Abstract
Introduction
To identify the potential factors responsible for the individual variability of dabigatran, we investigated the genetic variations associated with clinical outcomes and pharmacodynamics (PD) in Chinese patients with nonvalvular atrial fibrillation (NVAF).
Materials and methods
Chinese patients with NVAF taking dabigatran etexilate with therapeutic doses were enrolled. The primary (bleeding events) and secondary (thromboembolic and major adverse cardiac events) outcomes for a 2‐year follow‐up were evaluated. Peak and trough PD parameters (anti‐FIIa activity, activated partial thromboplastin time and prothrombin time) were detected. Whole‐exome sequencing, genome‐wide sequencing and candidate gene association analyses were performed.
Results
There were 170 patients with NVAF treated with dabigatran (110 mg twice daily) who were finally included. Two single‐nucleotide polymorphisms (SNPs) were significantly related with bleeding, which include UBASH3B rs2276408 (odds ratio [OR] = 8.79, 95% confidence interval [CI]: 2.99–25.83, p = 7.77 × 10−5 at sixth month visit) and FBN2 rs3805625 (OR = 8.29, 95% CI: 2.87–23.89, p = 9.08 × 10−5 at 12th month visit), as well as with increased trends at other visits (p < .05). Furthermore, minor allele carriers of 16 new SNPs increased PD levels, and those of one new SNP decreased PD values (p < 1.0 × 10−5). Lastly, 33 new SNPs were found to be associated with bleeding and PD among 14 candidate genes. Unfortunately, the low number of secondary outcomes precluded further association analyses.
Conclusions
Genetic variations indeed affected bleeding and PD in Chinese patients with NVAF treated with dabigatran. The functions of these suggestive genes and SNPs might further be explored and verified in more in vivo and in vitro investigations.
Keywords: atrial fibrillation, bleeding, dabigatran, genome‐wide association analysis, pharmacodynamics, whole‐exome sequencing
Genetic variations indeed affected outcomes of dabigatran in Chinese NVAF patients.
Minor allele carriers of UBASH3B rs2276408 and FBN2 rs3805625 increased bleeding risk.
Seventeen identified SNP polymorphisms were associated with pharmacodynamics.
Fourteen reported candidate genes were associated with bleeding and pharmacodynamics.
1. INTRODUCTION
Atrial fibrillation (AF) is becoming more prevalent globally in persistent arrhythmia of adults and is worthy of attention with its serious risk of stroke and all‐cause mortality. 1 , 2 Direct‐acting oral anticoagulant, dabigatran, inhibits factor IIa activity directly and has been recommended as one of the preferable therapies for individuals with nonvalvular AF (NVAF) to prevent stroke or systemic embolism (SE) in the global guidelines. 3 , 4 With generic drug development and drug price reductions in China, dabigatran is becoming more widely prescribed for patients with NVAF in the recent years. 5 Whereas, the controversial issues between the fixed‐dose regimens and its variations on pharmacokinetics (PK), pharmacodynamics (PD) and clinical outcomes have always been discussed. Previous studies in healthy participants demonstrated interindividual coefficient of variations (CVs) in PK and PD being 8.4%–46.0% and usually below 20%, respectively. 6 , 7 Besides, in the real world of patients with AF administered with recommended doses of dabigatran, the trough and peak plasma levels of interpatient CVs were 63.8% and 50.9%, respectively, and intrapatient CVs were 32.9% and 39.5%, respectively. 8 Furthermore, Asian patients receiving dabigatran 110 or 150 mg twice daily had a lower incidence of stroke and SE, haemorrhagic stroke and all‐cause mortality compared with non‐Asian patients. 9
So far, except for the meaningful factors responsible for individual variability in dabigatran, including age, sex, weight, food and renal function, 10 , 11 , 12 we could not ignore the pharmacogenomic influence on this issue. 13 , 14 , 15 , 16 , 17 Considering mainly the metabolism characteristics, previous studies were mainly devoted to verify the impact of genetic variations in the ABCB1 and CES1 polymorphism on dabigatran in different populations. ABCB1 gene encodes P‐glycoprotein (P‐gp) whose substrates, including dabigatran, and CES1 gene encode carboxylesterase 1, which primarily activates and hydrolyzes the prodrug (dabigatran etexilate) in hepatocytes. 13 However, whether in healthy participants or in AF populations, the significant impact of these two genes on the outcomes were not totally consistent (Table S1). Besides, there were only two researches that involved other genes except ABCB1 and CES1 before 2022, and no significant effect on PD and clinical outcomes was found. 18 , 19
Considering the different distribution of genes and analytical association methods, 20 , 21 we previously performed a pharmacogenomic study in 118 healthy Chinese volunteers to investigate the meaningful single‐nucleotide polymorphisms (SNPs) associated with dabigatran metabolism. 22 For further exploring the related genetic variations in patients, we planned to carry out a nationwide multicentre prospective cohort study in Chinese patients with NVAF by whole‐exome sequencing and genome‐wide association (GWA) analyses. We hope to seek for more significant markers on dabigatran efficacy and safety and improve the individualized anticoagulant therapy regimen in AF.
2. MATERIALS AND METHODS
2.1. Study design
This prospective research was conducted in a nationwide multicentre of six hospitals in China. The protocol registered in ClinicalTrial.org (NCT03161496) was approved by the ethics committees of all the hospitals. After introduction of the detailed information about the research, written informed consent was obtained from each patient before enrolment.
Adult patients with NVAF who had planned to take or had been taking dabigatran etexilate (brand name: Pradaxa) were recruited from September 2017 to May 2020. To ensure a steady drug concentration before blood sample collection, patients who had planned to take drug were required not to take it within 1 month before the study. On the other hand, patients who had been taking it were required to administer it continuously for more than 1 week prior to the beginning of the study. The exclusion criteria were as follows: (i) an immunodeficiency disease history, including positive human immunodeficiency virus (HIV) indices; (ii) co‐medications including CYP3A4 strong inhibitors and P‐gp inhibitors, and CYP3A4 strong inducers and P‐gp inducers (examples of detailed drugs showed in ClinicalTrial.org) within 14 days before dabigatran treatment; (iii) severe abnormal liver function (Child–Pugh B/C and liver cirrhosis) or renal function (estimated glomerular filtration rate [eGFR] <30 ml/min*1.73 m2); and (iv) any dabigatran contraindications, including hypersensitivity, active bleeding, history of intracranial and gastrointestinal (GI) haemorrhage within previous 6 months, and any major operations in the past 30 days.
After enrolment, each patient was required to take dabigatran regularly at therapeutic doses (110 or 150 mg twice daily) prescribed by his/her physician. They were also required to provide blood samples for the detection of genotypes and PD parameters after reaching steady drug concentrations (regularly taking dabigatran daily at least 72 h), and were followed up regularly for 2 years. Moreover, other related information was obtained from medical records, including demographics, clinical examinations indices, co‐medications and comorbidities at the time of enrolment. CHA2DS2‐VASc and HAS‐BLED scoring systems were used to evaluate the risks of thromboembolism and haemorrhage, respectively. The formula of eGFR determined was the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. 23
2.2. Pharmacodynamic evaluation
PD outcomes were mainly evaluated via trough and peak prothrombin time (PT), activated partial thromboplastin time (APTT) and anti‐FIIa activity. After reaching a steady state of dabigatran and without any dose adjustments, blood samples (2.7 ml) were taken in sodium citrate (3.2% v/v) tubes at time points >10 h after previous dose for detecting trough PD and 2 h after dosing for peak PD, respectively. Within 1 h after collection, blood samples were centrifuged for 15 min in 2500 × g at room temperature. The plasma samples were then stored at −80°C at each centre and all the samples were transferred to Peking University First Hospital for PD tests within 6 months. Through automated multiparameter haemostasis analyzer (Sysmex CS‐2100i), validated Coagulation or Chromogenic Method Kits were used to measure PT, APTT and anti‐FIIa activity. The detailed and validated methods of determining these parameters have been published in our previous studies. 22 , 24
2.3. Genotyping
Blood samples (6 ml) for genotype tests were taken in EDTA‐K2 tubes when collecting trough PD samples. Subsequently, the whole‐blood samples from all centres were stored at less than −60°C till genotype tests were conducted in the same institution (CapitalBio Technology Co., Ltd, Beijing, China). After genomic DNA preparation and quality assessment, whole‐exome sequencing was used for genotyping, which has also been published in our previous study. 22 Agilent SureSelect Human All Exon V6 Kit (Agilent Technologies Inc., USA) was used to create the whole‐exome library, and an Illumina NovaSeq 6000 sequencer (Illumina, USA) was used for paired‐end sequencing (2 × 150 bp). With 200‐bp extensions on either end, 283 350 SNPs found in exons underwent variant filtering and prediction. Missing rate more than 10%, minor allele frequency (MAF) less than 5% and Hardy–Weinberg equilibrium p‐value <10−6 were used as exclusion criteria of SNPs. Principal component analysis (PCA) was used in PLINK 1.09 to perform a stratified population evaluation and eliminate outlier samples (nine samples). Eventually, 75 630 SNPs were retained for correlation analysis of PD and clinical outcomes.
2.4. Follow‐up and clinical outcomes
After enrolment, all patients were followed up through telephone call or office appointment at 1, 6, 12 and 24 months; follow‐up was completed once they discontinued dabigatran. At every visit, the clinical outcomes, treatment compliance and co‐medications were required to be collected in detail with the medical records. The primary outcome was any bleeding event defined by the criterion of Bleeding Academic Research Consortium (BARC). 25 Major bleeding was determined as BARC types 3, 4 and 5 events, and BARC types 1 and 2 events were considered as minor bleeding. The secondary outcome was the incidence of thromboembolic events (TEs), including myocardial infarction, stroke or transient ischaemic attack, SE, pulmonary embolism, and all‐cause mortality, and major adverse cardiac events (MACEs) comprising cardiac death, myocardial infarction, stroke, stent thrombosis and repeated revascularization. Two independent doctors who were blinded to the findings of genotyping and PD tests assessed every event.
