Abstract
Nontypeable Haemophilus influenzae (NTHi) is an opportunistic pathogen associated with otitis media and the exacerbation of chronic bronchitis. This study reports the vaccine potential of three peptides representing conserved regions of the NTHi P5 outer membrane protein which have been fused to a promiscuous measles virus F protein T-cell eptitope (MVF). The peptides correspond to a region in surface loop one (MVF/L1A), the central region of loop four (MVF/L4), and a C-terminal region homologous to peptide 10 of OprF from Pseudomonas aeruginosa (MVF/H3). Immunization of rats with MVF/H3 was the most efficacious in significantly reducing the number of viable NTHi in both the broncho-alveolar lavage fluid (74%) and lung homogenates (70%), compared to control rats. Importantly, despite significantly increased rates of clearance, immunization with MVF/H3 elicited poor antibody responses, suggesting that cell-mediated rather than humoral responses play an important role in the enhanced clearance of NTHi in this model.
While nontypeable Haemophilus influenzae (NTHi) forms a minor component of the nasopharyngeal microbiota, it is also associated with recurrent infections of mucosal surfaces, in particular otitis media in children and the exacerbation of chronic bronchitis in middle-aged and elderly patients (reviewed in reference 21). The recurrent nature of NTHi infections is attributed to heterogeneity in immunodominant surface-exposed epitopes of outer membrane proteins (OMPs). These OMPs are thought to stimulate antibodies that fail to cross-protect against infection with heterologous NTHi strains (7). Recent interest directed at identifying conserved NTHi epitopes that could be included in a vaccine has recently focused on the heat-modifiable OMP, P5.
Molecular analysis of the variation in the electrophoretic mobility of P5 (17, 28) showed that this variation was a result of heterology in regions that are thought to be surface-exposed loops of an eight-stranded β-barrel (5, 31). Interestingly, loop one of this structure appears to fall into subclasses with a GINNNGAIK amino acid motif in a longer loop in some strains (31). Loop four appears to demonstrate the most homology of the other three surface-exposed loops. The C terminus of P5 is highly conserved (5, 25, 31) and contains a region proposed as a peptidoglycan binding domain (12). However, while the conserved nature of this domain suggests a periplasmic location that is shielded from the immune pressure of the host, the structure of this region of P5 is not clearly defined.
While immunization with P5 confers partial protection against transtubular challenge in chinchillas, this protection is strain specific (25), which further supports the notion that the immunodominant surface epitopes are variable. Notably, P5 has been proposed as a mediator in the binding of NTHi to sialic acid-containing oligosaccharides of respiratory mucin (19, 23, 24). Yet, in order for P5 to play a role as an adhesin, surface-exposed regions of the protein may need to be selectively conserved. Therefore, by using synthetic peptides, it may be possible to target an immune response to these conserved, functionally constrained regions of P5. In addition, peptide antibodies specific for regions of P5 associated with adhesion may also impair the pathogenicity of NTHi.
Recently, the efficacy of immunization with two synthetic peptides, R117-G135 (LB1) and Y163-T180 (LB2), which correspond to loop three and the first two-thirds of loop four, respectively (31), was assessed (1). While immunization with LB1 enhanced the clearance of NTHi from the nasopharynx of adenovirus-infected chinchillas, the heterogeneity that occurs in loop three (31) suggests that the immune response induced by this peptide may not be cross-protective. Recently, the problem of heterogeneity in LB1 was addressed by incorporating three representative sequences of this region into a chimeric peptide (2). However, a recent study of the sequence variation in P5 from Australian NTHi isolates (31) suggests that a greater number of variants may need to be considered for a cross-protective vaccine.
Peptides analogous to regions of the P5-homologous Pseudomonas aeruginosa OprF outer membrane protein have shown great promise as protective antigens. Antibodies raised against two OprF peptides (peptides 9 and 10) were shown to enhance opsonophagocytosis (10) and were as efficacious as OprF in reducing pulmonary lesions after P. aeruginosa challenge in a chronic mouse model (8). In addition, mice immunized with these peptides had higher survival rates in an acute model of pneumonia than mice immunized with carrier or other peptides (9). Although both peptides were significantly protective, peptide 10 was more efficacious than peptide 9. Interestingly, these peptides correspond to C-terminal regions in OprF, which in P5 and OmpA are proposed as being periplasmic. This result has contributed to debate in the literature regarding the topology of the C terminus of both OmpA and OprF and the suggestion that some of the C terminus may fold back across the outer membrane to be surface exposed (26, 27). Paradoxically, despite peptide 10-specific antibody being opsonophagocytic, flow cytometry has shown that this antibody labeled whole cells of P. aeruginosa poorly in comparison to peptide 9-specific antibody (11).
