Abstract
Aims
Clozapine is licensed for treatment-resistant psychosis and remains underutilised. This may berelated to the stringent haematological monitoring requirements that are mandatory in most countries. We aimed to compare guidelines internationally and develop a novel Stringency Index. We hypothesised that the most stringent countries would have increased healthcare costs and reduced prescription rates.
Method
We conducted a literature review and survey of guidelines internationally. Guideline identification involved a literature review and consultation with clinical academics. We focused on the haematological monitoring parameters, frequency and thresholds for discontinuation and rechallenge after suspected clozapine-induced neutropenia. In addition, indicators reflecting monitoring guideline stringency were scored and visualised using a choropleth map. We developed a Stringency Index with an international panel of clozapine experts, through a modified-Delphi-survey. The Stringency Index was compared to health expenditure per-capita and clozapine prescription per 100 000 persons.
Results
One hundred twocountries were included, from Europe (n = 35), Asia (n = 24), Africa (n = 20), South America (n = 11), North America (n = 7) and Oceania and Australia (n = 5). Guidelines differed in frequency of haematological monitoring and discontinuation thresholds. Overall, 5% of included countries had explicit guidelines for clozapine-rechallenge and 40% explicitly prohibited clozapine-rechallenge. Furthermore, 7% of included countries had modified discontinuation thresholds for benign ethnic neutropenia. None of the guidelines specified how long haematological monitoring should continue. The most stringent guidelines were in Europe, and the least stringent were in Africa and South America. There was a positive association (r = 0.43, p < 0.001) between a country's Stringency Index and healthcare expenditure per capita.
Conclusions
Recommendations on how haematological function should be monitored in patients treated with clozapine vary considerably between countries. It would be useful to standardise guidelines on haematological monitoring worldwide.
Key words: Adverse drug reaction, national guidelines, safety, stringency index, tolerability, treatment discontinuation, treatment-resistant schizophrenia
Introduction
Clozapine is licensed for the treatment of patients with schizophrenia who have failed to respond to two other antipsychotic medications, and is the only treatment that is effective in this subgroup, which is described as showing treatment resistance (Oloyede et al., 2021a). Recently, there has been interest amongst national regulatory bodies and academics to expand clozapine use in treatment-resistant psychosis (TRP). This interest reflects an increased acknowledgement of its underutilisation, despite sustained evidence indicating its superior therapeutic benefits in this subgroup (Land et al., 2017; Vermeulen et al., 2019; Bhavsar et al., 2020). Clozapine use is limited in part by the need for regular blood monitoring and the fear of severe neutropenia, a side effect that occurs in approximately 0.4% of treated patients, which can be fatal if undetected (Amsler et al., 1977; Kelly et al., 2018; Xiao-Hong et al., 2020; Oloyede et al., 2021a). Other factors associated with clozapine's underuse include adverse drug reactions such as weight gain, hypersalivation and acute hypersensitivity reactions such as clozapine-related drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (de Filippis et al., 2020; Parkes et al., 2022).
A common strategy in healthcare to overcome health disparities and improve quality of care, while ensuring patient safety, is through developing evidence-based guidelines (Kredo et al., 2016). Over recent decades, the number of guidelines for schizophrenia, including haematological monitoring in the context of clozapine treatment have increased in both high and middle-income countries (Warnez and Alessi-Severini, 2014; Nielsen et al., 2016). Several recent investigations have shown that excessively rigid guidelines, that prioritise risk minimisation without balancing this against the superior efficacy of clozapine, can lead to clozapine being withheld from patients for whom it represents their only hope of recovery (Schulte, 2006; Myles et al., 2018, 2019; Whiskey et al., 2019; Schulte et al., 2020; Oloyede et al., 2021a, 2021b). Preliminary investigations and existing literature suggest there are marked variations in key recommendations around monitoring between countries (Nielsen et al., 2016; Bachmann et al., 2017; Whiskey et al., 2021).
The aim of the present study was to provide a comprehensive review of these guidelines, comparing the stringency of clozapine haematological monitoring parameters and frequency, thresholds for discontinuation and rechallenge restrictions, with the extent of use.
Materials and methods
We identified and compared national guidelines for clozapine haematological monitoring to determine the level of variability in different countries. National or sub-national guidelines on prescribing, stopping and restarting clozapine were categorised as either regulations or recommendations. Conditions in guidelines for prescribing, stopping and restarting clozapine that were mandatory were defined as ‘regulations’. For example, in the United Kingdom (UK) haematologicalmonitoring is mandated by the marketing authorisation of clozapine. Non-mandatory conditions were defined as ‘recommendations’.
To capture all guidelines, we used two approaches in parallel . The first was to search the literature for published international guidelines, and then hand-search the references of identified guidelines. The second approach was to directly contact clinicians or academics in each country who were active in psychosis research. To compare the content of the guidelines, information was categorised into three domains: haematological monitoring parameters, criteria for clozapine discontinuation and restrictions for rechallenge after suspected clozapine-induced neutropenia. Clozapine Rechallenge was defined as restarting clozapine treatment after meeting country-specific discontinuation criteria for suspected clozapine-induced neutropenia/agranulocytosis. Clozapine-induced neutropenia was defined as the country-specific neutrophil threshold for clozapine discontinuation. Indicators reflecting stringency of monitoring around clozapine use were scored and plotted on a choropleth map. The relationship between regulatory stringency and health expenditure and clozapine utilisation rates was evaluated using a scatter plot and Pearson's correlation.
Search strategy and data extraction
Embase, Medline, PsychInfo and PubMed were searched up to 1st January 2021. The search terms, inclusion and exclusion criteria can be found in Supplementary Material (Table 1). The following search terms were used: Treatment*resistant psychosis* OR Treatment*refractory psychosis* OR Treatment*resistant schizophrenia OR Treatment*refractory schizophrenia OR clozapine AND algorithm OR guide* OR implementation OR monitor* protocol*. Exclusion criteria included non-specific worldwide or continental guidelines. Inclusion criteria were nationally or regionally recognised guidelines developed by their governing body. The most recent version was selected if the guidelines were published in multiple versions. To determine eligibility, two authors (E.O and G.B) screened the titles, abstracts or summaries, followed by a full-text review and discrepancies were resolved by consensus. In addition, the references to guidelines were manually searched. The title and abstract were screened and the full text was reviewed to confirm eligibility.
As national or sub-national guidelines may not be published in academic journals, guidelines were also identified by personal communication with academic researchers in the field. Personal communication was prioritised for timely data collection as response times with regulatory bodies were slower during the initial stages of data collection. Researchers in the fields were initially selected based on authorship of key papers in the field identified by consensus (Falkai et al., 2005; Gaebel et al., 2005; Nielsen et al., 2016; Bachmann et al., 2017; Howes et al., 2017; Siskind et al., 2020; Wagner et al., 2020; de Leon et al., 2021) or membership of relevant organisations related to psychosis (e.g. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis [TRRIP] Working Group). Attempts were made to contact the first and senior authors to provide guidelines. Alternatively, email requests were made to psychiatry associations to provide contact details of an appropriate academic or clinician. The following psychiatry associations were contacted: African Association of Psychiatrists and Allied Professions, Asian Federation of Psychiatric Association and The Royal Australian and New Zealand College of Psychiatrists.
Data contributors were asked to confirm the information extracted and summarised in a table. Where national guidelines were not available, sub-national guidelines were requested from academic researchers. Furthermore, academic researchers were asked to confirm if guidelines previously identified from the literature review were the most recent version. In addition, academic researchers were asked to confirm whether information was referenced from the summaries of product characteristics (SmPC) of manufacturers providing clozapine in the respective country.
