Abstract
Despite the proposed early life origins of attachment style and its implications for risk for psychopathology, little is known about its neurodevelopmental course. Adolescence represents a key transition period when neural substrates of emotion regulation and reward undergo dramatic maturational shifts. Thus, maladaptive coping strategies associated with insecure attachment styles may have an exaggerated effect during adolescence. The current study, therefore, examined the neural correlates of insecure attachment in a diverse sample of adolescents using a frustrative non-reward task (i.e. repeatedly being denied an expected reward). Although there were no significant interactions in the whole-brain activation averaged over the course of the task, the use of complementary analytic approaches (connectivity, change in activation over the course of the task) revealed widespread alterations associated with avoidant attachment during the immediate reaction to, and ensuing recovery from, being denied a reward. Most strikingly, increased avoidant attachment, adjusting for anxious attachment, predicted functional connectivity and change in activity over time in amygdala–prefrontal and frontostriatal networks to reward blocked vs received trials. These patterns were in the opposite direction compared to those exhibited by adolescents lower in avoidant attachment. The findings suggest that negative emotional experiences, such as receiving frustrating feedback, may be uniquely aversive internal experiences for avoidantly attached adolescents and provide preliminary evidence that early coping strategies may persist into adolescence in the form of altered emotion- and reward-related neural patterns.
Keywords: attachment, frustrative non-reward, adolescence, brain, emotion regulation
Attachment style develops as a result of a child’s early experiences with a caregiver (Bowlby, 1969/1982). If caregivers are not responsive, sensitive or available to restore the child’s sense of security, the result can be a greater degree of insecure attachment, which is associated with maladaptive coping styles when faced with new threats. In particular, individuals who are avoidantly attached exhibit coping strategies marked by avoidance, such as excessively tamping down on emotional responses; in contrast, anxiously attached individuals show coping strategies marked by approach responses, such as excessive reassurance seeking (Ainsworth, 1985; Mikulincer et al., 2003). In addition, traumatic events, especially ones perpetrated by the caregiver, can have detrimental effects on the quality of children’s attachment toward the caregiver (Breidenstine et al., 2011). However, the neural correlates of attachment style are less well understood, especially over developmentally meaningful periods. The current study, therefore, examined relationships between attachment style and neural activity in response to a frustrative non-reward task in a sample of adolescents who have experienced trauma.
During adolescence, numerous intense social, ecological and developmental changes repeatedly put coping strategies, including those associated with insecure attachment styles, to the test (Cicchetti and Rogosch, 2002; Casey et al., 2010a; Wiggins and Monk, 2013). Adolescence is marked by the rapid, yet disjointed, development of subcortical reward- and emotion-related structures (i.e. amygdala and striatum) and prefrontal/frontal emotion regulation-related structures (Gogtay et al., 2004). The maturational ‘mismatch’ of these structures is reflected in the intensification of emotional experiences, as relatively less mature prefrontal/frontal regions are less effective at downregulating amygdala and striatum activity (Casey et al., 2010b). At the same time, the amygdala and striatum are particularly sensitive to reward during adolescence, exhibiting outsized responses to both receiving and being blocked from rewards (Casey et al., 2010b; Galvan, 2010). In particular, maladaptive attachment styles resulting from traumatic experiences may exaggerate these developmental emotion/reward processing alterations (Vrticka and Vuilleumier, 2012). Frustrative non-reward, characterized by angry emotional responses to being denied an expected reward (i.e. reward blocked), may be a particularly relevant probe for reward and emotion processing during adolescence, as it incorporates elements of both of these highly relevant developing constructs (Amsel, 1958). Indeed, youths with trauma histories show altered neural processing during frustrative non-reward in amygdala/temporal and posterior regions (Hodgdon et al., 2021). Thus, the combination of reward hypersensitivity and poor emotion regulation abilities makes adolescence a key transition period when coping strategies learned from past experiences, such as maladaptive avoidance and approach responses, may have outsized contributions to psychological well-being. Although reward hypersensitivity and exaggerated responses to frustrative non-reward are conceptually distinct, there is evidence in other clinical populations that suggest that adolescents who place an increased emphasis on rewards also exhibit altered reactions to being denied such rewards (Alloy and Nusslock, 2019).
A multitude of neuroimaging studies linking attachment style with neural activity to emotional tasks have been conducted in adults (Gillath et al., 2005; Vrticka et al., 2008, 2012; DeWall et al., 2012). However, to the best of our knowledge, fewer studies have examined similar relationships in adolescent samples, and of those that have been conducted, the results are mixed. While some findings provide evidence that insecure attachment is related to greater reactivity in subcortical regions associated with emotion and reward (e.g. amygdala and ventral striatum), other findings suggest the opposite (i.e. insecure attachment is related to lowered reactivity in subcortical regions) (Debbane et al., 2017; van Hoof et al., 2019). In the only neuroimaging study that directly investigated the relationship between reward and attachment style in adolescents, insecure attachment styles (i.e. avoidant, dependent/resistant and controlling/insecure-other) were related to increased activation when receiving rewards (i.e. points during the Balloon Analogue Risk Task) in the anterior cingulate cortex, bilateral anterior insula, left ventrolateral prefrontal cortex and bilateral dorsal striatum (McCormick et al., 2019). In the other direction, there is evidence that, when receiving incongruent social feedback, greater avoidant attachment was associated with less amygdala, hippocampal and caudate activation. Similarly, in preadolescents, there is some evidence that avoidant attachment style is associated with decreased activation in the amygdala, cingulate cortex, prefrontal cortex and striatum in response to pictures of attachment-related distress (Choi et al., 2018). Taken together, these studies suggest that avoidant attachment is associated with a neural profile characterized by dysfunction in emotion and reward regions; however, more research is needed, especially in adolescents.
