Abstract
The cerebellum is one-third the size of the cerebrum yet holds twice the number of neurons. Historically, its sole function was thought to be in the calibration of smooth movements through the creation and ongoing modification of motor programs. This traditional viewpoint has been challenged by findings showing that cerebellar damage can lead to striking changes in non-motor behavior, including emotional changes. In this manuscript, we review the literature on clinical and subclinical affective disturbances observed in individuals with lesions to the cerebellum. Disorders include pathological laughing and crying, bipolar disorder, depression and mixed mood changes. We propose a theoretical model based on cerebellar connectivity to explain how the cerebellum calibrates affect. We conclude with actionable steps for future researchers to test this model and improve upon the limitations of past literature.
Keywords: cerebellum, affect, bipolar disorder, depression, pseudobulbar affect
Introduction
The traditional view of the cerebellum is that it is engaged solely in motor-related actions, such as the coordination of voluntary movements, gait, posture and speech (Holmes, 1939; Glickstein, 1992, 1993; Brodal and Bjaalie, 1997; Fine et al., 2002; Ito, 2002). However, interest has shifted to how the cerebellum might contribute to non-motor functions (Strick et al., 2009). These investigations were prompted in part by Schmahmann and colleagues’ seminal work reporting that some individuals, after sustaining a cerebellar injury to the posterior lobe of the cerebellum, develop ‘Cerebellar Cognitive Affective Syndrome’ (CCAS) (Schmahmann, 1998; Schmahmann and Sherman, 1998). Deficits associated with this disorder include flattened affect, reduced emotional expressivity, disinhibited, childish behavior and changes in linguistic output. A few years later, studies were published describing a similar constellation of altered cognition, affective and social behavior in children following resection of cerebellar tumors (Riva and Giorgi, 2000).
These studies were not the first to report changes in affect following cerebellar damage. Some early studies reported unexpected emotional problems resulting from damage to the cerebellum, but interpretations were complicated by the prevailing thought at the time that the cerebellum’s function was restricted to smoothing and coordinating movement (Schmahmann, 1996). Early nineteenth century case studies described patients with cerebellar agenesis, atrophy and degeneration in whom intellectual, social and emotional deficits were severe enough to saturate their personalities (Schmahmann, 2000). Later, Kutty and Prendes (1981) found pervasive behavioral and emotional changes since age 11 in another patient with cerebellar degeneration. The patient was described as belligerent and unmanageable and suffered from severe psychotic episodes that required medication and hospitalization. Similarly, Hamilton et al. (1983) published three case studies in which patients were diagnosed with bipolar disorder and schizophrenia: two of the cases involved cerebellar degeneration, while the third case involved a cerebellar tumor. Genís et al. (1995) investigated 22 patients with spinocerebellar ataxia type 1 (SCA1), a specific expression of a rare genetic disorder involving progressive cerebellar dysfunction (Opal and Ashizawa, 1998), and reported manic-like symptoms later in life including euphoria, emotional lability, nocturnal shouting and crying and irritability. These early studies suggest a cerebellar role in affect, but it remains unclear how prevalent these outcomes are and what mechanism produces them.
In this manuscript, we review the lesion literature on the cerebellum and affect with a focus on mood disorders. Given the heterogeneity of mental disorders, it is now recognized that mapping transdiagnostic symptoms to their underlying brain circuitry can have a greater translational impact than focusing on categorical disorders. Thus, we investigated a diverse range of affective changes, both diagnostic and subthreshold, associated with cerebellar lesions. Second, we provide a mechanistic framework for the cerebellum’s role in affective processes. Through this model, we seek to bridge the gap between the psychological and neurological literature studies to evaluate the cerebellum’s role in affect-related dysregulation. Finally, we provide suggestions for future research, including actionable steps for investigators based on the limitations of prior work.
Details of our search method are shown in Figure 1A. We used PubMed and Google Scholar search engines with one of three primary key words: ‘mood’, ‘emotion’ or ‘affect’. We then combined each primary key word with one of four secondary search terms: ‘cerebellum/cerebellar lesion’, ‘posterior fossa’, ‘Joubert’s syndrome’ or ‘Dandy–Walker’. These disorder terms were searched for individually because even though they involve cerebellar abnormalities, these cases are not reliably returned using the secondary term ‘cerebellum/cerebellar lesion’. We then supplemented our search by examining relevant publications from bibliographies of papers returned using the primary search method. Our goal was to cast our net widely.
Fig. 1.
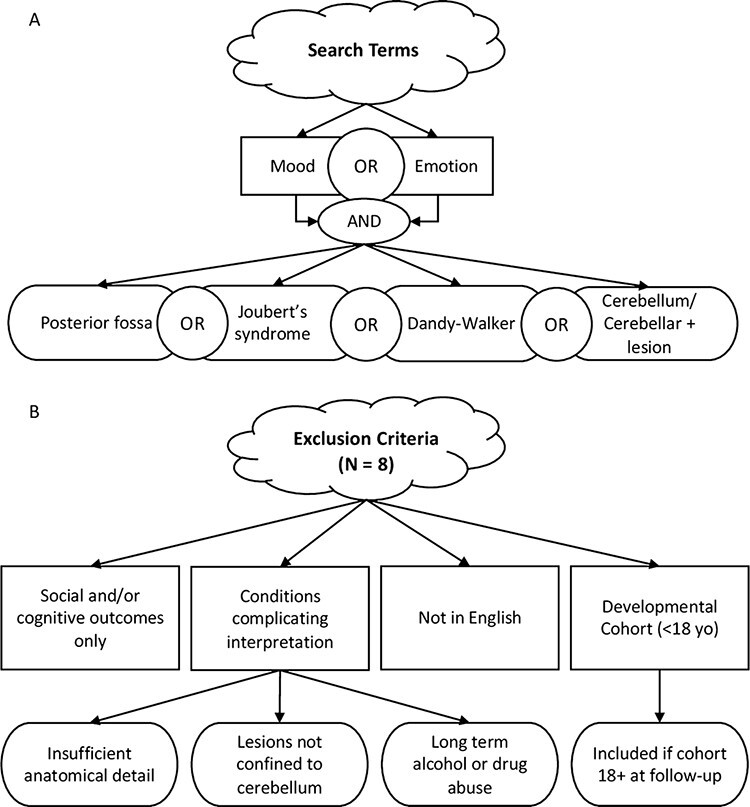
Schematic diagram of how studies were selected for review. (A) Search terms. (B) Exclusion criteria.
Included publications were either original (i.e., case reports) or secondary research describing adult patients with focal or degenerative lesions experiencing changes in affect, at both diagnostic and subthreshold severity levels. Importantly, we included patients with degenerative cerebellar disorders, in contrast to past reviews that excluded them (Lupo et al., 2019), because we felt that this exclusion is too restrictive, as a large proportion of cases of cerebellar damage in adults are due to degenerative disorders. Cases that were presented without personal or family history of psychiatric disease were assumed to be due to cerebellar dysfunction. Additionally, we included cases in which the reported symptoms were below the threshold for a diagnosis in order to capture the full range of affective impairment or change in cerebellar patients. For example, our search revealed some reports describing manic and bipolar symptoms in cerebellar patients that were not included in a prior review (Lupo et al., 2019) potentially because no formal diagnosis was made in these cases (e.g. Genís et al., 1995; Stone et al., 2001). Note that a transdiagnostic approach is congruous with the Research Domain Criteria (RDoC) framework advocated by the US National Institute of Mental Health. Exclusion criteria and counts of excluded publications are illustrated in Figure 1B. We excluded pediatric cases of cerebellar lesions because they are typically more severe than adult cases due to the protracted development of this brain region (Stoodley and Limperopoulos, 2016). We identified a total of 46 studies that fit our criteria. Full results of our search are presented in Table 1.
Table 1.