Stringency index
We developed a novel index to quantify the stringency of haematological monitoring during clozapine treatment. We used a modified Delphi methodology to arrive at a consensus. This methodology is well established for measuring international variability in clinical practice in other medical conditions (Hale et al., 2020). Full details are provided in appendix 3.
Analysis and visualisation
The overall Stringency Index was plotted on a choropleth map to visualise variations between countries (Datawrapper, 2022). The Stringency Index was compared to health expenditure per capita (2018 constant in US dollars) (WHO, 2022) and clozapine prescription per 100 000 persons in countries with published data using scatter plots and Pearson correlation coefficient produced in R (R Core Team, 2022). Descriptive statistics were used to evaluate variability of the stringency indices by continent. The interquartile range (IQR) method was used to identify outliers in each continent, defined as values more than 1.5 times the IQR.
Results
The search and selection of guidelines
In total, 954 records were identified through the search of Embase, Medline, PsychInfo and PubMed. After de-duplication and title and abstract screening, 15 guidelines were screened for eligibility. Guidelines from seven countries were included (Disayavanish et al., 2000; Gaebel et al., 2005; Schulte et al., 2010; SIGN, 2013; NICE, 2014; Galletly et al., 2016; Remington et al., 2017; Keepers et al., 2020; Japanese Society of Neuropsychopharmacology, 2021). These were from Australia, Canada, Japan, Netherlands, New Zealand, Thailand and the UK. Only the Australian, New Zealand and Dutch guidelines provided specific monitoring requirements and parameters for clozapine in the guidelines identified from the literature search. In addition, 132 clinicians and academics were approached directly, yielding a further 95 guidelines. The survey response rate for guideline identification was 98% (130 respondents). A total of 102 countries were included in the final review (see Supplementary Material, Table 2 for summary). The response rate from clinicians and academics for data confirmation was 73% (95 respondents). The data source was mandatory regulations (national or sub-national) in 40 (39%) of the countries and recommendations in 60 (59%) countries.
Haematological monitoring
Guidelines from 92 (90%) countries included routine haematological monitoring. This was mandatory (i.e., ‘no blood, no drug’) in 42 (45%) countries. Guidelines from 85 countries (83%) included both the white cell count (WCC) and the absolute neutrophil count (ANC) in this monitoring. Only five (5%) countries mandated or recommended ANC monitoring, based on United States (US) Food and Drug Administration (FDA) regulation revisions in 2015. These countries were Chile, Israel, Lebanon, South Africa and the US. Two countries (Armenia and Colombia) recommended WCC monitoring but not ANC monitoring. None of the countries provided explicit recommendations about when it was appropriate to stop haematological monitoring. In the Netherlands many psychiatrists and individuals receiving clozapine treatment agree to off-label use, where monitoring is stopped, or reduced to four times a year (Schulte et al., 2010). Seven (7%) countries have modified clozapine monitoring criteria for those diagnosed with benign ethnic neutoprenia (BEN). These countries were Canada, Iceland, Israel, Qatar, South Africa, United Kingdom and the USA.
Clozapine discontinuation
Sixty-two (61%) countries recommended clozapine discontinuation for a specified criterion based on haematological thresholds. Recommendation for treatment interruption or discontinuation after a below threshold haematological reading differed between countries. For example, 31 (30%) countries did not have explicit guidance regarding thresholds requiring clozapine discontinuation and were dependent on clinician judgement. Eight countries (in Asia and Europe) adopted a graded approach dependent on the length of treatment. The lowest ANC threshold for discontinuation was 0.5 mm3/L in Taiwan, while the highest was 1.5 mm3/L (in several countries). The lowest threshold WCC threshold for discontinuation was 1.0 mm3/L in Taiwan, while the highest limit was 4.0 mm3/L (in Armenia).
Clozapine rechallenge
Forty-one (40%) countries prohibited clozapine rechallenge after suspected clozapine-induced neutropenia. Seven (7%) countries partially restricted clozapine rechallenge. Specifically, guidelines from three countries (Argentina, Singapore, Turkey) recommended clozapine rechallenge based on the previous ANC/WCC count not indicating severe neutropenia. Brazil and Qatar required consultation with a haematologist prior to rechallenge. Australia, Canada and the United Kingdom required a manufacturer off-licence agreement. An off-licence agreement indicates that the use of clozapine is outside of the marketing authorisation and that the benefits of clozapine treatment outweigh any possible risks to the patient. In practice, this often involves liaison with a haematologist but this is not a pre-requisite.
Clozapine haematological monitoring stringency Index
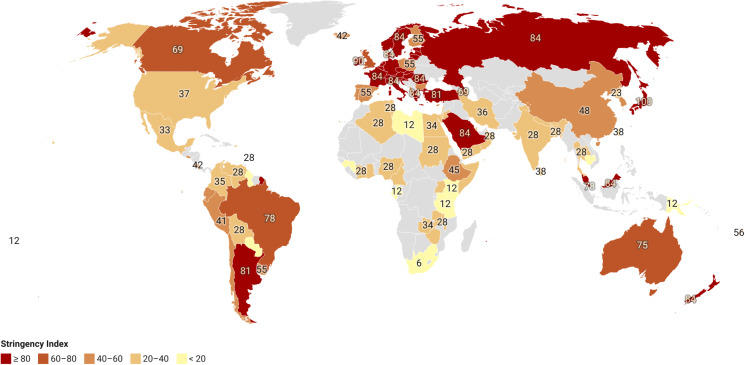
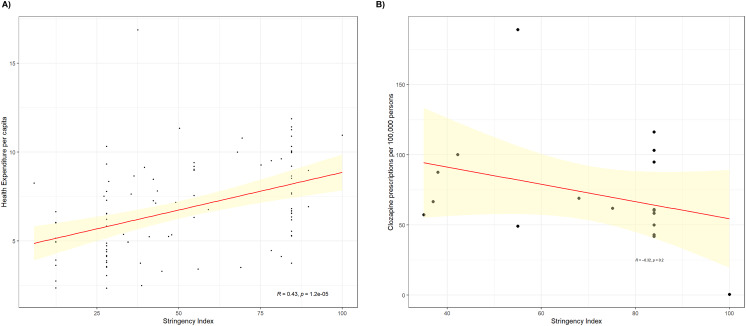
Figure 1 plots the clozapine Stringency Index for each country on a choropleth map. Within continents, Africa, North America followed by Europe showed the least variability between member countries as measured by standard deviation (Supplementary Material, Table 3).Asia showed the greatest international variability. Outliers in Asia were Japan (where stringency was scored 100), while Iceland and Bulgaria (where stringency was scored 42 and 43) were outliers in Europe. There was a nonsignificant negative correlation (R = −0.32, p = 0.2) between a country's Stringency Index and clozapine prescription rates per 100 000 persons (Fig. 2a). In contrast, there was a significant positive correlation (R = 0.43, p < 0.001) between a country's Stringency Index and healthcare expenditure per capita (Fig. 2b).
Fig. 1.
Choropleth map of clozapine haematological monitoring Stringency Index. Red indicates highest stringency and yellow the lowest stringency. Grey indicates no data.
Fig. 2.
(a) Scatter plot and Pearson's correlation coefficient for the clozapine Stringency iIndex and health expenditure per capita. Data was not available for Hong Kong, North Korea, Somalia and Taiwan. (b) Scatter plot and Pearson's correlation coefficient for the clozapine Stringency Index and clozapine utilisation rates in 18 countries. The yellow shaded area indicates confidence interval at the 95% level.