This handful of functional magnetic resonance imaging (fMRI) studies on attachment in adolescents leave several crucial areas ripe for investigation. First, only one of the paradigms examined neural responses to reward, despite the large role that reward plays in adolescence. Second, almost all of these studies focused on mean brain activation; yet, such psychological phenomena almost certainly recruit networks of regions, necessitating a complementary connectivity approach, beyond single regions. Third, although the brain activation that these studies examined was averaged over the entire task, emotional coping responses are dynamic processes that unfold over the course of multiple events. Finally, the majority of work on attachment, particularly in adolescents, has not explored neural differences within racially/ethnically diverse adolescent samples, potentially limiting generalizability.
To address these gaps, the goal of this preliminary study was to characterize the neural correlates of insecure attachment styles in a predominantly Hispanic/Latinx sample of adolescents with a history of multiple traumas, using a frustrative non-reward fMRI paradigm. Our novel, adolescent-friendly frustrative non-reward task allowed us to examine the unfolding process of the emotional response to blocked rewards—i.e. the feedback period which reflects the immediate reaction to being blocked from receiving a reward—as well as the ensuing recovery period, when individuals must regulate themselves by applying coping strategies and recovering post-feedback to perform on the next trial. Although avoidant and anxious attachment styles are commonly conceptualized as response styles that are opposites of each other (avoidant vs approach with high negative affect), it could be the case that the relationship between these attachment styles is more nuanced. It is entirely possible that individuals may endorse either style in varying levels depending on the context and may even exhibit responses that are representative of one style, while also exhibiting responses representative of the other style. Thus, while we did not expect avoidant and anxious attachment scores to be negatively correlated across participants because people who engage in one insecure reaction may also engage in the other insecure reaction at various times, we chose an analytic strategy to address both the theoretical conceptualization of these insecure types as opposites, as well as the possibility that individuals may endorse both types of behaviors: focusing on avoidant attachment and statistically adjusting for anxious attachment.
Based on the regions identified in the prior studies (Debbane et al., 2017; Choi et al., 2018; McCormick et al., 2019; van Hoof et al., 2019) and the demonstrated role of reward probes (McCormick et al., 2019), we expected that avoidant attachment would be associated with increased amygdala and ventral striatum connectivity with the prefrontal cortex in response to being blocked from vs receiving a reward during the immediate reaction period, as well as in the ensuing recovery period. Moreover, in line with coping strategies associated with avoidant attachment (Ainsworth, 1985; Mikulincer et al., 2003), it was expected that as avoidantly attached participants are repeatedly blocked from vs receiving a reward over the course of the task, amygdala and ventral striatum activation would more steeply decrease both in the immediate response to the blocked reward and in the ensuing recovery period before the next opportunity to get a reward.
Methods
Participants
Data were collected from N = 31 adolescents aged 12–18 years old (M = 14.53, s.d. = 1.74). The sample included treatment-seeking participants with complex trauma, i.e. more than one adverse childhood experience (Felitti et al., 1998) as part of a larger trauma-focused Cognitive Behavioral Therapy study (Hodgdon et al., 2021; Yang et al., 2021). The current study uses data collected during the initial baseline visit of this larger study. Participants were recruited from a local middle/high school in southeast San Diego through recommendations from school counselors and were predominantly Hispanic/Latinx and of relatively lower socioeconomic status, reflective of the sociodemographics of the surrounding area (http://www.gompersprep.org/about/directors-letter/school-accountability-report/). Detailed participant characteristics are given in Table 1. All adolescents gave written assent, parents gave written permission and families received monetary compensation for participation. The University of California San Diego Institutional Review Board, in joint agreement with the San Diego State University Institutional Review Board, approved all procedures.
Table 1.
Sample characteristics
| N | 27 | ||
|---|---|---|---|
| Age (M, s.d.) | 14.04 (1.72) | ||
| % | n | ||
| Female | 55.56 | 15 | |
| Race/ethnicity | |||
| African-American/Black | 11.11 | 3 | |
| Asian/Pacific Islander | 7.41 | 2 | |
| Hispanic/Latino/a/x | 81.48 | 22 | |
| Attachment style (CAPAI) | M | s.d. | Range |
| Anxious | 2.68 | 1.02 | 1.11–4.61 |
| Avoidant | 2.99 | 1.32 | 1.00–5.17 |
| Adverse childhood experiences (ACEs) | M | s.d. | Range |
| Total number of ACEs | 3.21 | 2.65 | 0–11 |
Notes: Data for ACEs were collected via both the parent and child report. The total number of ACEs was calculated by taking either the parent or child report, whichever was greater, due to documented concerns of underreporting ACEs by both children and their parents (Hardt and Rutter, 2004; Holt et al., 2009; Hungerford et al., 2010).
Attachment style
Attachment style with the primary caregiver was measured via self-report prior to fMRI acquisition using the Comprehensive Adolescent-Parent Attachment Inventory (CAPAI; Steiger and Moretti, 2005), a developmentally appropriate version of the widely used Experiences in Close Relationships Scale (Brennan et al., 1998). The CAPAI is comprised of two subscales that measure attachment anxiety (18 items, e.g. ‘I need a lot of reassurance that I am loved by my parent’) and attachment avoidance (18 items, e.g. ‘I find it difficult to depend on my parent’). Participants were instructed to complete the CAPAI based on their relationship with the parent or caregiver that played the most important part in raising them. Items are rated on a 1–7 scale, where higher scores indicated more insecure attachment. Four subjects were excluded due to missing CAPAI data, leaving a final analytic sample of n = 27.