2.5. Statistical analyses
Genome‐wide analyses for PD parameters and clinical outcomes were performed using linear regression and logistic regression methods, respectively, in PLINK1.09, assuming an additive genetic model. 26 PCA was conducted to correct possible population stratification and exclude any outliers. 27 Covariates of PD indices were also further adjusted for sex, creatinine, catheter ablation and mean platelet volume. Logistic regression models included sex and creatinine as independent variables. These clinical variables were selected, as they were associated with PD parameters and the clinical outcomes in univariate analyses and multivariate analyses. The genome‐wide p‐value significant threshold was set at .05/75 630 = 6.61 × 10−7 using a Bonferroni correction for successful replication. In addition, candidate gene association analyses were also used to verify meaningful genes related with PD and clinical outcomes of dabigatran. From prior pharmacogenomic researches (Table S1) and reviews, 13 , 17 a total of 25 candidate genes were chosen, including ABCB1, ABCC2, ABCG2, CES1, CES1P2, CYP1A2, CYP2A6, CYP2B6, CYP2C19, CYP2C8, CYP2C9, CYP2D6, CYP2J2, CYP3A4, CYP3A5, CYP4F2, FRAS1, SLC22A1, SLC4A4, SLCO1B1, SULT1A1, UGT1A1, UGT1A9, UGT2B7 and UGT2B15. The detailed methods about genome‐wide and candidate gene association analyses were similar to those used in our GWA study on healthy participants with dabigatran. 22 Regional plots were created in LocusZoom, 28 and additional graphics were generated using R. Except otherwise specified, continuous variables were presented as mean ± standard deviation, and a two‐sided p‐value of less than .05 was regarded as statistically significant.
3. RESULTS
3.1. Patient characteristics and outcomes
Overall, 170 patients with NVAF were included in our final genetic analysis. Basic characteristics and outcomes are showed in Table 1. The median age was 72 years, and 133 (78.2%) patients were aged 65 years or above. The median eGFR level was 73.6 ml/min/1.73 m2, and 167 (98.8%) of the levels were greater than or equal to 30 ml/min/1.73 m2. The dosage regimen of dabigatran was 110 mg twice daily for all. The median and maximum scores of CHA2DS2‐VASc were 2 and 8, and 155 (91.2%) scores were greater than or equal to 2. The median and maximum scores of HAS‐BLED were 4 and 5, and 64 (37.6%) of the scores were greater than or equal to 3. The median follow‐up duration was 12 months, and 150 (88.8%) visits were in greater than or equal to 6 months. A total of 151 (88.8%) and 155 (91.2%) data points were obtained for trough and peak PD tests, respectively, and 156 points for peak anti‐FIIa activity. The trough levels of all three PD parameters were significantly lower than each peak level (all p < .001). Bleeding events during follow‐up had a higher incidence compared with TEs and MACEs. The detailed clinical outcomes from each follow‐up visit are displayed in Table S2. There were only two major bleedings reported at visit on the 12th month, which were haematuria intervened by surgery and intracranial haemorrhage. Most of the remaining minor bleedings contained gingival, subcutaneous, nasal, pharyngeal, conjunctival, GI bleeding, microscopic haematuria and haematuria without intervention. Some patients experienced more than two types of bleeding. For TEs and MACEs, the events were focused on ischaemic strokes, haemorrhagic strokes, myocardial infarction, SE and repeated revascularization. Moreover, no patients suffered more than two types of the above events. Considering the low incidence of TEs and MACEs as well as that of major bleedings, we only performed GWA analysis for primary outcomes of all cumulative bleeding events.
TABLE 1.
Basic characteristics and outcomes of patients included in genome‐wide association analysis
| Variables | Results |
|---|---|
| Baseline characteristics | |
| Total patients | 170 |
| Regimen of dabigatran | 110 mg (n = 170), twice daily |
| Female, n (%) | 67 (39.4) |
| Age (years) | 72.0 (65.8, 79.0) |
| Bodyweight (kg) a | 70.7 ± 12.51 |
| BMI (kg/m2) a | 25.4 ± 3.69 |
| CREA (μmol/L) a | 83.8 ± 19.51 |
| eGFR (ml/min/1.73 m2) a | 73.6 (58.6, 88.6) |
| ALT (IU/L) a | 19.0 (14.0, 27.4) |
| AST (IU/L) a | 20.0 (16.0, 25.0) |
| HGB (g/L) a | 136.0 (121.5, 147.0) |
| PLT (109/L) a | 195.0 (162.0, 239.0) |
| MPV (fl) a | 9.4 (8.4, 10.9) |
| EF (%) a | 63.9 (58.8, 70.6) |
| CHA2DS2‐VASc score | 2.0 (1.75, 3.0) |
| HAS‐BLED score | 4.0 (3.0, 5.0) |
| Comorbidities, n (%) | |
|---|---|
| Hypertension | 121 (71.2) |
| Diabetes mellitus | 58 (34.1) |
| Stroke or TIA | 56 (32.9) |
| Coronary heart disease | 76 (44.7) |
| Myocardial infarction | 7 (4.1) |
| Heart failure | 27 (15.9) |
| PCI | 14 (8.2) |
| Catheter ablation a | 39 (34.8) |
| Co‐medications, n (%) | |
|---|---|
| ACEIs or ARBs a | 73 (43.5) |
| Beta‐blockers a | 95 (56.9) |
| Calcium channel blockers a | 64 (38.3) |
| Statins a | 103 (61.7) |
| Antiplatelet drugs a | 19 (11.3) |
| Nitrate esters a | 14 (12.1) |
| Proton‐pump inhibitors a | 33 (19.8) |
| Pharmacodynamic parameters | ||
|---|---|---|
| Trough levels | Peak levels | |
| Patients with tests, n (%) | 151 | 155 |
| Anti‐IIa activity (ng/ml) | 68.16 ± 60.34 | 150.62 ± 128.00 b |
| APTT (s) | 39.55 ± 9.90 | 48.29 ± 13.73 |
| PT (s) | 13.72 ± 6.11 | 14.09 ± 3.62 |
| Events, n (%) | |||
|---|---|---|---|
| Bleeding | TE | MACE | |
| 0–1 month (n = 169) | 7 (4.1) | 2 (1.2) | 0 (0.0) |
| 2–6 months (n = 149) | 11 (7.4) | 2 (1.3) | 2 (1.3) |
| 7–12 months (n = 122) | 12 (9.8) | 3 (2.5) | 4 (3.3) |
| 13–24 months (n = 51) | 3 (5.9) | 2 (3.9) | 2 (3.9) |
Note: For the continuous variables in baseline characteristics, data in normal distribution are shown as ‘mean ± SD’, and in skewed distribution are shown as ‘median (25, 75 percentiles)’.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; ARB, angiotensin receptor blockers; AST, aspartate aminotransferase; BMI, body mass index; CREA, creatinine; EF, ejection fraction; eGFR, estimate glomerular filtrate rate; HGB, haemoglobin; MACE, major adverse cardiac event; MPV, mean platelet volume; PCI, percutaneous coronary intervention; PLT, platelet; PT, prothrombin time; TE, thromboembolic event; TIA, transient ischaemic attack.
The number of the variable was the number of observed values, excluding missing values.
The number of peak anti‐IIa activity tests was 156.
3.2. Effects of suggestive genetic variations on bleeding events of dabigatran
In the light of modest sample size and incidence rates of this exploratory genetic study, the genome‐wide p‐value significant threshold was adjusted to 1.0 × 10−4 for screening suggestive genetic variations on cumulative bleeding events at each follow‐up. Eventually, two SNPs were related to bleeding events, including UBASH3B rs2276408 (odds ratio [OR] = 8.79, 95% confidence interval [CI]: 2.99–25.83, p = 7.77 × 10−5 at the 6th month visit) and FBN2 rs3805625 (OR = 8.29, 95% CI: 2.87–23.89, p = 9.08 × 10−5 at the 12th month visit). The detailed information of these genetic variations on bleeding is showed in Table 2. Besides, Figure 1 presents regional association plots within 500 kilobases, and Figure 2 displays Manhattan plots as well as quantile–quantile (Q–Q) plots. Except for the above‐selected visit, the minor allele carriers of both of these two SNPs significantly increased the risk of bleeding events at visits on 6, 12 and 24 months (p < .05). At their first month visit, the minor allele carriers of UBASH3B rs2276408 also had an increased risk of bleeding (p < .05). We also performed analysis of these genetic variations on peak and trough PD (Table S3). It was only UBASH3B rs2276408 that is significantly associated with peak anti‐FIIa activity (p = .024). The minor allele carriers showed a significantly higher peak anti‐FIIa level, and this same trend was also present in peak APTT, peak PT and trough PT with no significant differences.
TABLE 2.
Effects of suggestive genetic variations on bleeding events of patients treated with dabigatran
| Gene | UBASH3B | FBN2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | rs2276408 | rs3805625 | ||||||||||
| CHR | 11 | 5 | ||||||||||
| Func | Intronic | Intronic | ||||||||||
| Bleeding events, n (%) | GENO | A1A1 | A1A2 | A2A2 | OR (95% CI) | p‐Value | GENO | A1A1 | A1A2 | A2A2 | OR (95% CI) | p‐Value |
| 1 month | 2/30/137 | 1(50.0) | 3(10.0) | 3(2.2) | 5.66 (1.61–19.93) | 6.9 × 10−3 | 3/30/136 | 0(0.0) | 3(10.0) | 4(3.0) | 2.17 (0.60–7.85) | .239 |
| 6 months | 1/29/119 | 1(100.0) | 9(31.0) | 6(5.0) | 8.79 (2.99–25.83) | 7.77 × 10−5 | 2/26/121 | 1(50.0) | 6(23.1) | 9(7.4) | 3.80 (1.43–10.11) | 7.55 × 10−3 |
| 12 months | 1/28/93 | 1(100.0) | 10(35.7) | 13(14.0) | 4.10 (1.62–10.39) | 2.90 × 10−3 | 2/23/97 | 2(100.0) | 10(43.5) | 12(12.4) | 8.29 (2.87–23.89) | 9.08 × 10−5 |
| 24 months | 1/15/47 | 1(100.0) | 10(66.7) | 15(31.9) | 4.34 (1.29–14.57) | .017 | 2/16/45 | 2(100.0) | 11(68.8) | 13(28.9) | 9.31 (2.30–37.59) | 1.74 × 10−3 |
Note: UBASH3B SNP rs2276408: A1 = T, A2 = C; FBN2 SNP rs3805625: A1 = T, A2 = G.