Discussion
We found marked international variability in the recommendations for haematological monitoring during clozapine treatment, the discontinuation of treatment and clozapine rechallenge. Moreover, only 7% of countries have modified clozapine monitoring criteria for patients with BEN. There was a direct correlation (R = 0.43, p < 0.001) between a country's Stringency Index and healthcare expenditure per capita. To our knowledge, this is the largest study to assess national differences in clozapine haematological monitoring guidelines, and the first to compare haematological thresholds for discontinuation between countries. Our findings complement a previous study by Nielsen et al., 2016 that investigated broader aspects of international guidelines of clozapine use (Nielsen et al., 2016), and are of particular interest in the context of growing concerns about the underutilisation of clozapine in TRP. Several authors have called for the easing of restrictive guidelines, such as those mandating lifelong, frequent haematological monitoring or prohibiting rechallenge after haematological values fall below a particular threshold (Schulte et al., 2020; Siskind and Nielsen, 2020; Oloyede et al., 2021a).
Geographical variations in haematological monitoring requirements
Clinical guidelines represent an important step towards the dissemination and implementation of evidence-based clinical practice, and this includes clozapine treatment in TRP (Woolf et al., 1999). In our review, we found that the dissemination of haematological monitoring guidelines waslower in low- and middle-income countries. Moreover, as demonstrated in Fig. 1, there was a non-significant positive correlation between a country's Stringency Index and healthcare expenditure per capita. This variability in guideline availability may be attributed to reduced resources in these countries and/or a lower rate of clozapine prescription (Woolf et al., 1999).
The observed geographical variations in monitoring standards could reflect ethnic differences in the risk of clozapine-induced blood dyscrasias (de Leon et al., 2021). Nonetheless, there is mixed evidence regarding an increased risk in Asian populations (Munro et al., 1999; Shapiro et al., 1999; Sing et al., 2017; Xiao-Hong et al., 2020). There is clearer evidence regarding BEN, a phenotype seen predominantly in populations of African ancestry who have low ANC values of less than 1.5 mm3/L without an increase in adverse clinical outcomes (Oloyede et al., 2021b), which is associated with the Duffy-Null genotype (Legge et al., 2019). From our review, many national guidelines mentioned identifying BEN in liaison with a haematologist in their guidance but did not include modified monitoring parameters. Moreover, contrary to expectations, in continents where BEN prevalence is reportedly highest (Africa and Middle East) there was little or no mention of BEN in monitoring guidelines (Supplementary Material, Table 2 and Fig. 1) (Manu et al., 2016). Revisions concerning these monitoring parameters are warranted to overcome racial and ethnic disparities in clozapine use, particularly in countries where a high frequency of inter-ethnic admixture exists (Oloyede et al., 2021b; de Freitas et al., 2022). This is also emphasised by evidence suggesting that benign neutropenia (i.e. constitutional neutropenia) also occurs in Caucasian and Chinese populations (Cutting and Lang, 1964; Kyle and Linman, 1968; Dancey and Brubaker, 1980; Mant et al., 1987; Pathak et al., 2009). On a practical level, the identification of BEN may be complicated in some countries due to limited access to haematologists, however, the emergence of cost-effective genetic tests may improve this (Oloyede et al., 2021b).
Utilisation rates and clozapine-induced agranuloctyosis mortality rates
While our data provides a clearer perspective on clozapine haematological monitoring guidelines internationally, one important consideration that remains unanswered is the impact of these variations on clozapine utilisation rates. In a previous study, Bachmann et al., 2017 compared clozapine usage internationally,(Bachmann et al., 2017) and a similar study was conducted by Whiskey et al., 2021, reporting clozapine usage in the UK (Whiskey et al., 2021). Combining data from both studies, clozapine usage rates were highest in Finland, New Zealand, Iceland and the Netherlands (Bachmann et al., 2017). Interestingly, as shown in Fig. 2, these countries were among those with the least stringent clozapine guidelines. Conversely, clozapine use is significantly lower in Japan which until recently has relatively strict national guidelines. These data suggest that the stringency of monitoring is broadly related to usage rates, and this assertion is further supported by evidence that frequent monitoring is a factor that leads both patients and clinicians to discontinue clozapine (Black et al., 1996; Legge et al., 2016). Furthermore, previous studies have suggested that flexible neutrophil monitoring may contribute to long-term clozapine maintenance (Davis et al., 2014; Ingimarsson et al., 2016). Nevertheless, such conclusions are limited by the absence of data for clozapine utilisation rates in some countries included in our study.
Beyond clozapine usage rates, the safety implications of flexible haematological monitoring, particularly around the risk of severe neutropenia, are equally important (Boxer, 2012). Encouragingly, recent meta-analytic data found no significant difference in the prevalence of clozapine-induced severe neutropenia across 12 countries in five continents, with or without strict monitoring (Xiao-Hong et al., 2020). Therefore, these data would seemingly suggest that achieving optimum stringency of monitoring does not affect mortality rates secondary to clozapine-induced severe neutropenia. Notably, an early analysis suggested reduced mortality with the implementation of clozapine national registries with mandatory haematological monitoring requirements. However this study was flawed due to an assumption that 1% of patients treated with clozapine develop severe neutropenia with expected fatality rates of 20% (based on the antidepressant mianserin) (Honigfeld, 1996). However, meta-analytic evidence suggests that the rate of severe neutropenia is closer to 0.4% and estimated that fatalities are closer to 10%, even without strict monitoring, thus overestimating the impact of stringent monitoring (Myles et al., 2018; Xiao-Hong et al., 2020). Moreover, a recent case series has demonstrated how monitoring schemes should aim to identify true clozapine-induced severe neutropenia as opposed to threshold-defined nominal severe neutropenia (Taylor et al., 2022). In addition, several recent meta-analyses have shown that the risk of clozapine-induced severe neutropenia is highest in the first 6 months (Myles et al., 2018; Myles et al., 2019). Cumulatively, this casts doubts on the clinical utility of stringent haematological monitoring beyond the first six months of treatment, especially when considering the impact of premature discontinuation of clozapine on morbidity (Schulte, 2006; Shrivastava and Shah, 2009; Rettenbacher et al., 2010; Myles et al., 2018, 2019; Luykx et al., 2020; Schulte et al., 2020; Siskind and Nielsen, 2020; Johannsen et al., 2022).
Clozapine rechallenge criteria
The current literature emphasises the need to encourage continued clozapine treatment in responsive patients when it is safe to do so (Shah et al., 2018; Luykx et al., 2020). Nevertheless, there are occasions where treatment discontinuation is necessary, and this includes the case of a true clozapine-induced blood dyscrasia (Legge et al., 2016; Blackman and Oloyede, 2021; Blackman et al., 2021). Studies suggest that treatment is often interrupted in the absence of strong evidence of a haematological aberration (Davis et al., 2014; Oloyede et al., 2021a). This raises the question of whether it is appropriate to rechallenge such patients with clozapine. Encouragingly, most studies indicate that rechallenging is feasible when the neutropenia is not severe or emerged within the first few months of treatment (Manu et al., 2012; Meyer et al., 2015; Silva et al., 2020; Oloyede et al., 2021a). However, our review found that only a small minority of countries (5%) permit rechallenge, with the majority either imposing a lifelong prohibition on rechallenge after suspected clozapine-induced blood dyscrasias or providing no guidance on the issue. Balanced criteria from a mental and physical health perspective for clozapine rechallenge such as those used in Turkey and Singapore, based on the index ANC count, can conceivably achieve optimal outcomes for patients in regard to safety and therapeutic benefits (Schulte et al., 2010).