The aims of the investigations described here are to extend the study of Bakaletz and coworkers in determining the protective efficacy of immunization with synthetic P5 peptides fused to a promiscuous T-cell epitope (1). Peptides encompassing the GINNNGAIK motif in loop one, the central region of loop four, and the region in P5 that corresponds to peptide 10 in OprF were examined.
Design and synthesis of the chimeric P5 peptides.
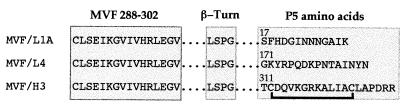
The sequences of the peptides (Fig. 1) were based on the sequence of P5 from NTHi strain UC19 (31). Peptide L1A encompasses the amino acid motif GINNNGAIK, which is found in loop one in a subset of NTHi strains, including UC19 (31); L4 encompasses the highly conserved central region of loop four; and H3 encompasses a region in P5 that is homologous to OprF peptide 10 from P. aeruginosa (10). The H3 sequence, which was found to be conserved in all the NTHi P5 proteins previously analyzed (5, 25, 31), was identified from the alignment of P5 from NTHi strain UC19 and OprF from P. aeruginosa strain PAO1 (GenBank accession no. M94078). In comparison, the homologous region in OmpA has only 75% identity with H3 (31). The sequences of H3, peptide 10, and the corresponding region in OmpA are shown in Fig. 2. The peptides also contained the measles virus F protein (22) promiscuous T-cell epitope (MVF) and a linker region composed of a 4-residue (LSPG) β-turn (15). Peptides were synthesized and purified and by the Biomolecular Resource Facility (Australian National University, Canberra City, Australian Capital Territory, Australia). In order to enhance antigenicity (18), multiple antigenic peptides were prepared by coupling the N-terminal cysteine of the peptides to polylysine (Auspep, Parkville, Victoria, Australia). Notably, the peptide MVF/H3 contains two intrachain cysteine residues which are the only cysteines that occur both in P5 and OmpA. As complete denaturation of P5 requires a reducing agent and there appears to be no evidence for a polymeric structure formed by these proteins, it seemed likely that these cysteines form an intrachain disulfide bond. Therefore, before coupling, the N-terminal cysteine in MVF/H3 was protected with an acetamido methyl group and an intrachain disulfide bridge was formed by oxidation with K3Fe(CN)6. After deprotection of the N-terminal cysteine, this peptide was then coupled to polylysine. Following coupling, peptides were dialyzed against 0.1 M NH4HCO3 and then lyophilized.
FIG. 1.
Sequences of the chimeric P5 peptides. Peptides were composed of MVF, a linker composed of a four-amino-acid β-turn and amino acids corresponding to regions in P5. Numbering above the P5 sequences refers to the amino acid number in P5, and the line below MVF/H3 indicates the intrachain disulfide bridge. The N-terminal cysteine of the peptides was used for coupling to a polylysine core to form multiple antigenic peptides.
FIG. 2.
Alignment of the P5 H3 peptide from NTHi isolate UC19 with OprF peptide 10 from P. aeruginosa and the corresponding region in OmpA from E. coli. In order to identify the peptide 10-homologous region in P5, the sequences of P5 and OprF, together with OmpA, were aligned with the PileUp program (Genetics Computer Group, University of Wisconsin, accessed through the Australian National Genomic Information Service). Gaps in the sequence, to create the alignment, are indicated with dots, and the numbers refer to the amino acid number in the preprotein.
Clearance of UC19 NTHi after immunization with the P5 chimeric peptides.