The attachment anxiety and attachment avoidance subscales of the CAPAI were calculated by taking the average of the 18 items (rated 1–7 by the participant) pertaining to each subscale. One subject was missing partial data for the attachment avoidance subscale, and four additional subjects were missing partial data for both subscales. These subjects had data for at least 83% of the items on both subscales, warranting their inclusion. Scores for these subjects were calculated by taking the average of items that had data, done separately for each subscale. Detailed statistics for both subscales are given in Table 1. On both subscales, higher scores were indicative of more avoidant/anxious attachment. Both subscales demonstrated good reliability with Cronbach’s alphas of 0.86 and 0.90 for the attachment anxiety and attachment avoidance subscales, respectively. The attachment avoidance and attachment anxiety subscales were not significantly correlated to each other (r = 0.29, P = 0.140).
fMRI data acquisition
Participants were scanned on a 3.0 T Siemens MRI with a 30-channel head coil. To improve temporal and spatial resolution, multiband procedures were used, which allowed for better inference of neural correlates of attachment. A high-resolution T1-weighted magnetization prepared rapid gradient echo sequence with prospective motion correction (MPRAGE PROMO, repetition time (TR) = 2.3 s; echo time (TE) = 2.98 ms; slice thickness = 1.0 mm; voxel size = 1.0 × 1.0 × 1.0 mm; matrix = 256 × 256 mm; flip angle = 9°; field of volume = 256 × 256 mm) was acquired for coregistration with functional images. Blood oxygen level-dependent (BOLD) images were acquired while participants completed the frustrative non-reward task (TR = 0.8 s; TE = 30.8 ms; slice thickness = 2.4 mm; voxel size = 2.4 × 2.4 × 2.4 mm; matrix = 90 × 90 mm; flip angle = 52°; field of volume = 216 × 216 mm; 60 slices; multiband acceleration = 6; number of TRs = 358; 3 runs of 4 minutes, 46 seconds).
Frustrative non-reward fMRI task
Adolescents completed a well-validated adolescent-friendly monetary incentive delay paradigm in which participants hit a piñata to win stars that translated into cash prizes, modified to elicit frustrative non-reward (Helfinstein et al., 2013; Dougherty et al., 2018). The participants first performed three runs of the non-rigged version of the task in which they received accurate feedback for hitting a target. In the subsequent three runs, participants completed a rigged version of the task, such that after hitting the target, the reward was blocked 60% of the time. Thus, participants are blocked from a reward they had earned, eliciting feelings of frustration. In total, the participants completed six runs of the task inside the scanner; however, this study focuses on the data collected from the last three runs of the task where the feedback was rigged. We do not directly compare conditions between the non-rigged (i.e. first three runs) and rigged (i.e. last three runs) versions of the task as, due to the frustration induction, baselines may differ.
For detailed information regarding trial structure, see Figure 1. Briefly, trials began with a variable duration anticipation period, during which time the participants were presented with a cue that a target (piñata) was about to appear. When the target appeared, the participant pressed a button to hit the target (target presentation period) and was then presented with feedback: either the piñata broke open and they received stars into their basket or it swung away and they were blocked from receiving the stars (feedback period). This feedback period represents the immediate reaction to receiving or being blocked from a reward. Sixty percent of hits had no reward; misses always had no reward. After a jittered intertrial interval, the next (n + 1) trial began with another anticipation period. This anticipation period in the n + 1 trial represents the ensuing recovery period, as participants attempt to regulate their emotions to cope with the frustration of missing out on a reward and adjust their expectations for the next trial. Thus, the conditions of interest for the immediate reaction period were ‘reward blocked’ (no reward even though they hit the target, i.e. rigged hit) and ‘reward received’ (non-rigged hit) and for the ensuing recovery period were ‘after reward blocked’ (after rigged hit) and ‘after reward received’ (after non-rigged hit). Miss trials were modeled in all first-level analyses but, due to their low number, excluded from second-level analyses as in prior work using a similar task (Deveney et al., 2013; Tseng et al., 2019).
Fig. 1.

fMRI frustrative non-reward task. In this novel task, a modified version of an adolescent-friendly monetary incentive delay task (Dougherty et al., 2018), participants first saw a cue (piñata dropping for 2000 ms) alerting them that a target (piñata) with a possible reward was about to drop, followed by a variable duration (2500–5500 ms) delay period when participants waited for the target to drop (anticipation period). When the target was presented (initially at 500 ms but adjusted in real-time based on performance), participants were instructed the ‘hit’ the piñata by pressing a button on an MRI response box. After a delay, participants received feedback on whether they received a reward (piñata breaking and stars falling into the basket) or were blocked from a reward (piñata swings away, no stars in basket) (immediate reaction period). Forty percent of hits were followed by positive feedback (reward received), but for 60% of hits, feedback was rigged such that participants were denied a reward (reward blocked). All misses were followed by no reward. After a jittered intertrial interval (2500–5500 ms), the subsequent anticipation period was considered the ensuing recovery period.
fMRI data preprocessing
Imaging data were preprocessed using Analysis of Functional NeuroImages (AFNI; https://afni.nimh.nih.gov/afni), including slice time correction, functional image realignment to address head motion, nonlinear registration for spatial standardization to the Talairach template, spatial smoothing at 4 mm and voxel-wise scaling into units of percent signal change. TR pairs with framewise displacement >0.5 mm were censored, and all participants had average framewise displacement <0.15 mm.
fMRI data analysis
The goal of the functional connectivity analyses was to examine networks of brain regions associated with frustrative non-reward. Complementary analyses were also conducted to examine how reactions may build over repeated experiences of being blocked from receiving a reward, reflected in the change in activation over the course of the task (see Supplementary material for mean activation analyses).
First-level analyses
Functional connectivity.
Given the central role of the amygdala in emotion and reward processing, as well as the ventral striatum in reward, right and left masks of each of these regions, defined by the Talairach atlas, were applied to generate seeds for correlations with the rest of the brain. Generalized psychophysiological interaction analysis was used to calculate connectivity between these a priori seed regions and the rest of the brain during each of the task conditions of interest (immediate reaction period: reward blocked, reward received; ensuing recovery period: after reward blocked, after reward received). The output of this analysis was a set of connectivity images depicting voxel-wise correlations between the seed regions and the other regions of the brain during each of the task conditions vs baseline for use in group-level analyses. Of note, we modeled the target presentation period in this and all first-level analyses for accurate estimation of the BOLD response but excluded the target presentation from second-level analysis because the target presentation was not of interest. Additionally, across all first-level analyses, nuisance regressors reflecting head motion (x, y, z, roll, pitch, yaw) and polynomial modeling of scanner drift were included.
Change in activation over the course of the task.