Proposed solution: internationally standardised guidelines
Haematological Monitoring is an intrinsic component of treatment with clozapine (Farooq et al., 2019; Schulte et al., 2020). Our review shows that most countries require that this continues throughout the duration of treatment. This approach has been adopted in most SmPCs, despite the lack of supporting evidence. This practice began over 30 years ago after a group of patients in Finland developed severe neutropenia leading to eight deaths (Hippius, 1999; Crilly, 2007). While the early detection of clozapine-induced agranulocytosis (CIA) has undoubtedly avoided many clinical complications(Copolov et al., 1998; Munro et al., 1999; Deliliers, 2000), two issues remain outstanding: the haematological threshold for discontinuation, and for how long haematological monitoring is necessary (Atkin et al., 1996). Concerning the first issue, the haematological thresholds originally used by the clozapine patent holder appear to have been set with a margin of safety. However, the manufacturers have since acknowledged that these limits were arbitrarily defined and are not consistent with clinical and scientific knowledge of immune system functioning, and therefore may unnecessarily restrict access to treatment (O'Sullivan and Lynch, 1996). Regarding the second issue, shortly after the aforementioned events in Finland, a Sandoz-sponsored article proposed weekly haematological monitoring for the first 18 weeks, similar to previous recommendations for chlorpromazine (Pisciotta et al., 1958; Amsler et al., 1977; Anderman and Griffith, 1977). However, the basis of the view that monitoring should continue indefinitely is unclear. As described by Kleinerman in 1990, this controversy is not new (Kleinerman, 1990). In a letter to the manufacturers, authors described the monitoring practices as ‘clinically, scientifically and economically unjustified’. Indefinite monitoring is increasingly questioned from both a safety and a health economics perspective (Lee, 1990; Zhang et al., 1996; Shrivastava and Shah, 2009; Nooijen et al., 2011; Lahdelma and Appelberg, 2012; Cohen and Monden, 2013; Myles et al., 2018, 2019). Routine monitoring increases the likelihood of detecting transient fluctuations in neutrophil count that are unrelated to clozapine treatment, particularly when patients have been established on treatment for many years and have unrecognised haematological phenotypes such as benign neutropenia (Oloyede et al., 2021a, 2021b; Taylor et al., 2022). To this end, limiting monitoring to the first few months of treatment, as used in Bulgaria, Mexico and Colombia, is arguably the most evidence-based approach. While cases of late-onset CIA have been previously reported, these events are rare (Lahdelma and Appelberg, 2012; Cohen and Monden, 2013). Moreover, other medications that increase the risk of neutropenia, such as carbamazepine are not subject to the same monitoring requirements (Ibáñez et al., 2005).
So what could be the solution to restrictive monitoring guidelines? There is a clear need to balance the benefits of mandatory haematological monitoring against the risk that these become barriers to the initiation and continuity of clozapine treatment. Noteworthy initiatives to address this issue have been made. For example, due to the low incidence of CIA, the Netherlands Clozapine Collaboration group allows haematological monitoring for neutropenia to be stopped or reduced to 3-monthly monitoring (off-label) after the first 6 months of clozapine treatment (Cohen and Monden, 2013). This has not led to an increase in mortality secondary to clozapine-induced severe neutropenia (van der Klauw et al., 1998; Schulte, 2006). Furthermore, in 2015 the US FDA updated its clozapine guidelines, decreasing the ANC cut-off for clozapine cessation to a lower threshold compared to many other countries (Sultan et al., 2017; Oloyede et al., 2022). In addition, the requirements for monitoring WCC were removed (Whiskey et al., 2019) and patients with BEN were permitted to commence clozapine treatment under lower thresholds than in most countries.
It is paramount that regulatory bodies on a global scale take actions to improve access to the only proven treatment for this severely debilitating and costly disorder (Schulte et al., 2020). Our direct communications with academic experts in low- and middle-income countries revealed inequality in access to clozapine care due to costly haematological monitoring requirements, despite clozapine being listed as an essential drug by the World Health Organisation (Barbui and Purgato, 2014). This is further supported by a recent study by Todesco et al. who conducted a cross-country analysis of selection, availability, prices and affordability of essential medicines for mental health conditions. From their findings, clozapine was considered an essential medicine in most high-income countries, but only in a minority of low-income countries (Todesco et al., 2022). Consistent evidence has shown overly stringent monitoring requirements to be a prominent barrier to prescribing or utilising clozapine in patients with TRP (Farooq et al., 2019). The result of which are worse long-term outcomes for this debilitating disorder, including lower long-term all-cause mortality rates(Vermeulen et al., 2019), reduced violent offending(Bhavsar et al., 2020) and readmission rates (Land et al., 2017). This evidence merits that guidelines should take a more balanced approach in which mental, as well as physical health outcomes are considered. In this regard, we propose that an alignment of some of the aforementioned measures fosters this goal.
Collaborative efforts to standardise monitoring could help overcome the lack of haematological monitoring guidelines in some countries by providing accessible, evidence based monitoring guidelines. Such efforts may prove important to improve access to treatment (Barbui and Purgato, 2014). Notably, a similar collaborative approach to guideline development is seen in Europe with countries regulated by the European Medicines Agency. However, as some guidelines are not consistent with present evidence, countries such as Iceland and the Netherlands have taken steps to adopt monitoring standards that often run contrary to manufacturer recommendations to alleviate the effect of restrictive guidelines (Ingimarsson et al., 2016; de Leon et al., 2021). In particular, in Iceland, haematological monitoring after the first 18 weeks of treatment (when the risk of severe neutropenia is highest) (Alvir et al., 1993; Atkin et al., 1996) is conducted approximately every four months as opposed to recommended monthly intervals. Furthermore, there is evidence that clozapine can be safely continued even after ANC levels that would have mandated treatment discontinuation in other countries. From a clinical standpoint, recent literature has demonstrated that this reduced neutrophil measurement did not lead to more frequent cases of severe neutropenia (Ingimarsson et al., 2016). Assuming that the available scientific evidence underpinning haematological monitoring is broadly generalisable, it should be feasible to produce consistent, evidence-based international recommendations, irrespective of the country. With the need to reduce barriers to clozapine initiation, maintenance and increase patient acceptability, the revision and standardisation of prescribing and monitoring regulations across countries should be prioritised (Black et al., 1996; Kelly et al., 2018; Farooq et al., 2019).
Strengths and limitations
Several important limitations need to be considered. First, reviewed guidelines may not be representative of the situation in countries that were not reviewed in our study. Nevertheless, our study includes over 50% of countries worldwide, covering all populated continents, suggesting representability. Second, our review included four countries where only sub-national guidelines were available present. Therefore, it is plausible that there is considerable variation in practice between regions of the same country. We therefore consulted with at least two academics from different regions of these countries. However, our study is limited by reliance on academics for guidelines and data provision opposed to regulatory bodies. Third, the Stringency Index does not measure the effectiveness of any of the monitoring guidelines, therefore, it is not possible to make definite conclusions on which regulation should be favoured on an international scale. Rather, our data provide a basis for future empirical analyses across countries using a combination of regulatory parameters from different countries. The fourth concerns the exploration of the association between monitoring stringency and clozapine use. As only 18 countries were included, this may have been insufficiently powered. Furthermore, while health expenditure is a reliable measure of healthcare spending, it was not possible to quantify the spending on clozapine treatment management specifically. Caution should be exercised in attributing a causal relationship from this ecological study due to ecological fallacy and requires confirmation in individual-level case-control or cohort studies. Fifth, we have assumed that national guidelines on clozapine monitoring have a significant influence on clinical practice, but could not assess this directly. It is thus possible that clinical practice may vary from that recommended in guidelines, such as the off-label reduction in haematological monitoring seen in the Netherlands. Finally, our review focuses primarily on haematological monitoring in relation to agranulocytosis and not general tolerability nor all haematological aspects such as clozapine-related DRESS syndrome.
