Abstract
Background
/Objectives: Obesity is a risk factor for COVID-19 infection severity and mortality. Anti-obesity medications (AOM) are effective for weight loss. However, weight loss outcomes with AOM during the COVID-19 pandemic are yet to be described.
Subjects
/Methods: Between January 1, 2016, and June 30, 2021, a total of 966 patients were prescribed long-term FDA-approved AOMs at the Mayo Clinic. From these patients, 711 patients did not meet inclusion criteria. A total of 255 patients were included.
Interventions/methods
We performed a retrospective systematic review of electronic medical records and included patients who started a long-term FDA-approved AOM. We excluded patients with history of bariatric procedure, AOM prescription with lorcaserin, orlistat, semaglutide (approved for weight loss after the pandemic), or phentermine (short-term AOM), those taking ≥2 AOMs, <3 months of prescribed AOM, and/or pregnancy. Analysis was divided by 1)preCOVID-19: those who started an AOM before COVID-19 restrictions, 2)COVID-19: those who started an AOM during first quarter of 2020 after the establishment of COVID-19 restrictions. Our primary endpoint was the total body weight loss percentage (%TBWL) at 3, 6, and 12 months after AOM initiation.
Results
There was a statistical difference in TBWL% between the preCOVID-19 and COVID-19 group: 5.3 ± 3.5% vs 4 ± 3.0% (95% CI -2.4 to −0.2; p = 0.02) and 9.7 ± 7.2% vs 6.2 ± 4.7% (95% CI -5.7 to −1.3; p = 0.002) at 3 and 12 months, respectively. At 6 months, the TBWL% was 7.1 for the preCOVID-19 group compared to 6.2% for the COVID-19 (95% CI -2.5 to 0.7; p = 0.25).
Conclusion
With the possible exception of liraglutide, this study shows that weight loss outcomes to AOMs were inferior when prescribed during the routine clinical practice throughout COVID-19 pandemic, compared to the outcomes observed prior to the COVID-19 pandemic.
Keywords: Anti-obesity medications, COVID-19, Obesity, Pandemic
1. Introduction
Obesity is a chronic and relapsing disease, with a rising prevalence and a high economic burden [1]. It is estimated that 42.5% of U.S. adults have obesity (i.e., body-mass index [BMI] ≥30 kg/m2), including 9.0% with severe obesity (i.e., BMI ≥40 kg/m2) [[2], [3], [4]]. Recent studies demonstrate an attributed annual medical costs of individuals with obesity exceed $2000, which poses a substantial financial burden on the healthcare system [5]. Obesity is also associated with higher rates of mortality and worse outcomes due to associated weight-related comorbidities such as dyslipidemia, type 2 diabetes mellitus, hypertension, and some forms of cancer [6].
The spread of COVID-19 imposed many governmental restrictions putting billions of people into a lockdown which may have increased their engagement in obesogenic prone behaviors [7]. Closure of fitness facilities, stay-at home policies, and increased frequency of unhealthy snacking may also have minimized the effectiveness of weight management programs leading to a sustained weight gain even after lockdown restrictions were lifted [8,9]. Literature studies have reported an average mean weight gain of 1.5–3 kg in the general population, with greater weight gain in males and individuals with overweight and obesity [[10], [11], [12], [13]].
Weight management guidelines recommend an intensive and multicomponent approach for weight loss in patients living with obesity and overweight. This approach has traditionally relied on a multidisciplinary team and on-site, in-person clinical care [14]. In view of the social distancing mandates, COVID-19 presented a challenge to multidisciplinary weight management programs, where telehealth became a popular alternative to in-person visits [15]. Telemedicine has demonstrated a safe and successful intervention to populations of difficult access [16]. Current evidence has proven the efficacy of telemedicine for short-term follow-up and its impact on patients with obesity [17].
The use of anti-obesity medications (AOMs) is a reliable and efficacious intervention for weight control, aiming at improving quality of life and preventing the progression of weight-related comorbidities [18,19]. The Food and Drug Administration (FDA) has approved AOMs for long-term use for individuals with BMI ≥30 kg/m2 or ≥ 27 kg/m2 with weight-related comorbidities. These AOMs include: Phentermine-topiramate [PHEN-TOP], naltrexone-bupropion [NBSR], orlistat, liraglutide, and semaglutide [[20], [21], [22], [23], [24]]. To date, most of the weight loss outcomes are derived from a focused testing of AOMs under strict control settings of randomized clinical trials (RCT). Few real-world studies often report different weight loss outcomes compared to previous RCTs [2,18,19,21,[25], [26], [27]]. Furthermore, the impact of limited healthcare access, confinement policies, and COVID-19 infection in individuals with overweight and obesity managed with AOM for weight control remains unknown [28]. Although the field of obesity medicine is rapidly advancing prior and during the pandemic, we aim to examine in the real-world weight-loss outcomes of FDA approved long-term AOM before and after the COVID-19 pandemic.