To estimate whole-brain change in activation across repeated trials for each adolescent, a general linear model was specified using AFNI’s amplitude modulation (AM2) function using the same eight regressors. Along with estimating beta coefficients for the average activation during the task periods, this general linear model also calculated beta coefficients representing linear slopes, i.e. change in activation over the course of the task, accounting for the spacing and number of events. This analysis resulted in values representing change in brain activation over time for each task condition. Only the first run of the rigged task was used for this set of analyses because the amygdala and other threat/reward regions have been shown to habituate over the course of a few minutes (Sladky et al., 2012; Williams et al., 2013; Stevens et al., 2017).
Second-level analyses
Beta coefficient images were then used in separate group-level analyses for each type of individual-level analysis (connectivity, mean activation, change in activation) and for immediate reaction and ensuing recovery periods. Using AFNI’s 3dMVM function, degree of anxious attachment and degree of avoidant attachment were entered as quantitative between-subjects variables, and task condition (reward blocked and reward received for the immediate reaction period; after reward blocked and after reward received for the ensuing recovery period) was the within-subject variable. Because both anxious and avoidant attachment variables were included in the same models, our results represent the effects of avoidant attachment, above and beyond anxious attachment (see Supplementary material for mean activation results and results of anxious attachment above and beyond avoidant attachment). Thus, significant results reflect neural activity that is uniquely associated with avoidant attachment above and beyond anxious attachment. The results are also adjusted for age and gender by including these as covariates. The results that were further adjusted for potentially related constructs are displayed in Supplementary material.
Whole-brain thresholds (P < 0.05 corrected) were calculated using AFNI’s 3dClustSim command, which uses a mixed-model spatial autocorrelation function, and the first nearest neighbors bi-sided option, allowing for separate clusters of positive and negative voxels. This generated a height threshold of 0.005 and a k threshold of 64.5–70.9 voxels, which varied based on the analysis (see Supplementary material for details). A group mask representing the regions where 90% of the adolescents had valid data was used by 3dClustSim in its threshold calculation. A conservative approach of applying stringent cluster-level thresholding based on 3dClustsim was applied within the analysis. Significant clusters were extracted for illustrative post hoc decomposition in SPSS v.27. For main effects, activation and connectivity values were averaged across subjects and plotted to determine the direction of the effect. Estimated marginal means were calculated and plotted to decompose interaction effects.
Results
Associations with avoidant attachment (adjusted for anxious attachment)
In our sample, the anxious and avoidant subscales of the CAPAI were not significantly correlated/anticorrelated (r = 0.29, P = 0.140). The results discussed here represent patterns of brain activity associated with avoidant attachment after adjusting for anxious attachment.
Connectivity
Immediate reaction period.
Consistent with hypotheses, there was a significant avoidant attachment × condition interaction for connectivity between the right amygdala and the right inferior frontal gyrus during the immediate reaction period. Lower levels of avoidant attachment were associated with greater connectivity to the reward blocked vs received trials, whereas adolescents with higher levels of avoidant attachment showed little difference in activation between the conditions (Figure 2A.1.1; Table 2). The main effects of avoidant attachment were not significant for the immediate reaction period for right amygdala connectivity, and no contrasts with avoidant attachment were significant for left amygdala connectivity in the immediate reaction period.
Fig. 2.
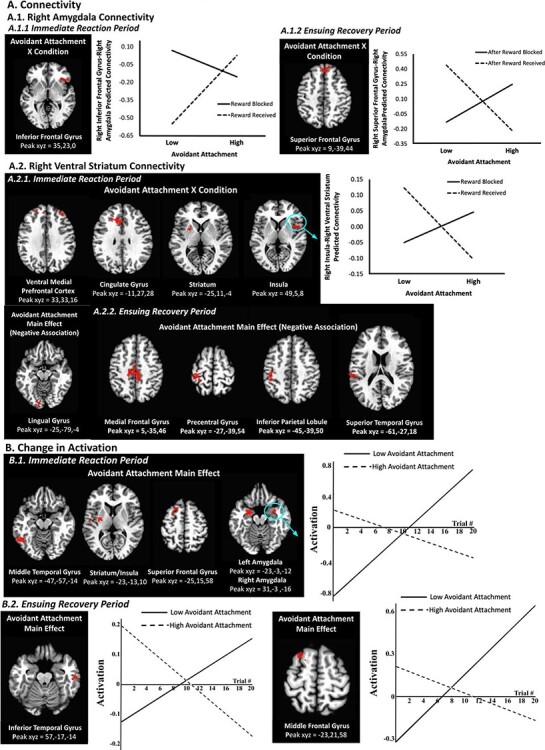
(A) The avoidant attachment × condition interaction predicts changes in right amygdala connectivity during the immediate reaction period (A.1.1) and the ensuing recovery period (A.1.2). The avoidant attachment × condition interaction predicts changes in right ventral striatum connectivity during the immediate reaction period (A.2.1), and avoidant attachment (main effect) negatively predicts changes in right ventral striatum connectivity during the ensuing recovery period (A.2.2). Avoidant attachment is plotted at 1 s.d. below the mean (‘low’) and 1 s.d. above the mean (‘high’). (B) Avoidant attachment (main effect) predicts changes in brain activation during both the immediate reaction period (B.1) and the ensuing recovery period (B.2). The ‘low avoidant attachment’ line depicts the changes in average brain activation over time for participants with avoidant attachment scores that were 1 s.d., or greater, below the mean; the ‘high avoidant attachment’ line depicts the changes in average brain activation over time for participants with avoidant attachment scores that were 1 s.d., or greater, above the mean. Brain images (threshold set at whole-brain corrected false probability rate of P < 0.05; axial view for all images) depict significant clusters. Only one cluster is plotted as an example when a contrast contains multiple regions with similar patterns.
Table 2.