Conclusion
There are wide variations in the guidelines for clozapine monitoring between countries. There is also a general lack of guidance on the duration of haematological monitoring, the discontinuation of clozapine in patients with BEN, and the restarting of clozapine following neutropenia. A single evidence-based and standardised international guideline, with more information on the three latter items could help to address the under-utilisation of clozapine in the management of patients with schizophrenia whilst simultaneously addressing safety concerns.
Acknowledgements
We would like to thank Dr Zoubir Benmebarek (Algeria), Dr Federico Daray (Argentina), Professor Arthur Mkrtchyan (Armania), Dr Roksana Zakharyan (Armenia), Professor Dan Siskind (Australia), Dr Olav Nielssen (Australia), Dr Alex Hofer (Austria), Professor Monika Edlinger (Austria), Dr Narmin Guliyeva (Azerbaijan), Dr Shirin Kazimon (Azerbaijan), Dr Yasir Arafat (Bangladesh), Dr Silvia Turjanski Loustric (Barbados), Professor Marc De Hert (Belgium), Dr Franciska Desplenter (Belgium), Dr Damber Kumar Nirola (Bhutan), Professor Guillermo Carlos Rivera Arroyo (Bolivia), Professor, Cristina Mariano Ruas (Brazil), Professor Pavlina Gateva (Bulgaria), Dr Eyoum Christian (Cameroon), Dr Annick Ndoumba (Cameroon), Dr Yap Boum (Cameroon), Dr Fair Karl Gwei Njuwa (Cameroon), Dr Alka Bhalla (Canada), Professor Gary Remington (Canada), Professor Silvia Alessi-Severini (Canada), Dr Cristián Mena Henriquez (Chile), Dr Si Tian-Mei (China), Dr Yutao Xiang (China), Professor Jorge Enrique Machado Alba (Colombia), Dr Adriana Patricia Bohórquez (Columbia), Dr Arturo E Arellano (Costa Rica), Dr Ivona Simunovic Filipcic (Croatia), Dr Maria NK Karanikola (Cyprus), Dr Libor Ustohal (Czech Republic), Dr Andrés Benavides (Ecuador), Professor Tarek A Okasha (Egypt), Dr Karim Abdul Aziz (Egypt), Dr Dina Aly El-Gabry (Egypt), Dr Fanny Elizabeth Rodriguez Elias (El Salvador), Mr Jana Lass (Estonia), Dr Minale Tareke (Ethiopia), Professor Solomon Teferra (Ethiopia), Dr Odille Chang (Fiji), Dr Kartika Goundar (Fiji), Dr Kiran Gaikwad (Fiji), Dr Leena Saastamoinen (Finland), Professor Hélène Verdoux (France), Dr Marie-Stella Marehin (Gabon), Dr Elias Wagner (Germany), Professor Christian Bachmann (Germany), Dr Irene Kretchy (Ghana), Dr Vasiliki Davidi (Greece), Dr Abdoulaye Sow (Guinea), Dr Noel Holder (Guyana), Professor Wing Chung Chang (Hong Kong), Dr Judit Lazáry (Hungary), Dr Oddur Ingimarsson (Iceland), Dr Anto Praveen Rajkumar Rajamani (India), Dr Sandeep Grover (India), Dr Mohammadreza Shalbafan (Iran), Dr Aviv Segev (Israel), Dr Amir Krivoy (Israel), Professor Ange-Eric Kouame-Assouan (Ivory Coast), Professor Ken Inada (Japan), Professor Mayyada Wazaify (Jordan), Dr Simon Githui (Kenya), Dr Samer El Hayek (Lebanon), Dr Joseph El Khoury (Lebanon), Dr Mansour Abdulshafea (Libya), Dr Genesis Chorwe-Sungani (Malawi), Dr Orwa Albitar (Malaysia), Mr Karl Schembri (Malta), Dr Myrthala Juarez Trevino (Mexico) Dr Ulloa Elena (Mexico), Dr Kamal Gautam (Nepal), Ms Amy Chan (New Zealand), Dr Oluyemi O Akanni (Nigeria), Dr Nosa Godwin Igbinomwanhia (Nigeria), Dr Jørgen Bramness (Norway), Professor Jimmi Nielsen (Norway), Dr Hamed Al Sinawi (Oman), Dr Florence Muga (Papua New Guinea), Professor Julio Torales (Paraguay), Dr Rubén Valle Rivadeneyra (Peru), Professor Paweł Zagożdżon (Poland), Dr António Pacheco Palha (Portugal), Professor Bernardo Barahona Corrêa (Portugal), Dr Monica Zolezzi (Qatar), Dr Ovais Wadoo (Qatar), Professor Petru Ifteni (Romania), Dr Oleg Kirilochev (Russia), Dr Jisha Lucca (Saudi Arabia), Dr Jimmy Lee Chee Keong (Singapore), Dr Marek Zelman (Slovakia), Dr Lubomira Izakova (Slovakia), Dr Jibriil Handule (Somalia), Dr Jade Bouwer (South Africa), Professor Lesley Robertson (South Africa), Dr Jaelim Cho (South Korea), Professor Young-chul Chung (South Korea), Dr Clemente Garcia (Spain), Professor Madhubhashinee Dayabandara (Sri Lanka), Professor Madhu Dayabandara (Sri Lanka), Ms Aneisha Lindy-Ann Prince (St Lucia), Dr Abdalla Abdelrahman (Sudan), Professor Johan Reutfors (Sweden), Professor Helle Kieler (Sweden), Professor Shih-Ku Lin (Taiwan), Professor Sylvia Kaaya (Tanzania), Dr Joel Ambikile Seme (Tanzania), Dr Chulaporn Limwattananon (Thailand), Dr Raphael Schulte (The Netherlands), Dr Dan Cohen (The Netherlands), Dr Amine Larnaout (Tunisia), Dr Rim Sellami (Tunisia), Professor Aygun Ertugrul (Turkey), Professor Elif Anil Yağcioğlu (Turkey), Dr Emmanuel Mwesiga (Uganda), Dr Juliet Nakku (Uganda), Dr Isaac Oloyede (United Kingdom), Dr Sarah Parry (United Kingdom), Dr Mauricio Toledo (Uruguay), Professor Marta Vázquez (Uruguay), Professor Trino Baptista (Venezuela), Dr Ebtesam Abood Saleh (Yemen), Dr Ravi Paul (Zambia), Mr Ephraim Phiri (Zambia), Dr Walter Mangezi (Zimbabwe), Dr Dixon Chibanda (Zimbabwe) for supporting this study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S204579602200066X.
click here to view supplementary material
Author's contributions
EO devised the study concept and were responsible for data acquisition. EO and GB extracted data. EO and GB carried out data analysis and produced the tables. EO wrote the original data of the manuscript with input from GB, JM, EW, DT and JM. All authors reviewed and edited the manuscript. All authors approved the final version of the manuscript for submission.
Financial support
The study did not receive direct funding.
Conflict of interest
All authors declare no conflict of interest.
Data and materials
Authors had free access to the study data. The data that support the findings of this study are available from the corresponding author, E.O., upon reasonable request.
Data availability
All data are available online as supplemental material of the present article.
