphocytes (data not shown).
FIG. 1.
Quantitation of splenic lymphocytes and CD4/CD8 ratios in infected and CD8+ T-cell-depleted mice. Groups (n = 4 to 6) of A/Sn and B10.A mice are as follows: saline-injected normal mice (Saline), P. brasiliensis-infected mice (Pb), infected mice treated with normal rat IgG (Pb + Normal IgG), and infected mice treated with anti-CD8+ T-cell MAb (Pb + α-CD8). Spleens were harvested at week 4 of the experiment. Data represent the numbers of positively fluorescent cells expressing forward- and side-scatter characteristics of lymphocytes.
TABLE 1.
Flow cytometric analysis of CD4+, CD8+, and Ig+ lung-infiltrating lymphocytes from P. brasiliensis-infected mice treated with anti-CD8+ T-cell MAb
| Mouse strain | Treatmenta | 106 cells (SE)b
|
CD4/CD8 ratio | |||
|---|---|---|---|---|---|---|
| Total | T CD4+ | T CD8+ | IG+ | |||
| A/Sn | Saline | 3.90 (0.63) | 0.90 (0.30) | 0.28 (0.07) | 1.47 (0.39) | 3.21 |
| Pb | 9.71 (1.51) | 1.39 (0.33) | 0.20 (0.06) | 4.27 (0.94) | 6.95 | |
| Pb + normal IgG | 9.90 (2.24) | 1.52 (0.20) | 0.26 (0.05) | 3.63 (0.90) | 5.85 | |
| Pb + α-CD8 | 9.78 (0.77) | 1.88 (0.14) | 0.07* (0.01) | 3.43 (0.20) | 26.86 | |
| B10.A | Saline | 5.67 (0.88) | 1.07 (0.23) | 0.95 (0.17) | 2.08 (0.28) | 1.24 |
| Pb | 22.67 (3.72) | 4.24 (1.05) | 2.89 (0.44) | 7.04 (1.97) | 1.47 | |
| Pb + normal IgG | 17.50 (1.26) | 3.65 (0.47) | 2.92 (0.34) | 5.19 (1.09) | 1.25 | |
| Pb + α-CD8 | 17.50 (1.26) | 4.43 (0.11) | 0.74* (0.10) | 6.68 (1.30) | 5.99 | |
Pb, P. brasiliensis infected; α-CD8, anti-CD8 T-cell MAb. To deplete CD8+ T cells, mice were treated with 150 μg of H-35 MAb i.p. at 1 day before infection with P. brasiliensis cells (day −1) and 100 μg weekly thereafter for 4 or 8 weeks. The normal IgG groups consisted of infected mice injected i.p. with normal rat IgG on the same schedule.
Lungs from control and infected mice were minced and digested with collagenase, and leukocytes were obtained after cell fractionation in Percoll. Values are for four to six mice tested at week 4 of infection. ∗, difference statistically significant (P < 0.05) compared to results obtained from P. brasiliensis-infected (Pb) and normal rat IgG-treated, infected mice (Pb + normal IgG).
Effect of in vivo depletion of CD8+ T cells on severity of infection.
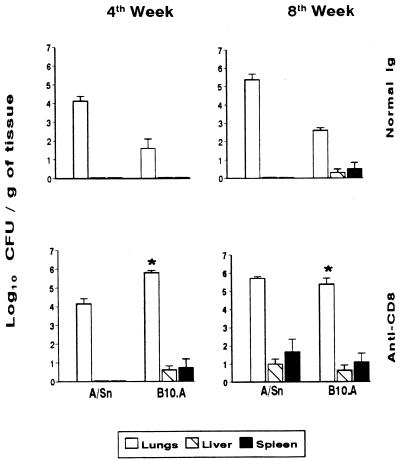
The evolution of disease in IgG-treated and CD8-depleted A/Sn and B10.A mice was monitored by CFU counts in the lungs, spleen, and liver at 4 and 8 weeks after infection (Fig. 2). Depletion of CD8+ T cells of resistant animals did not alter the infection pattern at the 4th week postinfection. The fungal load in the lungs (4.1472 ± 0.2740 log10 CFU/g of tissue) was similar to that found in the nondepleted A/Sn mice (4.1184 ± 0.2557 log10 CFU/g of tissue), and no fungal dissemination to other organs could be observed in either depleted or nondepleted mice. However, at the 8th week after infection, CD8+ T-cell-depleted A/Sn mice displayed fungal dissemination to the liver (0.9938 ± 0.2770 log10 CFU/g of tissue) and spleen (1.6480 ± 0.7098 log10 CFU/g of tissue) that could not be seen in nondepleted A/Sn mice. Although depletion of CD8+ T cells induced a disseminated pattern of disease, the pulmonary fungal load (5.7208 ± 0.0855 log10 CFU/g of tissue) was similar to that found in nondepleted animals (5.4815 ± 0.0627 log10 CFU/g of tissue).