Significant clusters resulting from connectivity analyses
| k | F (df = 1,22) | x | y | z | BA | Region |
|---|---|---|---|---|---|---|
| Right amygdala connectivity during the immediate reaction period | ||||||
| Avoidant attachment × condition | ||||||
| 100 | 30.44 | 35 | 23 | 0 | 47 | Inferior frontal gyrus |
| Avoidant attachment | ||||||
| No clusters | ||||||
| Right amygdala connectivity during the ensuing recovery period | ||||||
| Avoidant attachment × condition | ||||||
| 75 | 22.38 | 9 | 39 | 44 | 8 | Superior frontal gyrus |
| Avoidant attachment | ||||||
| No clusters | ||||||
| Left ventral striatum connectivity during the immediate reaction period | ||||||
| Avoidant attachment × condition | ||||||
| No clusters | ||||||
| Avoidant attachment | ||||||
| No clusters | ||||||
| Left ventral striatum connectivity during the ensuing recovery period | ||||||
| Avoidant attachment × condition | ||||||
| No clusters | ||||||
| Avoidant attachment | ||||||
| No clusters | ||||||
| Right ventral striatum connectivity during the immediate reaction period | ||||||
| Avoidant attachment × condition | ||||||
| 1053 | 32.11 | 33 | 33 | 16 | 32, 10, 9 | Ventromedial prefrontal cortex |
| 563 | 26.74 | −21 | −7 | 52 | 6 | Medial/middle frontal and precentral gyri |
| 416 | 26.61 | −11 | 27 | 28 | 32 | Cingulate gyrus |
| 201 | 32.46 | 49 | 5 | 8 | 13 | Insula |
| 177 | 28.41 | −25 | 11 | −4 | - | Striatum |
| 167 | 18.81 | 41 | −11 | 50 | 6 | Precentral gyrus |
| 95 | 25.41 | 23 | −23 | 36 | 31 | Cingulate gyrus |
| 94 | 19.32 | 23 | 1 | 40 | 6, 32, 24 | Middle frontal and cingulate gyri |
| 92 | 18.54 | 55 | −27 | 28 | 40 | Inferior parietal lobule |
| 72 | 21.95 | −25 | −89 | 8 | 18, 17, 19 | Cuneus, middle occipital gyrus |
| 70 | 16.65 | −35 | 39 | 28 | 9 | Superior/middle frontal gyri |
| Avoidant attachment | ||||||
| 115 | 17.54 | −25 | −79 | −4 | 1718 | Lingual Gyrus |
| Right ventral striatum connectivity during the ensuing recovery period | ||||||
| Avoidant attachment × condition | ||||||
| No clusters | ||||||
| Avoidant attachment | ||||||
| 287 | 23.76 | 5 | −35 | 46 | 6 | Medial frontal gyrus, paracentral lobule |
| 269 | 22.23 | −27 | −39 | 54 | 4 | Pre/postcentral gyrus |
| 116 | 23.63 | −45 | −39 | 50 | 40 | Inferior parietal lobule, postcentral gyrus |
| 86 | 24.48 | −61 | −27 | 18 | 40, 42 | Superior temporal and postcentral gyri |
BA = Brodmann area.
Note: Regions were identified by the Talairach–Tournoux atlas.
Furthermore, as expected, the avoidant attachment × condition interaction was significant during the immediate reaction period for right ventral striatum connectivity with multiple prefrontal/frontal regions (ventromedial and dorsolateral prefrontal cortices, cingulate and precentral gyri, insula) distributed across both hemispheres, as well as left striatum, right inferior parietal lobule and left cuneus (Figure 2A.2.1; Table 2). Across all of these clusters, patterns were similar: whereas lower levels of avoidant attachment were associated with less connectivity in response to reward blocked vs received trials, higher levels of avoidant attachment were associated with greater connectivity to reward blocked compared to reward received trials. In addition, there was a main effect of avoidant attachment for right ventral striatum connectivity with left lingual gyrus such that there was an inverse relationship between degree of avoidant attachment and connectivity across conditions (Figure 2A.2.1; Table 2). No contrasts with avoidant attachment were significant for left ventral striatum connectivity in the immediate reaction period.
Ensuing recovery period.
In line with our hypotheses, alterations in right amygdala–right inferior frontal gyrus connectivity were present in the ensuing recovery period (Figure 2A.1.2; Table 2). Here, the avoidant attachment × condition interaction was significant such that lower levels of avoidant attachment were associated with less amygdala–inferior frontal gyrus connectivity after reward blocked (vs received) trials, but higher levels of avoidant attachment were associated with the opposite pattern of greater connectivity. The main effects of avoidant attachment were not significant for the ensuing recovery period for right amygdala connectivity, and no contrasts with avoidant attachment were significant for left amygdala connectivity in the ensuing recovery period.
Some regions that were identified in the immediate reaction period additionally exhibited altered right ventral striatum connectivity in the recovery period, albeit only for the main effect and not for the avoidant attachment × condition interaction, partially supporting hypotheses. Thus, two prefrontal/frontal regions (medial prefrontal gyrus and paracentral gyrus), inferior parietal lobule and superior temporal gyrus, all in the left hemisphere, exhibited greater connectivity, regardless of condition, with right ventral striatum in adolescents with lower levels of avoidant attachment but less connectivity in adolescents with higher levels of avoidant attachment (Figure 2A.2.2; Table 2). No contrasts with avoidant attachment were significant for left ventral striatum connectivity during the ensuing recovery period.
Change in activation over the course of the task
Immediate reaction period.
Avoidant attachment × condition interactions were not significant for change in activation analyses in the immediate reaction period. However, there were multiple widespread significant clusters for the main effect of avoidant attachment during the immediate reaction period, spanning prefrontal/frontal, temporal and parietal cortices, as well as the basal ganglia and limbic system; specific regions included striatum, insula, dorsal prefrontal and temporal cortices (Figure 2B.1; Table 3). Clusters were predominantly in the left hemisphere, with the exception of the amygdala/parahippocampal gyri clusters, which were bilateral, and the right inferior parietal lobule. Across all clusters, patterns were similar: whereas adolescents with lower avoidant attachment increased their activation over the course of the task across conditions, adolescents with higher avoidant attachment decreased their activation, which is consistent with our hypotheses.
Table 3.