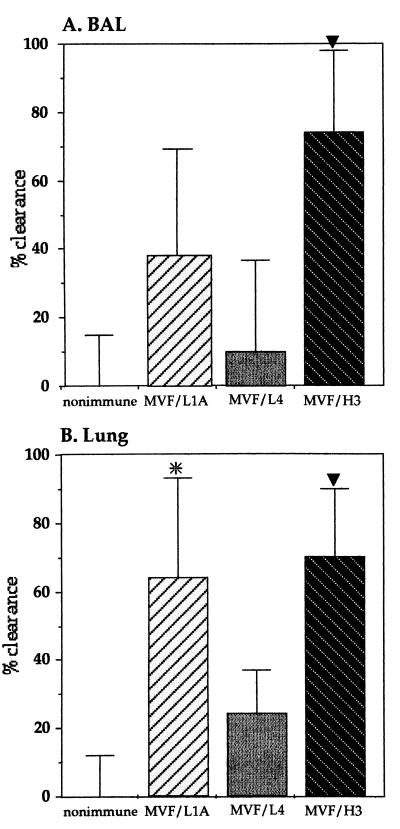
MVF/L1A, MVF/L4, and MVF/H3 were solubilized at a concentration of 6.5 mg/ml in 6 M guanidine-HCl and then diluted to 800 μg/ml with phosphate-buffered saline (PBS) and emulsified with an equal volume of incomplete Freund's adjuvant. Peyer's patch immunization was performed in Wistar rats as described previously (14, 29), with each animal receiving 20 μg of peptide. Control rats were unimmunized or immunized with the same concentration of incomplete Freund's adjuvant–PBS–guanidine-HCl as that used for the peptide-treated group. No differences in clearance were observed between these control groups. Animals were boosted 14 days later by intratracheal instillation of 20 μg of solubilized peptide in a total volume of 50 μl of PBS or with PBS–guanidine-HCl only. The procedure for assessing bacterial clearance of NTHi strain UC19 was performed 7 days after boosting as described previously (14, 29). Essentially, rats were intratracheally challenged with 5 × 108 CFU of UC19, and after 4 h, samples of broncho-alveolar lavage (BAL) fluid and lung homogenates were collected, serially diluted, and plated on chocolate agar to determine the viability of the NTHi remaining in the lungs. Significantly enhanced clearance was seen from the BAL fluid of rats immunized with MVF/H3 (74%, P < 0.005) and from the homogenized lungs of animals immunized with both MVF/H3 (70%, P < 0.005) and MVF/L1A (64%, P < 0.05), compared to the control animals (Fig. 3). Notably, while immunization with the MVF/H3 peptide significantly enhanced clearance from both BAL fluid and lung tissue, immunization with the MVF/L1A peptide enhanced significant clearance only from the homogenized lung sample. No significant enhancement of clearance was seen in the animals immunized with the MVF/L4 peptide.
FIG. 3.
Clearance of NTHi strain UC19 from BAL fluid (A) and homogenized lung (B) following challenge of animals immunized with the P5 chimeric peptides. Significant clearance was seen in the BAL fluid from MVF/H3-immunized rats and in the lung homogenates from MVF/L1A- and MVF/H3-immunized rats. ∗, P < 0.05; ▾, P < 0.005. The mean CFU recovered from the nonimmune group was given the value of 0% clearance. The percent clearance in the peptide-immunized groups was calculated as 100 minus the percent ratio of the mean CFU recovered from the immunized groups divided by the mean of the nonimmune group. Each group consisted of four to five animals, except for the control group, which consisted of eight animals. The error bars represent the standard errors of the mean expressed as percentages.
Peptide-specific antibodies in BAL fluid and serum.