2. Methods
2.1. Design and eligibility criteria
We performed a systematic review of electronic medical record (EMR) of patients from out-patient clinics (e.g., weight management clinic) from all the Mayo Clinic Health System sites. Informed consent was waived by the Institutional Review Boards (IRB) committee due to its minimal-risk nature. We included all patients who started a long-term AOM (PHEN-TOP, NBSR, and liraglutide) from January 1st, 2016, until June 30th, 2021. We abstracted our cohort population from Mayo Clinic Healthcare data through its medical record tool (MDE- Mayo Data Explorer). We screened patients using the electronic health record. Inclusion criteria included: 1) patients with a BMI ≥30 kg/m2 or ≥27 kg/m2 with at least one weight-related comorbidity; 2) patients prescribed an approved long-term AOM; 3) ≥3 months prescription of a long-term AOM. We excluded patients with a history of bariatric surgery, prior endoscopic procedure for weight loss (e.g., balloon, sleeve gastroplasty, transoral outlet reduction [TORe]), those taking ≥2 AOMs, history of previous or current malignancy, history of clinical trials for experimental weight loss interventions, pregnancy, and AOM prescription for lorcaserin (due to its discontinuation in 2020), orlistat (due to a restricted number of patients on this medication), semaglutide (due to its approval for weight loss after the pandemic), or phentermine (short-term AOM). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2.2. Data collection
We searched patient's comorbidities information based on ICD-10 codes. We abstracted from the EMR information about demographics, anthropometrics, including laboratory clinical values and weight in kg at 3, 6, and 12 months. We collected data from in-person and/or virtual encounters. For the 3- month data abstraction, we gave a timeframe of ±30 days after starting the AOM and for the 6- and 12- months data abstraction, we gave a timeframe of ±45 days after starting the AOM. We included patients prescribed phentermine-topiramate, naltrexone-bupropion, and liraglutide regardless of the dose achieved. We collected provider, dietitian, and psychology visits after the first day of AOM was started until AOM was suspended. We divided our cohort in 1) preCOVID-19: AOM prescribed from January 1st, 2016, to December 31st, 2020, i.e., 3 months prior to the establishment of COVID-19 restrictions and 2) COVID-19: AOM prescribed during and after COVID-19 restrictions were established.
2.3. Weight management program
The Mayo Clinic weight management program involves a multidisciplinary team that includes obesity medicine physicians, registered dietitians, advanced practice providers (physician assistants and nurse practitioners), and behavioral bariatric psychologists. Upon initial evaluation, patients are encouraged but not obligated to meet with a dietitian and the behavioral psychology team. All patients are encouraged but not obligated to participate in a standardized behavioral program. The general recommendations are to (1) reduce dietary intake to 1200–1500 calories per day for women and 1500–1800 calories per day for men, (2) achieve a goal of 10,000 steps or more per day and 150 min or more of moderate intensity activity per week, and (3) limit the consumption of liquid calories (e.g., sodas, juices, alcohol). Calorie restriction and counseling on activity might vary widely based on weight-related comorbidities and functional capacity. Some patients are prescribed AOMs. Patients were encouraged to return for follow-up visits 4–6 weeks after starting the medication and every 3 months thereafter. During each visit, providers recorded information on body weight and weight-related comorbidities, gathered information on medication adherence based on patients’ report and pharmacy data on prescriptions filled, and reported side effects of the AOMs.
2.4. Study endpoints
The primary endpoint was the total body weight loss percentage (%TBWL) at 3, 6, and 12 months after AOM initiation. %TBWL was calculated using the following formula: [100∗(weight at first visit – weight at each time point)]/weight at the first visit. Secondary endpoints included: (1) TBWL% at 3-, 6-, and 12-months by AOM type; (2) percentage of patients who achieved a TBWL of ≥5%, ≥10%, ≥15%, and ≥20%; (3) comparison between telemedicine (virtual) healthcare and in-person follow-up, and (4) metabolic changes at last follow up including: lipids [total cholesterol, low-density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides], fasting glucose, glycated hemoglobin (HbA1c), and systolic and diastolic blood pressures (SBP and DBP, respectively).
2.5. Statistical analysis
Baseline anthropometric and demographics were normally distributed and are summarized as mean ± standard deviation (SD). For continuous variables not normally distributed, data are summarized as median and interquartile ranges (IQR). Categorical data are presented as frequencies and percentages. We used two tail t-test to analyze the association of %TBWL at 3, 6, and 12 months, compared to baseline between the COVID-19 and preCOVID-19 groups. We performed a multiple regression analysis to obtain the effect between AOMs and %TBWL adjusted by BMI, age, and sex at baseline, and COVID-19 status, and follow up visits with providers, dietitians, and psychologists. Results were based on parameter estimates (PE) with 95% confidence intervals (95% CI) and significance values. Statistical significance was set at 2-sided p < 0.05. We used JMP®, Version 14.3.0 (SAS Institute Inc., Cary, NC, 1989–2019) to perform the statistical analysis.
