Abstract
Background
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, direct-to-patient, self-applied ECG patch use has substantially increased. There are limited data comparing clinic with self-applied electrocardiogram (ECG) patches.
Objective
The purpose of this study was to compare rates of ECG patch return, percentages of time patches yielded analyzable data (analyzable time), and percentages of prescribed time ECG patches were worn between clinic and self-applied ECG patches before and during COVID-19.
Methods
A retrospective analysis of patients prescribed an ECG patch during “pre-COVID” (March 1, 2019, through March 1, 2020) and “COVID” (April 4, 2020, through April 1, 2021) years was performed. ECG patch return rates, mean percentages of analyzable time, and mean percentages of prescribed wear time were compared between clinic and self-applied groups.
Results
Among the 29,093 ECG patch prescriptions (19% COVID self-applied), the COVID self-applied group had a lower return rate (90.8%) than did both clinic-applied groups (COVID: 97.1%; pre-COVID: 98.1%; P < .001). Among the 28,048 ECG patches (17.5% self-applied) returned for analysis, the COVID self-applied group demonstrated a lower mean percentage of analyzable time (95.9% ± 8.2%) than did both clinic-applied groups (COVID: 96.6% ± 6.6%; pre-COVID 96.6% ± 7.4%; P < .001). There were no differences in the mean percentage of prescribed wear time between groups (pre-COVID clinic-applied: 96.7% ± 34.3%; COVID clinic-applied: 97.4% ± 39.8%; COVID self-applied: 98.1% ± 52.1%; P = .09).
Conclusion
Self-applied ECG patches were returned at a lower rate and had a statistically lower percentage of analyzable time than clinic-applied patches. However, there were no differences between groups in mean percentages of prescribed wear time, and mean percentages of analyzable time were >95% in all groups.
Keywords: Remote, Telemedicine, Diagnosis, Rhythm monitor, Arrhythmia
Introduction
The use of telemedicine, with its associated reduction in in-person office visits, has increased dramatically since the start of the coronavirus disease 2019 (COVID-19) pandemic. Ambulatory ECG monitoring patches are a widely used diagnostic tool because of their ease of use and reliability in arrhythmia detection.1, 2, 3 Before the COVID-19 pandemic, electrocardiogram (ECG) patches were predominantly applied in a clinic setting by trained medical technicians. However, COVID-19 led to the widespread adoption of mailing ECG patches directly to patients for self-application in an attempt to maintain social distancing and prevent unnecessary exposures.
Prior research has validated the arrhythmia detection capability of ECG patches by comparing metrics with Holter monitors, the traditional standard for ambulatory ECG monitoring.1 , 4 However, in these validation studies, ECG patches were applied to patients in clinics by trained technicians. To date, the performance metrics surrounding self-applied ECG patch use are largely unknown. Because the self-application process includes multiple new steps subject to variability, including the mailing of patches to patients, patients effectively applying the patch, and patients activating the patch, an examination of the metrics surrounding this process would better inform current and future practice.
The goal of the present study was to compare the rate of patch return, the mean percentage of analyzable time (defined as the percentage of time the patch was worn that yielded analyzable data), and the mean percentage of prescribed time that the patch was worn between cohorts of patients who had clinic or self-applied ECG patches before and during the COVID-19 pandemic within a single health care system.