Abbreviations: A1, minor allele; A2, non‐minor allele; CHR, chromosome; CI, confidence interval; FBN2, fibrillin 2; Func, the region of the genome where the mutation is; GENO, number of each genotype (A1A1/A1A2/A2A2); OR, odds ratio; SNP, single‐nucleotide polymorphism; UBASH3B, ubiquitin‐associated and SH3 domain containing B.
FIGURE 1.
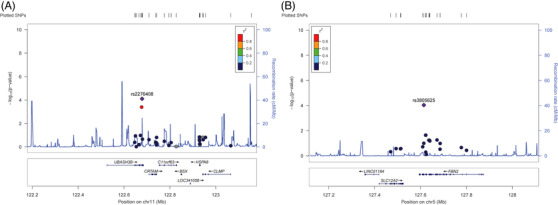
Regional association plots of suggestive single‐nucleotide polymorphisms (SNPs) on bleeding events of patients treated with dabigatran (A) UBASH3B SNP rs2276408 (B) FBN2 SNP rs3805625. Annotation: SNPs are presented as per their physical location and –log10 p‐values for association. The recombination rate is also shown in centimorgans per megabase (blue line) and the linkage disequilibrium (r 2) of each SNP with the SNP having the lowest p‐value.
FIGURE 2.
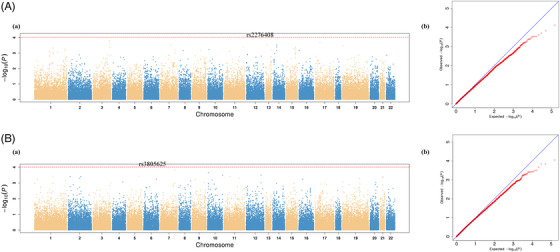
Manhattan and quantile–quantile plots of the association with bleeding events of patients treated with dabigatran. (A) UBASH3B SNP rs2276408. (B) FBN2 SNP rs3805625. Annotation: (a) Manhattan plot; (b) Quantile–quantile plot
3.3. Effects of suggestive genetic variations on the pharmacodynamics of dabigatran
Considering the sample size and measurement points of our experiment, the genome‐wide p‐value significant threshold was adjusted to 1.0 × 10−5 for screening suggestive genetic variations on PD. As a result, 17 suggestive SNPs of 14 genes met the inclusion criteria. The related effects of genetic variations on PD are presented in Table 3 (Manhattan plots and Q–Q plots are shown in Figures S1 and S2). For anti‐FIIa activity, there were four suggestive SNPs, including SNX7 rs9433747 (p = 1.70 × 10−7), BRD4 rs11669901 (p = 2.90 × 10−6), FLCN rs3744124 (p = 4.77 × 10−6) associated with peak level and UBAP1 rs1556439 (p = 6.69 × 10−6) associated with trough level. The minor allele carriers of all these SNPs had significantly higher PD levels. For APTT, IGLV3‐12 rs2073451 (p = 8.50 × 10−6) was associated with peak level, and LRRC8E rs3745382 (p = 8.41 × 10−6) and PTPLAD1 rs11539008 (p = 9.69 × 10−6) were associated with trough level. The minor allele of IGLV3‐12 rs2073451 was significantly associated with a lower value, whereas the minor alleles of LRRC8E rs3745382 and PTPLAD1 rs11539008 were both associated with the higher values. For PT, 10 SNPs were successfully screened, including one for peak level and nine for trough levels. Peak levels for the minor allele of ZNF230 rs12753 were significantly higher (p = 1.97 × 10−6). The minor alleles of the remaining nine SNPs were significantly associated with higher trough values, containing ANP32A rs12904108 (p = 7.13 × 10−7), SLC25A28 rs12252561 (p = 7.81 × 10−7), ABCC2 rs2273697 and rs4148395 (both p = 3.10 × 10−6), MYBPC1 rs11110942, rs3751246 and rs11110952 (all p = 4.46 × 10−6), CEP170B rs60001925 (p = 5.53 × 10−6) and GYPA rs145195209 (p = 9.24 × 10−6). Among the seven SNPs selected for anti‐FIIa activity and APTT, all had significant influences on more than one of PD indices (p < .05). For ZNF230 rs12753 associated with peak PT, it was significantly associated with peak anti‐FIIa activity and APTT (p < 1 × 10−3). To evaluate the effect of these suggestive SNPs on bleeding events, GWA analyses were performed (Table S4), and only the minor allele carriers of SNX7 rs9433747 had a significantly increased bleeding risk at the 24th month visit (OR = 4.76, 95% CI: 1.20–18.95, p = .027) with the same trends at visits on 1, 6 and 12 months.
TABLE 3.
Effects of suggestive genetic variations on the pharmacodynamics of dabigatran
| Gene | SNP | Genotypes a | Peak PD levels | Trough PD levels | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GENO b | FIIa (ng/ml) | p‐Value | FAPTT (s) | p‐Value | FPT (s) | p‐Value | GENO | GIIa (ng/ml) | p‐Value | GAPTT (s) | p‐Value | GPT (s) | p‐Value | |||
| SNX7 | rs9433747 | A1A1 | 0/28/127 | / | 1.70 × 10−7 | / | 1.88 × 10−3 | / | 6.41 × 10−5 | 0/27/124 | / | 0.025 | / | .582 | / | .828 |
| A1A2 | 228.26 ± 161.58 | 54.12 ± 13.62 | 15.14 ± 2.48 | 90.39 ± 70.74 | 40.50 ± 8.62 | 12.80 ± 1.30 | ||||||||||
| A2A2 | 133.62 ± 111.91 | 47.00 ± 13.37 | 13.86 ± 3.76 | 63.31 ± 56.42 | 39.35 ± 10.11 | 13.92 ± 6.67 | ||||||||||
| BRD4 | rs11669901 | A1A1 | 1/18/136 | 287.67 | 2.90 × 10−6 | 51.8 | .096 | 13.60 | .172 | 1/17/133 | 131.07 | 0.015 | 35.0 | .665 | 11.10 | .660 |
| A1A2 | 219.50 ± 172.25 | 52.03 ± 10.67 | 14.36 ± 2.25 | 98.71 ± 87.31 | 41.55 ± 8.01 | 12.83 ± 1.57 | ||||||||||
| A2A2 | 140.56 ± 117.38 | 47.77 ± 14.01 | 14.06 ± 3.75 | 63.78 ± 54.43 | 39.33 ± 10.09 | 13.86 ± 6.45 | ||||||||||
| FLCN | rs3744124 | A1A1 | 6/54/95 | 288.71 ± 193.56 | 4.77 × 10−6 | 65.37 ± 21.22 | 6.05 × 10−3 | 21.15 ± 12.97 | .169 | 6/51/94 | 135.92 ± 104.60 | 6.40E‐03 | 52.30 ± 13.05 | .064 | 17.73 ± 9.76 | .729 |
| A1A2 | 150.23 ± 135.44 | 47.15 ± 13.86 | 13.81 ± 2.43 | 67.13 ± 60.37 | 37.45 ± 9.39 | 13.42 ± 5.71 | ||||||||||
| A2A2 | 142.19 ± 111.94 | 47.86 ± 12.20 | 13.80 ± 1.97 | 64.39 ± 53.15 | 39.88 ± 9.22 | 13.63 ± 5.89 | ||||||||||
| UBAP1 | rs1556439 | A1A1 | 1/15/139 | 455.10 | .117 | 66.30 | .054 | 16.70 | .063 | 1/14/136 | 263.48 | 6.69E‐06 | 45.80 | .011 | 13.00 | .083 |
| A1A2 | 182.90 ± 109.48 | 53.83 ± 14.08 | 14.79 ± 2.06 | 117.20 ± 88.94 | 46.56 ± 11.97 | 17.54 ± 11.03 | ||||||||||
| A2A2 | 144.97 ± 126.69 | 47.56 ± 13.47 | 13.99 ± 3.73 | 61.67 ± 51.29 | 38.79 ± 9.35 | 13.33 ± 5.19 | ||||||||||
| IGLV3‐12 | rs2073451 | A1A1 | 30/74/51 | 120.01 ± 94.94 | .021 | 43.33 ± 11.40 | 8.50 × 10−6 | 13.42 ± 1.91 | 1.61 × 10−4 | 29/75/47 | 63.12 ± 47.87 | 0.638 | 37.56 ± 8.39 | .120 | 12.78 ± 1.82 | .904 |