References
- Alvir JMJ, Lieberman JA, Safferman AZ, Schwimmer JL and Schaaf JA (1993) Clozapine-induced agranulocytosis – incidence and risk factors in the United States. New England Journal of Medicine 329, 162–167. [DOI] [PubMed] [Google Scholar]
- Amsler HA, Teerenhovi L, Barth E, Harjula K and Vuopio P (1977) Agranulocytosis in patients treated with clozapine. A study of the Finnish epidemic. Acta Psychiatrica Scandinavica 56, 241–248. [DOI] [PubMed] [Google Scholar]
- Anderman B and Griffith RW (1977) Clozapine-induced agranulocytosis: a situation report up to August 1976. European Journal of Clinical Pharmacology 11, 199–201. [DOI] [PubMed] [Google Scholar]
- Atkin K, Kendall F, Gould D, Freeman H, Liberman J and O'Sullivan D (1996) Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. British Journal of Psychiatry 169, 483–488. [DOI] [PubMed] [Google Scholar]
- Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, Coma Fusté A, Furu K, Garuoliené K, Hoffmann F, Hollingworth S, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, López SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Piovani D, Reutfors J, Saastamoinen LK, Sato I, Schuiling-Veninga CCM, Shyu YC, Siskind D, Skurtveit S, Verdoux H, Wang LJ, Zara Yahni C, Zoëga H and Taylor D (2017) International trends in clozapine use: a study in 17 countries. Acta Psychiatrica Scandinavica 136, 37–51. [DOI] [PubMed] [Google Scholar]
- Barbui C and Purgato M (2014) Decisions on WHO's essential medicines need more scrutiny. BMJ: British Medical Journal 349, g4798. [DOI] [PubMed] [Google Scholar]
- Bhavsar V, Kosidou K, Widman L, Orsini N, Hodsoll J, Dalman C and MacCabe JH (2020) Clozapine treatment and offending: a within-subject study of patients with psychotic disorders in Sweden. Schizophrenia Bulletin 46, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LL, Greenidge LL, Ehmann T, Ganesan S and Honer WG (1996) A centralized system for monitoring clozapine use i British Columbia. Psychiatric services (Washington, DC) 47, 81–83. [DOI] [PubMed] [Google Scholar]
- Blackman G and Oloyede E (2021) Clozapine discontinuation withdrawal symptoms in schizophrenia. Therapeutic Advances in Psychopharmacology 11. doi: 10.1177/20451253211032053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman G, Oloyede E, Horowitz M, Harland R, Taylor D, MacCabe J and McGuire P (2021) Reducing the risk of withdrawal symptoms and relapse following clozapine discontinuation – is it feasible to develop evidence-based guidelines? Schizophrenia Bulletin 48, 176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer LA (2012) How to approach neutropenia. The American Society of Hematology Education Program Book 2012, 174–182. [DOI] [PubMed] [Google Scholar]
- Cohen D and Monden M (2013) White blood cell monitoring during long-term clozapine treatment. American Journal of Psychiatry 170, 366–369. [DOI] [PubMed] [Google Scholar]
- Copolov DL, Bell WR, Keks NA, Strazzeri DC, Benson WJ and Johnson GF (1998) Clozapine treatment in Australia: a review of haematological monitoring. Medical Journal of Australia 168, 495–497. [DOI] [PubMed] [Google Scholar]
- Crilly J (2007) The history of clozapine and its emergence in the US market: a review and analysis. History of Psychiatry 18, 39–60. [DOI] [PubMed] [Google Scholar]
- Cutting HO and Lang JE (1964) Familial benign chronic neutropenia. Annals of Internal Medicine 61, 876–887. [DOI] [PubMed] [Google Scholar]
- Dancey JT and Brubaker LH (1980) Neutrophil marrow in chronic benign idiopathic neutropenia. The American Journal of Medicine 68, 251–254. [DOI] [PubMed] [Google Scholar]
- Datawrapper (2022) Datawrapper. Available at https://app.datawrapper.de/ (Accessed 18th March).
- Davis MC, Fuller MA, Strauss ME, Konicki PE and Jaskiw GE (2014) Discontinuation of clozapine: a 15-year naturalistic retrospective study of 320 patients. Acta Psychiatrica Scandinavica 130, 30–39. [DOI] [PubMed] [Google Scholar]
- de Filippis R, Soldevila-Matías P, De Fazio P, Guinart D, Fuentes-Durá I, Rubio JM, Kane JM and Schoretsanitis G (2020) Clozapine-related drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a systematic review. Expert Review of Clinical Pharmacology 13, 875–883. [DOI] [PubMed] [Google Scholar]
- de Freitas DF, Patel I, Kadra-Scalzo G, Pritchard M, Shetty H, Broadbent M, Patel R, Downs J, Segev A, Khondoker M, MacCabe JH, Bhui K and Hayes RD (2022) Ethnic inequalities in clozapine use among people with treatment-resistant schizophrenia: a retrospective cohort study using data from electronic clinical records. Social Psychiatry and Psychiatric Epidemiology 57, 1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, Kopeček M, Švancer P, Olmos I, Ricciardi C, Iglesias-Garcia C, Iglesias-Alonso A, Spina E, Ruan CJ, Wang CY, Wang G, Tang YL, Lin SK, Lane HY, Kim YS, Kim SH, Rajkumar AP, González-Esquivel DF, Jung-Cook H, Baptista T, Rohde C, Nielsen J, Verdoux H, Quiles C, Sanz EJ, Las Cuevas C, Cohen D, Schulte PFJ, Ertuğrul A, Yağcıoğlu AEA, Chopra N, McCollum B, Shelton C, Cotes RO, Kaithi AR, Kane JM, Farooq S, Ng CH, Bilbily J, Hiemke C, López-Jaramillo C, McGrane I, Lana F, Eap CB, Arrojo-Romero M, Rădulescu F, Seifritz E, Every-Palmer S, Bousman CA, Bebawi E, Bhattacharya R, Kelly DL, Otsuka Y, Lazary J, Torres R, Yecora A, Motuca M, Chan SKW, Zolezzi M, Ouanes S, Berardis D, Grover S, Procyshyn RM, Adebayo RA, Kirilochev OO, Soloviev A, Fountoulakis KN, Wilkowska A, Cubała WJ, Ayub M, Silva A, Bonelli RM, Villagrán-Moreno JM, Crespo-Facorro B, Temmingh H, Decloedt E, Pedro MR, Takeuchi H, Tsukahara M, Gründer G, Sagud M, Celofiga A, Ristic DI, Ortiz BB, Elkis H, Pacheco Palha AJ, A LL, Fernandez-Egea E, Siskind D, Weizman A, Masmoudi R, Saffian SM, Leung JG, Buckley PF, Marder SR, Citrome L, Freudenreich O, Correll CU and Müller DJ (2021) An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry 35, e100773. [DOI] [PubMed] [Google Scholar]
- Deliliers GL (2000) Blood dyscrasias in clozapine-treated patients in Italy. Haematologica 85, 233–237. [PubMed] [Google Scholar]
- Disayavanish C, Srisurapanont M, Udomratn P, Disayavanish P, Kasantikul D, Netrakom P, Ngamtipwatthana T, Phornchirasilp S, Rangseekajee P, Samuthrsindh P, Sukanich P, Thiam-Kaew K and Visanuyothin T (2000) Guideline for the pharmacotherapy of treatment-resistant schizophrenia. Royal College of Psychiatrists of Thailand. Journal of the Medical Association of Thailand 83, 579–589. [PubMed] [Google Scholar]
- Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller H-J and Schizophrenia WTFoTGf (2005) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: acute treatment of schizophrenia. The World Journal of Biological Psychiatry 6, 132–191. [DOI] [PubMed] [Google Scholar]
- Farooq S, Choudry A, Cohen D, Naeem F and Ayub M (2019) Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. BJPsych Bulletin 43, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebel W, Weinmann S, Sartorius N, Rutz W and McIntyre JS (2005) Schizophrenia practice guidelines: international survey and comparison. British Journal of Psychiatry 187, 248–255. [DOI] [PubMed] [Google Scholar]
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, Kulkarni J, McGorry P, Nielssen O and Tran N (2016) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Australian & New Zealand Journal of Psychiatry 50, 410–472. [DOI] [PubMed] [Google Scholar]
- Hale T, Petherick A, Phillips T and Webster S (2020) Variation in government responses to COVID-19. Blavatnik school of government working paper 31.