Peptide-specific antibodies in serum and BAL fluid were measured by enzyme-linked immunosorbent assay (ELISA) (17). Microtiter wells were coated overnight at 4°C with 0.4 μg of peptide or with 0.2 μg of P5 that had been purified as previously described (31). The optimum peptide concentration used for coating was determined by using MVF/H3, as this peptide induced the highest serum antibody response. However, only one animal in each of the MVF/L1A- and MVF/H3-immunized groups (n = 4 and 5, respectively) responded with a low level of peptide-specific serum immunoglobulin G (IgG) (means ± standard errors of the means, 0.23 ± 0.06 and 13.12 ± 1.61 μg/ml, respectively). In addition, no animals in the MVF/L4-immunized group had detectable levels of MVF/L4-specific antibody and no peptide-specific antibodies could be detected in any of the control animals. Peptide-specific IgG or IgA was undetectable in the BAL fluid. To further investigate if peptide-specific antibodies were present, P5-reactive IgG in the sera of peptide-immunized and control animals was measured by ELISA. In addition, for comparison, P5-specific antibodies in a pool of serum from five animals immunized with purified P5 was also determined (Table 1). The animals that responded with detectable levels of MVF/L1A- or MVF/H3-specific serum IgG also had antibodies that cross-reacted with P5. All other animals had barely detectable levels of P5-reactive serum IgG. However, there were no significant differences in the levels of P5-reactive serum IgG between the animals that failed to respond with detectable peptide-specific antibody in the peptide-immunized groups, compared to the control group. High levels of P5-specific IgG were detected in the sera of the P5-immunized animals. Importantly, this demonstrated that L1A and H3 antibodies cross-reacted with P5 and suggested that the failure to detect peptide-specific antibodies in some animals was not due to inadequacies in the peptide-specific ELISA.
TABLE 1.
Comparison of P5- and OmpA-specific serum IgG in peptide- and P5-immunized rats
| Antigen and animala | P5-specific IgG (μg/ml) | OmpA-specific IgG (μg/ml) |
|---|---|---|
| N01 | 0.80 | 0.72 |
| N02 | 1.34 | 0.34 |
| N03 | 4.94 | 0.85 |
| N04 | 1.64 | 0.32 |
| MVF/L1A01 | 1.26 | 5.09 |
| MVF/L1A02b | 12.05 | 0.95 |
| MVF/L1A03 | 1.49 | 0.38 |
| MVF/L1A04 | 1.20 | 0.31 |
| MVF/L401 | 0.98 | 0.25 |
| MVF/L402 | 1.39 | 0.25 |
| MVF/L403 | 0.55 | 0.36 |
| MVF/L404 | 3.97 | 0.24 |
| MVF/H301b | 29.00 | 0.67 |
| MVF/H302 | 4.59 | 0.36 |
| MVF/H303 | 2.03 | 0.51 |
| MVF/H304 | 1.03 | 0.55 |
| MVF/H305 | 3.05 | 1.94 |
| P5c | 97.90 ± 18.32 | 24.84 ± 6.73 |
Antigen used for immunization and animal number. N represents nonimmune animals.
Animals with detectable MVF/L1A- or MVF/H3-specific serum IgG.
The antigen-specific IgG in the P5-immunized animals was for a pool of serum from five rats.
This sporadic production of peptide-specific antibodies within the peptide-immunized groups is intriguing. A recent report suggested that skewing of the immune response could be influenced by several factors, including the genotype of the major histocompatibility complex (MHC), the avidity with which peptides are bound, and the strength of the interaction formed with the T-cell receptor (4). High affinity appears to favor a cell-mediated Th1 type response, while low affinity favors an antibody helper Th2 type response. Therefore, the sporadic production of antibody within the MVF/L1A- and MVF/H3-immunized groups may be based on a similar rationale and dependent on the repertoire of the MHC haplotypes of the individual animal. This is especially relevant considering that the Wistar rats used in this study are an outbred strain and would be expected to have a diverse repertoire of MHC isotypes.
Relationship between peptide-specific antibody production and the enhanced clearance of NTHi.