3. Results
3.1. Patient selection
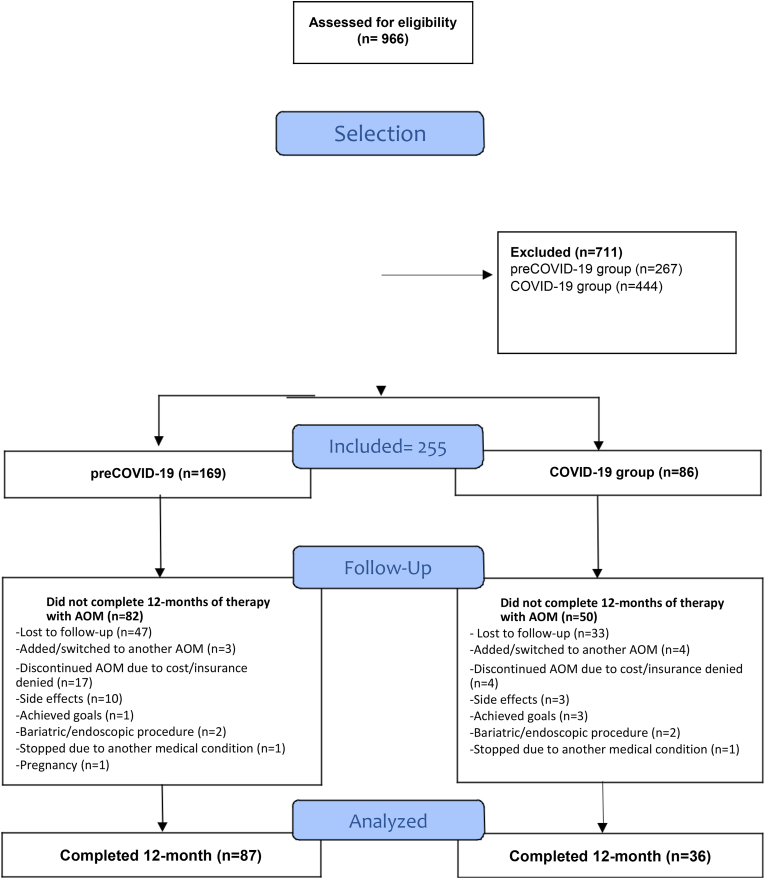
Between January 1, 2016, and June 30, 2021, a total of 966 patients were prescribed long-term FDA-approved AOMs at the Mayo Clinic. As shown in Fig. 1, 711 patients did not meet inclusion criteria. A total of 255 patients were included. From these, 116 completed 12 months of AOM use, 87 (51%) in the preCOVID-19 group and 36 (40%) in the COVID-19 group (p = 0.12). The overall reasons for discontinuation included: lost to follow-up (n = 80), AOM discontinued due to cost or insurance denial (n = 21), added/switched to another AOM (n = 7), side effects (n = 13), achieved goals (n = 4), bariatric/endoscopic procedure (n = 4), stopped by another medical condition (n = 2), and pregnancy (n = 1).
Fig. 1.
Follow-up flowchart.
3.2. Baseline characteristics
From the 255 patients who met inclusion and exclusion criteria, 75.6% were female with a mean age of 48.2 ± 12.9 years and mean BMI of 41.9 ± 8.5 kg/m2. Most patients, 55.3%, had obesity class 3 (BMI≥40 kg/m2) and were primarily White (96.1%). A total of 169 patients were identified in the preCOVID-19 group and 86 in the COVID-19 group. Similarly, there was an increased number of patients with obesity class 3 in the preCOVD-19 in comparison with the COVID-19 group: 59.8% vs. 46.5%; p = 0.04. There were no other differences in demographic and anthropometric baseline characteristics between COVID-19 and preCOVID-19 groups (Table 1).
Table 1.
Continuous data are summarized as mean and standard deviation (SD) or median and interquartile ranges (IQR). Categorical data are presented as frequencies and percentages. Significant p values are indicated in bold.
| All patients | preCOVID-19 | COVID-19 | p value | |
|---|---|---|---|---|
|
A. Baseline demographic information | ||||
| N (%) | 255 (100) | 169 (66.2) | 86 (33.7) | |
| Age, years (SD) | 48.2 (12.9) | 49.1 (13.1) | 46.6 (12.5) | 0.13 |
| Sex, Female (%) | 193 (75.6) | 126 (74.6) | 67 (77.9) | 0.55 |
| Race, White (%) | 245 (96.1) | 167 (98.8) | 78 (90.7) | 0.0008 |
|
Baseline clinical information | ||||
| Weight, kg (SD) | 119.6 (29.3) | 118 (23.8) | 122.7 (37.9) | 0.29 |
| BMI, kg/m2(SD) | 41.86 (8.5) | 41.7 (7.4) | 42.3 (10.2) | 0.63 |
| Overweight, n (%) | 11 (4.3) | 6 (3.6) | 5 (5.8) | 0.41 |
| Obesity Class 1, n (%) | 53 (20.8) | 33 (19.5) | 20 (23.3) | 0.49 |
| Obesity Class 2, n (%) | 50 (19.6) | 29 (17.2) | 21 (24.4) | 0.17 |
| Obesity Class 3, n (%) | 141 (55.3) | 101 (59.8) | 40 (46.5) | 0.04 |
| SBP, mmHg (SD) | 129 (15) | 129 (15) | 129 (15) | 0.90 |
| DBP, mmHg (SD) | 78 (22) | 78 (10) | 78 (12) | 0.80 |
| Glucose, mg/dL (SD) | 122 (52) | 129 (61) | 109 (29) | 0.03 |
| HbA1c, % (SD) | 7.0 (1.7) | 7.1 (1.8) | 6.5 (1.2) | 0.11 |
| Total Cholesterol, mg/dL (SD) | 179 (49) | 175 (40) | 186 (61) | 0.32 |
| Total Triglycerides, mg/dL (SD) | 151 (78) | 160 (84) | 133 (63) | 0.06 |
| LDL-cholesterol, mg/dl (SD) | 103 (40) | 96 (32) | 115 (51) | 0.04 |
| HDL-cholesterol, mg/dl (SD) | 50 (14) | 48 (14) | 53 (13) | 0.08 |
|
B. Comorbidities | ||||
| Dyslipidemia, n (%) | 153 (60) | 106 (62.7) | 47 (54.7) | 0.35 |
| Diabetes mellitus, n (%) | 80 (31.4) | 58 (34.3) | 22 (25.6) | 0.15 |
| Prediabetes, n (%) | 23 (9.1) | 13 (7.7) | 10 (11.6) | 0.36 |
| Hypertension, n (%) | 123 (48.2) | 81 (47.9) | 42 (48.8) | 0.69 |
| GERD, n (%) | 66 (25.9) | 44 (26) | 22 (25.6) | 0.93 |
| Obstructive sleep apnea, n (%) | 92 (36.1) | 63 (37.3) | 29 (33.7) | 0.58 |
| Degenerative joint disease, n (%) | 83 (32.5) | 67 (39.6) | 16 (18.6) | 0.0009 |
| NAFLD, n (%) | 19 (7.5) | 13 (7.7) | 6 (7.0) | 0.89 |
|
C. Medication | ||||
| Phentermine/topiramate, n (%) | 124 (48.6) | 92 (54.4) | 32 (37.2) | 0.01 |
| Naltrexone/bupropion, n (%) | 59 (23.1) | 31 (18.3) | 28 (32.6) | 0.01 |
| Liraglutide, n (%) | 72 (28.2) | 46 (27.2) | 26 (30.2) | 0.66 |
All p values < 0.05 were considered significant.