Lesion literature search findings
| Authors | Lesion site | Etiology | N | Findings |
|---|---|---|---|---|
| Pseudobulbar affect | ||||
| Parvizi and Schiffer (2007) | Midline | Cyst | 1 | Frequent inappropriate crying 5+ years coinciding with worsening tremor |
| Famularo et al. (2007) | Vermis and 4th ventricle | Tumor | 1 | 4 month hx of syncope following intense pathological laughter |
| Parvizi et al. (2001) | R WM | Stroke | 1 | Pathological laughter and crying of 1+ year duration |
| Kim and Choi-Kwon (2000) a | All areas | Stroke | 148 | Poststroke emotional incontinence in 22.2% of patients w/CB lesions |
| Doorenbos et al. (1993) | L anterior lobe (cortex and WM), FN, anterior & posterior interposed CB nuclei and superior CB peduncle | Hemorrhage | 1 | Forced, involuntary pathological laughter spurred by a CB tremor in left arm and leg when initiating movement |
| Manic and bipolar symptoms | ||||
| Lupo et al. (2018) | L lobules VI, Crus I, IX; posterior vermis | Anyeurism rupture | 1 | Mania, emotional lability, disinhibited laughter and anger |
| Yadalam et al. (1985) | 4th ventricle, vermis and CB cortices | Familial cerebellar degeneration | 2 | Two sisters: one diagnosed with schizoaffective disorder, predominantly manic; the other diagnosed with manic-depressive illness |
| Matsumoto et al. (2020) | L hemisphere | Tumor | 1 | Met criteria for mania 5 months postoperatively |
| Garg et al. (2019) b | Vermis, superior CB peduncles, brainstem and 4th ventricle | Joubert’s Syndrome | 1 | Unprovoked screaming, increased talking, energy and aggression, decreased need for sleep and food, poorly groomed with inappropriate laughter, restless, spontaneous singing and dancing, irritable and labile affect, delusions of persecution. Two discrete episodes ∼2 months each; bipolar disorder diagnosis |
| Genís et al. (1995) | Middle CB peduncles | SCA1 | 22 | Euphoria, emotional lability-near end of life in most. Nocturnal shouting and crying, irritability and aggressiveness |
| Jagadesan et al. (2014) | L hemisphere | Stroke | 1 | Excessive and spontaneous talking, quarreling, sleepless night, accelerated psychomotor activity, euphoric and irritable mood, grandiose thoughts and distractibility. Lacked insight into MI |
| Kutty and Prendes (1981) | Prepontine, pontocerebellar and 4th ventricle | Neurodegeneration | 1 | Behavioral/emotional changes since age 11: belligerent, unmanageable and psychotic breakdowns requiring hospitalization and medication |
| Stone et al. (2001) | All areas | SCA8 | 2 | P #1 had labile mood, aggression, was methodical and inflexible, lacked insight and deficient on several cognitive domains. Mother (P #2) had labile mood, was inflexible and irresponsible |
| Das et al. (2015) c | Inferior medial R hemisphere and posterolateral/dorsolateral medulla | Stroke | 1 | Hypomanic sx within a month after stroke at age 86 and then had 3 worsening manic episodes before stabilizing on psychotropic medication and dying at age 90 |
| Eudo et al. (2012) | R paramedial cerebellum | Stroke | 1 | 81-year-old man presented with 2 week manic episode revealing a CB stroke; no prior hx of manic sx, one depressive episode at age 57 after losing job |
| Kozak et al. (2015) | Posterior inferior hemispheres | Stroke | 1 | Hypomanic episode began 2nd day after hospital admission for stroke. Follow up at 6 months unremarkable |
| Batmaz et al. (2017) | All areas, posterior fossa and lateral ventricles | DWM | 1 | DWM diagnosis in childhood. Presented with complaints of decreased need for sleep, irritability and recently increased speed of thoughts. Loud fast speech, grandiose attitude, irritable mood and elevated affect, prone to anger. No insight; diagnosed with bipolar disorder |
| Bakhla et al. (2010) | Vermis, posterior fossa and 4th ventricle | DWM | 1 | Presented with hyperactivity, over-talkativeness, tall claims, decreased need for sleep and aggressive behavior. Hx of 2 manic episodes without any depressive episode. dipolar I disorder diagnosis |
| Turan et al. (2010) | Posterior fossa | DWC; mega cisterna magna | 1 | Hx of manic sx; presented with inappropriate and euphoric affect, flight of ideas, irritability, aggressive behaviors and psychotic sx. Increase in self-esteem, immersion in projects, shopping, energy and talking. Decreased need for sleep. Partial insight. Hx of depression in father. At follow-up, euthymic state, no more hallucinations or delusions |
| Marques (2019) | Vermis and posterior fossa | DWV; mega cisterna magna | 1 | 20 year hx of ‘paranoid schizophrenia’- fluctuant cliniphilia, irritable mood, impulsive behavior, but no psychotic sx |
| Slattery et al. (2010) | All areas | Neurodegeneration | 1 | Developed bipolar affective disorder in 7 years following paraneoplastic CB degeneration diagnosis |
| Li et al. (2008) | Vermis and 4th ventricle | DWS | 1 | Admitted to hospital because of severe depressive episode. Presented with psychomotor retardation, depressed mood, anhedonia, insomnia and anorexia. Diagnosed with bipolar II disorder because of hyperactive and elevated mood 1 month prior |
| Can et al. (2014) | Vermis, hemispheres and 4th ventricle | DWV; mega cisterna magna | 1 | 12 year hx of Bipolar I Disorder presenting with manic and depressive sx. Neurologic and bipolar sx both began around age 20 |
| Kalayam et al. (1994) | L hemisphere | Tumor | 1 | Long hx of recurrent mania, but no family hx. Manic and depressive periods |
| Lauterbach, (1996) d | R hemisphere, overall atrophy and R midbrain at inferolateral border of substantia nigra | Mixed | 15 | 20% subjects developed bipolar disorders after focal CB circuit lesions |
| Mariën et al. (2008) | All areas, especially vermis | Hypoplasia (Gillespie’s syndrome) | 1 | Impulsiveness, inappropriate behavior, facetiousness, mild euphoria and foul language |
| Mariën et al. (2009) | R CB/pons | Stroke | 1 | Disinhibition, overfamiliarity, confabulations and perseverations |
| Blunted affect and depressive symptoms | ||||
| Annoni et al. (2003) | L FN and 4th ventricle | Stroke | 1 | Affectively indifferent, depressed and apathetic; abnormal SCR for negative and positive reinforcement |
| Belser-Ehrlich et al. (2020) | Vermis and 4th ventricle | DWS | 1 | Father reported blunted affect and apathy and irresponsible lifestyle, patient denied concerns; abnormal startle eyeblink, but normal valence ratings |
| Gottwald et al. (2004) | Mixed areas, including vermis | Tumor and hematoma | 21 | P’s mildly depressed, reported feeling significantly more dejected, decreased motivation and initiative and tiredness |
| Kim et al. (2017) | Mixed areas | Stroke | 24 | 30% occurrence of depression in patients with first-time isolated CB stroke |
| Klinke et al. (2010) | Not specified | SCA1, SCA2, SCA3 and SCA6 | 32 | Mildly depressed mood in 75% of SCA6 patients, 50% of SCA1 patients, 33% SCA2 patients and 13% of SCA3 patients. Similar scores in 14% of controls |
| Liszewski et al. (2004) e | Not specified | Neurodegeneration | 133 | Most common psychiatric manifestation was depression (major and nonmajor) |
| Lo et al. (2016) | Not specified | SCA1, SCA2, SCA3 and SCA6 | 300 | Comorbid depression occurred at an average rate of 26% across SCA types. Suicidal ideation was common in 52% of patients and more frequent in SCA3 (65%) |
| Clausi et al. (2019) | All areas | Mixed | 38 | 26% of CB patients were affected by depressive sx. Depressed CB patients had similar scores to depressed patients without CB pathology |
| Fancellu et al. (2013) | Not specified | SCA1 and SCA2 | 42 | SCA patients showed significantly higher depression and apathy scores than controls. No significant change in psychiatric status after 2 years |
| Exner et al. (2004) | SCA and PICAf territories | Stroke | 11 | PICA subjects scored significantly higher on measures of emotional withdrawal and blunted affect compared to SCA subjects |
| Orsi et al. (2011) g | Not specified | SCA1, SCA2, SCA6 and SCA 8 | 33 | 72% patients had subthreshold depression and 47% mild disorder. Marginally significant increase in depression compared to controls |
| Mixed mood symptoms | ||||
| Schmahmann and Sherman (1998) | Vermis | Mixed | 20 | Changes ranging from passivity and blunting of emotion to disinhibited and child-like behavior |
| Bobo et al. (2009) | L hemisphere | Stroke | 1 | Presented 2× yearly with recurrent mania, delirium and prominent catatonic features; at 10 month follow-up, no sx |
| Paulus et al. (2004) | L posterior | Stroke | 1 | Agitated, anxious, emotionally stressed and had panic disorder |
| Rolfs et al. (2003) | Not specified | SCA17 | 15 | 40% developed a psychiatric condition: schizophrenic sx, paranoia, mania, hallucinations, depression and anxiety |
| Baillieux et al. (2010) | L and R hemispheres and vermis | Mixed | 18 | Frontal-like behavioral abnormalities in 45% of patients ranging from apathy and flat affect to disinhibition syndrome, as well as obsessive-compulsive tendencies |
| Baillieux et al. (2006) | Posterior fossa and R hemisphere | Tumor | 1 | Initial post-operative euphoria followed by persistent emotional indifference, apathy and social withdrawal as well as excessive eating and cigarette smoking |
| Hickey et al. (2018) | Superior CB peduncles and vermis | Joubert’s syndrome | 3 | Mood dysregulation, depression, anxiety, aggression, suicidal depression and psychotic sx |
| Hamilton et al. (1983) | CB/pontine region; R CB hemisphere/posterior fossa | Neurodegeneration and tumor | 3 | Patients were diagnosed with bipolar disorder and schizophrenia |
Note. SCA1: Spinocerebellar ataxia type 1. FN: Fastigial nucleus. CB: Cerebellum/cerebellar. Sx: Symptoms. L: Left. R: Right. WM: White matter. MI: Mental illness. Hx: History. SCR: Skin conductance response. DWM: Dandy–Walker malformation. DWC: Dandy–Walker complex. DWV: Dandy–Walker variant. DWS: Dandy–Walker syndrome. VPT: Very preterm. PBA: Pseudobulbar affect.