| A1A2 | 151.86 ± 134.17 | 47.54 ± 13.55 | 14.10 ± 4.64 | 68.02 ± 65.70 | 39.37 ± 10.16 | 13.54 ± 5.30 | ||||||||||
| A2A2 | 166.76 ± 131.18 | 52.29 ± 13.98 | 14.47 ± 2.37 | 71.48 ± 57.36 | 41.06 ± 10.00 | 14.60 ± 8.42 | ||||||||||
| LRRC8E | rs3745382 | A1A1 | 4/29/122 | 213.08 ± 107.13 | 9.22 × 10−3 | 50.10 ± 14.00 | .067 | 13.70 ± 2.05 | .282 | 3/28/120 | 184.95 ± 105.36 | 1.74E‐03 | 52.07 ± 19.68 | 8.41 × 10−6 | 16.90 ± 5.43 | .143 |
| A1A2 | 172.76 ± 143.83 | 54.62 ± 15.02 | 15.33 ± 6.85 | 78.56 ± 69.50 | 45.13 ± 10.62 | 15.17 ± 8.64 | ||||||||||
| A2A2 | 143.35 ± 122.89 | 46.72 ± 12.88 | 13.80 ± 2.17 | 62.81 ± 52.45 | 37.94 ± 8.53 | 13.30 ± 5.25 | ||||||||||
| PTPLAD1 | rs11539008 | A1A1 | 0/22/133 | / | .209 | / | 3.09 × 10−3 | / | .012 | 0/21/130 | / | 1.41E‐03 | / | 9.69 × 10−6 | / | .024 |
| A1A2 | 162.47 ± 135.75 | 54.38 ± 16.79 | 14.80 ± 2.81 | 105.75 ± 92.08 | 47.90 ± 38.20 | 17.63 ± 11.98 | ||||||||||
| A2A2 | 148.56 ± 126.02 | 47.28 ± 12.83 | 13.97 ± 3.70 | 62.08 ± 50.65 | 10.65 ± 9.04 | 13.09 ± 4.12 | ||||||||||
| ZNF230 | rs12753 | A1A1 | 11/52/92 | 144.02 ± 176.03 | 1.86 × 10−4 | 48.35 ± 19.92 | 2.99 × 10−4 | 14.84 ± 3.28 | 1.97 × 10−6 | 10/49/92 | 50.26 ± 65.69 | 0.414 | 37.10 ± 9.68 | .820 | 12.75 ± 0.97 | .760 |
| A1A2 | 156.90 ± 126.10 | 50.95 ± 13.29 | 14.23 ± 2.12 | 82.24 ± 73.63 | 40.16 ± 9.34 | 14.38 ± 8.21 | ||||||||||
| A2A2 | 147.77 ± 121.26 | 46.78 ± 12.73 | 13.92 ± 4.23 | 62.60 ± 49.07 | 39.50 ± 10.12 | 13.48 ± 4.95 | ||||||||||
| ANP32A | rs12904108 | A1A1 | 5/41/109 | 103.61 ± 108.62 | .841 | 47.04 ± 12.89 | .391 | 13.20 ± 1.58 | .277 | 5/40/106 | 56.31 ± 27.70 | 0.477 | 42.04 ± 9.95 | .143 | 26.82 ± 18.10 | 7.13 × 10−7 |
| A1A2 | 162.36 ± 137.36 | 49.73 ± 10.94 | 14.25 ± 2.23 | 77.40 ± 65.59 | 41.22 ± 8.57 | 14.15 ± 6.91 | ||||||||||
| A2A2 | 148.36 ± 123.95 | 47.80 ± 14.59 | 14.07 ± 4.05 | 65.23 ± 58.69 | 38.81 ± 10.23 | 12.94 ± 3.25 | ||||||||||
| SLC25A28 | rs12252561 | A1A1 | 9/44/102 | 173.09 ± 85.29 | .796 | 54.33 ± 10.10 | .291 | 14.34 ± 1.47 | .219 | 8/43/100 | 105.80 ± 71.81 | 0.226 | 47.69 ± 10.39 | .0499 | 23.50 ± 15.12 | 7.81 × 10−7 |
| A1A2 | 129.74 ± 101.74 | 48.70 ± 10.35 | 13.91 ± 1.75 | 69.65 ± 73.56 | 40.56 ± 8.22 | 13.92 ± 6.59 | ||||||||||
| A2A2 | 157.56 ± 139.00 | 47.57 ± 15.03 | 14.14 ± 4.26 | 64.50 ± 50.93 | 38.47 ± 10.13 | 12.85 ± 3.26 | ||||||||||
| ABCC2 | rs2273697 | A1A1 | 3/21/131 | 209.74 ± 74.86 | .598 | 51.83 ± 11.05 | .441 | 14.20 ± 1.36 | .426 | 2/21/128 | 98.32 ± 10.57 | 0.052 | 44.70 ± 6.60 | .286 | 32.10 ± 19.20 | 3.10 × 10−6 |
| A1A2 | 161.40 ± 104.82 | 49.48 ± 9.88 | 13.84 ± 1.87 | 95.65 ± 96.79 | 42.03 ± 11.47 | 17.13 ± 11.46 | ||||||||||
| A2A2 | 147.55 ± 131.37 | 48.01 ± 14.24 | 14.13 ± 3.84 | 63.17± 50.64 | 39.07 ± 9.54 | 12.88 ± 2.92 | ||||||||||
| ABCC2 | rs4148395 | A1A1 | 3/21/131 | 209.74 ± 74.86 | .598 | 51.83 ± 11.05 | .441 | 14.20 ± 1.36 | .426 | 2/21/128 | 98.32 ± 10.57 | 0.052 | 44.70 ± 6.60 | .286 | 32.10 ± 19.20 | 3.10 × 10−6 |
| A1A2 | 161.40 ± 104.82 | 49.48 ± 9.88 | 13.84 ± 1.87 | 95.65 ± 96.79 | 42.03 ± 11.47 | 17.13 ± 11.46 | ||||||||||
| A2A2 | 147.55 ± 131.37 | 48.01 ± 14.24 | 14.13 ± 3.84 | 63.17 ± 50.64 | 39.07 ± 9.54 | 12.88 ± 2.92 | ||||||||||
| MYBPC1 | rs11110942 | A1A1 | 7/61/87 | 112.94 ± 83.09 | .739 | 50.53 ± 6.80 | .708 | 13.77 ± 1.03 | .776 | 7/59/85 | 67.07 ± 49.26 | 0.239 | 44.83 ± 9.39 | .292 | 24.00 ± 17.42 | 4.46 × 10−6 |
| A1A2 | 146.66 ± 107.43 | 47.03 ± 10.69 | 13.75 ± 1.90 | 75.23 ± 72.71 | 39.38 ± 9.01 | 13.85 ± 5.41 | ||||||||||
| A2A2 | 156.34 ± 141.97 | 48.99 ± 15.75 | 14.35 ± 4.51 | 63.34 ± 49.96 | 39.24 ± 10.35 | 12.79 ± 3.31 | ||||||||||
| MYBPC1 | rs3751246 | A1A1 | 7/62/86 | 112.94 ± 83.09 | .739 | 50.53 ± 6.80 | .708 | 13.77 ± 1.03 | .776 | 7/60/84 | 67.07 ± 49.26 | 0.270 | 44.83 ± 9.39 | .240 | 24.00 ± 17.42 | 4.46 × 10−6 |
| A1A2 | 147.79 ± 106.92 | 47.22 ± 10.71 | 13.79 ± 1.90 | 75.13 ± 72.11 | 39.59 ± 9.08 | 13.87 ± 5.37 | ||||||||||
| A2A2 | 155.65 ± 142.63 | 48.87 ± 15.80 | 14.33 ± 4.53 | 63.26 ± 50.26 | 39.08 ± 10.31 | 12.76 ± 3.32 | ||||||||||
| MYBPC1 | rs11110952 | A1A1 | 7/63/85 | 112.94 ± 83.09 | .739 | 50.53 ± 6.80 | .708 | 13.77 ± 1.03 | .776 | 7/60/84 | 67.07 ± 49.26 | 0.270 | 44.83 ± 9.39 | .240 | 24.00 ± 17.42 | 4.46 × 10−6 |
| A1A2 | 147.07 ± 106.22 | 47.35 ± 10.68 | 13.81 ± 1.89 | 75.13 ± 72.11 | 39.59 ± 9.08 | 13.87 ± 5.37 | ||||||||||
| A2A2 | 156.26 ± 143.35 | 48.79 ± 15.88 | 14.32 ± 4.56 | 63.26 ± 50.26 | 39.08 ± 10.31 | 12.76 ± 3.32 | ||||||||||
| CEP170B | rs60001925 | A1A1 | 3/34/118 | 109.85 ± 14.90 | .852 | 58.70 ± 5.77 | .443 | 14.43 ± 1.81 | .400 | 3/30/118 | 32.15 ± 21.05 | 0.481 | 44.93 ± 2.95 | .113 | 24.67 ± 15.45 | 5.53 × 10−6 |
| A1A2 | 147.58 ± 132.20 | 49.34 ± 12.49 | 14.24 ± 2.09 | 71.14 ± 55.42 | 41.79 ± 10.38 | 15.79 ± 10.33 | ||||||||||
| A2A2 | 152.50 ± 127.65 | 47.72 ± 14.03 | 14.04 ± 3.96 | 68.31 ± 61.65 | 38.85 ± 9.72 | 12.92 ± 3.08 | ||||||||||
| GYPA | rs145195209 | A1A1 | 2/21/132 | 110.87 ± 8.19 | .280 | 71.35 ± 17.55 | .604 | 15.70 ± 3.30 | .818 | 1/22/128 | 60.08 | 0.601 | 46.50 | .920 | 46.50 | 9.24 × 10−6 |
| A1A2 | 148.31 ± 163.25 | 44.61 ± 11.77 | 13.60 ± 1.94 | 63.45 ± 75.96 | 39.28 ± 9.41 | 14.66 ± 8.22 | ||||||||||
| A2A2 | 151.59 ± 121.54 | 48.52 ± 13.53 | 14.14 ± 3.79 | 69.03 ± 57.18 | 39.54 ± 9.97 | 13.30 ± 4.84 | ||||||||||
Abbreviations: A1, minor allele; A2, non‐minor allele; GENO, number of each genotype (A1A1/A1A2/A2A2); SNP, single‐nucleotide polymorphism.