Methods
Patients
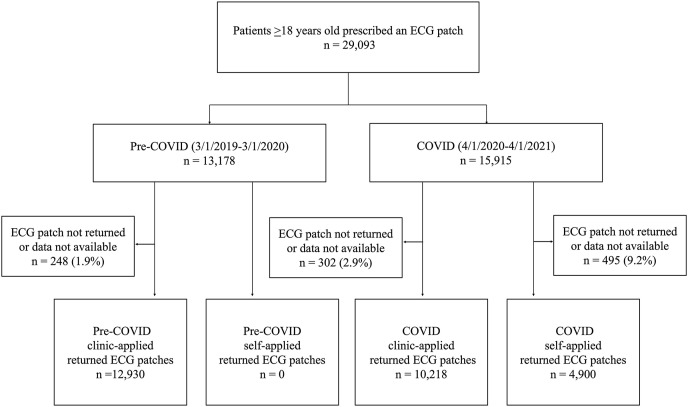
All patients 18 years and older prescribed an iRhythm Zio XT ECG (San Francisco, CA) patch by a Northwestern Memorial Healthcare (Chicago, IL) provider between March 1, 2019, and March 1, 2020, and between April 4, 2020, and April 1, 2021, were eligible for inclusion in this study. Patients with an ECG patch prescription between March 1, 2019, and March 1, 2020, were designated as the “pre-COVID” cohort and those prescribed an ECG patch between April 4, 2020, and April 1, 2021, were designated as the “COVID” cohort. Patients were further categorized according to whether their ECG patch was applied by a technician in clinic or self-applied at home (Figure 1 ).
Figure 1.
Flow of patient inclusion. CONSORT diagram detailing exclusion criteria and the allocation of 29,093 patients prescribed an ECG patch by a Northwestern Medicine provider between March 1, 2019, and April 1, 2021, into cohorts on the basis of type of patch application and time period. Data were unavailable if patients returned the ECG patch without collection of data. In the pre-COVID clinic-applied, COVID clinic-applied, and COVID self-applied cohorts, 33 ECG patches, 41 ECG patches, and 102 ECG patches, respectively, were returned without data available. Additionally, 6 ECG patches in the COVID clinic-applied cohort and 1 ECG patch in the COVID self-applied cohort were successfully returned with data available but not included in the final analysis because the ECG report was not available at the time of data extraction for this study. COVID = coronavirus disease; ECG = electrocardiogram.
ECG patch and its application
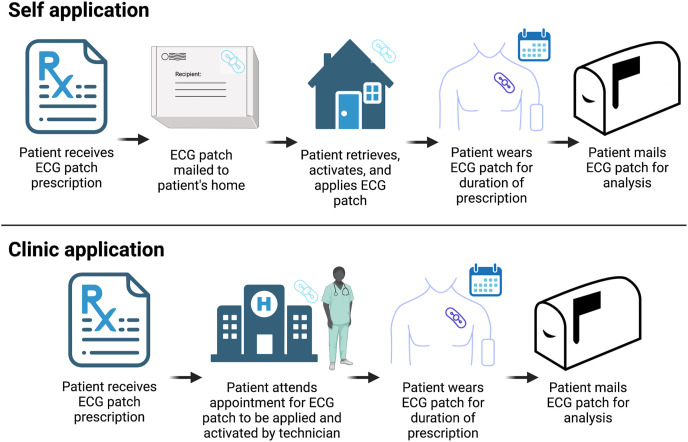
The iRhythm Zio XT ECG patch is a small (2 in × 5 in) adhesive continuous ECG monitor. In the self-application process, after a patch is prescribed, iRhythm mails a patch and an instruction manual regarding self-application to the patient. The patient must receive the patch in the mail and prepare their skin by shaving, abrading, and cleaning the patch site according to instructions provided. Then, the patient must remove the adhesive covering, apply the device, activate the device, wear the patch, and return the patch in the mail after the prescribed wear time. When ECG patches are applied in the clinic, patients present to the clinic for a visit with a trained technician who shaves, abrades, and cleans the patient’s skin and then applies and activates the patch in a manner similar to the self-application process. During this visit, the technician also describes precautions the patient should follow while the patch is worn.
In both clinic and self-applied groups, patients are provided with an addressed and postage-paid box to return the patch. At the conclusion of the prescribed wear time, all patients are instructed to mail the patch to iRhythm for analysis (Figure 2 ). When the patch is received in the mail by iRhythm, arrhythmia adjudication occurs via iRhythm’s Food and Drug Administration–approved arrhythmia processing algorithm, and the results are confirmed by a certified cardiographic technician.5 The rhythm data processed by the algorithm must be above a specified signal-to-noise ratio for the processing algorithm to accurately distinguish various rhythms. Analysis results and rhythm tracings are then sent to the ordering institution for physician overread.