Symptoms arose 2–4 months after stroke onset.
Unclear whether damage is to deep brain nuclei, white matter tracts or a combination of damage to the brainstem and cerebellum.
Unclear whether damage is to deep brain nuclei, white matter tracts or a combination of damage to the cerebellum, pons and medulla.
Unclear whether damage is to deep brain nuclei, white matter tracts or a combination of damage to the cerebellum and midbrain.
44% of cases met criteria for basal ganglia involvement. There was no significant difference in depression frequency between cases with and without basal ganglia involvement.
PICA: posterior inferior cerebellar artery.
Unclear whether damage is to deep brain nuclei, white matter tracts or a combination of damage to the cerebellum and midbrain.
Broad categories of disordered affect
Pseudobulbar affect following cerebellar damage
Pseudobulbar affect is a rare, neurological syndrome involving involuntary emotional expression somewhat disconnected from actual emotional experience and inappropriate to the context (Lauterbach et al., 2013). It has been depicted by characters in several films such as The Joker and Parasite. We found five articles describing pseudobulbar affect resulting from cerebellar lesions. For instance, one case study described a patient with a midline cerebellar cyst who displayed frequent and inappropriate crying (Parvizi and Schiffer, 2007), while another case study described a patient with intense pathological laughter, who was revealed to have a tumor affecting the vermis and fourth ventricle (Famularo et al., 2007).
Manic and bipolar symptoms following cerebellar damage
Symptoms of mania can include, but are not limited to, a natural state of rapid speech, decreased need for sleep, racing thoughts, an increase in goal-directed activity, psychomotor agitation, elevated mood, dysregulated affect and impulsivity. Manic and hypomanic episodes, the latter typically being less persistent and impairing, are necessary for diagnoses of bipolar I and II disorders, respectively (Dailey and Saadabadi, 2020). Our search found many studies that reported new manic or bipolar symptoms following cerebellar damage (n = 22). Notably, these patients’ symptoms tended to arise later in life, far past the median onset age of 25 for bipolar disorder (Dailey and Saadabadi, 2020).
Some cases describe both mania and pseudobulbar affect. For example, Garg et al. (2019) detail a patient with Joubert’s syndrome, a rare genetic disease characterized by cerebellar hypoplasia and malformations in the brainstem (often termed the ‘molar tooth’ sign on radiological reads of magnetic resonance imaging (MRI) scans; Joubert et al., 1969). This patient was diagnosed with bipolar disorder and experienced unprovoked screaming, inappropriate laughter and spontaneous singing and dancing. Because this patient had no family history of psychiatric disorder nor any episodes prior to age 24, it is highly likely that the behavioral disturbances and affect dysregulation were linked to the underdevelopment of the cerebellum. Cases such as this could suggest a similar underlying mechanism to pseudobulbar affect and mania, but to our knowledge this relationship has not yet been explored.
Other cases describe manic and bipolar symptoms in the absence of pseudobulbar affect. For instance, one patient met the criteria for mania five months after an operation to remove a left cerebellar hemisphere tumor (Matsumoto et al., 2020). Another study describes a patient who suddenly presented with excessive and spontaneous talking, quarreling, sleeplessness and euphoria but also irritable mood. It was discovered that they had had a stroke in the left cerebellar hemisphere (Jagadesan et al., 2014). Another group (Delle Chiaie et al., 2015) compared the incidence of psychiatric comorbidity following the onset of neurological disease between 16 individuals with cerebellar pathology and 13 individuals with Parkinson’s disease (see Figure 2). They found that bipolar disorders were diagnosed in 31% of patients with cerebellar disease but were not found in patients with Parkinson’s disease. The cerebellar group exhibited an overall higher incidence of psychiatric diagnoses (81%) than the Parkinson’s patients (62%). While larger samples are needed to replicate these results, this study offers two suggestions pertinent to our investigation. First, it suggests that mania is overrepresented in cerebellar disease given that the incidence of bipolar disorder ranges from 1% to 3% across the general population (Rowland and Marwaha, 2018). Second, this study provides preliminary evidence that affective changes in cerebellar patients are not merely caused by an emotional reaction to disease or disability (Westbrook and Viney, 1982), as cerebellar patients experienced psychiatric disorders at higher rates than patients with Parkinson’s disease. Instead, mood disorders observed in patients with the cerebellar disease may be due to some other underlying mechanism of affect dysregulation.
Fig. 2.
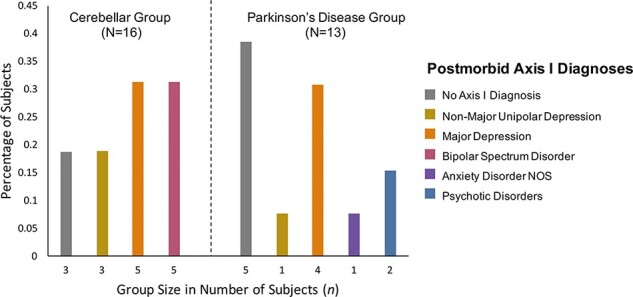
Data from Delle Chiaie et al. (2015, 2015), replotted to show the percentage of individuals with cerebellar damage and diagnosed axis 1 psychiatric disorders after the onset of neurological disease. Note that compared to a group of individuals with Parkinson’s disease, the incidence of bipolar spectrum disorder (rightmost bar within cerebellar group; n = 5) is very high following cerebellar damage.