- Hippius H (1999) A historical perspective of clozapine. Journal of Clinical Psychiatry 60(suppl. 12), 22–23. [PubMed] [Google Scholar]
- Honigfeld G (1996) Effects of the clozapine national registry system on incidence of deaths related to agranulocytosis. Psychiatric Services 47, 52–56. [DOI] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, Bloomfield MA, Bressan RA, Buchanan RW, Carpenter WT, Castle DJ, Citrome L, Daskalakis ZJ, Davidson M, Drake RJ, Dursun S, Ebdrup BH, Elkis H, Falkai P, Fleischacker WW, Gadelha A, Gaughran F, Glenthøj BY, Graff-Guerrero A, Hallak JE, Honer WG, Kennedy J, Kinon BJ, Lawrie SM, Lee J, Leweke FM, MacCabe JH, McNabb CB, Meltzer H, Möller HJ, Nakajima S, Pantelis C, Reis Marques T, Remington G, Rossell SL, Russell BR, Siu CO, Suzuki T, Sommer IE, Taylor D, Thomas N, Üçok A, Umbricht D, Walters JT, Kane J and Correll CU (2017) Treatment-resistant schizophrenia: Treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. American Journal of Psychiatry 174, 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez L, Vidal X, Ballarín E and Laporte J-R (2005) Population-based drug-induced agranulocytosis. Archives of Internal Medicine 165, 869–874. [DOI] [PubMed] [Google Scholar]
- Ingimarsson O, MacCabe JH, Haraldsson M, Jónsdóttir H and Sigurdsson E (2016) Clozapine treatment and discontinuation in Iceland: a national longitudinal study using electronic patient records. Nordic Journal of Psychiatry 70, 450–455. [DOI] [PubMed] [Google Scholar]
- Japanese Society of Neuropsychopharmacology (2021) Japanese society of neuropsychopharmacology: “guideline for pharmacological therapy of schizophrenia”. Neuropsychopharmacology Reports 41, 266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen C-F, Petersen TS, Nielsen J, Jørgensen A, Jimenez-Solem E and Fink-Jensen A (2022) Clozapine-and non-clozapine-associated neutropenia in patients with schizophrenia: a retrospective cohort study. Therapeutic Advances in Psychopharmacology 12. doi: 10.1177/20451253211072341. PMID: 35273789; PMCID: PMC8902187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, Servis M, Walaszek A, Buckley P, Lenzenweger MF, Young AS, Degenhardt A and Hong SH (2020) The American psychiatric association practice guideline for the treatment of patients with schizophrenia. American Journal of Psychiatry 177, 868–872. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Freudenreich O, Sayer MA and Love RC (2018) Addressing barriers to clozapine underutilization: a national effort. Psychiatric Services 69, 224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman MJ (1990) Controversy grows over monitoring system for new schizophrenia drug. JAMA 264, 2488–2491. [DOI] [PubMed] [Google Scholar]
- Kredo T, Bernhardsson S, Machingaidze S, Young T, Louw Q, Ochodo E and Grimmer K (2016) Guide to clinical practice guidelines: the current state of play. International Journal for Quality in Health Care 28, 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle RA and Linman JW (1968) Chronic idiopathic neutropenia: a newly recognized entity? New England Journal of Medicine 279, 1015–1019. [DOI] [PubMed] [Google Scholar]
- Lahdelma L and Appelberg B (2012) Clozapine-induced agranulocytosis in Finland, 1982–2007: long-term monitoring of patients is still warranted. Journal of Clinical Psychiatry 73, 837–842. [DOI] [PubMed] [Google Scholar]
- Land R, Siskind D, McArdle P, Kisely S, Winckel K and Hollingworth SA (2017) The impact of clozapine on hospital use: a systematic review and meta-analysis. Acta Psychiatrica Scandinavica 135, 296–309. [DOI] [PubMed] [Google Scholar]
- Lee H (1990) Pharmacists outraged by clozaril system. Journal of Pharmacy Practice 3, 6–8. [Google Scholar]
- Legge SE, Hamshere M, Hayes RD, Downs J, O'Donovan MC, Owen MJ, Walters JTR and MacCabe JH (2016) Reasons for discontinuing clozapine: a cohort study of patients commencing treatment. Schizophrenia Research 174, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge SE, Pardiñas AF, Helthuis M, Jansen JA, Jollie K, Knapper S, MacCabe JH, Rujescu D, Collier DA, O'Donovan MC, Owen MJ and Walters JTR (2019) A genome-wide association study in individuals of African ancestry reveals the importance of the Duffy-null genotype in the assessment of clozapine-related neutropenia. Molecular Psychiatry 24, 328–337. [DOI] [PubMed] [Google Scholar]
- Luykx JJ, Stam N, Tanskanen A, Tiihonen J and Taipale H (2020) In the aftermath of clozapine discontinuation: comparative effectiveness and safety of antipsychotics in patients with schizophrenia who discontinue clozapine. British Journal of Psychiatry 217, 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant MJ, Gordon PA and Akabutu JJ (1987) Bone marrow granulocyte reserve in chronic benign idiopathic neutropenia. Clinical & Laboratory Haematology 9, 281–288. [DOI] [PubMed] [Google Scholar]
- Manu P, Sarpal D, Muir O, Kane JM and Correll CU (2012) When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophrenia Research 134, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manu P, Sarvaiya N, Rogozea LM, Kane JM and Correll CU (2016) Benign ethnic neutropenia and clozapine use: a systematic review of the evidence and treatment recommendations. Journal of Clinical Psychiatry 77, e909–e916. [DOI] [PubMed] [Google Scholar]
- Meyer N, Gee S, Whiskey E, Taylor D, Mijovic A, Gaughran F, Shergill S and MacCabe JH (2015) Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. Journal of Clinical Psychiatry 76, e1410–e1416. [DOI] [PubMed] [Google Scholar]
- Munro J, O'Sullivan D, Andrews C, Arana A, Mortimer A and Kerwin R (1999) Active monitoring of 12,760 clozapine recipients in the UK and Ireland. Beyond pharmacovigilance. British Journal of Psychiatry 175, 576–580. [DOI] [PubMed] [Google Scholar]
- Myles N, Myles H, Xia S, Large M, Kisely S, Galletly C, Bird R and Siskind D (2018) Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatrica Scandinavica 138, 101–109. [DOI] [PubMed] [Google Scholar]
- Myles N, Myles H, Xia S, Large M, Bird R, Galletly C, Kisely S and Siskind D (2019) A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Australian & New Zealand Journal of Psychiatry 53, 403–412. [DOI] [PubMed] [Google Scholar]
- NICE (2014) Psychosis and schizophrenia in adults: prevention and management. NICE guidelines. [PubMed]
- Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang YT, Schulte PF, Correll CU and Taylor D (2016) Worldwide differences in regulations of clozapine use. CNS Drugs 30, 149–161. [DOI] [PubMed] [Google Scholar]
- Nooijen PMM, Carvalho F and Flanagan RJ (2011) Haematological toxicity of clozapine and some other drugs used in psychiatry. Human Psychopharmacology: Clinical and Experimental 26, 112–119. [DOI] [PubMed] [Google Scholar]
- Oloyede E, Casetta C, Dzahini O, Segev A, Gaughran F, Shergill S, Mijovic A, Helthuis M, Whiskey E, MacCabe JH and Taylor D (2021a) There is life after the UK clozapine central non-rechallenge database. Schizoprehnia Buletin 47, 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloyede E, Dzahini O, Barnes N, Mijovic A, Gandhi S, Stuart-Smith S, de Witte T, Taylor D and Whiskey E (2021b) Benign ethnic neutropenia: an analysis of prevalence, timing and identification accuracy in two large inner-city NHS hospitals. BMC Psychiatry 21, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloyede E, Whiskey E, Casetta C, Dzahini O, Dunnett D, Gandhi S, Gaughran F, Shergill S, McGuire P, MacCabe JH and Taylor D (2022) Relaxation of the criteria for entry to the UK clozapine central Non-rechallenge database: a modelling study. The Lancet Psychiatry 9, 636–644. [DOI] [PubMed] [Google Scholar]