SNX7 SNP rs9433747: A1 = G, A2 = A; BRD4 SNP rs11669901: A1 = A, A2 = G; FLCN SNP rs3744124: A1 = T, A2 = C; UBAP1 SNP rs1556439: A1 = T, A2 = C; IGLV3‐12 SNP rs2073451: A1 = G, A2 = A; LRRC8E SNP rs3745382: A1 = A, A2 = G; PTPLAD1 SNP rs11539008: A1 = A, A2 = G; ZNF230 SNP rs12753: A1 = A, A2 = C; ANP32A SNP rs12904108: A1 = T, A2 = A; SLC25A28 SNP rs12252561: A1 = C, A2 = G; ABCC2 SNP rs2273697: A1 = A, A2 = G; ABCC2 SNP rs4148395: A1 = A, A2 = G; MYBPC1 SNP rs11110942: A1 = G, A2 = C; MYBPC1 SNP rs3751246: A1 = T, A2 = C; MYBPC1 SNP rs11110952: A1 = C, A2 = T; CEP170B SNP rs60001925: A1 = C, A2 = CGCAGGA; GYPA SNP rs145195209: A1 = A, A2 = AT.
As the number of peak anti‐FIIa activity was 156 and the number of peak APTT and PT was 155, the genotype of extra one that had only peak anti‐FIIa activity was: SNX7 SNP rs9433747: A2A2; BRD4 SNP rs11669901: A2A2; FLCN SNP rs3744124: A2A2; UBAP1 SNP rs1556439: A2A2; IGLV3‐12 SNP rs2073451: A1A2; LRRC8E SNP rs3745382: A2A2; PTPLAD1 SNP rs11539008: A1A2; ZNF230 SNP rs12753: A1A2; ANP32A SNP rs12904108: A2A2; SLC25A28 SNP rs12252561: A2A2; ABCC2 SNP rs2273697: A2A2; ABCC2 SNP rs4148395: A2A2; MYBPC1 SNP rs11110942: A2A2; MYBPC1 SNP rs3751246: A2A2; MYBPC1 SNP rs11110952: A2A2; CEP170B SNP rs60001925: A2A2; GYPA SNP rs145195209: A1A2.
3.4. Candidate gene association analysis
Except for the three candidate genes not detected (CES1P2, CYP3A4 and UGT2B15), correlation analyses were performed for the remaining 22 reported genes and 148 detected SNPs, and we identified a total of 14 candidate genes and 33 new SNPs. The impacts of reported genes on bleeding and PD of dabigatran are summarized in Table 4 and Table S5. A total of six genes and 10 SNPs showed an effect on bleeding, including ABCG2 rs2231165, CYP2A6 rs8192720 and rs8192726, CYP2B6 rs3745276 and rs3745277, CYP2J2 rs3738474, FRAS1 rs17003071, SLCO1B1 rs2291076, rs2306283 and rs4149032 (p < .05). Among these SNPs, ABCG2 rs2231165, CYP2A6 rs8192720 and SLCO1B1 rs4149032 were associated with bleeding at more than one visit. A total of 11 genes and 25 SNPs had the positive effects on PD (p < .05), except three genes (CYP2A6, CYP2J2 and SLCO1B1). There were two genes and six SNPs (ABCG2 rs2231142, rs2231148 and rs2231156, CES1 rs112236246, rs2244614 and rs3217164) associated with more than one PD parameter. Only one SNP (ABCG2 rs2231156) was identified for the association with both bleeding and PD. For reported CES1 rs2244613 associated with bleeding, no association was found in our study. Further, these reported SNPs associated with PD were also found to be negative in our study, including ABCG2 rs2231138, CYP2B6 rs2279342, CYP2C19 rs12769205, rs3758580 and rs4244285, CYP3A5 rs15524 and rs4646453, FRAS1 rs6835769, SLC4A4 rs138389345, SLCO1B1 rs11045748, SULT1A1 rs9282862 and UGT1A9 rs12466997.
TABLE 4.
Positive effects of candidate genes on bleeding and the pharmacodynamics of dabigatran
| SNP | Gene | Bleeding 1 month | Bleeding 6 months | Bleeding 12 months | Bleeding 24 months | FIIa (ng/ml) | FAPTT (s) | FPT (s) | GIIa (ng/ml) | GAPTT (s) | GPT (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| p‐Values | |||||||||||
| rs2235013a | ABCB1 | 0.102 | 0.572 | 0.396 | 0.112 | 0.021 | 0.087 | 0.094 | 0.496 | 0.430 | 0.786 |
| rs2235033a | ABCB1 | 0.102 | 0.572 | 0.396 | 0.112 | 0.021 | 0.087 | 0.094 | 0.496 | 0.430 | 0.786 |
| rs2273697 a | ABCC2 | / | 0.249 | 0.701 | 0.990 | 0.598 | 0.441 | 0.426 | 0.052 | 0.286 | 3.10E‐06 |
| rs4148395 a | ABCC2 | / | 0.249 | 0.701 | 0.990 | 0.598 | 0.441 | 0.426 | 0.052 | 0.286 | 3.10E‐06 |
| rs2231142 b | ABCG2 | 0.352 | 0.691 | 0.273 | 0.227 | 0.111 | 0.017 | 0.016 | 7.21E‐03 | 1.79E‐03 | 0.705 |
| rs2231148 a | ABCG2 | 0.683 | 0.568 | 0.892 | 0.960 | 0.201 | 0.083 | 0.032 | 0.028 | 0.811 | 0.129 |
| rs2231156 a | ABCG2 | 0.165 | 0.823 | 0.782 | 0.457 | 0.222 | 0.030 | 0.118 | 5.96E‐04 | 3.91E‐03 | 0.559 |
| rs2231165 a | ABCG2 | 0.242 | 7.07E‐03 | 0.083 | 0.049 | 0.921 | 0.219 | 0.823 | 0.605 | 0.021 | 0.457 |
| rs112236246 a | CES1 | 0.758 | 0.902 | 0.427 | 0.454 | 0.322 | 0.283 | 0.783 | 0.150 | 0.028 | 0.027 |
| rs2244614 a | CES1 | 0.763 | 0.910 | 0.441 | 0.348 | 0.342 | 0.459 | 0.925 | 0.157 | 0.040 | 0.025 |
| rs3217164 a | CES1 | 0.763 | 0.910 | 0.441 | 0.348 | 0.342 | 0.459 | 0.925 | 0.157 | 0.040 | 0.025 |
| rs4646427 a | CYP1A2 | 0.259 | 0.084 | 0.435 | 0.449 | 0.161 | 0.097 | 0.037 | 0.517 | 0.625 | 0.558 |
| rs8192720 a | CYP2A6 | 0.222 | 0.081 | 0.017 | 0.030 | 0.621 | 0.905 | 0.788 | 0.697 | 0.958 | 0.340 |
| rs8192726 a | CYP2A6 | 0.441 | 0.797 | 0.136 | 0.018 | 0.293 | 0.584 | 0.944 | 0.457 | 0.142 | 0.605 |
| rs8192719 a | CYP2B6 | 0.667 | 0.991 | 0.632 | 0.944 | 0.286 | 0.931 | 0.708 | 0.023 | 0.419 | 0.654 |
| rs3745275 a | CYP2B6, CYP2A13 | 0.690 | 0.419 | 0.183 | 0.210 | 0.014 | 0.602 | 0.274 | 0.251 | 0.156 | 0.904 |
| rs3745276 a | CYP2B6, CYP2A13 | 0.494 | 0.172 | 0.028 | 0.068 | 0.643 | 0.850 | 0.840 | 0.795 | 0.306 | 0.647 |
| rs3745277 a | CYP2B6, CYP2A13 | 0.041 | 0.141 | 0.159 | 0.984 | 0.459 | 0.048 | 0.119 | 0.696 | 0.738 | 0.832 |
| rs7249735 a | CYP2B6, CYP2A13 | 0.835 | 0.378 | 0.246 | 0.122 | 0.011 | 0.638 | 0.240 | 0.124 | 0.270 | 0.945 |
| rs1058932 a | CYP2C8 | 0.580 | 0.235 | 0.126 | 0.099 | 0.515 | 0.832 | 0.603 | 0.148 | 0.923 | 0.011 |
| rs11572078 a | CYP2C8 | 0.580 | 0.235 | 0.126 | 0.099 | 0.515 | 0.832 | 0.603 | 0.148 | 0.923 | 0.011 |
| rs2275622 a | CYP2C8 | 0.983 | 0.628 | 0.241 | 0.208 | 0.339 | 0.882 | 0.875 | 0.259 | 0.997 | 0.016 |
| rs17847029 | CYP2C9 | 0.141 | 0.813 | 0.701 | 0.453 | 0.043 | 0.083 | 0.088 | 0.931 | 0.882 | 0.860 |
| rs3738474 | CYP2J2 | 0.035 | 0.111 | 0.234 | 0.538 | 0.940 | 0.809 | 0.718 | 0.854 | 0.648 | 0.737 |
| rs11937525 | FRAS1 | 0.184 | 0.334 | 0.819 | 0.855 | 0.289 | 0.259 | 0.120 | 0.182 | 0.398 | 0.023 |
| rs17003071 | FRAS1 | 0.945 | 0.704 | 0.0492 | 0.199 | 0.566 | 0.893 | 0.897 | 0.713 | 0.465 | 0.298 |
| rs17003160 | FRAS1 | 0.261 | 0.753 | 0.759 | 0.680 | 0.633 | 0.553 | 0.538 | 0.678 | 0.935 | 0.028 |
| rs398092530 | FRAS1 | 0.242 | 0.858 | 0.619 | 0.552 | 0.569 | 0.532 | 0.381 | 0.923 | 0.919 | 0.039 |
| rs1062677 | SLC4A4 | / | 0.713 | 0.456 | 0.735 | 0.441 | 0.715 | 0.764 | 0.022 | 0.200 | 0.765 |
| rs2291076 a | SLCO1B1 | 0.121 | 0.166 | 0.193 | 0.043 | 0.402 | 0.523 | 0.897 | 0.112 | 0.589 | 0.617 |
| rs2306283 a | SLCO1B1 | 0.121 | 0.154 | 0.164 | 0.043 | 0.686 | 0.826 | 0.854 | 0.129 | 0.634 | 0.685 |
| rs4149032 a | SLCO1B1 | 0.471 | 0.217 | 0.047 | 0.022 | 0.924 | 0.660 | 0.899 | 0.138 | 0.274 | 0.085 |
| rs79527462 | SULT1A1 | 0.599 | 0.999 | 0.527 | 0.176 | 0.083 | 0.471 | 0.053 | 0.013 | 0.179 | 0.630 |
Abbreviations: ABCB1, ATP‐binding cassette subfamily B member 1; ABCC2, ATP‐binding cassette subfamily C member 2; ABCG2, ATP binding cassette subfamily G member 2; CES1, carboxylesterase 1; CYP1A2, cytochrome P450 family 1 subfamily A member 2; CYP2A6, cytochrome P450 family 2 subfamily A member 6; CYP2B6, cytochrome P450 family 2 subfamily B member 6; CYP2A13, cytochrome P450 family 2 subfamily A member 13; CYP2AJ2, cytochrome P450 family 2 subfamily J member 2; CYP2C8, cytochrome P450 family 2 subfamily C member 8; CYP2C9, cytochrome P450 family 2 subfamily C member 9; SLC4A4, solute carrier family 4 member 4; SLCO1B1, solute carrier organic anion transporter family member 1B1; SULT1A1, sulfotransferase family 1A member 1.