Significant clusters resulting from whole-brain change in activation analyses
| k | F (df = 1,22) | x | y | z | BA | Region |
|---|---|---|---|---|---|---|
| Change in activation during the immediate reaction period | ||||||
| Avoidant attachment × condition | ||||||
| No clusters | ||||||
| Avoidant attachment | ||||||
| 330 | 31.5 | −47 | −57 | −14 | 37 | Middle/Inferior temporal and fusiform gyri |
| 218 | 28.62 | −27 | −37 | 46 | 40 | Inferior parietal lobule |
| 191 | 29.56 | −25 | 15 | 58 | 6 | Superior/middle frontal gyri |
| 176 | 36.51 | 29 | −17 | −14 | 36 | Parahippocampal gyrus |
| 150 | 24.47 | −29 | −77 | 28 | 19 | Cuneus |
| 143 | 24.87 | −29 | −5 | 62 | 6 | Middle frontal/precentral gyri |
| 140 | 45.48 | −23 | −3 | −12 | - | Amygdala |
| 131 | 23.72 | −19 | −31 | −16 | 36, 35 | Parahippocampal gyrus |
| 115 | 23.32 | −27 | −63 | 52 | 7 | Superior parietal lobule |
| 97 | 30.6 | −59 | −23 | −14 | 21 | Middle temporal gyrus |
| 95 | 21.4 | −23 | −13 | 10 | - | Striatum, insula |
| 86 | 34.47 | 31 | −3 | −16 | 71 | Amygdala |
| 81 | 28.85 | −47 | −23 | 36 | 2 | Postcentral gyrus |
| 79 | 21.81 | 11 | −67 | −10 | - | Cerebellum |
| 67 | 23.18 | 59 | −29 | 24 | 40 | Inferior parietal lobule |
| Change in activation during the ensuing recovery period | ||||||
| Avoidant attachment × condition | ||||||
| No clusters | ||||||
| Avoidant attachment | ||||||
| 66 | 20.81 | 57 | −17 | −14 | 21 | Inferior temporal gyrus |
| 66 | 31.00 | −23 | 21 | 58 | 6 | Middle frontal gyrus |
BA = Brodmann area.
Note: Regions were identified by the Talairach–Tournoux atlas.
Ensuing recovery period.
As with responses to the immediate reaction period, avoidant attachment × condition interactions were not significant for change in activation analyses in the ensuing recovery period. Instead, the main effect of avoidant attachment was significant in the left middle frontal and right inferior temporal gyri (Figure 2B.2; Table 3). Consistent with hypotheses, and the pattern in response to the immediate reaction period, adolescents with lower avoidant attachment increased their activation over time, whereas adolescents with higher avoidant attachment decreased activation over time.
Discussion
This study was one of the first to examine neural correlates of attachment style in adolescents and the only one to do so using a frustrative non-reward paradigm with network analyses in a sample of treatment-seeking adolescents with trauma. Our theoretically driven (Cicchetti and Rogosch, 2002; Casey et al., 2010a; Wiggins and Monk, 2013) developmental approach emphasizes that early influences unfold and unpack over time, as maturing brain systems come ‘online’ and social/environmental changes (e.g. in school, peers, romantic relationships and life transitions) make vulnerabilities, such as avoidant attachment style, acquired early in development more relevant or apparent later in development. In this vein, individual differences in attachment style, which may reflect differences in emotion regulation strategies learned during infancy, relate to neural alterations in adolescence.
The complementary analytic approaches (functional connectivity and change in activation) revealed widespread differences in neural activity associated with avoidant attachment style that would have remained obscured if only a single technique were used. Furthermore, the use of multiple analysis techniques reduced the likelihood that the results are due to artifacts or idiosyncrasies of a single analytic approach. For example, analyzing the change in activation over the course of the task allowed us to identify the granular changes in brain responses over time that are not available using traditional activation analyses, as used in prior work (Vrticka et al., 2014; Debbane et al., 2017; McCormick et al., 2019). In both types of analyses, there was converging evidence, suggesting that avoidant attachment, adjusting for anxious attachment, is associated with emotion regulation strategies characterized by emotional disengagement or distancing from the source(s) of negative emotional events. We discuss each pattern of results separately.
When looking at functional connectivity during the task, avoidant attachment-related prefrontal–amygdala and frontostriatal connectivity alterations in the immediate reaction period persisted into the ensuing recovery period. That is, connectivity alterations in similar areas related to emotion regulation in the immediate reaction period also emerged during the recovery period (Table 2). These alterations may reflect a protracted emotional recovery from the negative emotional event (e.g. frustration when reward is blocked), as adolescents with avoidant attachment style may find negative emotional events to be particularly evocative and struggle to restore neutral mood.
Analyses examining the change in activation over the course of the task also support the view that adolescents with avoidant attachment find negative emotional events to be particularly evocative. At the beginning of the frustrative non-reward task, adolescents with higher compared to lower levels of avoidant attachment initially evidenced greater activation in regions that often associated with emotion (amygdala, insula, striatum, parahippocampal gyrus and superior frontal gyrus; Lindquist et al., 2016). However, adolescents with higher avoidant attachment decreased their activation as the task progressed, whereas those with lower avoidant attachment increased their activation. These patterns suggest that adolescents with high levels of avoidant attachment initially have outsized reactions to emotional stress but quickly tamp down those reactions to the point that they end up showing hypoactivation, relative to adolescents with lower avoidant attachment.
In sum, abundant differences in emotion- and reward-related neural patterns were identified in adolescents with higher vs lower levels of avoidant attachment during the frustrative non-reward task, consistent with prior findings using other attachment probes in preadolescents and adolescents (Vrticka et al., 2014; Choi et al., 2018). For instance, in prefrontal and amygdala networks, those higher in avoidant attachment showed the opposite pattern of neural responses immediately after the reward was blocked vs received as well as in the ensuing recovery period, compared to those lower in avoidant attachment. These considerable alterations in emotion- and reward-related brain regions may belie the emotionally distanced, low-affect exterior theorized to be most characteristic of avoidant attachment, suggesting that negative emotional events, such as frustrative non-reward, may be aversive internal experiences for avoidantly attached adolescents. Moreover, it is possible that altered recruitment of emotion regulation prefrontal/frontal regions signifies greater effort necessary to maintain avoidance strategies in the face of frustrative non-reward. Experiencing negative emotional events, such as frustrative non-reward, that are evocative to the point of being aversive may motivate individuals to avoid (rather than approach) the source of the negative emotions. Of note, whereas avoidant attachment style is theorized to be the opposite of anxious attachment style, people may engage in behaviors and responses from one or both attachment styles at various times. Indeed, avoidant attachment and anxious attachment were not significantly negatively correlated in our sample. Thus, our results represent neural associations of avoidant attachment, above and beyond anxious attachment.