FIG. 2.
Effect of in vivo depletion of CD8+ T cells in genetically resistant (A/Sn) and susceptible (B10.A) mice i.t. infected with 106 P. brasiliensis yeast cells. Recovery of CFU from lungs, liver, and spleen of infected mice treated with normal rat IgG or rat MAb anti-CD8+ T cells is shown. The bars depict means ± standard errors of log10 CFU obtained from groups of 8 to 10 mice at weeks 4 and 8 after infection. ∗, significantly different (P < 0.05) from normal IgG-treated group.
B10.A mice were precociously affected by MAb H-35: at week 4 after infection, depleted animals presented significantly higher numbers of CFU in the lungs (5.8093 ± 0.0855 log10 CFU/g of tissue) than did nondepleted mice (1.5925 ± 0.5133 log10 CFU/g of tissue). Also, it was observed that the disease was disseminated to the liver (0.6245 ± 0.2051 log10 CFU/g of tissue) and spleen (0.73605 ± 0.4658 log10 CFU/g of tissue) in the depleted group but not in the infected, nondepleted B10.A mice. At week 8 postinfection, MAb-treated B10.A animals displayed an exacerbation of pulmonary infection (5.3801 ± 0.3309 log10 CFU/g of tissue) compared to their nondepleted counterparts (2.1785 ± 0.0855 log10 CFU/g of tissue). The extrapulmonary dissemination, however, was not markedly different in the two groups of B10.A mice: animals treated with the MAbs presented 0.6278 ± 0.2882 and 1.0861 ± 0.4859 log10 CFU/g of hepatic and splenic tissues, respectively, and the nondepleted group showed 0.3000 ± 0.1968 log10 CFU/g of hepatic tissue and 0.5075 ± 0.3324 log10 CFU/g of splenic tissue.
Effect of CD8+ T-cell depletion on pulmonary lesions.
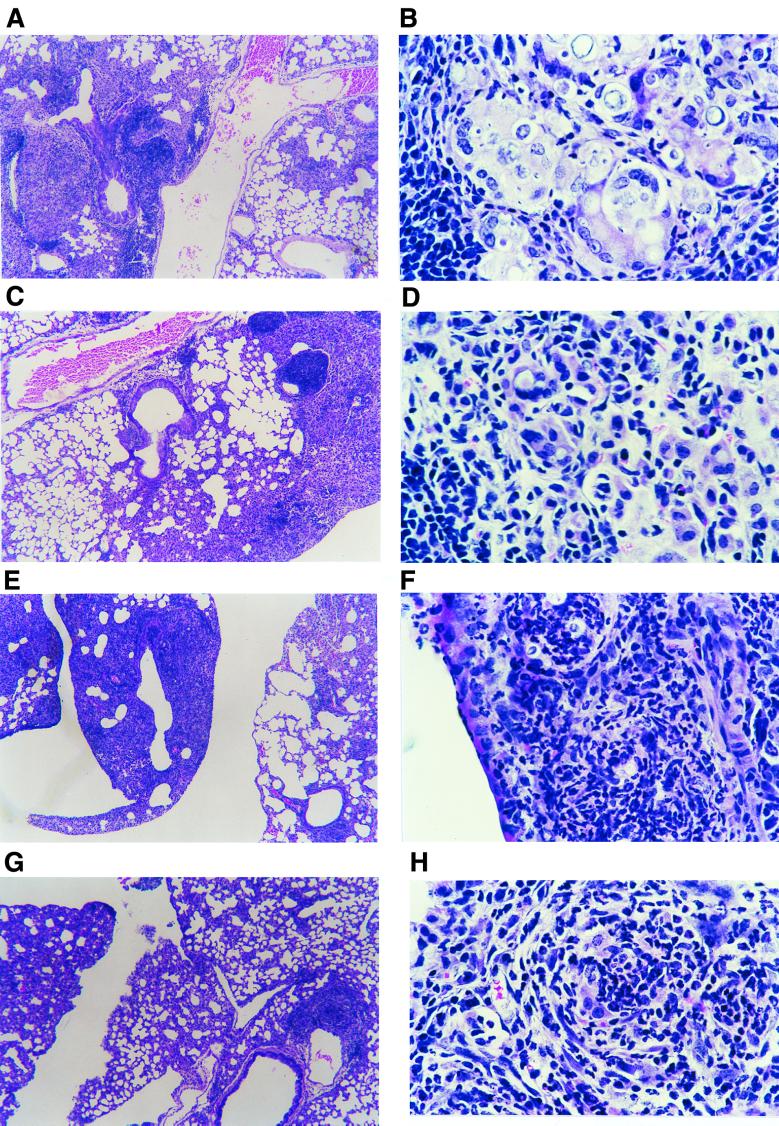
The increased fungal load and extrapulmonary dissemination observed in CD8+ T-cell-depleted mice suggested that this T-cell subpopulation could interfere, at least in susceptible mice, in the morphology of pulmonary lesions. Thus, we analyzed lung sections from IgG-treated and anti-CD8 MAb-treated A/Sn and B10.A mice at week 4 postinfection. All studied mice presented circumscribed granulomatous lesions scattered in the lungs, localized preferentially around the bronchi, bronchioles, and blood vessels. In both groups of A/Sn mice (anti-CD8 MAb or rat IgG treated), the patterns of the lesions were similar, with few granulomas and preservation of the pulmonary parenchyma (Fig. 3A and C). The cellular compositions of the lesions were also qualitatively the same, with multinucleated giant cells often containing P. brasiliensis yeasts and the presence of a peripheral sheet of lymphocytes and plasmocytes, although in the CD8-depleted