Depressive symptoms following cerebellar damage
Depression is one of the most common mental illnesses. Symptoms include but are not limited to feelings of sadness, anhedonia, sleep troubles and fatigue (The National Institute of Mental Health, T. N. I. o. M., 2018). While reported less frequently than mania in cerebellar patients, depressive symptoms and blunted affect have been observed in the absence of other affect changes (n = 11 studies). Two publications (Annoni et al., 2003; Belser-Ehrlich et al., 2020) describe case studies of blunted affect and apathy following cerebellar damage as well as abnormal physiological measures of emotion modulation, including skin conductance responses and startle eyeblink. Another study of 24 patients with first-time isolated cerebellar stroke found a 30% occurrence of depressive disorder diagnoses (Kim et al., 2017). Note that this incidence is similar to the 31% incidence for both depressive and bipolar disorders in the aforementioned study by Delle Chiaie et al. (2015). While the incidence of depression diagnoses in that study was the same across patient groups (cerebellar disease and Parkinson’s disease), the consistency in depressive symptom incidence across cerebellar studies here (Delle Chiaie et al., 2015; Kim et al., 2017) is notable and may serve a role in predictions for future work. We note that recent findings have provided evidence for close interactions between the cerebellum and basal ganglion in both motor and reward processing (Bostan and Strick, 2018; Carta et al., 2019), and there is emerging evidence for cerebellar dysfunction in Parkinson’s disease [O’Shea et al., 2022]. It is possible that the depression seen in both Parkinson’s and cerebellar disease is related to diminished dopamine levels, modulated directly by the basal ganglion and indirectly by the cerebellum.
Mixed mood symptoms following cerebellar damage
Last, eight studies described mixed mood symptoms in cerebellar patients. To illustrate, Schmahmann and Sherman’s (1998) original publication on the emerging CCAS diagnosis details 20 patients, all with damage to the midline cerebellar vermis, whose affective changes ranged from passivity and blunted affect to disinhibited and child-like behavior, reflecting affective lability. Another study found that 40% of patients in a sample of individuals with spinocerebellar ataxia type 17 (SCA17), a rare genetic disorder involving the cerebellum, developed a psychiatric condition, but diagnoses ranged from paranoia, mania and hallucinations to depression and anxiety (Rolfs et al., 2003). Further, a case study of a patient with Dandy–Walker syndrome, a congenital brain malformation affecting the cerebellum, described a patient with a 9 year history of psychiatric symptoms with inconsistent diagnoses until they were diagnosed with psychosis not otherwise specified (Buonaguro et al., 2014). Interestingly, another case study described a left cerebellar hemisphere stroke patient who presented about twice yearly with recurrent mania, delirium and prominent catatonic features (Bobo et al., 2009; see also Mariën et al. 2013). The diagnostic messiness described in this section is not unique to individuals with cerebellar damage; it is estimated that 40% of patients with bipolar disorder experience mixed episodes, sometimes with psychotic features (Fagiolini et al., 2015).
Mood disorders in psychiatric sample populations: any role for the cerebellum?
Differences in cerebellar thickness, density or volume have been reported in individuals with traditionally defined psychiatric disorders. This has been reported in individuals with schizophrenia (Heath et al., 1979; Lippmann et al., 1982; Weinberger et al., 1983; Jurjus et al., 1994; Ichimiya et al., 2001; Keller et al., 2003; Yeganeh-Doost et al., 2011; Laidi et al., 2015; Moberget et al., 2018) and bipolar disorder, although findings are mixed (Adler et al., 2007; Baldaçara et al., 2011; Brambilla et al., 2002; DelBello et al., 1999; Demirgören et al., 2019; Dewan et al., 1988; Eker et al., 2014; Kim et al., 2013; Laidi et al., 2015; Lippmann et al., 1982; Lupo et al., 2021; Mills et al., 2005; Monkul et al., 2008; Nasrallah et al., 1982; Rieder et al., 1983). Reduced cerebellar volume, especially in the vermis, has been reported in individuals with unipolar depression (Shah et al., 1992; Escalona et al., 1993; Brambilla et al., 2002; Frodl et al., 2008; Peng et al., 2011; Schutter et al., 2012; Yucel et al., 2013). At a general level, these findings corroborate the lesion findings suggesting transdiagnostic linkages between the cerebellum and affect regulation.
What biological mechanism could explain these changes in thickness, density or volume in a neurologically normal population? One mechanism that has been proposed for thicker/thinner cerebellar volumes in bipolar patients is an inflammatory process that destroys synapses or cells (Kim and Choi-Kwon, 2000). Unfortunately, it is very difficult to measure neural inflammation in vivo. Another potential explanation (that is not mutually exclusive) rests on changes in cerebral metabolism. Radiologists have observed ‘crossed cerebellar diaschisis’—depressed metabolism in a cerebellar hemisphere secondary to dysfunction in the contralateral frontal lobe (Patronas et al., 1984). Prolonged depressed metabolism could starve cerebellar parenchyma of nutrients and ultimately cause cell death and volume loss. If true, the altered cerebellar volume observed in mood disorders should be preceded by hypometabolism. Intriguingly, several studies have reported cerebellar hypometabolism in bipolar disorder (Wu et al., 2021).
The cerebellum and emotional regulation: a theoretical model
Our review finds that changes in mood following cerebellar disease are quite common. Studies report pathological laughing and crying, manic and bipolar symptoms, blunted affect and depressive symptoms as well as mixed mood changes. Although there is considerable variability in the psychiatric symptoms and syndromes that are associated with cerebellar damage, manic and bipolar symptoms (n = 22) and blunted affect and depressive symptoms (n = 11) were the most common.
How can we extend these findings to mood disorders, more generally? In psychiatric parlance, the observed changes in mood map onto the symptom of ‘affective instability’, ‘mood lability’ or ‘emotional dysregulation’ that is defined in terms of the oscillation of, intensity of and/or the inability to regulate affect/mood (Marwaha et al., 2014). Affect dysregulation is transdiagnostic symptom and a known risk factor for and feature of a number of mental illnesses including unipolar and bipolar depression and schizophrenia (Broome et al., 2015; Fernandez et al., 2016; Marwaha et al., 2018).
How should we conceptualize the role of the cerebellum in transdiagnostic affect dysregulation? It is widely held that the cerebellum is an associative learning machine that learns an expected state for a given context, hence its role in all kinds of habit and procedural learning (Bellebaum and Daum, 2011). It is also widely believed that the cerebellum continuously checks for deviations from the learned expected state (Leggio and Molinari, 2015; Ivry, 2020). If a deviation is detected that exceeds a given threshold defined by the context, the cerebellum will refine the behavioral output—whether the response is overreaching to a coffee cup or crying too long and too loudly during a Hallmark movie. The detection of a deviation results in a recalibration of the internal model/expected state (Pierce and Péron, 2020). In other words, cells in the cerebellum tune the behavioral response to a sensory stimulus such that the response is dampened or heightened in a context-specific manner (Schmahmann, 2000, Vaaga et al., 2020). Thus without the cerebellum, the sensory → behavioral output link would be an on–off step function.
This tuning mechanism can be used to explain reward hyper- or hyposensitivity in bipolar disorder. Abnormally persistent goal-directed or reward-seeking behavior is a diagnostic criterion for mania (American Psychiatric Association, 2013), while diminished responsivity to reward and broad-ranging deficits in goal-directed behaviors are important features of depression (Paulus, 2015; Rizvi et al., 2018). One prominent model of bipolar disorder, the ‘Reward Hypersensitivity Model’ (see Figure 3A), argues that a key part of the pathophysiology of bipolar disorder is a hypersensitivity to rewards with receipt of rewards being associated with hyperactivation of reward circuits and nonreceipt of rewards being associated with deactivation of reward circuits (Johnson et al., 2012; Alloy et al., 2015). In individuals with vulnerabilities to affective disorders, hyperactivation of reward circuits is hypothesized to lead to manic episodes and deactivation of reward circuits is hypothesized to lead to depressive episodes. In this model, the responsiveness of reward system functioning to reward activating and deactivating events would be related to nucleus accumbens–frontal lobe connectivity (e.g. Damme et al., 2017).
Fig. 3.