Figure 2.
Process of self- vs clinic application of ECG patches. Patients in the self-application group receive an ECG monitor and instruction manual in the mail; apply, activate, and wear the patch; and then return the patch in the mail after their completed wear time. Patients in the clinic-application group schedule an appointment, a technician applies the patch, the patient receives instructions, and then returns the patch in the mail after their completed wear time. ECG = electrocardiogram.
Data acquisition
Data were collected from the prospectively maintained iRhythm de-identified commercial electronic health record of demographic and ECG rhythm data. This study was deemed exempt by the Institutional Review Board at Northwestern University.
Study end points
The primary metrics analyzed in this study included the ECG patch return rate, the mean percentage of analyzable time, and the mean percentage of ECG patch wear time.
The return rate was calculated as follows: (patches returned to iRhythm with data available/prescribed patches) × 100. ECG patches could be returned to iRhythm but not have data available for various reasons, including a patient failed to activate the patch, the device failed to activate, the device was applied incorrectly, or the device detached from the patient.
The mean percentage of analyzable time reflects the quality of data collected by the ECG patch. The arrhythmia adjudication algorithm designates data above the necessary signal-to-noise ratio as “analyzable.” The percentage of analyzable time was calculated as follows: (time that data were above the necessary signal-to-noise ratio/patient wear time) × 100. The mean percentage of analyzable time was then calculated for each cohort.
The percentage of prescribed wear time was calculated as follows: (actual patient wear time/prescribed wear time) × 100. The mean percentage of prescribed wear time was then calculated for each cohort. It was possible for patients to wear the patch longer than the prescribed wear time, which resulted in a wear time percentage greater than 100%. ECG patches that were not returned were not included in the calculation of the wear time or the analyzable time metrics.
Statistical analysis
Patient demographic characteristics and prescription durations were compared between groups by using analysis of variance for continuous data and χ2 test for categorical data. Differences in the ECG patch return rates, mean percentages of analyzable time, and mean percentages of prescribed wear time were compared between groups using analysis of variance. Post hoc analyses were performed to assess specific between-group differences.
Kruskal-Wallis tests and Cox proportional hazard modeling were performed as appropriate to investigate whether demographic or clinical variables (age, gender, and prescription duration) had significant effects on the between-group differences observed. Cox regression analysis was then used to determine whether differences in study end points between cohorts were still significant after controlling for the confounding effects of clinical and demographic variables. Post hoc analysis was performed to assess specific between-group differences.
A threshold of P < .05 was considered statistically significant. Numerical results are reported as mean ± SD, median (interquartile range), or number (percentage). All analyses were performed using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC).
Results
In total, 29,093 patients (13,178 [45%] pre-COVID clinic-applied, 10,520 [36%] COVID clinic-applied, and 5395 [19%] COVID self-applied) were prescribed an ECG patch over the 2 time periods. Of these, 28,048 patients (mean age 59.3 ± 17.7 years; 15,591 [55.6%] female) wore and returned an ECG patch with data available for analysis (Figure 1).
The most common indications for ECG monitoring were palpitations (28.6%) and atrial fibrillation (19.1%) (Table 1 ). The median monitoring duration of the 28,048 patches returned for analysis was 14 days (3–14 days), and 54.3% of all prescriptions in the study were for 14 days. The mean duration of time between device registration and activation in the self-application group was 8.1 ± 12.2 days.
Table 1.