BMI, body mass index; DBP, diastolic blood pressure; GERD, gastroesophageal reflux disease; HbA1c, Hemoglobin A1c; HDL, High-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; SBP, systolic blood pressure.
Diagnosis of obesity comorbidities was based on diagnoses listed by clinicians.
Dyslipidemia was the predominant comorbidity found in the study cohort (60%), followed by hypertension (48.2%) and obstructive sleep apnea (OSA) (36.1%). In term of comorbidities, no significant difference was found except for degenerative joint disease, which was more predominant in the preCOVID-19 group compared to the COVID-19 group: 39.6% vs. 18.6%, p = 0.009.
Mean values of glucose, HbA1c, LDL-cholesterol, HDL-cholesterol, total cholesterol, and triglycerides at baseline are presented in Table 1. There were no differences in laboratory results between both groups, except for glucose and LDL-cholesterol. Pre-COVID-19 had a median glucose of 129 ± 69 mg/dl in comparison with the COVID-19 group 109 ± 29 mg/dl (p = 0.03). LDL-cholesterol in the COVID-19 group was higher in comparison with the preCOVID-19 group: 115 ± 51 vs. 96 ± 32 mg/dl, p = 0.04.
3.2.1. Prescribed antiobesity medications
In the entire cohort, the most frequent prescribed medication was phentermine-topiramate (48.6%) followed by liraglutide (28.2%), and bupropion-naltrexone (23.1%). Phentermine-topiramate was prescribed at 7.5–46 mg daily in 77.1% of patients, while 6% received 11.25–69 mg, and 16.9% received 15–92 mg. For liraglutide, a weight loss dose ≥1.8 mg (high dose), was achieved in 90.3% of patients. When stratified by our two groups, 84.6% patients in the COVID-19 group achieved a dose >1.8 mg, whereas 93.5% patients in the preCOVID-19 group achieved the same dose range (p = 0.21). All patients taking bupropion/naltrexone received 16–180 mg twice daily.
3.3. Changes in total body weight loss outcomes
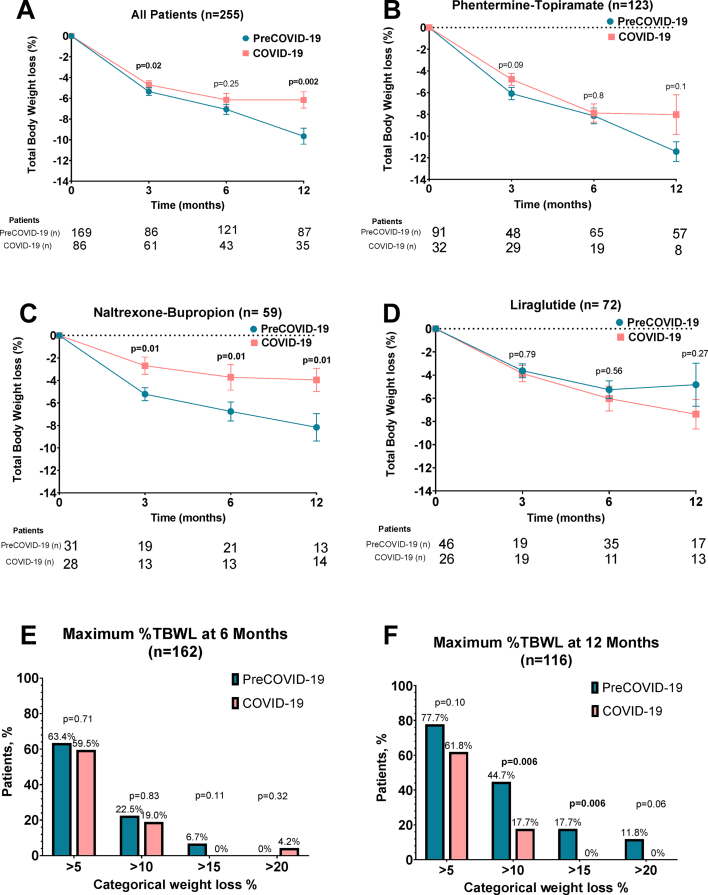
The entire cohort had a mean of 8.6 ± 6.7 %TBWL at one year. The COVID-19 group lost significantly less TBWL% compared to the preCOVID-19 group at 3 and 12 months (Fig. 2A). At 3 months, the COVID-19 group lost 0.65% less weight compared to the preCOVID-19 group [% TBWL 4.7% vs. 5.35%; 95% CI -2.4 to −0.2; p = 0.02]. At 12 months, COVID-19 group lost 3.6% less weight compared to preCOVID-19 group [% TBWL 6.16% vs 9.66%; 95% CI -5.7 to −1.3; p = 0.02] (Fig. 2A). There was no significant difference in %TBWL at 6 months (TBWL 7.07% for pre-COVID-19 vs 6.15% for COVID-19 group; 95% CI -2.5 to 0.7; p = 0.25) for the preCOVID-19 group (see Fig. 2A).