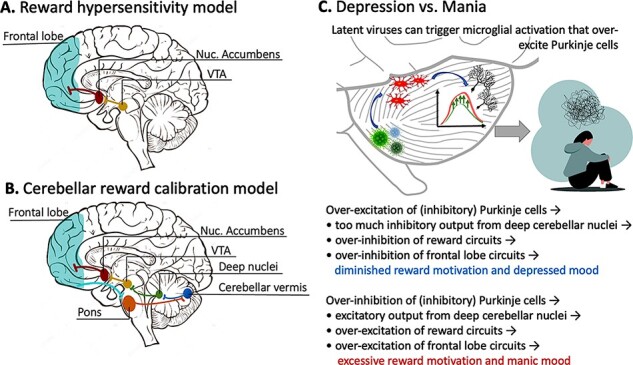
(A) A schematic depiction of the neural circuit described in the Reward Hypersensitivity Model of bipolar disorder. (B) A biologically inspired expansion of the Reward Hypersensitivity Model, described in this paper. Tract-tracing studies have shown that the frontal lobe receives input from the cerebellum and also sends input to the cerebellum via the corticopontocerebellar pathway. This allows for close communication between regions of the frontal lobe and the cerebellum. (C) How Purkinje cell firing patterns can be perturbed by toxins or pathogens, ultimately leading to changes in reward motivation and mood (top). An abbreviated description of perturbed signaling pathways (bottom).
Reward- and emotion-related processes are known to be intricately intertwined (Sander and Nummenmaa, 2021), with distinct reward processing components of reward anticipation and consummation being linked to wanting/approach motivation and liking/pleasure, respectively (Nguyen et al., 2021). Rewards evoke dopaminergic signals projecting from the ventral tegmental area (VTA) to the striatum that encode information about the discrepancy between reward expectancies and actual reward outcomes to facilitate goal-directed behavior (Schultz, 2016). Reward-related dopamine transmission within this circuit modulates reinforcement learning (Pessiglione et al., 2006; Kroemer et al., 2016), and reinforcement learning processes are thought to engage discrete affective states (e.g., approach and avoidance states and emotional states) that influence longer-term mood (Mendl et al., 2010; Eldar et al., 2021). Studies of patients with mood disorders have shown associations between blunted reward processing mechanisms (e.g., prediction error signaling) and depressed mood (Kumar et al., 2008, 2018) and heightened reward processing associated with manic symptoms (Bermpohl et al., 2010; O’Sullivan et al., 2011; Nusslock et al., 2012). Research on the ‘Reward Hypersensitivity Model’ has focused on nucleus accumbens–frontal lobe interactions (e.g. Damme et al., 2017). We propose an expanded version of this model grounded in known anatomical connections. In this model, reward hyper- or hyposensitivity is caused by perturbed cerebellar tuning of a polysynaptic circuit (see Figure 3B) that includes portions of the cerebellum that upregulate or downregulate dopamine output in the VTA, which has been robustly demonstrated in rodents (Carta et al., 2019). This, in turn, modulates the nucleus accumbens and, finally, portions of the frontal lobe. There is a known polysynaptic pathway from the cerebellum to medial prefrontal cortex; the cerebellum might create a model of the value of rewarding things, while the ventromedial prefrontal cortex stores the reward value. This region then provides feedback, via the corticopontocerebellar pathway, to the cerebellar cortex and then the entire process repeats itself.
Biological substrates of the model
For ease of discussion, throughout this paper, we have referred to the cerebellum as if it were a unitary structure. However, just like the cerebral cortex, the cerebellar cortex has a functional topography. The region that has been most consistently associated with emotion (broadly defined) is the cerebellar vermis. The vermis has anatomical connections with brainstem nuclei that control autonomic functions, such as heart rate and respiration (Stoodley and Schmahmann, 2010). Studies in rodents have found that vermal–amygdala connections are important for fear-related learning (Supple et al., 1987; Sacchetti et al., 2002; Zhu et al., 2011; Strata, 2015). Note that the cerebellum does not store emotional response patterns; therefore, lesions to the entire vermis do not destroy affective experience (Schmahmann and Sherman, 1998). Rather, emotions are expected to be dysregulated and labile. Thus, perturbation of the vermis, its connections to the deep nuclei or the output paths of the deep nuclei should ripple through this system, potentially causing mania, depression or rapid vacillations and lability (Minichino et al., 2014) depending on which groups of cells are affected and whether cells are being excessively excited or inhibited (see Figure 3C).
What biological mechanism might cause cells in the cerebellum to be excessively excited or inhibited? One study found that artificial inhibition of cerebellar Purkinje cells, which are inhibitory, in rodents can cause abnormal sociability and increased repetitive grooming behaviors (Tsai et al., 2012). Another study (Yamamoto et al., 2019) showed the opposite: when Purkinje cells become overexcited by neural immune cells called microglia, animals become less social and move less, mimicking depressive symptoms. Microglia become more active in the presence of a pathogen, and one study found elevated levels of Human herpes virus (HHV) (specifically, HHV-6A and HHV-6B) in postmortem cerebellar tissue (specifically in Purkinje cells), of individuals with major depression and bipolar disorder relative to healthy control brain tissue (Prusty et al., 2018; see Figure 3C). The authors noted that the affected cells were dysmorphic. Interestingly, this potentially explains the MRI findings mentioned earlier of cerebellar volume loss in the brains of individuals with major depression and bipolar disorder.
Testing the model
Our model is testable in human samples. For example, the model predicts that individuals with and at risk for bipolar disorder should have altered functional and structural connectivity between the cerebellar vermis and brain regions depicted in Figure 3B. The model also predicts that if noninvasive brain stimulation over the vermis is given in the context of arousing stimuli (like a positively valenced video), it should relatively increase or decrease (depending on the stimulation parameters) emotional responses such as heart rate, pupillary response and/or laughter in healthy samples.
Summary
In this paper, we reviewed the lesion literature on affective disturbances following lesions to the cerebellum. Compared to other review articles, we included a wider range of studies with the goal of presenting a more representative spectrum of the affective disturbances observed after cerebellar insult. In addition, we provided a testable model (see Figure 3) that explains the modulatory influence of the cerebellum on affect, as well as how damage to this structure can account for both mania and depression symptoms. Open questions can be found in Box 1.
Box 1. Questions for Future Research.
Why isn’t the cerebellum activated more consistently in functional magnetic resonance imaging (fMRI) studies of emotion? Our laboratory and others have found that emotional stimuli activate the cerebellar vermis (Metoki et al., 2021) (Lane et al., 1997; Garrett and Maddock, 2006; Turner et al., 2007; Baumann and Mattingley, 2012; Schraa-Tam et al., 2012; Keren‐Happuch et al., 2014; Guell et al., 2018); however, the majority of studies do not report this. One possibility is a methodological choice made at the level of image acquisition because it is common in fMRI studies to set the field of view so that the lower half of the cerebellum is sacrificed (Schlerf et al., 2014).
It has been suggested that the cerebellum is important for cognitive sequencing in domains such as social cognition (Leggio and Molinari, 2015; Van Overwalle et al., 2021). Relatedly, it has been proposed that the cerebellum is crucially involved in making moment-to-moment predictions about what’s next (Leggio and Molinari, 2015; Ivry, 2020). This suggests that its role in affective processes may be better captured by using dynamic stimuli, such as emotionally evocative film clips, rather than static images, which are more commonly used in fMRI studies (Trautmann et al., 2009; Van Overwalle et al., 2021). Our model assumes that the cerebellum is more generally involved in creating and utilizing internal models, including models about reward outcomes, across various functional domains.
Is the cerebellum’s involvement in emotion calibration restricted to regulation of bodily sensations such as heart rate, breathing rate and physical sensations of shock and fear? The cerebellum has traditionally been implicated in motor learning and calibration of movements that result in smooth, automatic actions. One possibility is that the cerebellum is involved in bodily aspects of emotion calibration but not cognitive aspects, such as reappraisal.
Apart from overt brain damage from stroke or tumor, what might cause cerebellar dysfunction? The literature suggests a few possibilities. Cerebellar Purkinje cells and microglia are particularly sensitive to toxic exposure, with the best-known example being alcohol toxicity (Victor, 1990; Sullivan et al., 1995). Another possibility is viral exposure accompanied by inflammation. Cerebellar microglia are more motile and have enhanced immune signaling compared to microglia in the cerebrum, suggesting that the cerebellum might be especially prone to the negative consequences of inflammation (Stoessel and Majewska, 2021).
Is there any link between ‘early’ cerebellar perturbation and mood disorders? The effects of cerebellar damage early in life are far more devasting and of longer duration than the effects of lesions in adulthood (Stoodley and Limperopoulos, 2016). Also, microglia change over the course of development and developing cerebellar microglia are particularly sensitive to environmental insult and thus may play a role in neuropsychiatric disease (Stoessel and Majewska, 2021).