Patient demographic characteristics and monitoring indications
| Characteristic | Overall (N = 28,048) |
Pre-COVID clinic-applied (n = 12,930) | COVID clinic-applied (n = 10,218) | COVID self-applied (n = 4900) | P |
|---|---|---|---|---|---|
| Age (y) | 59.3 ± 17.7 | 59.5 ± 17.6 | 59.8 ± 17.9 | 57.9 ± 17.5 | <.001 |
| Male gender | 12,457 (44.4) | 5883 (45.5) | 4369 (42.8) | 2205 (45.0) | <.001 |
| Indications | |||||
| Palpitations | 8,032 (28.6) | 3694 (28.6) | 2995 (29.3) | 1343 (27.4) | <.001 |
| Atrial fibrillation | 5,362 (19.1) | 2531 (19.6) | 1747 (17.1) | 1084 (22.1) | <.001 |
| Other | 14,654 (52.2) | 6705 (51.9) | 5476 (53.6) | 2473 (50.5) | <.001 |
| Prescription duration (d) | 14 (3–14) | 14 (3–14) | 14 (3–14) | 14 (7–14) | <.001 |
Values are presented as mean ±SD, median (interquartile range), or n (%).
COVID = coronavirus disease.
ECG patch return rate
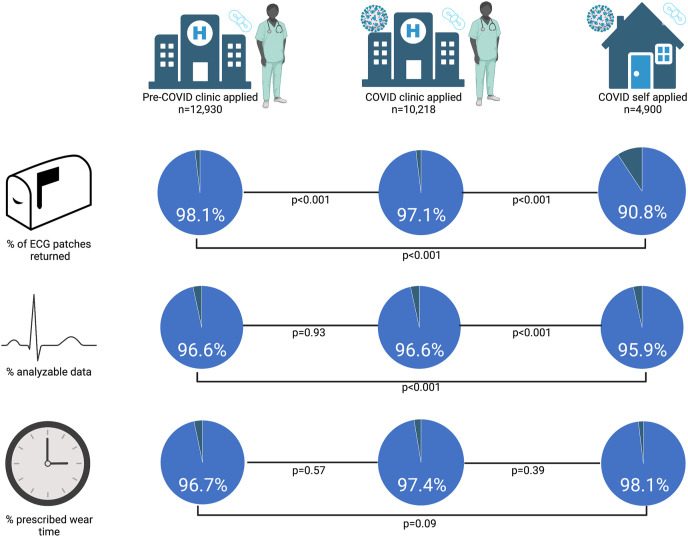
In total, 28,048 ECG patches (96.4%) were returned to iRhythm with data available for analysis. The ECG patch return rates in the pre-COVID clinic-applied, COVID clinic-applied, and COVID self-applied cohorts were 98.1%, 97.1%, and 90.8%, respectively (P < .001) (Figure 3 ). The COVID clinic-applied return rate was significantly lower than the pre-COVID clinic-applied return rate (P < .001). Furthermore, the self-applied return rate was significantly lower than both the pre-COVID and COVID clinic-applied return rates (P < .001 for both comparisons) (Figure 3).
Figure 3.
Comparison of clinic vs self-application of ECG patches. A total of 28,048 patients returned their ECG patch with data available for analysis. The COVID self-applied group had a lower rate of patch return than did both clinic-applied groups. Both the clinic application and self-application groups yielded mean percentages of analyzable time >95% and wore the patch for >95% of the prescribed monitoring duration. There were significant differences in the mean percentage of analyzable time, but no differences in the mean percentage of prescribed wear time between groups. ECG patch return rates were calculated from a total of 29,093 ECG patch prescriptions (45% pre-COVID clinic-applied; 36% COVID clinic-applied; 19% COVID self-applied) in the study period. The mean percentage of analyzable time and mean percentage of prescribed wear time metrics were calculated from a total of 28,048 ECG patches returned with data available. COVID = coronavirus disease; ECG = electrocardiogram.
In univariate analysis, age and prescription duration were found to be significant predictors of return rate (P < .001 for both variables) whereas gender was not (P = .51) (Table 2 ). After using Cox proportional hazards modeling to account for the potential confounding effects of age and prescription duration, there remained significant differences between cohorts in return rate (P < .001). Post hoc analysis demonstrated significant differences between each comparison of the pre-COVID clinic-applied, COVID clinic-applied, and COVID self-applied cohorts (Table 3 ).
Table 2.