group the multinucleated giant cells were more frequent and larger than those observed in the IgG-treated group (Fig. 3B and D). In B10.A mice, the depletion of CD8+ T cells also did not change the pattern of granulomas but increased the intensity of the inflammatory reaction (Fig. 3E and G). Actually, B10.A mice depleted of CD8+ T cells showed more severe pulmonary involvement than all the other studied groups, as verified by the increased size of granulomas and their fungal load. The lesions observed in B10.A mice were formed by foci of macrophages and polymorphonuclear leukocytes around Pb18 yeasts; in the CD8-depleted group the polymorphonuclear cell infiltrate was more prominent than in the control group (Fig. 3F and H). In this mouse strain, the presence of multinucleated giant cells was less intense than that observed in A/Sn mice.
FIG. 3.
Photomicrographs of granulomatous lesions from mice i.t. infected with 106 P. brasiliensis cells and treated with anti-CD8 MAb or normal rat IgG. Shown are, respectively, panoramic views (hematoxylin and eosin, ×52) and details (hematoxylin and eosin, ×435) of lesions developed by A/Sn mice depleted of CD8+ T cells (A and B), A/Sn mice treated with normal rat IgG (C and D), B10.A mice depleted of CD8+ T cells (E and F), and normal IgG-treated B10.A mice (G and H). Notice the higher number and size of multinucleated giant cells in the pulmonary lesions of CD8-depleted A/Sn mice (B) and the more intense polymorphonuclear infiltrate in the lesions of CD8-depleted B10.A mice (F).
Effect of in vivo depletion of CD8+ T cells on DTH response.
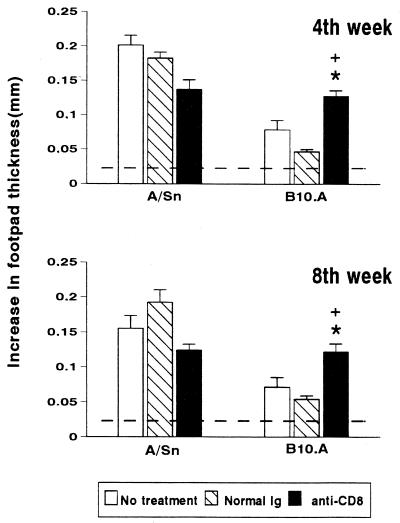
To assess the influence of CD8+ T cells on the DTH response during pulmonary PCM, CD8+ T-cell-depleted A/Sn and B10.A animals were evaluated at weeks 4 and 8 after infection. The reactions of these animals were compared to three nondepleted control groups: normal (noninfected) mice, infected mice, and mice infected and treated with normal rat IgG. As shown in Fig. 4, infected A/Sn and B10.A mice, regardless of the treatment, always presented significant DTH responses compared to normal mice at the two assayed points postinfection. Depletion of CD8+ T cells in A/Sn mice induced a slightly lower DTH reaction compared to their infected, nondepleted counterparts, at both the 4th and 8th week postinfection. Susceptible mice displayed the opposite behavior: depletion of CD8+ T cells induced DTH responses at week 4 and 8 postinfection that were significantly more intense than those found in nontreated or normal rat IgG-treated, infected controls.