This phenomenon has its parallels in psychopathology literature. Individuals with autism spectrum disorder show heightened sensitivity to emotional faces to the extent that they find the faces more aversive (Swartz et al., 2013) and thus avoid eye contact (Dalton et al., 2005). Whereas initial observations of individuals with autism spectrum disorder suggested that they did not find faces to be salient, as they attended away from faces (Ashwin et al., 2007), it was in fact the opposite—looking into another’s eyes was so evocative and thus, aversive, that individuals with autism spectrum disorder avoided faces (Monk et al., 2010; Weng et al., 2011; Swartz et al., 2013). Despite notable differences between those with autism spectrum disorder and those high on avoidant attachment, it may be the case that those who are avoidantly attached find negative emotional events, potentially including frustrative non-reward as used in the current paradigm, so evocative and aversive that they avoid them.
This study had several limitations. First, as this study was preliminary, the sample size was small. However, such a modest sample size precludes us from detecting small effects and, importantly, from examining avoidant attachment × anxious attachment interactions. Much work has treated avoidant and anxious attachment as opposite ends of the same spectrum (and our results for anxious attachment, displayed in Supplementary material, are in the opposite direction to avoidant attachment, supporting this notion) (Fraley and Waller, 1998). Nevertheless, these avoidant vs anxious attachment styles may also represent different dimensions. A larger sample with the representation of all combinations of high and low levels of both avoidant and anxious attachment will be necessary for interaction analyses. Second, the primary contrast of interest was ‘reward blocked’ vs ‘reward received’ after a correct hit, and trials where participants missed the target were excluded (for which they received no reward) due to very few trials of this type. Although in line with prior work using similar frustration tasks (Deveney et al., 2013; Tseng et al., 2019), this approach prevented us from determining whether attachment-related neural activation during reward blocked trials was due to the denial of a reward that participants perceived they had earned (i.e. negative prediction error) or due to an absence of a reward more generally. Task parameters were designed with the constraints inherent to scanning adolescents (e.g. limited time in scanner and limited number of trials), but future studies may wish to explore tasks that can more fully tap into the negative prediction error vs non-reward comparison.
To conclude, this preliminary study provides evidence consistent with the notion that early life experiences that shape attachment style have effects that persist into adolescence in the form of altered emotion reactivity and regulation neural patterns. The study population was composed of trauma-exposed adolescents from racial/ethnic minority groups often understudied in neuroscience and attachment research, so the results may generate hypotheses for diverse populations in future research. Overall, the findings contribute to research that delineates the neural underpinnings of attachment style—relevant for many forms of psychopathology—that unfold across the lifespan.
Supplementary Material
Acknowledgements
We thank the administration and staff of Gompers Preparatory Academy, including Director Vincent Riveroll, Assistant Director Lisa Maples, Ms Victoria Canto and the rest of the counseling department for assistance with recruitment. We gratefully acknowledge Drs Feion Villodas and Miguel Villodas, both from San Diego State University and the Healthy Child and Family Development Laboratory for aiding with recruitment. We thank the Translational Emotion Neuroscience and Development Laboratory for assistance in data collection and the San Diego State University MRI Center for help in scanning. We are grateful to the families who participated.
Contributor Information
Marvin Yan, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Elizabeth A Hodgdon, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Ruiyu Yang, Department of Psychology, San Diego State University, San Diego, CA 92120, USA.
Qiongru Yu, San Diego Joint Doctoral Program in Clinical Psychology, San Diego State University/University of California, San Diego, CA 92120, USA.
Tristen K Inagaki, Department of Psychology, San Diego State University, San Diego, CA 92120, USA; San Diego Joint Doctoral Program in Clinical Psychology, San Diego State University/University of California, San Diego, CA 92120, USA.
Jillian L Wiggins, Department of Psychology, San Diego State University, San Diego, CA 92120, USA; San Diego Joint Doctoral Program in Clinical Psychology, San Diego State University/University of California, San Diego, CA 92120, USA.
Funding
This work was supported by a Brain and Behavior Research Foundation Young Investigator Grant (#26802) to Dr J.L.W.
Conflict of interest
The authors have no conflicts of interest to report.
Supplementary data
Supplementary data are available at SCAN online.