Univariate analysis for ECG patch return rate and mean percentage of analyzable time
| Study end point | Variable | P |
|---|---|---|
| ECG patch return rate | Age | <.001 |
| Gender | .51 | |
| Prescription duration | <.001 | |
| Mean percentage of analyzable time | Age | <.001 |
| Gender | <.001 | |
| Prescription duration | <.001 |
ECG = electrocardiogram.
Table 3.
Cox regression analysis for ECG patch return rate and mean percentage of analyzable time
| Study end point | Post hoc comparisons | P |
|---|---|---|
| ECG patch return rate | Pre-COVID clinic-applied vs COVID self-applied | <.001 |
| COVID clinic-applied vs COVID self-applied | <.001 | |
| Pre-COVID clinic-applied vs COVID clinic-applied | <.001 | |
| Mean percentage of analyzable time | Pre-COVID clinic-applied vs COVID self-applied | <.001 |
| COVID clinic-applied vs COVID self-applied | <.001 | |
| Pre-COVID clinic-applied vs COVID clinic-applied | <.001 |
COVID = coronavirus disease; ECG = electrocardiogram.
Mean percentage of analyzable time
In the 28,048 patients who returned their ECG patch, the mean percentage of analyzable time was 96.5% ± 7.2%. While there was no significant difference in the mean percentage of analyzable time between the 2 clinic-applied cohorts (pre-COVID clinic-applied: 96.6% ± 6.6%; COVID clinic-applied: 96.6% ± 7.4%; P = .93), the mean percentage of analyzable time was significantly lower in the self-applied cohort (95.9% ± 8.2%) than in the clinic-applied cohorts (P < .001 for both comparisons) (Figure 3).
In univariate analysis, age, gender, and prescription duration were each found to be significant predictors of mean percentage of analyzable time (P < .001 for each variable) (Table 2). After using Cox proportional hazards modeling to account for the potential confounding effects of age, gender, and prescription duration, there were still significant differences between cohorts in the mean percentage of analyzable time (P < .001). Post hoc analysis demonstrated significant differences between each comparison of the pre-COVID clinic-applied, COVID clinic-applied, and COVID self-applied cohorts (Table 3).
Mean percentage of prescribed wear time
Of all the patients who returned their ECG patch, the mean percentage of wear time was 97.2% ± 39.9% of the prescribed duration. There were no significant differences in the mean percentage of prescribed wear time between the 3 cohorts (pre-COVID clinic-applied: 96.7% ± 34.3%; COVID clinic-applied: 97.4% ± 39.8%; COVID self-applied: 98.1% ± 52.1%; P = .09) (Figure 3).
Discussion
The results of the present study compare return rates and performance metrics of clinic and self-applied ECG patches. Compared with clinic application, self-application was associated with a lower ECG patch return rate and a lower mean percentage of analyzable time. Conversely, there were no significant differences in the mean percentage of prescribed wear time between groups. These results may help inform decisions regarding current and future practices surrounding ECG patch application and use.
Existing data on self-application
Most of the prior research demonstrating the efficacy and reliability of ECG patches has involved clinic application of patches by trained technicians.1 , 4 , 6 To date, limited data exist on the performance metrics of self-applied ECG patches. In a subcohort of the Multi-Ethnic Study of Atherosclerosis (MESA), the mean percentage of prescribed wear time and the mean percentage of analyzable time were compared between 15 patients in a self-applied group and 15 patients in a clinic-applied group. Similar to our study, results from the MESA cohort demonstrate no significant difference in the mean percentage of prescribed wear time between the 2 groups. However, results from the MESA cohort differ from the present analysis by demonstrating no significant difference in the mean percentage of analyzable time between groups.7 The present study expands on prior work by investigating metrics of self-applied ECG patches in a large cohort and by presenting data on ECG patch return rates.