Our review finds that changes in mood following cerebellar disease are quite common. Studies report pathological laughing and crying, manic and bipolar symptoms, blunted affect and depressive symptoms as well as mixed mood changes. Although there is considerable variability in the psychiatric symptoms and syndromes that are associated with cerebellar damage, manic and bipolar symptoms (n = 22) and blunted affect and depressive symptoms (n = 11) were the most common. The finding that is perhaps most striking is that the occurrence of mood disorder symptoms in cerebellar patients greatly exceeds the incidence rates of these disorders in the general population (Kessler et al., 2003; Rowland and Marwaha, 2018). The lesion findings are supported by MRI studies showing changes in cerebellar thickness, density or volume in individuals with a range of mood disorders. In addition, several PET studies have reported glucose hypometabolism in the cerebellum in bipolar disorder (e.g. Wu et al., 2021).
The lesion and neuroimaging findings were not sufficiently granular to specify which cerebellar regions are involved in affective modulation. However, the cerebellar vermis has long been regarded as the ‘limbic cerebellum’ in nonhuman animal research, and some lesion research supports this as well.
The reviewed findings strongly suggest a role for the cerebellum in the calibration of affect. Despite the fact that these findings are quite compelling—and we are not the first to report this (Adamaszek et al., 2017; Pierce and Péron, 2020)—textbooks on the brain and emotion do not include chapters on the cerebellum (Adolphs and Anderson, 2018; Gross, 2014), and dominant neuroscientific models of affect fail to discuss the cerebellum (Lindquist et al., 2012; Ochsner and Gross, 2013; Feldman-Barrett et al., 2016; Schore, 2016; Feldman-Barrett, 2020). This likely reflects the outdated view that the cerebellum is involved solely in balance, motor processing and various types of implicit learning, such as eyeblink conditioning (Strick et al., 2009). Our goal in writing this review was to bring the cerebellum to the attention of researchers interested in emotion research because we feel these findings are quite compelling.
Acknowledgements
We would like to thank Athanasia Metoki, Yin Wang, Katie Jobson, Aditya Bhise and Zachary Heffernan for their helpful insights during the planning stages of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Contributor Information
Madeleine R Frazier, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Linda J Hoffman, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Haroon Popal, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Holly Sullivan-Toole, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Thomas M Olino, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Ingrid R Olson, Department of Psychology, Temple University, Philadelphia, PA 19122, USA.
Funding
This work was supported by the National Institute of Health grants to I.R.O. (R01HD099165, R01 MH091113, R21 HD098509 and 2R56MH091113-11). The authors report no biomedical financial interests.
Conflict of interest
The authors declared that they had no financial or other conflict of interest with respect to their authorship or the publication of this article.
References
- Adamaszek M., D’Agata F., Ferrucci R., et al. (2017). Consensus paper: cerebellum and emotion. Cerebellum, 16(2), 552–76. [DOI] [PubMed] [Google Scholar]
- Adler C.M., DelBello M.P., Jarvis K., Levine A., Adams J., Strakowski S.M. (2007). Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biological Psychiatry, 61(6), 776–81. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Anderson D. (2018). The Neuroscience of Emotion: A New Synthesis. Princeton, NJ, Princeton University Press. [Google Scholar]
- Alloy L.B., Nusslock R., Boland E.M. (2015). The development and course of bipolar spectrum disorders: an integrated reward and circadian rhythm dysregulation model. Annual Review of Clinical Psychology, 11, 213–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn, Arlington, VA. [Google Scholar]
- Annoni J.-M., Ptak R., Caldara-Schnetzer A.-S., Khateb A., Pollermann B.Z. (2003). Decoupling of autonomic and cognitive emotional reactions after cerebellar stroke. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 53(5), 654–8. [DOI] [PubMed] [Google Scholar]
- Baillieux H., De Smet H.J., Lesage G., Paquier P., De Deyn P.P., Mariën P. (2006). Neurobehavioral alterations in an adolescent following posterior fossa tumor resection. Cerebellum, 5(4), 289–95. [DOI] [PubMed] [Google Scholar]
- Baillieux H., De Smet H.J., Dobbeleir A., Paquier P.F., De Deyn P.P., Mariën P. (2010). Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex, 46(7), 869–79. [DOI] [PubMed] [Google Scholar]
- Bakhla A.K., Sarkhel S., Kumar R. (2010). Bipolar I disorder in a patient with Dandy–Walker malformation. Psychiatry and Clinical Neurosciences, 64(2), 212–3. [DOI] [PubMed] [Google Scholar]
- Baldaçara L., Nery-Fernandes F., Rocha M., et al. (2011). Is cerebellar volume related to bipolar disorder? Journal of Affective Disorders, 135(1–3), 305–9. [DOI] [PubMed] [Google Scholar]
- Batmaz M., Balcik Z.E., Ürün Ö.Z.E.R., Yalçin B.H., Şakir Ö.Z.E.N. (2017). Dandy-Walker malformation presenting with affective symptoms. Archives of Neuropsychiatry, 54(3), 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. (2012). Functional topography of primary emotion processing in the human cerebellum. Neuroimage, 61(4), 805–11. [DOI] [PubMed] [Google Scholar]
- Bellebaum C., Daum I. (2011). Mechanisms of cerebellar involvement in associative learning. Cortex, 47(1), 128–36. [DOI] [PubMed] [Google Scholar]
- Belser-Ehrlich J., Lafo J.A., Mangal P., Bradley M., Wicklund M., Bowers D. (2020). Neurocognitive profile of a man with Dandy–Walker malformation: evidence of subtle cerebellar cognitive affective syndrome. The Clinical Neuropsychologist, 34(3), 591–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F., Kahnt T., Dalanay U., et al. (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping, 31(7), 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo W.V., Murphy M.J., Heckers S.H. (2009). Recurring episodes of Bell’s mania after cerebrovascular accident. Psychosomatics, 50(3), 285–8. [DOI] [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. (2018). The basal ganglia and the cerebellum: nodes in an integrated network. Nature Reviews Neuroscience, 19(6), 338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Barale F., Caverzasi E., Soares J.C. (2002). Anatomical MRI findings in mood and anxiety disorders. Epidemiologia E Psichiatria Sociale, 11(2), 88–99. [DOI] [PubMed] [Google Scholar]
- Brodal P., Bjaalie J.G. (1997). Salient anatomic features of the cortico-ponto-cerebellar pathway. Progress in Brain Research, 114, 227–49. [DOI] [PubMed] [Google Scholar]
- Broome M.R., Saunders K.E.A., Harrison P.J., Marwaha S. (2015). Mood instability: significance, definition and measurement. British Journal of Psychiatry, 207(4), 283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro E.F., Cimmarosa S., De Bartolomeis A. (2014). Dandy-Walker syndrome with psychotic symptoms: a case report. Rivisita Di Psichiatria, 49(2), 100–2. [DOI] [PubMed] [Google Scholar]
- Can S.S., Uğurlu G.K., Çakmak S. (2014). Dandy Walker variant and bipolar I disorder with graphomania. Psychiatry Investigation, 11(3), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I., Chen C.H., Schott A.L., Dorizan S., Khodakhah K. (2019). Cerebellar modulation of the reward circuitry and social behavior. Science, 363(6424), 1–4.doi: 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausi S., Lupo M., Olivito G., et al. (2019). Depression disorder in patients with cerebellar damage: awareness of the mood state. Journal of Affective Disorders, 245, 386–93. [DOI] [PubMed] [Google Scholar]
- Dailey M.W., Saadabadi A. (2020). Mania. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. [Google Scholar]