Fig. 2.
A. Total body weight loss at 3, 6 and 12 months in COVID-19 and PreCOVID-19 patients. B-D. Total body weight loss at 3, 6 and 12 months in COVID-19 and PreCOVID-19 patients by medication. E-F. Percentage of patients who achieved 5, 10, 15 and 20% TBWL at 6 and 12 months. Figures A–D are represented in mean and standard error.
3.4. Secondary end points
3.4.1. TBWL% at 3-, 6-, and 12-months analysis by AOM type
In patients prescribed phentermine-topiramate, we found no significant differences in % TBWL at 3, 6, or 12 months between COVID-19 and preCOVID-19 groups (Fig. 2B). There was a trend for higher weight loss in the preCOVID-19 group at 12 months, but the trend did not achieve significance.
Patients prescribed naltrexone-bupropion during COVID-19 had a significantly lesser TBWL% at all time points in comparison with those in the preCOVID-19 group (Fig. 2C).
In patients prescribed liraglutide, no significant differences in % TBWL were observed at 3, 6, or 12 months between COVID-19 and pre-COVID-19 group (Fig. 2D). There was a trend for greater weight loss in patients in the COVID-19 group. We performed a subanalysis in the liraglutide group by dose (≤1.8 mg low dose vs. ≥2.4 mg high dose). In the high dose subanalysis at 12 months, preCOVID-19 achieved a −13.9% vs −7.8% TBWL in the COVID-19 group (p = 0.66). In the low dose subanalysis at 12 months, preCOVID-19 achieved a −3.62% vs −6.0% TBWL in the COVID-19 group (p = 0.67) (Fig. 1S).
3.4.2. Proportion of patients achieving ≥5–20% TBWL
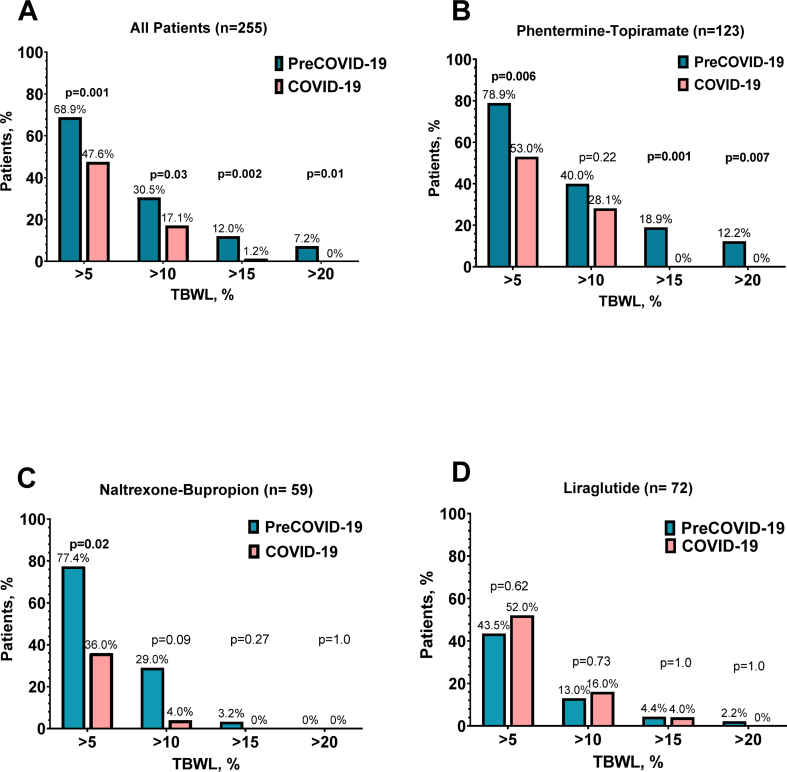
The percentages of patients achieving a TBWL of ≥5%, ≥10%, ≥15%, and 20% were significantly higher in the preCOVID-19 group in comparison to COVID-19 group (Fig. 2E-F and Fig. 3).
Fig. 3.
A. Percentage of patients who achieved 5, 10, 15 and 20% TBWL at AOM termination for all the medications. B-D. Percentage of patients who achieved 5, 10, 15 and 20% TBWL at AOM termination by medications.
When displayed by medication, phentermine-topiramate and naltrexone-bupropion led to a greater proportion of patients achieving a TBWL >5%, 15%, and 20% in the preCOVID-19 group compared to the COVID-19 group. No differences were observed between groups in patients prescribed liraglutide.
3.4.3. Multidisciplinary weight management program and telemedicine (Table 2)
Table 2.
Multidisciplinary provider visits. Continuous data are summarized as median and interquartile ranges (IQR).
| preCOVID-19 |
COVID-19 |
p value | |
|---|---|---|---|
| N = 169 | N = 86 | ||
| Number of visits with a physician, (IQR) | 2 (2–3) | 2 (1.75–3) | 0.02 |
| Patients with virtual physician visit, n (%) | 0 (0) | 30 (34.8) | <0.0001 |
| Patients with ≥1 dietitian visit, n (%) | 77 (45.6) | 23 (26.7) | 0.004 |
| Total no. of patients with virtual dietitian visit n (%) | 1 (0.6) | 11 (12.8) | <0.0001 |
| Patients with ≥1 psychologist visit, n (%) | 61 (36.1) | 15 (17.4) | 0.002 |
| Total no. of patients with virtual psychologist visit, n (%) | 0 (0) | 9 (10.4) | <0.0001 |
Significant p values are indicated in bold.
All p values < 0.05 were considered significant.