References
- Ainsworth M.D. (1985). Patterns of attachment. Clinical Psychologist, 38(2), 27–9. [Google Scholar]
- Alloy L.B., Nusslock R. (2019). Future directions for understanding adolescent bipolar spectrum disorders: a reward hypersensitivity perspective. Journal of Clinical Child & Adolescent Psychology, 48(4), 669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel A. (1958). The role of frustrative nonreward in noncontinuous reward situations. Psychological Bulletin, 55(2), 102–19. [DOI] [PubMed] [Google Scholar]
- Ashwin C., Baron-Cohen S., Wheelwright S., O’Riordan M., Bullmore E.T. (2007). Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger syndrome. Neuropsychologia, 45(1), 2–14. [DOI] [PubMed] [Google Scholar]
- Bowlby J. (1969/1982). Attachment and Loss, Vol. 1: Attachment. Attachment and Loss. New York: Basic Books. [Google Scholar]
- Breidenstine A.S., Bailey L.O., Zeanah C.H., Larrieu J.A. (2011). Attachment and trauma in early childhood: a review. Journal of Child & Adolescent Trauma, 4, 274–90. [Google Scholar]
- Brennan K.A., Clark C.L., Shaver P.R. (1998). Self-report measurement of adult attachment: an integrative overview. In: Simpson, J.A., Rholes, W.S., editors. Attachment Theory and Close Relationships. New York, NY: The Guilford Press, 46–76. [Google Scholar]
- Casey B.J., Duhoux S., Cohen M.M. (2010a). Adolescence: what do transmission, transition, and translation have to do with it? Neuron, 67(5), 749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., et al. (2010b). The storm and stress of adolescence: insights from human imaging and mouse genetics. Developmental Psychobiology, 52(3), 225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., Taylor M.J., Hong S.B., Kim C., Yi S.H. (2018). The neural correlates of attachment security in typically developing children. Brain and Cognition, 124, 47–56. [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A. (2002). A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology, 70(1), 6–20. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debbane M., Badoud D., Sander D., Eliez S., Luyten P., Vrticka P. (2017). Brain activity underlying negative self- and other-perception in adolescents: the role of attachment-derived self-representations. Cognitive, Affective & Behavioral Neuroscience, 17(3), 554–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney C.M., Connolly M.E., Haring C.T., et al. (2013). Neural mechanisms of frustration in chronically irritable children. American Journal of Psychiatry, 170(10), 1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWall C.N., Masten C.L., Powell C., Combs D., Schurtz D.R., Eisenberger N.I. (2012). Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience, 7(2), 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty L.R., Schwartz K.T.G., Kryza-Lacombe M., Weisberg J., Spechler P.A., Wiggins J.L. (2018). Preschool- and school-age irritability predict reward-related brain function. Journal of the American Academy of Child and Adolescent Psychiatry, 57(6), 407–17.e2. [DOI] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., et al. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–58. [DOI] [PubMed] [Google Scholar]
- Fraley R.C., Waller N.G. (1998). Adult attachment patterns: A test of the typological model. In: Simpson, J.A., Rholes, W.S., editors. Attachment Theory and Close Relationships. New York, NY: The Guilford Press, 77–114. [Google Scholar]
- Galvan A. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath O., Bunge S.A., Shaver P.R., Wendelken C., Mikulincer M. (2005). Attachment-style differences in the ability to suppress negative thoughts: exploring the neural correlates. Neuroimage, 28(4), 835–47. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J., Rutter M. (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–73. [DOI] [PubMed] [Google Scholar]
- Helfinstein S.M., Kirwan M.L., Benson B.E., et al. (2013). Validation of a child-friendly version of the monetary incentive delay task. Social Cognitive and Affective Neuroscience, 8(6), 720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgdon E.A., Yu Q., Kryza-Lacombe M., et al. (2021). Irritability-related neural responses to frustrative non-reward in adolescents with trauma histories: a preliminary investigation. Developmental Psychobiology, 63(6), e22167. [DOI] [PubMed] [Google Scholar]
- Holt M.K., Kantor G.K., Finkelhor D. (2009). Parent/child concordance about bullying involvement and family characteristics related to bullying and peer victimization. Journal of School Violence, 1, 42–63. [Google Scholar]
- Hungerford A., Ogle R.L., Clements C.M. (2010). Children’s exposure to intimate partner violence: relations between parent-child concordance and children’s adjustment. Violence and Victims, 25(2), 185–201. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick E.M., McElwain N.L., Telzer E.H. (2019). Alterations in adolescent dopaminergic systems as a function of early mother-toddler attachment: a prospective longitudinal examination. International Journal of Developmental Neuroscience, 78, 122–9. [DOI] [PubMed] [Google Scholar]
- Mikulincer M., Shaver P.R., Pereg D. (2003). Attachment theory and affect regulation: the dynamics, development, and cognitive consequences of attachment-related strategies. Motivation and Emotion, 27, 77–102. [Google Scholar]
- Monk C.S., Weng S.J., Wiggins J.L., et al. (2010). Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry and Neuroscience, 35(2), 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Hoflich A., Atanelov J., et al. (2012). Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by FMRI. PLoS One, 7(11), e50050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A., Moretti M. (2005). Convergent and discriminant validity of the Comprehensive Adolescent-Parent Attachment Inventory. In: Paper presented at the Poster presented at the annual meeting of the Canadian Psychological Association. Montreal, Canada. [Google Scholar]
- Stevens J.S., Kim Y.J., Galatzer-Levy I.R., et al. (2017). Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biological Psychiatry, 81(12), 1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. (2013). Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52(1), 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W.L., Deveney C.M., Stoddard J., et al. (2019). Brain mechanisms of attention orienting following frustration: associations with irritability and age in youths. American Journal of Psychiatry, 176(1), 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof M.J., Riem M., Garrett A., et al. (2019). Unresolved-disorganized attachment is associated with smaller hippocampus and increased functional connectivity beyond psychopathology. Journal of Traumatic Stress, 32(5), 742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P., Andersson F., Grandjean D., Sander D., Vuilleumier P. (2008). Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One, 3(8), e2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P., Bondolfi G., Sander D., Vuilleumier P. (2012). The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience, 7(5), 473–93. [DOI] [PubMed] [Google Scholar]
- Vrticka P., Sander D., Anderson B., Badoud D., Eliez S., Debbane M. (2014). Social feedback processing from early to late adolescence: influence of sex, age, and attachment style. Brain and Behavior, 4(5), 703–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrticka P., Vuilleumier P. (2012). Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience, 6, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S.J., Carrasco M., Swartz J.R., et al. (2011). Neural activation to emotional faces in adolescents with autism spectrum disorders. Journal of Child Psychology and Psychiatry, 52(3), 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Monk C.S. (2013). A translational neuroscience framework for the development of socioemotional functioning in health and psychopathology. Development and Psychopathology, 25(4 Pt 2), 1293–309. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Blackford J.U., Luksik A., Gauthier I., Heckers S. (2013). Reduced habituation in patients with schizophrenia. Schizophrenia Research, 151(1–3), 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Yu Q., Owen C.E., Aspe G.I., Wiggins J.L. (2021). Contributions of childhood abuse and neglect to reward neural substrates in adolescence. Neuroimage: Clinical, 32, 102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