- Damme K.S., Young C.B., Nusslock R. (2017). Elevated nucleus accumbens structural connectivity associated with proneness to hypomania: a reward hypersensitivity perspective. Social Cognitive and Affective Neuroscience, 12(6), 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Chopra A., Rai A., Kuppuswamy P.S. (2015). Late‐onset recurrent mania as a manifestation of Wallenberg syndrome: a case report and review of the literature. Bipolar Disorders, 17(6), 677–82. [DOI] [PubMed] [Google Scholar]
- DelBello M.P., Strakowski S.M., Zimmerman M.E., Hawkins J.M., Sax K.W. (1999). MRI analysis of the cerebellum in bipolar disorder: a pilot study. Neuropsychopharmacology, 21(1), 63–8. [DOI] [PubMed] [Google Scholar]
- Delle Chiaie R., Minichino A., Salviati M., et al. (2015). Bipolar spectrum disorders in patients with cerebellar lesions: a comparison with Parkinson’s disease. The Journal of Nervous and Mental Disease, 203(9), 725–9. [DOI] [PubMed] [Google Scholar]
- Demirgören B.S., Özbek A., Karabekir N.G., et al. (2019). Cerebellar volumes in early-onset bipolar disorder: a pilot study of a stereological measurement technique. Psychiatry and Clinical Psychopharmacology, 29(3), 293–7. [Google Scholar]
- Dewan M.J., Haldipur C.V., Lane E.E., Ispahani A., Boucher M.F., Major L.F. (1988). Bipolar affective disorder. I. Comprehensive quantitative computed tomography. Acta Psychiatrica Scandinavica, 77(6), 670–6. [DOI] [PubMed] [Google Scholar]
- Doorenbos D.I., Haerer A.F., Payment M., Clifton E.R. (1993). Stimulus-specific pathologic laughter: a case report with discrete unilateral localization. Neurology, 43(1), 229–30. [DOI] [PubMed] [Google Scholar]
- Eker C., Simsek F., Yılmazer E.E., et al. (2014). Brain regions associated with risk and resistance for bipolar I disorder: a voxel-based MRI study of patients with bipolar disorder and their healthy siblings. Bipolar Disorders, 16(3), 249–61. [DOI] [PubMed] [Google Scholar]
- Eldar E., Pessiglione M., Van Dillen L. (2021). Positive affect as a computational mechanism. Cobeha, 39, 52–7. [Google Scholar]
- Escalona P.R., Early B., McDonald W.M., et al. (1993). Reduction of cerebellar volume in major depression: a controlled MRI study. Depression, 1, 156–8. [Google Scholar]
- Eudo C., Beaufils E., Desmidt T., et al. (2012). First manic episode revealing cerebellar stroke. Journal of the American Geriatrics Society, 60(3), 588–9. [DOI] [PubMed] [Google Scholar]
- Exner C., Weniger G., Irle E. (2004). Cerebellar lesions in the PICA but not SCA territory impair cognition. Neurology, 63(11), 2132–5. [DOI] [PubMed] [Google Scholar]
- Fagiolini A., Coluccia A., Maina G., et al. (2015). Diagnosis, epidemiology and management of mixed states in bipolar disorder. CNS Drugs, 29(9), 725–40. [DOI] [PubMed] [Google Scholar]
- Famularo G., Corsi F.M., Minisola G., De Simone C., Nicotra G. (2007). Cerebellar tumour presenting with pathological laughter and gelastic syncope. European Journal of Neurology, 14(8), 940–3. [DOI] [PubMed] [Google Scholar]
- Fancellu R., Paridi D., Tomasello C., et al. (2013). Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. Journal of Neurology, 260(12), 3134–43. [DOI] [PubMed] [Google Scholar]
- Feldman-Barrett L., Lewis M., Haviland-Jones J. (2016). Handbook of Emotions, 4th edn. New York, NY: The Guilford Press. [Google Scholar]
- Feldman-Barrett L. (2020). Seven and a Half Lessons about the Brain. Boston, MA: Houghton Mifflin Harcourt. [Google Scholar]
- Fernandez K.C., Jazaieri H., Gross J.J. (2016). Emotion regulation: a transdiagnostic perspective on a new RDoC domain. Cognitive Therapy and Research, 40(3), 426–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine E.J., Ionita C.C., Lohr L. (2002). The history of the development of the cerebellar examination. Seminars in Neurology, 22(4), 375–84. [DOI] [PubMed] [Google Scholar]
- Frodl T.S., Koutsouleris N., Bottlender R., et al. (2008). Depression-related variation in brain morphology over 3 years: effects of stress? Archives of General Psychiatry, 65(10), 1156–65. [DOI] [PubMed] [Google Scholar]
- Garg S., Rakesh K., Khattri S., Mishra P., Krishna Tikka S. (2019). Bipolar disorder with intellectual disability in Joubert syndrome: a case report. Psychiatry and Clinical Neurosciences, 73(12), 761–2. [DOI] [PubMed] [Google Scholar]
- Garrett A.S., Maddock R.J. (2006). Separating subjective emotion from the perception of emotion-inducing stimuli: an fMRI study. Neuroimage, 33(1), 263–74. [DOI] [PubMed] [Google Scholar]
- Genís D., Matilla T., Volpini V., et al. (1995). Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology, 45(1), 24–30. [DOI] [PubMed] [Google Scholar]
- Glickstein M. (1992). The cerebellum and motor learning. Current Opinion in Neurobiology, 2(6), 802–6. [DOI] [PubMed] [Google Scholar]
- Glickstein M. (1993). Motor skills but not cognitive tasks. Trends in Neurosciences, 16(11), 450–451; discussion 453–454. [DOI] [PubMed] [Google Scholar]
- Gottwald B., Wilde B., Mihajlovic Z., Mehdorn H.M. (2004). Evidence for distinct cognitive deficits after focal cerebellar lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 75(11), 1524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (2014). Handbook of Emotion Regulation, 2nd edn. New York, NY: The Guilford Press. [Google Scholar]
- Guell X., Gabrieli J.D.E., Schmahmann J.D. (2018). Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage, 172, 437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N.G., Frick R.B., Takahashi T., Hopping M.W. (1983). Psychiatric symptoms and cerebellar pathology. The American Journal of Psychiatry, 140(10), 1322–6. [DOI] [PubMed] [Google Scholar]
- Heath R.G., Franklin D.E., Shraberg D. (1979). Gross pathology of the cerebellum in patients diagnosed and treated as functional psychiatric disorders. The Journal of Nervous and Mental Disease, 167(10), 585–92. [DOI] [PubMed] [Google Scholar]
- Hickey C.L., Sherman J.C., Goldenberg P., et al. (2018). Cerebellar cognitive affective syndrome: insights from Joubert syndrome. Cerebellum & Ataxias, 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. (1939). The cerebellum of man. Brain, 62, 1–30. [Google Scholar]
- Ichimiya T., Okubo Y., Suhara T., Sudo Y. (2001). Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biological Psychiatry, 49(1), 20–7. [DOI] [PubMed] [Google Scholar]
- Ito M. (2002). Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Annals of the New York Academy of Sciences, 978, 273–88. [DOI] [PubMed] [Google Scholar]
- Ivry R. (2020). Human Cerebellar Function in Multiple Task Domains. University of California Berkeley, Berkeley, CA, United States: National Institute of Health (NIH). [Google Scholar]
- Jagadesan V., Thiruvengadam K., Muralidharan R. (2014). Cerebellar stroke-manifesting as mania. Indian Journal of Psychological Medicine, 36(3), 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.L., Edge M.D., Holmes M.K., Carver C.S. (2012). The behavioral activation system and mania. Annual Review of Clinical Psychology, 8, 243–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert M., Eisenring J.J., Robb J.P., Andermann F. (1969). Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology, 19(9), 813–25. [DOI] [PubMed] [Google Scholar]
- Jurjus G.J., Weiss K.M., Jaskiw G.E. (1994). Schizophrenia-like psychosis and cerebellar degeneration. Schizophrenia Research, 12(2), 183–4. [DOI] [PubMed] [Google Scholar]
- Kalayam B., Young R.C., Tsuboyama G.K. (1994). Mood disorders associated with acoustic neuromas. The International Journal of Psychiatry in Medicine, 24(1), 31–43. [DOI] [PubMed] [Google Scholar]
- Keller A., Castellanos F.X., Vaituzis A.C., Jeffries N.O., Giedd J.N., Rapoport J.L. (2003). Progressive loss of cerebellar volume in childhood-onset schizophrenia. American Journal of Psychiatry, 160(1), 128–33. [DOI] [PubMed] [Google Scholar]