The SNP was detected and analyzed for variations associated with the pharmacodynamics in reported pharmacogenomic studies of dabigatran.
The SNP was detected and analyzed for variations associated with bleeding in reported pharmacogenomic studies of dabigatran.
4. DISCUSSION
We performed a nationwide multicentre prospective cohort study and genome‐wide pharmacogenetic analysis to identify the genetic variations on bleeding and PD of dabigatran in Chinese patients with NVAF. As far as we are aware, this is the first GWA study focusing on Chinese NVAF populations with dabigatran. Using whole‐exome sequencing and correlation analysis, we first screened two suggestive SNPs (UBASH3B rs2276408 and FBN2 rs3805625) associated with bleeding, which revealed that the minor allele carriers had higher bleeding risks at the sixth and 12th month visits, respectively. Moreover, the same significant trends for these SNPs were also presented at other visits. Second, there were four (SNX7 rs9433747, BRD4 rs11669901, FLCN rs3744124 and UBAP1 rs1556439), three (IGLV3‐12 rs2073451, LRRC8E rs3745382 and PTPLAD1 rs11539008) and 10 (ZNF230 rs12753, ANP32A rs12904108, SLC25A28 rs12252561, ABCC2 rs2273697 and rs4148395, MYBPC1 rs11110942, rs3751246 and rs11110952, CEP170B rs60001925 and GYPA rs145195209) SNPs that successfully exceeded the threshold to affect anti‐FIIa activity, APTT, and PT, respectively. Apart from the fact that the minor allele carriers of IGLV3‐12 rs2073451 show a lower PD value, the minor allele carriers of remaining 16 SNPs had higher PD levels. Lastly, we discovered 33 new relevant SNPs of 14 reported genes linked to bleeding and PD. A total of six genes (ABCG2, CYP2A6, CYP2B6, CYP2J2, FRAS1 and SLCO1B1) affected bleeding and 11 genes (ABCB1, ABCC2, ABCG2, CES1, CYP1A2, CYP2B6, CYP2C8, CYP2C9, FRAS1, SLC4A4 and SULT1A1) influenced PD. For the reported positive SNPs associated with bleeding or PD parameters, we found the opposite results in our study. As we collected as many indicators as possible and prolonged follow‐up time, our results deserved to be understandable. Our integrated studies, including previous similar study on healthy participants, 22 have comprehensively explored genetic variations associated with PK, PD and clinical outcomes in the Chinese populations treated with dabigatran.
Considering high inter‐ and intraindividual CVs of dabigatran as well as the greater risk of stroke/SE events, major bleeding and GI bleeding, 29 many studies have focused on exploring biomarkers on treatment. The prior trial showed that dabigatran's higher trough concentrations were linked to increased bleeding and decreased thromboembolic events. 10 The predicted peak and trough plasma levels of dabigatran in individuals with AF were 52–383 and 28–215 ng/ml, respectively, according to a European guideline. 30 However, PK measurement methods like high‐performance liquid chromatography or mass spectrometry took a long time, and the new method of chromogenic assay was not available in all areas. Therefore, PD indices determined qualitatively and quantitatively might be more conveniently accessible. Traditional coagulation indicators, including PT and APTT, were generally prolonged in a concentration‐dependent pattern for dabigatran patients. 30 , 31 , 32 , 33 Anti‐FIIa activity, as a specific test determined by chromogenic assay, has been recommended, and it demonstrated that peak anti‐FIIa activity could be an indicator for predicting bleeding outcomes of dabigatran. 32 , 33 Consequently, our study focused on genetic variation on the direct outcomes of bleeding and indirect outcomes of PD, including anti‐FIIa activity, APTT and PT.
The UBASH3B gene (ubiquitin‐associated and SH3 domain containing B, also known as TULA‐2, TULA2) is a protein‐coding gene and located in chromosome 11q24.1. The rs2276408 is intron variant of UBASH3B, which has a C base that becomes a T base (C>T). From the 1000 Genomes Project Phase 3 populations data, MAF (T) is 21% in all the populations, including 32% in American and 10% in East Asian. From the HPA RNA‐seq normal tissue data, UBASH3B gene mainly expresses in human spleen and placenta, as well as a small amount of distribution in kidney and liver. 34 These proteins encoded by this gene have the functions of preventing platelet‐derived growth factor receptor and epidermal growth factor receptor from being endocytosed. Previous studies have demonstrated that heparin‐induced thrombocytopenia and adverse drug reaction pathways were associated with UBASH3B gene. 35 , 36 The sole member of the T‐cell ubiquitin ligand (TULA) family among the protein tyrosine phosphatases found in the platelets of both mice and humans is TULA‐2. 37 This gene encodes this protein. As the platelet Fcγ‐receptor (FcγRIIa) is a causative factor of thrombin generation and thrombotic complications, 38 TULA‐2 may interfere with the platelet FcγRIIa cascade by dephosphorylating Syk. An in vivo study in mice revealed that lower levels of TULA‐2 enhanced platelet reactivity, worsened thrombocytopenia and thrombosis and reduced the time it took for tails to bleed. 35 Although dabigatran inhibits thrombin factor in coagulation cascade, thrombin also participates in platelet aggregation and finally results in thrombosis formation. 39 In our study, MAF (T) of UBASH3B rs2276408 is 10.0%, which is consistent with reported data. At the the sixth month visit, minor allele carriers had a significantly greater incidence rate of bleeding than noncarriers (TT 100.0%, TC 31.0% and CC 5.0%). Moreover, the trends in other visits of 1, 12 and 24 months also showed similar results. Besides, the minor allele carriers had a higher peak anti‐FIIa activity than noncarriers (TT 330.93 ± 44.08 ng/ml, TC 194.21 ± 145.66 ng/ml and CC 137.61 ± 119.31 ng/ml).
The FBN2 gene (fibrillin 2) is also a protein‐coding gene and located in chromosome 5q23.3. A G base is converted into a T base (G>T) in the rs3805625 mutation, which causes intron variation. From the 1000 Genomes Project Phase 3 populations data, MAF (T) is 2% in all the populations. Interestingly, it is almost expressed only in East Asian population (9%). In other populations, the T frequency is almost 0%, especially no mutations in American and African populations. From the HPA RNA‐seq normal tissue data, the expression level of FBN2 gene in human placenta is much higher, with very little in kidney and liver. 40 Whether mutations in this gene cause congenital contractural arachnodactyly remains controversy. 41 , 42 The extracellular matrix structural ingredient and calcium ion binding are gene ontology annotations connected to this gene. FBN1 (fibrillin 1), a significant paralog of this gene, has mutations linked to Marfan syndrome. The extracellular matrix gp (fibrillin‐1) encoded by FBN1 gene is a structural element of calcium‐binding microfilaments. It might mediate cell adhesion by interacting with the cell surface receptors integrins ITGAV:ITGB3 and ITGA5:ITGB1, 43 , 44 and subsequently bind heparin, resulting in assembly of microfibrils. 45 FBN1 gene involvement in the pathways of platelet activation was discovered in a recent work focused on a multilayer systems biology investigation of gastric cancer. 46 Besides, activated fibrinolysis, thrombin and platelet dysfunction are less well known but are indeed important features of Marfan syndrome. 47 The above information may provide us preliminary insight into exploring the effect of FBN2 gene on bleeding of drugs. According to our results, the MAF (T) of FBN2 rs3805625 is 10.6%, which is consistent with reported data. At the 12th month visit, the bleeding rates in minor allele carriers were significantly higher than noncarriers (TT 100.0%, TG 43.5% and GG 12.4%). Moreover, these increased trends were also presented in other visits on the 6th and 24th month.