ECG patch return rate
In the present analysis, patients returned ECG patches at a significantly lower rate during the COVID pandemic, with the lowest rate observed in the self-application group. These differences remained significant after controlling for the potential confounding variables included in this study. The differences in return rates between the pre-COVID and COVID time periods are potentially explained by COVID-specific factors, including interrupted mail pickup and patient social distancing during the beginning of the COVID-19 pandemic. Among other reasons, the lower return rate observed in the self-application cohort may have been secondary to unsuccessful mailing of the patch to patients, difficulty with the self-application process, or issues with mailing the patch back to iRhythm. Despite these differences that can be, at least partially, attributed to the specific time period of the study, health care professionals should be aware of the differences in return rates seen.
Outreach to patients who fail to return the ECG patch could prove to be a valuable method for improving return rates. In this study, patients in the clinic-applied cohort who failed to return their patch were contacted by a hospital employee via telephone. For patients in the self-applied cohort, there were multiple outreach processes that developed during the pandemic to contact patients who failed to return their patch, including automated end-of-wear reminder phone calls, smartphone app alerts, text messages, and phone calls from iRhythm employees. These processes were updated throughout the study period, with more outreach efforts occurring toward the end of the study period than at the start of the pandemic. Continued improvement in these outreach processes could lead to an increase in ECG patch return rates.
Mean percentage of analyzable time
Statistically significant differences in the mean percentage of analyzable time between clinic and self-application cohorts were also found in the present study. These significant differences remained after controlling for potential confounding effects of the variables included in this study. The decrease in the mean percentage of analyzable time seen in the self-application cohort may be related to suboptimal application of the patch by patients. However, despite a statistically significant result, the absolute differences in the mean percentage of analyzable time between groups were low (96.6% vs 96.6% vs 95.9%) and each cohort averaged >23 hours of analyzable time per day. Based on a 14-day wear time prescription (the most common duration prescribed in this study), the 0.7% decrease in analyzable time in the self-applied cohort equates to ∼10 fewer minutes of rhythm monitoring per day. As ECG patches have previously been validated for arrhythmia detection despite variable rates of analyzable time, it is likely that the mean percentages of analyzable time achieved in each cohort were sufficient for the detection of most arrhythmias.1, 2, 3
Mean percentage of prescribed wear time
Pre-COVID clinic-applied, COVID clinic-applied, and COVID self-applied cohorts of patients all wore their patches for a significant proportion (>95%) of their prescribed wear time, and there were no significant differences in the mean percentage of prescribed wear time between groups. This demonstrates that the method of ECG patch application is not associated with wear time in our sample.
Utility of self-application
Direct-to-patient, self-application of ECG patches may affect practice within health care systems, and there are many potential applications for this process. Among others, these include improved primary and secondary prevention efforts, expanded access to health care, and community-based research applications.8, 9, 10, 11, 12 In aggregate, the findings from this study provide valuable information to providers and researchers who use self-application of ECG patches. These results may help inform decisions regarding whether to apply ECG patches in the clinic or home, and they should also enable improved patient counseling before ECG patch prescription. Specifically, it is important for patients who are selected for self-application of ECG patches to be counseled on the importance of returning their patch on the basis of the lower rate of self-applied patch return demonstrated in the present analysis. Among those who do return their patches, it is encouraging that both clinic and self-applied patients achieve adequate durations of continuous rhythm monitoring for the detection of most arrhythmias. Indeed, it is likely that certain clinical scenarios will favor one application method over the other, and the flexibility to choose between methods should help providers adapt to an increasingly remote health care landscape.
Limitations
It is important to view the results of this study in the appropriate context. First, self-application of ECG patches at our institution was initiated at the beginning of the COVID-19 pandemic, and within 1 year, thousands of patients were part of the self-application process. Therefore, the metrics surrounding self-application in this study do not reflect a long-standing process, but one that was rapidly adopted and began during a tumultuous and unpredictable time. It is likely that the metrics surrounding self-application would differ if examined further from the onset of the pandemic. Second, these results reflect the performance metrics of 1 device in 1 health care system and geographical region. There may be variability across vendors, institutions, and regions, and future studies should replicate this analysis with different ECG patch vendors in different health care systems and regions. Third, because percentage of prescribed wear time was calculated as (actual patient wear time/prescribed wear time) ∗ 100, it is possible that select patients had >100% wear time, which may have affected the analysis. Fourth, in this study, the most common indications for ECG monitoring were reported in the Results section to provide readers with context surrounding the patient population. However, there were >100 unique indications, and these indications are based on user-entered responses and not clinical diagnoses. As these indications were not strictly defined, and because there were a multitude of different indications entered for patients in this study, indication for ECG monitoring was not included as a potential confounding variable in our analyses. Lastly, there are likely other variables that were not included in our analysis that affect the study end points, and future research into these factors could better inform which patients would be best suited for clinic vs self-application.