- O'Sullivan DP and Lynch KP (1996) Reply from clozaril patient monitoring service. British Medical Journal 313, 1262. [Google Scholar]
- Parkes S, Mantell B, Oloyede E and Blackman G (2022) Patients’ experiences of clozapine for treatment-resistant schizophrenia: a systematic review. Schizophrenia Bulletin Open 3, p.sgac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S, Chandra AB, Huang Y and Xu Y (2009) Etiology of incidental asymptomatic neutropenia and prevalence of benign ethnic neutropenia in patients of Chinese ethnicity. American Society of Hematology 114, 4515. [Google Scholar]
- Pisciotta AV, Ebbe S, Lennon EJ, Metzger GO and Madison FW (1958) Agranulocytosis following administration of phenothiazine derivatives. American Journal of Medicine 25, 210–223. [DOI] [PubMed] [Google Scholar]
- R Core Team (2022) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Remington G, Addington D, Honer W, Ismail Z, Raedler T and Teehan M (2017) Guidelines for the pharmacotherapy of schizophrenia in adults. The Canadian Journal of Psychiatry 62, 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenbacher MA, Hofer A, Kemmler G and Fleischhacker WW (2010) Neutropenia induced by second generation antipsychotics: a prospective investigation. Pharmacopsychiatry 43, 41–44. [DOI] [PubMed] [Google Scholar]
- Schulte PF (2006) Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Annals of Pharmacotherapy 40, 683–688. [DOI] [PubMed] [Google Scholar]
- Schulte PFJ, Cohen D, Bogers JPAM, van Dijk D and Bakker B (2010) A Dutch guideline for the use of clozapine. Australian and New Zealand Journal of Psychiatry 44, 1055–1056. [DOI] [PubMed] [Google Scholar]
- Schulte PFJ, Bogers J, Bond-Veerman SRT and Cohen D (2020) Moving forward with clozapine. Acta Psychiatrica Scandinavica 142, 75–77. [DOI] [PubMed] [Google Scholar]
- Shah P, Iwata Y, Plitman E, Brown EE, Caravaggio F, Kim J, Nakajima S, Hahn M, Remington G, Gerretsen P and Graff-Guerrero A (2018) The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: a systematic review. Psychiatry Research 268, 114–122. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Issaragrisil S, Kaufman DW, Anderson T, Chansung K, Thamprasit T, Sirijirachai J, Piankijagum A, Porapakkham Y, Vannasaeng S, Leaverton PE and Young NS (1999) Agranulocytosis in Bangkok, Thailand: a predominantly drug-induced disease with an unusually low incidence. Aplastic Anemia study group. American Journal of Tropical Medicine and Hygiene 60, 573–577. [DOI] [PubMed] [Google Scholar]
- Shrivastava A and Shah N (2009) Prescribing practices of clozapine in India: results of a opinion survey of psychiatrists. Indian Journal of Psychiatry 51, 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGN Guidelines (2013) 131 Management of Schizophrenia: A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network. [Google Scholar]
- Silva E, Higgins M, Hammer B and Stephenson P (2020) Clozapine rechallenge and initiation despite neutropenia: a practical, step-by-step guide. BMC Psychiatry 20, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing CW, Wong IC, Cheung BM, Chan JC, Chu JK and Cheung CL (2017) Incidence and risk estimate of drug-induced agranulocytosis in Hong Kong Chinese. A population-based case–control study. Pharmacoepidemiology and Drug Safety 26, 248–255. [DOI] [PubMed] [Google Scholar]
- Siskind D and Nielsen J (2020) Clozapine: a fine balance. Acta Psychiatrica Scandinavica 141, 175–177. [DOI] [PubMed] [Google Scholar]
- Siskind D, Honer WG, Clark S, Correll CU, Hasan A, Howes O, Kane JM, Kelly DL, Laitman R and Lee J (2020) Consensus statement on the use of clozapine during the COVID-19 pandemic. Journal of Psychiatry & Neuroscience: JPN 45, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan RS, Olfson M, Correll CU and Duncan EJ (2017) Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. The Journal of Clinical Psychiatry 78, 0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D, Vallianatou K, Whiskey E, Dzahini O and MacCabe J (2022) Distinctive pattern of neutrophil count change in clozapine-associated, life-threatening agranulocytosis. Schizophrenia 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todesco B, Ostuzzi G and Barbui C (2022) Mapping the selection, availability, price and affordability of essential medicines for mental health conditions at a global level. Epidemiology and Psychiatric Sciences 31, E22. doi: 10.1017/S2045796022000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Klauw MM, Wilson JHP and Stricker BHC (1998) Drug-associated agranulocytosis: 20 years of reporting in The Netherlands (1974–1994). American Journal of Hematology 57, 206–211. [DOI] [PubMed] [Google Scholar]
- Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, Sutterland AL, Correll CU and de Haan L (2019) Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophrenia Bulletin 45, 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E, Kane JM, Correll CU, Howes O, Siskind D, Honer WG, Lee J, Falkai P, Schneider-Axmann T, Hasan A and Group TW (2020) Clozapine combination and augmentation strategies in patients with schizophrenia – recommendations from an international expert survey among the Treatment Response and Resistance in Psychosis (TRRIP) working group. Schizophrenia Bulletin 46, 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnez S and Alessi-Severini S (2014) Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry 14, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiskey E, Dzahini O, Ramsay R, O'Flynn D, Mijovic A, Gaughran F, MacCabe J, Shergill S and Taylor D (2019) Need to bleed? Clozapine haematological monitoring approaches a time for change. International Clinical Psychopharmacology 34, 264–268. [DOI] [PubMed] [Google Scholar]
- Whiskey E, Barnard A, Oloyede E, Dzahini O, Taylor D and Shergill SS (2021) An evaluation of the variation and underuse of clozapine in the United Kingdom. Acta Psychiatrica Scandinavica 143, 339–347. [DOI] [PubMed] [Google Scholar]
- Woolf SH, Grol R, Hutchinson A, Eccles M and Grimshaw J (1999) Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ (Clinical Research ed.) 318, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2022) Global Health Expenditure Database. Available at http://apps.who.int/nha/database [Google Scholar]
- Xiao-Hong L, Xiao-Mei Z, Lu L, Zheng W, Shi-bin W, Wen-wang R, Wang S, Ng CH, Ungvari GS and Wang G (2020) The prevalence of agranulocytosis and related death in clozapine-treated patients: a comprehensive meta-analysis of observational studies. Psychological Medicine 50, 583–594. [DOI] [PubMed] [Google Scholar]
- Zhang M, Owen RR, Pope SK and Smith GR (1996) Cost-effectiveness of clozapine monitoring after the first 6 months. Archives of General Psychiatry 53, 954–958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S204579602200066X.
click here to view supplementary material
Data Availability Statement
All data are available online as supplemental material of the present article.