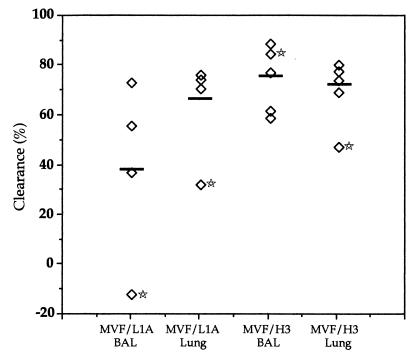
To determine the efficacy of peptide-specific antibody, a comparison was made between the clearance in antibody-responding and -nonresponding rats (Fig. 4). The numbers of viable NTHi in both the BAL fluid and lung homogenates of the MVF/L1A-specific antibody-responding rat and the lung homogenates of the MVF/H3-specific antibody-responding rat had the lowest clearance of viable NTHi within each respective group. In addition, the animal producing MVF/L1A-specific antibody had more viable NTHi recovered from the BAL fluid than the animals in the control group. Importantly, these results suggest that processes other than humoral-orchestrated mechanisms can mediate the pulmonary clearance of NTHi in this model. In addition, the manner in which NTHi is cleared may be related to early events in B-cell activation and the preference for either a Th1 or Th2 bias. The switch to the production of IgG2a, the antibody isotype associated with Th1 type responses, is thought to require sequential clonal proliferation of B cells to secrete the Th2 type antibodies, IgG1 and IgM, followed by a switch toward Th1 type cytokine production. However, the presence of a strong gamma interferon-mediated Th1 type response in the absence of any Th2 type component appears to suppress antigen-specific antibody production (3, 16). Clearly, further studies are required to analyze the relevant cytokine levels and whether immunization with the MVF/H3 peptide stimulates high gamma interferon levels and both a concomitant suppression of antibody production and the stimulation of strong cell-mediated Th1 responses. Notably, previous studies have suggested that humoral-mediated responses are in fact not critical for the clearance of NTHi from the lungs of rats immunized via the gut-respiratory regime. While immunization with low doses of killed NTHi was ineffective at eliciting detectable antigen-specific antibody responses, it was sufficient for enhancing pulmonary clearance (29). In addition, adoptive transfer of T lymphocytes from immunized rats has the capacity to accelerate the clearance of NTHi from the lungs of challenged animals (30). Studies concerned with the assessment of the NTHi-protective immune responses elicited in response to immunization with purified proteins have also demonstrated that enhanced clearance does not necessarily correlate with the antigen-specific antibody titer (13). If T-cell-mediated responses orchestrate the enhanced killing of NTHi in immune animals; the question remains as to the specificity of this response. While analysis of the immune responses that are efficacious in clearing P. aeruginosa from the rat lung suggested the importance of cell-mediated responses, it appears from studies with the adoptive transfer of T cells that this response is also antigen specific (6). However, it seems that the only way that this specificity can be generated in the absence of antigen-stimulated B cells is via T-cell epitopes. While the P5 peptides in the present study contained a promiscuous T-cell epitope that has been shown to overcome haplotype restriction of T-cell epitopes in mice and humans, it is unknown if the same response is generated in rats. Notably, the MVF epitope per se does not appear to have significantly influenced the clearance of NTHi, as no significant protection was seen in the MVF/L4-immunized group of rats. Moreover, in a previous study, immunization with a chimeric peptide corresponding to the N-terminal region of loop four combined with the MVF epitope (LB2) also failed to inhibit nasopharyngeal colonization in chinchillas (1). It therefore seems likely that the P5-based regions of the peptides influenced the efficacy of the immune responses that were generated and that the enhanced clearance was mediated by antigen-specific cell-mediated responses. However, further analysis of the proliferative responses of antigen-specific lymphocytes will be required to confirm this conclusion.
FIG. 4.
Comparison of the clearance in P5 peptide-specific antibody-responding and -nonresponding rats. The percent clearance was calculated for individual animals as described in the legend for Fig. 3. The mean of the nonimmune group was given the value of 0% clearance. The animals with peptide-specific serum IgG are indicated with stars, and the means of each group are indicated by bars. Notably, the animal producing MVF/L1A-specific antibody had more viable NTHi in the BAL fluid than the nonimmune animals.
Cross-reactivity of peptide-specific antibodies with OmpA.