There was a difference in the number of provider visits (in-person or via telemedicine) between groups: 3 (2–3) for preCOVID-19 vs. 2 (1.75–3) for COVID-19, p = 0.02. No participants in the preCOVID-19 group had an appointment via telemedicine, while 36.6% of participants in the COVID-19 group had at least 1 or more virtual encounter (p < 0.0001).
The proportion of patients with at least one dietitian encounter was higher in the preCOVID-19 group compared to COVID-19 group: 45.6% vs. 26.7%, p = 0.004. When stratified by group, 92.3% of the virtual dietitian visits happened in the COVID-19 group (p=<0.0001).
Similarly, the proportion of patients with at least one bariatric psychology visit was higher in the preCOVID-19 vs. COVID-19 group: 36.1% vs. 17.4%, p = 0.002. Telemedicine visit modality was predominantly in the COVID-19 group (p=<0.0001).
3.4.4. Baseline metabolic changes (Table 2S)
At last follow-up visit, there were no significant difference between groups in terms of clinical improvements in glucose, hemoglobin A1c, SBP, DBP or in the lipid panel (total cholesterol, HDL-cholesterol, LDL-cholesterol, or triglycerides) when compared between groups.
3.4.5. Multiple regression analysis
To assess what variables predict %TBWL at 12 months, we performed regression analysis. Variables considered included: starting an AOM during COVID-19 pandemic, BMI at baseline, and number of visits with a physician, dietitian, and psychologist. Multiple regression analysis showed that the following variables predicted a lower %TBWL at 12 months: starting an AOM in the COVID-19 cohort (PE [95% CI]: −1.45; p = 0.03) and a lower number of visits with a physician (PE [95% CI]: 1.83; p = 0.003). When adding the type of medication to the variables in the multiple regression analysis, the only variable that predicted a lower TBWL% was lower number of visits with a physician (PE [95% CI]: −1.30; p = 0.03).
4. Discussion
The COVID-19 pandemic affected individuals’ eating and lifestyle habits with multiple studies reporting an increase in weight [29]. Most of the research to date has focused on weight gain and negative health consequences of COVID-19 in the general population. However, obesity treatment strategies, as AOM and its weight loss outcomes, have not been assessed. Therefore, in this study, we report weight loss outcomes to AOM in a real-world setting during COVID-19 pandemic. This retrospective study of adult population revealed that the use of approved long-term AOM during the COVID-19 pandemic had a different impact on weight loss outcome when compared to a population prescribed before the pandemic. We report a significant lower %TBWL of about 0.65% and 3.6% at 3- and 12-months follow-up respectively, for those who were prescribed an AOM during the COVID 19 pandemic in comparison with those who were prescribed an AOM before the pandemic.
The %TBWL achieved among patients prescribed an AOM before the COVID-19 pandemic showed similar results to other studies evaluating the efficacy of AOM ranging from 2.9% to 6.8% [25,26,[30], [31], [32], [33], [34]]. Our results showed a weight loss response very similar of about 5.3% at 3 months and 9.8% at 12-month follow-up.
In our study, the AOM response by medication during COVID-19 pandemic represented important differences to previously reported data [25]. Phentermine-topiramate showed the greatest %TBWL with 11.4% and 8.0% before and during the COVID-19 pandemic at 12 months, respectively. These overall robust response of phentermine-topiramate is clinically less in the COVID-19 group when compared to other studies, where the highest %TBWL at 12 months can reach about 12% at 1 year [25]. The weight loss response of naltrexone-bupropion before and during the COVID-19 pandemic at 12 months was 6.7 vs 3.7% TBWL, respectively, with significantly less %TBWL in all timepoints in the COVID-19 group, when compared to the preCOVID-19 group and published studies [33]. Interestingly, only naltrexone-bupropion achieved significant differences when compared between both groups. The etiology behind this seen difference is not well understood. The weight loss response to liraglutide at 12 months follow-up before and during the COVID-19 pandemic was 4.8 vs. 7.4 %TBWL, respectively. Previously reported data have reported a weight loss as high as 7.4 kg and a 8% TBWL at 12 months [24,32]. The discrepancy in the results (greater weight loss during the pandemic) is explained by our secondary analysis. When the liraglutide group response was divided into ≤1.8 mg vs ≥ 2.4 mg dose, the preCOVID-19 group achieved a greater weight loss at ≥2.4 mg whereas the COVID-19 group achieved greater weight loss at doses ≤1.8 mg. We do not have any explanation as what this difference may be related to. The sample is small, and no further conclusions can be drawn from the current data.
The proportion of patients achieving weight loss outcomes of >5%, >10%, >15%, and >20% before the pandemic was 77.7%, 44.7%. 17.7% and 11.8% and is similar to those observed in multicenter clinical studies [25]. In our cohort, categorical weight loss outcomes (5–20% TBWL) during the COVID-19 pandemic showed a lower proportion of patients achieving the same outcomes, with significant differences in those who achieved >10% and >15% TBWL.
While significant weight loss was observed in both groups, only modest improvements were observed in the individual's metabolic parameters. It is reported that for certain comorbidities (e.g., hypertension, dyslipidemia, and diabetes type 2) modest weight loss of 5–10% is required to prevent their progression or development [35,36]. Whereas other comorbidities (OSA or NAFLD) require a greater weight loss to translate into a clinical improvement [37].
We hypothesized that prescribing an AOM will lead to a significantly less weight loss during the pandemic due to a limited standard medical care and changes in social behaviors due to the pandemic. The importance of these findings relies on its contribution towards a better understanding of the impact a stressful situation and how contributory factors such as this one can influence obesity therapies and their weight loss outcomes. To date, no former studies have approached the study of weight loss outcomes of AOM during the COVID-19 pandemic or other similar related situation, hence the importance of our findings.