For suggestive genes and SNPs associated with PD, the detailed characteristics are shown in Table S6. Most genes express in kidney and liver except IGLV3‐12 and MYBPC1. The MAFs of these SNPs in our study are almost consistent with that in East Asian population. Interestingly, MAFs of some SNPs in East Asian population were higher or lower compared with those in American and European populations. To our knowledge, all these genes are protein‐coding genes, and some genes have demonstrated the therapeutic potential in cardiovascular disease. 48 , 49 There is, however, little knowledge of how these genes and SNPs affect platelet or coagulation function. Previous pharmacoproteomic investigation in a standardized murine model found that the inductive effect of exosomes, which were abundant with coagulation factors, might be decreased by BRD4 inhibitors. 50 Besides, a rat study revealed that a BRD4 inhibitor might reduce clinical platelet counts. 51 Glycophorins A (GYPA) and B are the major sialoglycoproteins of human erythrocyte membrane. Response to elevated platelet cytosolic Ca2+ is the related pathway of GYPA gene. According to the study on coronary high‐signal intensity plaques (HIPs), which was immunoreactive for GYPA and fibrin, intraplaque bleeding occurred much more in HIPs than in non‐HIPs. 52 The ABCC2 gene mainly encodes multidrug‐resistance‐associated protein 2, and previous studies of genetic variation on dabigatran showed different results. 18 , 22 Our similar study in 118 Chinese healthy participants revealed ABCC2 rs2273697 and rs4148395 affected AUC, and C max as well as rs717620 had no influence on PK and PD. 22 Another study in 107 Spanish healthy participants found there was no association between ABCC2 rs2273697 and rs717620 and PK. 18 In this study, which focused on Chinese patients with NVAF, the minor allele (A) carriers of ABCC2 rs2273697 and rs4148395 had a higher trough PT than noncarriers (AA 32.10 ± 19.20 s, AG 17.13 ± 11.46 s and GG 12.88 ± 2.92 s).
Previous pharmacogenomic studies about the association between CES1 rs2244613 and bleeding of dabigatran in patients with AF was controversial. Two studies performed in European and Chinese patients 20 , 21 showed that the allele (C) carriers had a lower risk of bleeding, especially minor bleeding. On the other hand, other two studies 19 , 53 as well as our results found no association. Besides, another SNP, ABCB1 rs1045642, which was reported the allele (T) carriers had an increased risk of bleeding in patients after total knee arthroplasty with dabigatran, 54 had no association with bleeding in patients with AF, including our results. Further, our result of ABCG2 rs2231142 was negative for bleeding, which was consistent with previous study. 19 Our identified six genes with bleeding were only explored for association with PK/PD in previous studies of healthy participants, and most had negative results except SLCO1B1 rs4149032 associated with maximum plasma concentration. 22 There were no associations detected in previous studies for our identified 11 genes and 25 SNPs associated with PD. Besides, all these reported positive SNPs with PD had negative outcomes in our study. 22 We found ABCG2 rs2231156 had a significant impact on bleeding and PD; however, the prior study only discovered the positive result in time to peak concentration of healthy participants. 22
Some limitations in our study must be discussed. First, although the sample size of our study was larger than few other researches (Table S1), the number of patients included (n = 170) was still limited, and our results should be verified in other studies with more patients. Second, the dabigatran regimen was 110 mg twice daily for all and might decrease bleeding events. The reasons were considered that the formulations of 150 and 75 mg were approved later than that of 110 mg in China and the reduced‐dose anticoagulants were more used in Asian population. 55 Consequently, more different doses should be included in future studies. Third, the incidence rates of TEs and MACEs were relatively low. This might be also associated with sample size as well as loss to follow‐up. Future studies should be performed to ensure completion of visits. Fourth, considering the protection of participants from the perspective of the ethics committee, PK parameters were not included. Peak and trough plasma levels of dabigatran might be also meaningful for predicting bleeding risk, and the technology of concentration measurement should be improved in future. Finally, the mechanisms of thrombosis and haemorrhage in our new‐found suggestive genes and SNPs were not well understood. More future in vivo and in vitro pharmacogenomic and proteomic studies that explore and validate our results are required. Together with shortcomings that we already mentioned, we suggested that overall results of this explorative study should be more circumspective.
5. CONCLUSIONS
Genetic variations have a potential influence on bleeding risk and PD of dabigatran in Chinese patients with NVAF. The suggestive two SNPs (UBASH3B rs2276408 and FBN2 rs3805625) associated with bleeding as well as 17 new SNPs of 14 genes (SNX7, BRD4, FLCN, UBAP1, IGLV3‐12, LRRC8E, PTPLAD1, ZNF230, ANP32A, SLC25A28, ABCC2, MYBPC1, CEP170B and GYPA) associated with PD may be novel targets for anticoagulation therapy. Considering the explorative nature of our study, how the functions of these SNPs on mechanism of anticoagulant work, as well as whether these genetic variations affect other populations or outcomes of dabigatran, must be investigated in future via in vitro and in vivo researches.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Table S1 Previous pharmacogenomic studies and candidate genes reported for dabigatran
Table S2 Detailed clinical outcomes of patients treated with dabigatran at all follow‐up visits
Table S3 Effects of UBASH3B rs2276408 and FBN2 rs3805625 on the pharmacodynamics of dabigatran
Table S4 Effects of suggestive SNPs associated with PD parameters on bleeding events of patients treated with dabigatran
Table S5 Negative effects of candidate genes on bleeding and the pharmacodynamics of dabigatran
Table S6 Characteristics of suggestive genes and SNPs associated with PD parameters
Figure S1 Manhattan plots of association with pharmacodynamic parameters
Figure S2 Quantile–quantile plots of association with pharmacodynamic parameters
Xiang Q, Xie Q, Liu Z, et al. Genetic variations in relation to bleeding and pharmacodynamics of dabigatran in Chinese patients with nonvalvular atrial fibrillation: A nationwide multicentre prospective cohort study. Clin Transl Med. 2022;12:e1104. 10.1002/ctm2.1104
Qian Xiang and Qiufen Xie are the co‐first authors.
DATA AVAILABILITY STATEMENT
The data presented in the study are deposited in the National Population Health Data Center (NPHDC) repository, accession number 10.12213/11.A0028.202009.338.V1.0 (https://www.ncmi.cn/phda/dataDetails.html?type=project_data&id=CSTR:A0006.11.A0028.202009.338.V1.0‐V1.0).
REFERENCES
- 1. Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NA. The state of the art: atrial fibrillation epidemiology, prevention, and treatment. Mayo Clin Proc. 2016;91(12):1778‐1810. 10.1016/j.mayocp.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 2. Lin HJ, Wolf PA, Kelly‐Hayes M, et al. Stroke severity in atrial fibrillation. the framingham study. Stroke. 1996;27:1760‐1764. 10.1161/01.str.27.10.1760 [DOI] [PubMed] [Google Scholar]
- 3. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125‐e151. 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 5. Zhou H, Nie X, Jiang M, Dong W. Cost‐effectiveness of anticoagulants for preventing stroke in patients with non‐valvular atrial fibrillation in mainland China. J Clin Pharm Ther. 2022;47(4):523‐530. 10.1111/jcpt.13575 [DOI] [PubMed] [Google Scholar]
- 6. Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292‐303. 10.1111/j.1365-2125.2007.02899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47(1):47‐59. 10.2165/00003088-200847010-00005 [DOI] [PubMed] [Google Scholar]
- 8. Chan NC, Coppens M, Hirsh J, et al. Real‐world variability in dabigatran levels in patients with atrial fibrillation. J Thromb Haemost. 2015;13(3):353‐359. 10.1111/jth.12823 [DOI] [PubMed] [Google Scholar]
- 9. Lee LH. DOACs — advances and limitations in real world. Thromb J. 2016;14(Suppl 1):17. 10.1186/s12959-016-0111-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY trial (randomized evaluation of long‐term anticoagulation therapy). J Am Coll Cardiol. 2014;63(4):321‐328. 10.1016/j.jacc.2013.07.104 [DOI] [PubMed] [Google Scholar]
- 11. Fawzy AM, Lip GYH. Pharmacokinetics and pharmacodynamics of oral anticoagulants used in atrial fibrillation. Expert Opin Drug Metab Toxicol. 2019;15(5):381‐398. 10.1080/17425255.2019.1604686 [DOI] [PubMed] [Google Scholar]
- 12. Wieland E, Shipkova M. Pharmacokinetic and pharmacodynamic drug monitoring of direct‐acting oral anticoagulants: where do we stand? Ther Drug Monit. 2019;41(2):180‐191. 10.1097/FTD.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 13. Raymond J, Imbert L, Cousin T, et al. Pharmacogenetics of direct oral anticoagulants: a systematic review. J Pers Med. 2021;11(1):37. 10.3390/jpm11010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanuri SH, Kreutz RP. Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019;9(1):7. 10.3390/jpm9010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cullell N, Carrera C, Muiño E, Torres N, Krupinski J, Fernandez‐Cadenas I. Pharmacogenetic studies with oral anticoagulants. Genome‐wide association studies in vitamin K antagonist and direct oral anticoagulants. Oncotarget. 2018;9(49):29238‐29258. 10.18632/oncotarget.25579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ašić A, Marjanović D, Mirat J, Primorac D. Pharmacogenetics of novel oral anticoagulants: a review of identified gene variants & future perspectives. Per Med. 2018;15(3):209‐221. 10.2217/pme-2017-0092 [DOI] [PubMed] [Google Scholar]