Because the equivalent region in OmpA is 75% homologous to the H3 region in P5 (Fig. 2), it was important to determine whether immunization with MVF/H3 stimulated an antibody response that cross-reacted with OmpA. Certainly an undesirable feature in an antigen is the induction of an immune response that depletes a component of the normal microbiota. To address this issue, a crude preparation of OmpA was purified from Escherichia coli. Essentially, strain TG1 (Amersham, Buckinghamshire, Little Chalfont, United Kingdom) was lyzed, and total cell proteins were solubilized in PBS containing 0.3% (wt/vol) sodium dodecyl sulfate (SDS). Insoluble material was removed by centrifugation at 12,000 × g for 15 min. The concentration of SDS in the soluble fraction was then reduced to 0.03% (wt/vol) by dilution with PBS, and OmpA was precipitated by incubating overnight at 4°C. This method resulted in a preparation of OmpA that was approximately 90 to 95% pure as determined by SDS-polyacrylamide gel electrophoresis (data not shown). The identity of OmpA (20) was confirmed by N-terminal analysis of the protein from an appropriate gel fragment. ELISA, using OmpA-coated plates, was then used to determine the cross-reactivity of the antibody produced by the immunized rats. The cross-reactivity of the serum from the peptide-immunized animals with both P5 and OmpA demonstrated that although MVF/L1A- and MVF/H3-specific antibodies cross-reacted with P5, similar cross-reactivity was not seen with OmpA (Table 1). Notably, while some animals from both the control and peptide-immunized groups had slightly elevated OmpA-reactive serum IgG levels, compared to their cohorts, the reactivity of serum containing either MVF/L1A- or MVF/H3-specific antibodies was not higher than that in the peptide-specific antibody-nonresponding cohorts. This suggested that the poor clearance observed, especially from the lung homogenates of the peptide-specific antibody-responding animals was not associated with impairment of the integrity of the lymphoid tissue in the gut and a resulting increase in peptide-cross-reactive antibodies due to stimulation by OmpA. Importantly, these results demonstrate that there was no cross-reactivity between MVF/H3-specific antibodies and OmpA. However, future work is required to determine if MVF/H3-specific cell-mediated responses similarly lack cross-reactivity with OmpA. In contrast, OmpA-reactive IgG was detected in the serum from animals immunized with P5, albeit at a lower titer than the titer of P5 antibody specific for P5. Therefore, it seems that while some antigenic cross-reactivity is observed between these proteins, a considerable proportion of the P5-specific antibodies were directed toward regions of P5 that are not conserved in OmpA.
In summary, while infection or systemic immunization may bias toward strain-specific humoral responses, it is possible that mucosal-directed immunization may be more efficacious in stimulating cell-mediated responses. A corollary to this conclusion is that more emphasis may need to be placed on the study of conserved, not necessarily surface-exposed epitopes as potential vaccine components presented in an immunization regime that stimulates strong cell-mediated responses. As significantly enhanced clearance of NTHi was seen after immunization with the MVF/H3 peptide and as the corresponding region in P5 is well conserved, it appears that this peptide warrants further investigation as a NTHi vaccine component.
Acknowledgments
We are extremely grateful to Graeme Cox for the generous provision of experimental resources and to Kerry McAndrew of the Biomolecular Resource Facility, John Curtin School of Medical Research, Australian National University, for peptide synthesis and the preparation of the multiple antigenic peptides. D.C.W. was supported by a University of Canberra Postgraduate Research Award and a Collaborative Research Scholarship provided by the John Curtin School of Medical Research.
REFERENCES
- 1.Bakaletz L O, Leake E R, Billy J M, Kaumaya P T P. Relative immunogenicity and efficacy of two synthetic chimeric peptides of fimbrin as vaccinogens against nasopharyngeal colonization by nontypeable Haemophilus influenzae. Vaccine. 1997;15:955–961. doi: 10.1016/s0264-410x(96)00298-8. [DOI] [PubMed] [Google Scholar]
- 2.Bakaletz L O, Kennedy B-J, Novotny L A, Duquesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect Immun. 1999;67:2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boom W H, Liano D, Abbas A K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B cells. J Exp Med. 1988;167:1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunkley M L, Clancy R L, Cripps A W. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83:362–369. [PMC free article] [PubMed] [Google Scholar]
- 7.Faden H, Bernstein J, Brodsky L, Stanievich J, Krystofik D, Shuff C, Hong J J, Ogra P L. Otitis media in children. I. The systemic immune response to nontypable Haemophilus influenzae. J Infect Dis. 1989;160:999–1004. doi: 10.1093/infdis/160.6.999. [DOI] [PubMed] [Google Scholar]