A probable aspect that may have impacted the weight loss outcome response to AOM is possibly related to the difference regarding the frequency and type of visit (e.g., telemedicine vs. in-person) of provider, dietitian, and psychology visits. It is known that a weight loss multidisciplinary intervention is associated with a greater and more clinically significant and sustained weight loss compared with standard of care [[38], [39], [40]]. Although we showed that the pandemic was a independent predictor of weight loss on multiple regression analysis, after taking into consideration the type of medication, the number of visits with an obesity medicine provider was the strongest predictor of response. Visits with additional members of the multidisciplinary team did not independently affect the differences observed in weight loss outcomes before and during the pandemic in this report. It is important to note that with the advent of technologies, telemedicine has become a popular alternative to conventional in-person visits to achieve significant and sustained weight loss. Consequently, depending on the patient propensity to obesity, telemedicine could be geared towards prevention of weight gain rather than weight loss in patients at high risk of weight gain during high stress situations such as the COVID-19 pandemic [29,41,42].
This study has several limitations. First, as this is a retrospective study, not all variables were available for all patients at all time points. Furthermore, we rely on chart documentation which sometimes is not accurate and comprehensive. Consequently, we could not report data on relevant outcomes such as changes in the prevalence of weight-related metabolic risk factors (prediabetes, diabetes, HTN, HLD) or changes in the number of medications used for these diseases. Similarly, due to this, data reported on metabolic outcomes do not account for potential implementation or adjustment of drugs altering these parameters during the period of using AOM. Second, demographically, our cohort is predominantly female and White which limits the generalization of these data to other populations. Third, weight and visits data were abstracted through in-person and virtual (self-report) follow-up. This is especially relevant for the COVID-19 group, where a telemedicine medical setting was preferentially opted, altering the regularity of standard medical care, therefore EMR records [43]. The retrospective nature of this report does not allow to determine the accuracy of EMR recording, therefore the impact of this factor on weight loss outcomes remains undetermined. Fourth, due to limited number of prescriptions, some AOM were left out, including well proven medications such as orlistat due to no prescriptions during COVID pandemic and semaglutide due to being approved during the pandemic and not having a control in the pre-pandemic era. One could argue that we could have used patients prescribed semaglutide at diabetes doses, however, the doses for weight loss are higher (2.4 mg weekly versus 1 mg weekly, as of the submission of this manuscript). Finally, another limitation includes the lack of assessment of the social and mental health factors that may contribute to the difference observed (e.g., access to exercise facilities, stress, anxiety, depression). Studying these factors may help explain the decrease in weight loss outcomes of the COVID-19 group. However, this was limited due to the retrospective nature of our study.
With the possible exception of liraglutide, this study shows that weight loss outcomes to AOMs were inferior when prescribed during the routine clinical practice throughout COVID-19 pandemic, compared to the outcomes observed prior to the COVID-19 pandemic. Further studies are needed to understand whether this observation is due to changes in care delivery during the pandemic or due to individual factors such as stress, decreased physical activity, remote working, among others.
Author contribution
Concept and design: De la Rosa, Hurtado, Acosta. Acquisition, analysis, or interpretation of data: De la Rosa, Ghusn, Sacoto, Campos, Cifuentes, Busebee, Hurtado, Acosta. Drafting of the manuscript: De la Rosa, Ghusn, Sacoto, Campos, Cifuentes, Hurtado, Acosta. Critical revision of the manuscript for important intellectual content: De la Rosa, Ghusn, Sacoto, Campos, Cifuentes, Acosta, Hurtado. Statistical analysis: De la Rosa, Campos, Cifuentes, Ghusn, Acosta, Hurtado. Administrative, technical, or material support: Acosta, Hurtado.
Ethical review
The submission represents original work. The submission does not involve any human test subjects or volunteers.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Deidentified data will be made available for the journal upon request.
Declaration of competing interest
Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences; he served as a consultant for Rhythm Pharmaceuticals, General Mills, Amgen, Bausch Health, RareDiseases. The rest of authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
All authors had full access to all the data and statistical analyses.
Dr. Hurtado had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- AOM
anti-obesity medications
- PHEN-TOP
phentermine-topiramate
- NBSR
naltrexone-bupropion sustained release
- BMI
body-mass index
- %TBWL
total body weight loss percentage
- FDA
Food and Drug Administration
- RCT
randomized clinical trials
- EMR
electronic medical record
- IRB
Institutional Review Boards
- PE
parameter estimates
- TORe
transoral outlet reduction
- MDE
Mayo Data Explorer
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- HbA1c
glycated hemoglobin
- OSA
obstructive sleep apnea
- NFLD
non-alcoholic fatty liver disease
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- SD
standard deviation
- IQR
interquartile ranges
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.obpill.2022.100046.
Contributor Information
Alan De la Rosa, Email: alan_dv@live.com.mx.
Wissam Ghusn, Email: wissamghosn777@gmail.com.
Daniel Sacoto, Email: danielsacoto93@gmail.com.
Alejandro Campos, Email: angel.camposrodriguez@bmc.org.
Lizeth Cifuentes, Email: lizethc177@gmail.com.
Fauzi Feris, Email: fauzifj@gmail.com.
Bradley Busebee, Email: Busebee.Bradley@mayo.edu.
Gerardo Calderon, Email: Calderon.Gerardo@mayo.edu.
Andres Acosta, Email: Acosta.Andres@mayo.edu.