Conclusion
In an attempt to promote physical distancing, COVID-19 prompted a rapid adoption of mailing ECG patches to patients for self-application. Before this study, the performance metrics of self-applied ECG patches were largely unknown. The findings presented in the present study demonstrate a lower rate of patch return in patients in the self-applied cohort. However, there were no differences between groups in the mean percentage of prescribed wear time, and despite statistically significant differences, the mean percentage of analyzable time was >95% in both clinic and self-application groups. The results of the present study may help inform current and future decision making for providers, researchers, and health care organizations engaged in arrhythmia detection.
Footnotes
Funding Sources: This research did not receive grants, contracts, or other financial support from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: Dr Passman receives research support from the American Heart Association (#18SFRN34250013), research support and speaker fees from Medtronic, research support from Abbott, and royalties from UpToDate. Dr Knight receives honoraria for speaking or consulting from Abbott, Biosense Webster, Biotronik, Boston Scientific, CVRx, Medtronic, and Philips. Dr Hsu, Mr Wilk, Dr Crosson, and Ms Lenane are employees of iRhythm Technologies. The remaining authors have no disclosures to report.
References
- 1.Barrett P.M., Komatireddy R., Haaser S., et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127:95.e11–95.e17. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heckbert S.R., Austin T.R., Jensen P.N., et al. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51:997–1002. doi: 10.1016/j.jelectrocard.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber D., Sattar A., Drigalla D., Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med. 2014;15:194–198. doi: 10.5811/westjem.2013.11.18973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg M.A., Samuel M., Thosani A., Zimetbaum P.J. Use of a noninvasive continuous monitoring device in the management of atrial fibrillation: a pilot study. Pacing Clin Electrophysiol. 2013;36:328–333. doi: 10.1111/pace.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eysenck W., Freemantle N., Sulke N. A randomized trial evaluating the accuracy of AF detection by four external ambulatory ECG monitors compared to permanent pacemaker AF detection. J Interv Card Electrophysiol. 2020;57:361–369. doi: 10.1007/s10840-019-00515-0. [DOI] [PubMed] [Google Scholar]
- 6.Turakhia M.P., Hoang D.D., Zimetbaum P., et al. Diagnostic utility of a novel leadless arrhythmia monitoring device. Am J Cardiol. 2013;112:520–524. doi: 10.1016/j.amjcard.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M.J., Roetker N.S., Folsom A.R., Alonso A., Heckbert S.R., Chen L.Y. Feasibility of using a leadless patch monitor in community cohort studies: the Multi-ethnic Study of Atherosclerosis. Pacing Clin Electrophysiol. 2018;41:1389–1390. doi: 10.1111/pace.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhouli M., Alqahtani F., Aljohani S., Alvi M., Holmes D.R. Burden of atrial fibrillation-associated ischemic stroke in the United States. JACC Clin Electrophysiol. 2018;4:618–625. doi: 10.1016/j.jacep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Gladstone D.J., Spring M., Dorian P., et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 10.Healey J.S., Connolly S.J., Gold M.R., et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 11.Kaura A., Sztriha L., Chan F.K., et al. Early prolonged ambulatory cardiac monitoring in stroke (EPACS): an open-label randomised controlled trial. Eur J Med Res. 2019;24:25. doi: 10.1186/s40001-019-0383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhubl S.R., Waalen J., Edwards A.M., et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA. 2018;320:146–155. doi: 10.1001/jama.2018.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]