- 8.Gilleland L B, Gilleland H E J. Synthetic peptides representing two protective linear B-cell epitopes of outer membrane protein F of Pseudomonas aeruginosa elicit whole-cell-reactive antibodies that are functionally pseudomonad specific. Infect Immun. 1995;63:2347–2351. doi: 10.1128/iai.63.6.2347-2351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes E E, Gilleland H E J. Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against P. aeruginosa infection in a murine acute pneumonia model. Vaccine. 1995;13:1750–1753. doi: 10.1016/0264-410x(95)00166-x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes E E, Gilleland L B, Gilleland H E J. Synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa that elicit antibodies reactive with whole cells of heterologous immunotype strains of P. aeruginosa. Infect Immun. 1992;60:3497–3503. doi: 10.1128/iai.60.9.3497-3503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes E E, Matthews-Greer J M, Gilleland H E J. Analysis by flow cytometry of surface-exposed epitopes of outer membrane protein F of Pseudomonas aeruginosa. Can J Microbiol. 1996;42:859–862. doi: 10.1139/m96-109. [DOI] [PubMed] [Google Scholar]
- 12.Koebnik R. Proposal for a peptidoglycan-associating alpha-helical motif in the C-terminal regions of some bacterial cell-surface proteins. Mol Microbiol. 1995;16:1269–1270. doi: 10.1111/j.1365-2958.1995.tb02348.x. [DOI] [PubMed] [Google Scholar]
- 13.Kyd J M, Cripps A W. Modulation of antigen-specific T and B cell responses influence bacterial clearance of non-typeable Haemophilus influenzae from the lung in a rat model. Vaccine. 1996;14:1471–1478. doi: 10.1016/s0264-410x(96)00034-5. [DOI] [PubMed] [Google Scholar]
- 14.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lairmore M D, DiGeorge A M, Conrad S F, Trevino A V, Lal R B, Kaumaya P T P. Human T-lymphotropic virus type 1 peptides in chimeric and multivalent constructs with promiscuous T-cell epitopes enhance immunogenicity and overcome genetic restriction. J Virol. 1995;69:6077–6089. doi: 10.1128/jvi.69.10.6077-6089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong K H, Ramsay A J, Boyle D B, Ramshaw I A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeb M R, Smith D H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980;30:709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y-A, Clavijo P, Galantino M, Shen Z-Y, Liu W, Tam J P. Chemically unambiguous peptide immunogen: preparation, orientation and antigenicity of purified peptide conjugated to the multiple antigen peptide system. Mol Immunol. 1991;28:623–630. doi: 10.1016/0161-5890(91)90131-3. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto N, Bakaletz L O. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla Eustachian tube and middle ear. Microb Pathog. 1996;21:343–356. doi: 10.1006/mpat.1996.0067. [DOI] [PubMed] [Google Scholar]
- 20.Movva N R, Nakamura K, Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980;143:317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- 21.Murphy T F, Apicella M A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Partidos C D, Steward M W. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990;71:2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- 23.Reddy M S, Bernstein J M, Murphy T F, Faden H S. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect Immun. 1996;64:1477–1479. doi: 10.1128/iai.64.4.1477-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy M S, Murphy T F, Faden H S, Bernstein J M. Middle ear mucin glycoprotein: purification and interaction with nontypable Haemophilus influenzae and Moraxella catarrhalis. Otolaryngol Head Neck Surg. 1997;116:175–180. doi: 10.1016/S0194-59989770321-8. [DOI] [PubMed] [Google Scholar]
- 25.Sirakova T, Kolattukudy P E, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun. 1994;62:2002–2020. doi: 10.1128/iai.62.5.2002-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stathopoulos C. An alternative topological model for Escherichia coli OmpA. Protein Sci. 1996;5:170–173. doi: 10.1002/pro.5560050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugawara E, Steiert M, Rouhani S, Nikaido H. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J Bacteriol. 1996;178:6067–6069. doi: 10.1128/jb.178.20.6067-6069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Alphen L, Riemens T, Poolman J, Zanen H C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983;155:878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace F J, Clancy R L, Cripps A W. An animal model demonstration of enhanced clearance of nontypeable Haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989;140:311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- 30.Wallace F J, Cripps A W, Clancy R L, Husband A J, Witt C S. A role for intestinal lymphocytes in bronchus mucosal immunity. Immunology. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Webb D C, Cripps A W. Secondary structure and interstrain variability in the P5 outer membrane protein of nontypeable Haemophilus influenzae isolated from diverse anatomical sites. J Med Microbiol. 1998;47:1059–1067. doi: 10.1099/00222615-47-12-1059. [DOI] [PubMed] [Google Scholar]