FIG. 4.
Effects of treatment with MAb anti-CD8+ T cells on DTH responses of resistant (A/Sn) and susceptible (B10.A) mice i.t. infected with 106 P. brasiliensis yeast cells. Animals were untreated, treated with normal rat IgG, or treated with rat anti-CD8+ T-cell MAb. At weeks 4 and 8 after infection, mice (8 to 10 animals per group) were injected intrafootpad with a soluble yeast antigen 24 h before measurement of the footpad response. The bars depict means ± standard errors of footpad swelling. The horizontal lines denote means ± 2 standard deviations (95% confidence interval) of noninfected A/Sn and B10.A mice submitted to the footpad test (n = 19). ∗, significantly different (P < 0.05) from untreated group; +, significantly different (P < 0.05) from normal IgG-treated group.
Effects of in vivo depletion of CD8+ T cells on specific humoral immune responses.
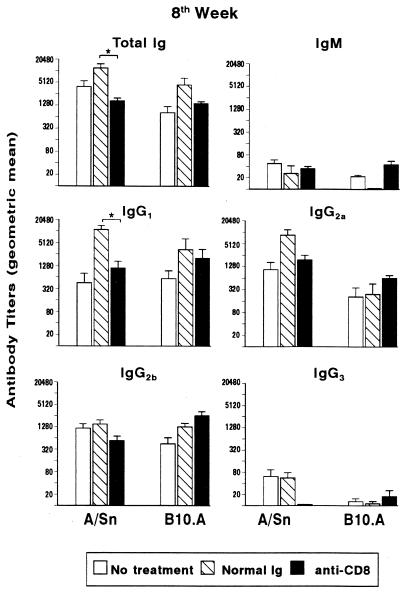
Cytokines produced by CD4+ and CD8+ T cells play an important role in regulation of the humoral immune response and isotype switching (23, 38, 39). Moreover, previous studies from our group have demonstrated that the humoral immune response against most P. brasiliensis components is T-cell dependent (7). Thus, we studied the influence of the integrity of the CD8+ T-cell subset on specific antibody production during pulmonary infection of mice treated with control IgG or H-35 MAb. At 4 and 8 weeks postinfection, sera were collected and assayed for their contents of specific total Ig, IgM, IgG1, IgG2a, IgG2b, and IgG3. Control animals treated with normal rat IgG commonly presented higher levels of specific anti-P. brasiliensis antibodies than did nontreated controls, although the difference was usually not significant (Fig. 5). At week 4 of infection, no significant differences were found between normal IgG-treated and CD8-depleted groups (data not shown). When treatment was given for 8 weeks, antibody titers were usually higher than those verified at week 4 postinfection. A decrease in total Ig and IgG1 levels was observed in A/Sn MAb-treated mice compared to their normal rat IgG-treated counterparts. On the other hand, prolonged CD8+ T-cell depletion in B10.A mice did not induce significant differences in isotype titers relative to normal IgG-treated animals.
FIG. 5.
Levels of anti-P. brasiliensis-specific antibodies in sera of resistant (A/Sn) and susceptible (B10.A) mice at 8 weeks after i.t. infection with 106 yeast cells. Infected mice were untreated, treated with normal rat IgG, or treated with anti-CD8+ T-cell MAb. Sera were assayed for total Ig, IgM, IgG1, IgG2a, IgG2b, and IgG3 by using an isotype-specific ELISA, as detailed in Materials and Methods. The bars depict geometric means ± standard errors of serum titers (8 to 10 animals per group). ∗, significantly different (P < 0.05) from normal IgG-treated groups.
DISCUSSION
Data determined by CFU counts in the lungs, liver, and spleen of i.t. infected mice clearly demonstrated that, during experimental P. brasiliensis infection, depletion of CD8+ T cells induces a more severe and/or disseminated disease in both resistant and susceptible mice. Depletion of CD8+ T cells of A/Sn mice during 4 weeks did not alter the degree of infection, thus suggesting that CD8+ T cells may not play an important role at the initial phases of the disease of resistant animals. However, CD8+ T cells appear to be involved in later control of pulmonary infection, hampering the escape of fungal cells to other organs, since prolonged depletion (8 weeks) resulted in extrapulmonary fungal dissemination, although the pulmonary infection remained unaffected. In susceptible mice, however, CD8+ T cells seem to have a protective role already at the beginning of the infection, since at the 4th week postinfection CD8+ T-cell-depleted mice presented an exacerbated pulmonary infection and precocious fungal dissemination to the liver and spleen; this profile was maintained after prolonged times of depletion.