Maria D. Hurtado, Email: Hurtado.mariadaniela@mayo.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., et al. Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ. 2015;18(12):1020–1028. doi: 10.3111/13696998.2015.1067623. [DOI] [PubMed] [Google Scholar]
- 2.Gadde K.M., Atkins K.D. The limits and challenges of antiobesity pharmacotherapy. Expet Opin Pharmacother. 2020;21(11):1319–1328. doi: 10.1080/14656566.2020.1748599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden C.L., et al. Trends in obesity prevalence by race and hispanic origin—1999-2000 to 2017-2018. JAMA. 2020;324(12):1208–1210. doi: 10.1001/jama.2020.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., et al. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49(3):810–823. doi: 10.1093/ije/dyz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cawley J., et al. Direct medical costs of obesity in the United States and the most populous states. Journal of Managed Care & Specialty Pharmacy. 2021;27(3):354–366. doi: 10.18553/jmcp.2021.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelaal M., le Roux C.W., Docherty N.G. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5(7) doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding D., et al. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine; 2020. Is the COVID-19 lockdown nudging people to be more active: a big data analysis; pp. 1183–1184. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani S., Dellen M.R.v., Cooper J.A. Longitudinal weight gain and related risk behaviors during the COVID-19 pandemic in adults in the US. Nutrients. 2021;13(2):671. doi: 10.3390/nu13020671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 10.Maltoni G., et al. Nutr Metab Cardiovasc Dis; 2021. Gender differences in weight gain during lockdown due to COVID-19 pandemic in adolescents with obesity; pp. 2181–2185. [DOI] [PubMed] [Google Scholar]
- 11.Zachary Z., et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14(3):210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrini M., et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12(7):2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan M.A., Smith J.E.M. Covibesity,” a new pandemic. Obesity medicine. 2020;19 doi: 10.1016/j.obmed.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curry S.J., et al. Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(11):1163–1171. doi: 10.1001/jama.2018.13022. [DOI] [PubMed] [Google Scholar]
- 15.Lohnberg J.A., et al. Rapid conversion to virtual obesity care in COVID-19: impact on patient care, interdisciplinary collaboration, and training. Obesity Science & Practice. 2022;8(1):131–136. doi: 10.1002/osp4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ufholz K., Bhargava D. A review of telemedicine interventions for weight loss. Current Cardiovascular Risk Reports. 2021;15(9):17. doi: 10.1007/s12170-021-00680-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alencar M.K., et al. The efficacy of a telemedicine-based weight loss program with video conference health coaching support. J Telemed Telecare. 2019;25(3):151–157. doi: 10.1177/1357633X17745471. [DOI] [PubMed] [Google Scholar]
- 18.Apovian C.M., et al. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava G., Apovian C.M. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14(1):12–24. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 20.Jensen M.D., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol. 2014;63(25 Part B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Daneschvar H.L., Aronson M.D., Smetana G.W. FDA-approved anti-obesity drugs in the United States. Am J Med. 2016;129(8):879.e1–879.e6. doi: 10.1016/j.amjmed.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Gadde K.M., et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 23.Hollander P., et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36(12):4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pi-Sunyer X., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 25.Calderon G., et al. Effectiveness of anti-obesity medications approved for long-term use in a multidisciplinary weight management program: a multi-center clinical experience. Int J Obes. 2021:1–9. doi: 10.1038/s41366-021-01019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A.K., Singh R. Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs. Expet Rev Clin Pharmacol. 2020;13(1):53–64. doi: 10.1080/17512433.2020.1698291. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya K., et al. The benefit of short-term weight loss with anti-obesity medications in real-world clinical practice. Endocr Pract. 2019;25(10):1022–1028. doi: 10.4158/EP-2019-0081. [DOI] [PubMed] [Google Scholar]
- 28.Stephenson J. More US adults report insurance coverage loss associated with COVID-19 recession. JAMA Health Forum. 2020;1(7) doi: 10.1001/jamahealthforum.2020.0855. e200855-e200855. [DOI] [PubMed] [Google Scholar]
- 29.Minsky N.C., et al. Managing obesity in lockdown: survey of health behaviors and telemedicine. Nutrients. 2021;13(4):1359. doi: 10.3390/nu13041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaccard E., et al. Evidence-based precision medicine is needed to move toward general internal precision medicine. J Gen Intern Med. 2018;33(1):11–12. doi: 10.1007/s11606-017-4149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allison D.B., et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity. 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astrup A., et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36(6):843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenway F.L., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 34.Wadden T.A., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 35.Sjöström C.D., et al. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36(1):20–25. doi: 10.1161/01.hyp.36.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein D.J. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord: journal of the International Association for the Study of Obesity. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 37.Ryan D.H., Yockey S.R. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Current obesity reports. 2017;6(2):187–194. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tapsell L.C., et al. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7(7):e014533. doi: 10.1136/bmjopen-2016-014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iłowiecka K., et al. The long-term dietitian and psychological support of obese patients who have reduced their weight allows them to maintain the effects. Nutrients. 2021;13(6):2020. doi: 10.3390/nu13062020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castelnuovo G., et al. Psychology research and behavior management; 2017. Cognitive behavioral therapy to aid weight loss in obese patients: current perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuk J.L., et al. Predictors of weight loss and weight gain in weight management patients during the COVID-19 pandemic. Journal of Obesity. 2021:2021. doi: 10.1155/2021/4881430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowley W., et al. Medical weight management prevents COVID-19 pandemic-related weight gain. Obesity. 2021:124–125. [Google Scholar]
- 43.Morrison C., et al. Weight loss outcomes with telemedicine during COVID-19. Obesity. 2021:191–192. doi: 10.3389/fendo.2022.793290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data will be made available for the journal upon request.