- 17. Shnayder NA, Petrova MM, Shesternya PA, et al. Using pharmacogenetics of direct oral anticoagulants to predict changes in their pharmacokinetics and the risk of adverse drug reactions. Biomedicines. 2021;9(5):451. 10.3390/biomedicines9050451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zubiaur P, Saiz‐Rodríguez M, Ochoa D, et al. Effect of sex, use of pantoprazole and polymorphisms in SLC22A1, ABCB1, CES1, CYP3A5 and CYP2D6 on the pharmacokinetics and safety of dabigatran. Adv Ther. 2020;37(8):3537‐3550. 10.1007/s12325-020-01414-x [DOI] [PubMed] [Google Scholar]
- 19. Lähteenmäki J, Vuorinen AL, Pajula J, et al. Pharmacogenetics of bleeding and thromboembolic events in direct oral anticoagulant users. Clin Pharmacol Ther. 2021;110(3):768‐776. 10.1002/cpt.2316 [DOI] [PubMed] [Google Scholar]
- 20. Paré G, Eriksson N, Lehr T, et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation. 2013;127(13):1404‐1412. 10.1161/CIRCULATIONAHA.112.001233 [DOI] [PubMed] [Google Scholar]
- 21. Ji Q, Zhang C, Xu Q, Wang Z, Li X, Lv Q. The impact of ABCB1 and CES1 polymorphisms on dabigatran pharmacokinetics and pharmacodynamics in patients with atrial fibrillation. Br J Clin Pharmacol. 2021;87(5):2247‐2255. 10.1111/bcp.14646 [DOI] [PubMed] [Google Scholar]
- 22. Xie QF, Li Y, Liu ZY, et al. SLC4A4, FRAS1, and SULT1A1 genetic variations associated with dabigatran metabolism in a healthy Chinese population. Front Genet. 2022;13:873031. 10.3389/fgene.2022.873031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu ZY, Xie QF, Xiang Q, et al. Anti‐Fxa‐IIa activity test in Asian and its potential role for drug adherence evaluation in patients with direct oral anticoagulants: a nationwide multi‐center synchronization study. Cardiovasc Diagn Ther. 2020;10(5):1293‐1302. 10.21037/cdt-20-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736‐2747. 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 26. Sabourin J, Nobel AB, Valdar W. Fine‐mapping additive and dominant SNP effects using group‐LASSO and fractional resample model averaging. Genet Epidemiol. 2015;39(2):77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abegaz F, Chaichoompu K, Génin E, et al. Principals about principal components in statistical genetics. Brief Bioinform. 2019;20(6):2200‐2216 [DOI] [PubMed] [Google Scholar]
- 28. Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome‐wide association scan results. Bioinformatics. 2010;26(18):2336‐2337. 10.1093/bioinformatics/btq419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Z, Ma L, Zhang H, et al. Comparison of non‐vitamin K antagonist oral anticoagulants on bleeding and thrombosis. J Clin Pharm Ther. 2021;46(6):1729‐1742. 10.1111/jcpt.13514 [DOI] [PubMed] [Google Scholar]
- 30. Heidbuchel H, Verhamme P, Alings M, et al. Updated european heart rhythm association practical guide on the use of nonvitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17(10):1467‐1507. 10.1093/europace/euv309 [DOI] [PubMed] [Google Scholar]
- 31. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2):e44S‐e88S. 10.1378/chest.11-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16(2):209‐219. 10.1111/jth.13912 [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, Zhang H, Xie Q, et al. Different coagulation indicators in predicting clinical outcomes for patients with direct oral anticoagulants: a systematic review and meta‐analysis. Clin Ther. 2020;42(10):2066‐2081.e9. 10.1016/j.clinthera.2020.08.00 [DOI] [PubMed] [Google Scholar]
- 34. Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Mol Cell Proteomics. 2014;13(2):397‐406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Abraham S, Renna S, et al. TULA‐2 (T‐cell ubiquitin ligand‐2) inhibits the platelet Fc receptor for IgG IIA (FcγRIIA) signaling pathway and heparin‐induced thrombocytopenia in mice. Arterioscler Thromb Vasc Biol. 2016;36(12):2315‐2323. 10.1161/ATVBAHA.116.307979 [DOI] [PubMed] [Google Scholar]
- 36. Miller E, Norwood C, Giles JB, et al. PharmGKB summary: heparin‐induced thrombocytopenia pathway, adverse drug reaction. Pharmacogenet Genomics. 2022;32(3):117‐124. 10.1097/FPC.0000000000000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsygankov AY. TULA‐family proteins: a new class of cellular regulators. J Cell Physiol. 2013;228:43‐49. 10.1002/jcp.24128 [DOI] [PubMed] [Google Scholar]
- 38. Perdomo J, Leung HHL, Ahmadi Z, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin‐induced thrombocytopenia. Nat Commun. 2019;10(1):1322. 10.1038/s41467-019-09160-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discov. 2020;19:333‐352. 10.1038/s41573-020-0061-0 [DOI] [PubMed] [Google Scholar]
- 40. Fagerberg L, Hallström BM, Oksvold P, et al. Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Mol Cell Proteomics. 2014;13(2):397‐406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park ES, Putnam EA, Chitayat D, Child A, Milewicz DM. Clustering of FBN2 mutations in patients with congenital contractural arachnodactyly indicates an important role of the domains encoded by exons 24 through 34 during human development. Am J Med Genet. 1998;78(4):350‐355. 10.1002/(sici)1096-8628(19980724)78:4<350::aid‐ajmg9>3.3.co;2‐P [DOI] [PubMed] [Google Scholar]
- 42. Maya I, Kahana S, Agmon‐Fishman I, et al. Based on a cohort of 52,879 microarrays, recurrent intragenic FBN2 deletion encompassing exons 1–8 does not cause Beals syndrome. Eur J Med Genet. 2020;63(10):104008. 10.1016/j.ejmg.2020.104008 [DOI] [PubMed] [Google Scholar]
- 43. Bax DV, Bernard SE, Lomas A, et al. Cell adhesion to fibrillin‐1 molecules and microfibrils is mediated by alpha 5 beta 1 and alpha v beta 3 integrins. J Biol Chem. 2003;278(36):34605‐34616. 10.1074/jbc.M303159200 [DOI] [PubMed] [Google Scholar]
- 44. Jovanovic J, Takagi J, Choulier L, et al. alphaVbeta6 is a novel receptor for human fibrillin‐1. Comparative studies of molecular determinants underlying integrin‐rgd affinity and specificity. J Biol Chem. 2007;282(9):6743‐6751. 10.1074/jbc.M607008200 [DOI] [PubMed] [Google Scholar]
- 45. Tiedemann K, Bätge B, Müller PK, Reinhardt DP. Interactions of fibrillin‐1 with heparin/heparan sulfate, implications for microfibrillar assembly. J Biol Chem. 2001;276(38):36035‐36042. 10.1074/jbc.M104985200 [DOI] [PubMed] [Google Scholar]
- 46. Salarikia SR, Kashkooli M, Taghipour MJ, Malekpour M, Negahdaripour M. Identification of hub pathways and drug candidates in gastric cancer through systems biology. Sci Rep. 2022;12(1):9099. 10.1038/s41598-022-13052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Kodolitsch Y, Demolder A, Girdauskas E, et al. Features of Marfan syndrome not listed in the Ghent nosology — the dark side of the disease. Expert Rev Cardiovasc Ther. 2019;17(12):883‐915. 10.1080/14779072.2019.1704625 [DOI] [PubMed] [Google Scholar]
- 48. Lin S, Du L. The therapeutic potential of BRD4 in cardiovascular disease. Hypertens Res. 2020;43(10):1006‐1014. 10.1038/s41440-020-0459-4 [DOI] [PubMed] [Google Scholar]
- 49. Monteagudo S, Cornelis FMF, Wang X, et al. ANP32A represses Wnt signaling across tissues thereby protecting against osteoarthritis and heart disease. Osteoarthritis Cartilage. 2022;30(5):724‐734. 10.1016/j.joca.2022.02.615 [DOI] [PubMed] [Google Scholar]
- 50. Zhao Y, Tian B, Sun H, et al. Pharmacoproteomics reveal novel protective activity of bromodomain containing 4 inhibitors on vascular homeostasis in TLR3‐mediated airway remodeling. J Proteomics. 2019;205:103415. 10.1016/j.jprot.2019.103415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collins TA, Hattersley MM, Yates J, Clark E, Mondal M, Mettetal JT. Translational modeling of drug‐induced myelosuppression and effect of pretreatment myelosuppression for AZD5153, a selective BRD4 inhibitor. CPT Pharmacometrics Syst Pharmacol. 2017;6(6):357‐364. 10.1002/psp4.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuroiwa Y, Uchida A, Yamashita A, et al. Coronary high‐signal‐intensity plaques on T‐weighted magnetic resonance imaging reflect intraplaque hemorrhage. Cardiovasc Pathol. 2019;40:24‐31. 10.1016/j.carpath.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 53. Sychev D, Skripka A, Ryzhikova K, et al. Effect of CES1 and ABCB1 genotypes on the pharmacokinetics and clinical outcomes of dabigatran etexilate in patients with atrial fibrillation and chronic kidney disease. Drug Metab Pers Ther. 2020;35(1):20190029. 10.1515/dmpt-2019-0029 [DOI] [PubMed] [Google Scholar]
- 54. Sychev DA, Levanov AN, Shelekhova TV, et al. The impact of ABCB1 (rs1045642 and rs4148738) and (rs2244613) gene polymorphisms on dabigatran equilibrium peak concentration in patients after total knee arthroplasty. Pharmgenomics Pers Med. 2018;11:127‐137. 10.2147/PGPM.S169277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shen NN, Zhang C, Hang Y, et al. Real‐world prevalence of direct oral anticoagulant off‐label doses in atrial fibrillation: an epidemiological meta‐analysis. Front Pharmacol. 2021;12:581293. 10.3389/fphar.2021.581293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Previous pharmacogenomic studies and candidate genes reported for dabigatran
Table S2 Detailed clinical outcomes of patients treated with dabigatran at all follow‐up visits
Table S3 Effects of UBASH3B rs2276408 and FBN2 rs3805625 on the pharmacodynamics of dabigatran
Table S4 Effects of suggestive SNPs associated with PD parameters on bleeding events of patients treated with dabigatran
Table S5 Negative effects of candidate genes on bleeding and the pharmacodynamics of dabigatran
Table S6 Characteristics of suggestive genes and SNPs associated with PD parameters
Figure S1 Manhattan plots of association with pharmacodynamic parameters
Figure S2 Quantile–quantile plots of association with pharmacodynamic parameters
Data Availability Statement
The data presented in the study are deposited in the National Population Health Data Center (NPHDC) repository, accession number 10.12213/11.A0028.202009.338.V1.0 (https://www.ncmi.cn/phda/dataDetails.html?type=project_data&id=CSTR:A0006.11.A0028.202009.338.V1.0‐V1.0).