The decrement in splenic and pulmonary CD8+ T cells in resistant and susceptible mice given anti-CD8+ T-cell MAb ranged from 62.8 to 77.7%. Despite the incomplete elimination of CD8+ T cells in P. brasiliensis-infected mice, at week 4 the number of pulmonary yeast cells increased more than 10,000-fold in susceptible mice, demonstrating that CD8+ T cells play at an early time an important role in the clearance of P. brasiliensis. A similar degree of CD8+ T-cell depletion, however, did not affect the fungal burden in the lungs of resistant animals, showing a more prominent participation of this T-cell subpopulation in the susceptible strain.
An important observation was that the inflammatory influx into the lungs of susceptible mice was composed of CD4+ and CD8+ T cells, whereas in resistant mice only the former subpopulation appears in slightly increased numbers. This picture agrees with CFU data at week 4 of infection when depletion of CD8+ T cells affected fungal pulmonary loads only of susceptible mice. Altogether these data appear to indicate that the pulmonary protective immune responses mounted by B10.A mice involve the contribution of CD4+ and CD8+ T cells, whereas in A/Sn mice the CD4+ T-cell subset is mainly involved.
Imbalances in T-cell subsets have also been described for human PCM. In T-cell subpopulations in the peripheral blood of patients with acute or chronic forms of PCM, a significant fall in the CD4/CD8 T-cell ratio was detected (2, 30). Some authors observed decreased numbers of CD4+ and CD8+ T cells (30), whereas others reported a decrease only in the CD4+ subset (2). The mucous and skin granulomatous lesions of PCM patients presented an elevated CD4/CD8 T-cell ratio due to high numbers of infiltrating CD4+ T cells; this ratio in the blood, however, was very low (29). These findings showed that the phenotype of cells found in the blood does not reflect the subsets infiltrating the lesions.
In an analysis of the histopathological data obtained at 4 weeks postinfection, two intensities of lesions were detected: more pronounced in B10.A mice depleted of CD8+ T cells and less pronounced in the three other groups studied. Thus, the increase in the severity of the lesions and in the number of yeasts present in the lungs of depleted animals confirm the protective role of CD8+ T cells in the susceptible strain. This picture was not found in resistant A/Sn mice, in which the absence of CD8+ T cells did not affect the number and intensity of pulmonary inflammatory lesions. Even in susceptible mice, absence of CD8+ T cells did not cause remarkable alterations in the patterns of pulmonary lesions, such as those caused by the depletion of gamma interferon (IFN-γ), which resulted in complete disorganization of the pulmonary structure (13). The data presented herein are in accordance with those reported by Deepe (15), who studied the role of CD8+ T cells in murine histoplasmosis; no differences in the histopathological patterns of splenic and hepatic lesions were detected between depleted and control mice. As already shown, multinucleated giant cells were found more consistently in resistant than in susceptible mice. Analogously to what was reported by Hill in experimental cryptococcosis (18), most of these cells contained ingested fungi, suggesting a protective role of giant cells in experimental PCM.
In humans (reviewed in reference 4), as well as in our isogenic murine model of PCM (reviewed in reference 8), positive DTH reactions correlate with less severe disease. Regarding the involvement of CD8+ T cells in the specific DTH reaction, it became clear from our studies that in vivo depletion of CD8+ T cells does not alter significantly the DTH responses of resistant animals but significantly increases these reactions in susceptible mice. These results suggest that CD8+ T-cell subsets could be suppressing, at least partially, the DTH reactions in susceptible animals and confirm the studies by Jimenez-Finkel and Murphy (20), demonstrating that intravenous treatment of mice with P. brasiliensis antigens induces production of suppressor T cells that impair specific DTH responses. Thus, unexpectedly, in B10.A mice, protective immunity and DTH responses are dissociated traits. Our results suggest that CD8+ T cells could be involved in both protection against P. brasiliensis (perhaps by mean of their cytotoxic activity or ability to secrete IFN-γ) and negative regulation of DTH responses. This dual role exerted by CD8+ T cells in susceptible mice deserves further investigation using purified T-cell subsets. In murine leishmaniasis, using susceptible BALB/c mice Liew et al. (24) have also observed a dissociation between protective immunity and DTH responses. They reported that previous subcutaneous immunization with irradiated promastigotes of Leishmania major induced an increased DTH reactivity in parallel with more severe disease. The influence of CD8+ T cells on DTH reactions was also observed in studies with other fungal diseases. Depletion of CD8+ T cells during experimental cryptococcosis is associated with suppression of specific DTH responses (28), whereas depletion of these cells does not alter the onset or maintenance of DTH reactions in histoplasmosis (15).
High levels of circulating antibodies are directly related to the severity of PCM, although their pathophysiologic significance is still unknown (4, 10). When antibody production was analyzed herein, it was observed that treatment with normal IgG stimulated production of higher antibody levels independently of the mouse strain. A recent publication (40) reported that high concentrations of normal Ig induce the activation of CD4+ T cells and B cells. This fact could explain, at least partially, the increments in antibody levels observed in this study. When IgG-treated controls were compared to CD8-depleted mice, no significant differences in antibody levels were detected except for the decreased levels of total Ig and IgG1 in strain A/Sn at week 8 of infection, indicating that CD8+ T cells are not primarily involved in control of antibody production. In a previous work using athymic and euthymic BALB/c mice (7), it was verified that the majority of P. brasiliensis antigens are T-cell-dependent components since only T-cell-sufficient mice were able to produce anti-P. brasiliensis antibodies. The results obtained herein confirm the CD4+ T-cell subset as the major regulator of antibody production, since depletion of CD8+ T cells only peripherally affected antibody production. Indeed, in vivo CD4+ T-cell depletion experiments practically abrogate antibody production by resistant and susceptible mice after P. brasiliensis infection (L. E. Cano and V. L. G. Calich, unpublished data).
It is well known that exogenous foreign protein antigens are presented to CD4+ T cells by class II major histocompatibility complex (MHC) molecules, whereas CD8+ T lymphocytes recognize endogenous peptides bound to class I MHC molecules. Despite the preferential endosomal behavior of P. brasiliensis yeast cells (5), the experiments described herein indicate that fungal antigens probably can be presented by MHC class I molecules. Moreover, our results suggest that, early in disease, antigen processing by antigen-presenting cells of susceptible animals may be different from that of resistant mice. Although many aspects of CD8+ T-cell recruitment, activation, and interaction with other cells remain to be better elucidated, our results clearly show, for the first time, that CD8+ T cells are protective in experimental PCM and that the genetic pattern of the host is a determinant in the intensity of CD8+ T-cell activation.
ACKNOWLEDGMENTS
We are grateful to R. G. Landgraf and B. P. Albe for technical assistance. We are also grateful to G. B. Huffnagle for expert orientation in obtaining lung-infiltrating lymphocytes.
C. Arruda was a recipient of a fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and L. E. Cano was supported by a Ph.D. fellowship (92/0962-9) from FAPESP. This work was supported by grants from FAPESP and CNPq.
REFERENCES
- 1.Adams J, Follett D, Hamilton H, Czuprynski C H. Effects of administration of anti-CD4+ and anti-CD8+ monoclonal antibodies on Mycobacterium paratuberculosis infection in intragastrically challenged mice. Immunol Lett. 1993;35:183–190. doi: 10.1016/0165-2478(93)90089-k. [DOI] [PubMed] [Google Scholar]
- 2.Bava A J, Mistchenko A S, Palacios M F, Estevez M E, Tiraboshi N I, Sen L, Negroni R, Diez R A. Lymphocyte subpopulations and cytokine production in paracoccidioidomycosis patients. Microbiol Immunol. 1991;35:167–174. doi: 10.1111/j.1348-0421.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 3.Berliner M D, Reca M E. Vital staining of Histoplasma capsulatum with Janus Green B. Sabouraudia. 1966;5:26–29. [PubMed] [Google Scholar]
- 4.Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummer E, Hanson L H, Restrepo A, Stevens D A. Intracellular multiplication of Paracoccidioides brasiliensis in macrophages: killing and restriction of multiplication by activated macrophages. Infect Immun. 1989;57:2289–2294. doi: 10.1128/iai.57.8.2289-2294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger E, Miyaji M, Sano A, Calich V L G, Nishimura K, Lenzi H L. Histopathology of paracoccidioidomycotic infection in athymic and euthymic mice: a sequential study. Am J Trop Med Hyg. 1996;55:235–242. doi: 10.4269/ajtmh.1996.55.235. [DOI] [PubMed] [Google Scholar]
- 7.Burger E, Xidieh C F, Sano A, Kashino S S, Vaz C A C, Calich V L G, Singer-Vermes L M, Nishimura K, Miyaji M. Paracoccidioides brasiliensis infection in nude mice: studies with isolates differing in virulence and definition of their T-dependent and T-independent components. Am J Trop Med Hyg. 1996;55:391–398. doi: 10.4269/ajtmh.1996.55.391. [DOI] [PubMed] [Google Scholar]
- 8.Calich V L G, Vaz C A C, Burger E. Immunity to Paracoccidioides brasiliensis infection. Res Immunol. 1998;149:407–416. doi: 10.1016/s0923-2494(98)80764-5. [DOI] [PubMed] [Google Scholar]
- 9.Calich V L G, Singer-Vermes L M, Siqueira A M, Burger E. Susceptibility and resistance of inbred mice to Paracoccidioides brasiliensis. Br J Exp Pathol. 1985;66:585–594. [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo Z P, Cano L E. Humoral immunity. In: Franco M F, Lacaz C S, Restrepo A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 187–201. [Google Scholar]
- 11.Camargo Z P, Taborda C P, Rodrigues E G, Travassos L R. The use of cell-free-antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol. 1991;29:31–38. [PubMed] [Google Scholar]
- 12.Cano L E, Singer-Vermes L M, Vaz C A C, Russo M, Calich V L G. Pulmonary paracoccidioidomycosis in resistant and susceptible mice: relationship among progression of infection, bronchoalveolar cell activation, cellular immune response, and specific isotype patterns. Infect Immun. 1995;63:1777–1783. doi: 10.1128/iai.63.5.1777-1783.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cano L E, Kashino S S, Arruda C, André D, Xidieh C F, Singer-Vermes L M, Vaz C A C, Burger E, Calich V L G. Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect Immun. 1998;66:800–806. doi: 10.1128/iai.66.2.800-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castañeda E, Brummer E, Pappagianis D, Stevens D A. Impairment of cellular but not humoral immune response in chronic pulmonary and disseminated paracoccidioidomycosis in mice. Infect Immun. 1988;56:1771–1777. doi: 10.1128/iai.56.7.1771-1777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deepe G S., Jr Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J Immunol. 1994;152:3491–3500. [PubMed] [Google Scholar]
- 16.Fava Netto C, Vegas V S, Sciannaméa I M, Guarnieri E D B. Antigeno polissacarídico do Paracoccidioides brasiliensis. Estudo do tempo de cultivo do P. brasiliensis necessário ao preparo do antígeno. Rev Inst Med Trop São Paulo. 1969;11:177–181. [PubMed] [Google Scholar]
- 17.Fazioli R A, Singer-Vermes L M, Kashino S S, Burger E, Franco M, Moschardi-Bacchi M, Calich V L G. Delayed-type hypersensitivity response in an isogenic murine model of paracoccidioidomycosis. Mycopathologia. 1994;126:137–146. doi: 10.1007/BF01103767. [DOI] [PubMed] [Google Scholar]
- 18.Hill, J. Q. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J. Exp. Med. 175:1685–1695. [DOI] [PMC free article] [PubMed]
- 19.Huffnagle G B, Yates J L, Lipscomb M. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991;173:793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez-Finkel B E, Murphy J W. Characterization of efferent T suppressor cells induced by Paracoccidioides brasiliensis-specific afferent T suppressor cells. Infect Immun. 1988;56:744–750. doi: 10.1128/iai.56.4.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashino S S, Calich V L G, Burger E, Singer-Vermes L M. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia. 1985;92:173–178. doi: 10.1007/BF00437630. [DOI] [PubMed] [Google Scholar]
- 22.Kashino S S, Singer-Vermes L M, Calich V L G, Burger E. Alterations in the pathogenicity of one Paracoccidioides brasiliensis isolate do not correlate with its in vitro growth. Mycopathologia. 1990;111:173–180. doi: 10.1007/BF02282801. [DOI] [PubMed] [Google Scholar]
- 23.Kemeny D M, Noble A, Holmes B J, Diaz-Sanchez D. Immune regulation: a new role for CD8+ T cells. Immunol Today. 1994;15:107–110. doi: 10.1016/0167-5699(94)90152-X. [DOI] [PubMed] [Google Scholar]
- 24.Liew F Y, Singleton A, Cillari E, Howard J G. Prophylactic immunization against experimental leishmaniasis. V. Mechanism of the anti-protective blocking effect induced by subcutaneous immunization against Leishmania major infection. J Immunol. 1985;135:2102–2107. [PubMed] [Google Scholar]
- 25.McEwen J G, Bedoya V, Patiño M M, Salazar M E, Restrepo A. Experimental murine paracoccidioidomycosis induced by inhalation of conidia. J Med Vet Mycol. 1987;25:165–175. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- 26.McKinney M M, Parkinson A. A simple non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987;96:271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 27.Mendes R P. The gamut of clinical manifestations. In: Franco M F, Lacaz C S, Restrepo A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press; 1994. pp. 233–258. [Google Scholar]
- 28.Mody C H, Cheng G H, Jackson C, Curtis J L, Toews G B. Depletion of murine CD8+ T cells in vivo decreases clearance of a moderately virulent strain of Cryptococcus neoformans. J Lab Clin Med. 1993;121:765–773. [PubMed] [Google Scholar]
- 29.Moscardi-Bacchi M, Soares A, Mendes R, Marques S, Franco M. In situ localization of T lymphocyte subsets in paracoccidioidomycosis. J Med Vet Mycol. 1989;27:149–158. [PubMed] [Google Scholar]
- 30.Mota N G S, Peraçoli M T S, Mendes R P, Gattass C R, Marques S A, Soares A M V C, Izato I C, Rezkallah-Iwasso M T. Mononuclear cell subsets in patients with different clinical forms of paracoccidioidomycosis. J Med Vet Mycol. 1988;26:105–111. doi: 10.1080/02681218880000151. [DOI] [PubMed] [Google Scholar]
- 31.Mota N G S, Rezkallah-Iwasso M T, Peraçoli M T S, Audi R C, Mendes R P, Marcondes J, Marques S A, Dillon N L, Franco M F. Correlation between cell-mediated immunity and clinical forms of paracoccidioidomycosis. Trans R Soc Trop Med Hyg. 1985;79:765–772. doi: 10.1016/0035-9203(85)90112-9. [DOI] [PubMed] [Google Scholar]
- 32.Muller I, Kropf P, Louis J A, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun. 1994;62:2575–2581. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musatti C C, Rezkallah M T, Mendes E, Mendes N F. In vivo and in vitro evaluation of cell-mediated immunity in patients with paracoccidioidomycosis. Cell Immunol. 1976;24:365–378. doi: 10.1016/0008-8749(76)90220-3. [DOI] [PubMed] [Google Scholar]
- 34.Phillips S M, Lin J, Galal N, Tung A S, Linette G P, Perrin P J. Resistance in murine schistosomiasis is contingent on activated IL-2 receptor-bearing L3T4+ lymphocytes, negatively regulated by Lyt-2+ cells, and uninfluenced by the presence of IL-4. J Immunol. 1991;146:1335–1340. [PubMed] [Google Scholar]
- 35.Restrepo A. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. J Med Vet Mycol. 1985;23:323–334. [PubMed] [Google Scholar]
- 36.Singer-Vermes L M, Caldeira C B, Burger E, Calich V L G. Experimental murine paracoccidioidomycosis: relationship among dissemination of the infection, humoral and cellular immune responses. Clin Exp Immunol. 1993;94:75–79. doi: 10.1111/j.1365-2249.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer-Vermes L M, Ciavaglia M C, Kashino S S, Burger E, Calich V L G. The source of the growth-promoting factor(s) affects the plating efficiency of Paracoccidioides brasiliensis. J Med Vet Mycol. 1992;30:261–264. doi: 10.1080/02681219280000331. [DOI] [PubMed] [Google Scholar]
- 38.Snapper C M, Marcu K B, Zelazowsky P. Immunoglobulin class switch: beyond accessibility. Immunity. 1997;6:217–223. doi: 10.1016/s1074-7613(00)80324-6. [DOI] [PubMed] [Google Scholar]
- 39.Snapper C M, Paul W E. Interferon-γ and B stimulatory factor-I reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 40.Sunblad A, Marcos M A R, Malanchere E, Castro A, Haury M, Huetz F, Nobrega A, Freitas A, Coutinho A. Observation on the mode of actions of normal immunoglobulin at high doses. Immunol Rev. 1994;139:125–158. doi: 10.1111/j.1600-065x.1994.tb00860.x. [DOI] [PubMed] [Google Scholar]
- 41.Zar J H. Biostatistical analysis. 2nd ed. Upper Saddle River, N.J: Prentice Hall, Inc.; 1984. [Google Scholar]