- Keren‐Happuch E., Chen S.H., Ho M.H., Desmond J.E. (2014). A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human Brain Mapping, 35(2), 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., et al. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA, 289(23), 3095–105. [DOI] [PubMed] [Google Scholar]
- Kim D., Cho H.B., Dager S.R., et al. (2013). Posterior cerebellar vermal deficits in bipolar disorder. Journal of Affective Disorders, 150(2), 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Choi-Kwon S. (2000). Poststroke depression and emotional incontinence: correlation with lesion location. Neurology, 54(9), 1805–10. [DOI] [PubMed] [Google Scholar]
- Kim N.Y., Lee S.C., Shin J.-C., Park J.E., Kim Y.W. (2017). Voxel-based lesion symptom mapping analysis of depressive mood in patients with isolated cerebellar stroke: a pilot study. Neuroimage: Clinical, 13, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke I., Minnerop M., Schmitz-Hübsch T., et al. (2010). Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum, 9(3), 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak H.H., Uca A.U., Uğuz F. (2015). Hypomanic episode improved spontaneously after isolated acute cerebellar infarct: a case report. Journal of Stroke and Cerebrovascular Diseases, 24(1), e11–3. [DOI] [PubMed] [Google Scholar]
- Kroemer N.B., Lee Y., Pooseh S., Eppinger B., Goschke T., Smolka M.N. (2016). L-DOPA reduces model-free control of behavior by attenuating the transfer of value to action. BioRxiv, 186(October 2018), 086116. [DOI] [PubMed] [Google Scholar]
- Kumar P., Waiter G., Ahearn T., Milders M., Reid I., Steele J.D. (2008). Abnormal temporal difference reward-learning signals in major depression. Brain, 131(8), 2084–93. [DOI] [PubMed] [Google Scholar]
- Kumar P., Goer F., Murray L., et al. (2018). Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology, 43(7), 1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty I., Prendes J. (1981). Psychosis and cerebellar degeneration. The Journal of Nervous and Mental Disease, 169(6), 390–1. [DOI] [PubMed] [Google Scholar]
- Laidi C., d’Albis M.A., Wessa M., et al. (2015). Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatrica Scandinavica, 131(3), 223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Ahern G.L., Schwartz G.E., Davidson R.J. (1997). Neuroanatomical correlates of happiness, sadness, and disgust. The American Journal of Psychiatry, 154(7), 926–33. [DOI] [PubMed] [Google Scholar]
- Lauterbach E.C. (1996). Bipolar disorders, dystonia, and compulsion after dysfunction of the cerebellum, dentatorubrothalamic tract, and substantia nigra. Biological Psychiatry, 40(8), 726–30. [DOI] [PubMed] [Google Scholar]
- Lauterbach E.C., Cummings J.L., Kuppuswamy P.S. (2013). Toward a more precise, clinically-informed pathophysiology of pathological laughing and crying. Neuroscience and Biobehavioral Reviews, 37(8), 1893–916. [DOI] [PubMed] [Google Scholar]
- Leggio M., Molinari M. (2015). Cerebellar sequencing: a trick for predicting the future. Cerebellum, 14(1), 35–8. [DOI] [PubMed] [Google Scholar]
- Li H., Chen Q., Liu W. (2008). Adult asymptomatic case of Dandy–Walker syndrome associated with bipolar disorder. Acta Neuropsychiatrica, 20(3), 170–1. [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. Behavioral and Brain Sciences, 35(3), 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann S., Manshadi M., Baldwin H., Drasin G., Rice J., Alrajeh S. (1982). Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients. The American Journal of Psychiatry, 139(5), 667–8. [DOI] [PubMed] [Google Scholar]
- Liszewski C.M., O’Hearn E., Leroi I., Gourley L., Ross C.A., Margolis R.L. (2004). Cognitive impairment and psychiatric symptoms in 133 patients with diseases associated with cerebellar degeneration. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(1), 109–12. [DOI] [PubMed] [Google Scholar]
- Lo R.Y., Figueroa K.P., Pulst S.M., et al. (2016). Depression and clinical progression in spinocerebellar ataxias. Parkinsonism & Related Disorders, 22, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M., Olivito G., Siciliano L., et al. (2018). Evidence of cerebellar involvement in the onset of a manic state. Frontiers in Neurology, 9, 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M., Siciliano L., Leggio M. (2019). From cerebellar alterations to mood disorders: a systematic review. Neuroscience and Biobehavioral Reviews, 103, 21–8. [DOI] [PubMed] [Google Scholar]
- Lupo M., Olivito G., Gragnani A., et al. (2021). Comparison of cerebellar grey matter alterations in bipolar and cerebellar patients: evidence from voxel-based analysis. International Journal of Molecular Sciences, 22(7), 2–4.doi: 10.3390/ijms22073511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P., Brouns R., Engelborghs S., et al. (2008). Cerebellar cognitive affective syndrome without global mental retardation in two relatives with Gillespie syndrome. Cortex, 44(1), 54–67. [DOI] [PubMed] [Google Scholar]
- Mariën P., Baillieux H., De Smet H.J., et al. (2009). Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex, 45(4), 527–36. [DOI] [PubMed] [Google Scholar]
- Mariën P., De Smet H.J., Wijgerde E., Verhoeven J., Crols R., De Deyn P.P. (2013). Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex, 49(1), 284–300. [DOI] [PubMed] [Google Scholar]
- Marques J.G. (2019). Twenty years of misdiagnosis of schizophrenia in a patient with Dandy-Walker variant syndrome. General Psychiatry, 32(1), e100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwaha S., He Z., Broome M., et al. (2014). How is affective instability defined and measured? A systematic review. Psychological Medicine, 44(9), 1793–808. [DOI] [PubMed] [Google Scholar]
- Marwaha S., Price C., Scott J., et al. (2018). Affective instability in those with and without mental disorders: a case control study. Journal of Affective Disorders, 241(July), 492–8. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ayani N., Kitabayashi Y., Narumoto J. (2020). New‐onset mania in an elderly patient five months after acoustic neuroma resection. Bipolar Disorders, 22, 768–70.doi: 10.1111/bdi.12970. [DOI] [PubMed] [Google Scholar]
- Mendl M., Burman O.H.P., Paul E.S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proceedings of the Royal Society B: Biological Sciences, 277(1696), 2895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metoki A., Wang Y., Olson I.R. (2021). The social cerebellum: a large-scale investigation of functional and structural specificity and connectivity. Cerebral Cortex (New York, N.Y.: 1991), 32, 991–2.doi: 10.1093/cercor/bhab260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N.P., Delbello M.P., Adler C.M., Strakowski S.M. (2005). MRI analysis of cerebellar vermal abnormalities in bipolar disorder. American Journal of Psychiatry, 162(8), 1530–2. [DOI] [PubMed] [Google Scholar]
- Minichino A., Bersani F.S., Trabucchi G., et al. (2014). The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Rivista Di Psichiatria, 49(3), 124–31. [DOI] [PubMed] [Google Scholar]
- Moberget T., Doan N.T., Alnæs D., Kaufmann T., Córdova-Palomera A., Lagerberg T.V.KaSP . (2018). Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Molecular Psychiatry, 23(6), 1512–20. [DOI] [PubMed] [Google Scholar]
- Monkul E.S., Hatch J.P., Sassi R.B., et al. (2008). MRI study of the cerebellum in young bipolar patients. Progress in Neuro-psychopharmacology & Biological Psychiatry, 32(3), 613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah H.A., McCalley-Whitters M., Jacoby C.G. (1982). Cerebral ventricular enlargement in young manic males. A controlled CT study. Journal of Affective Disorders, 4(1), 15–9. [DOI] [PubMed] [Google Scholar]
- Nguyen D., Naffziger E.E., Berridge K.C. (2021). Positive affect: nature and brain bases of liking and wanting. Current Opinion in Behavioral Sciences, 39, 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Almeida J.R., Forbes E.E., et al. (2012). Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders, 14(3), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea I.M., Popal H.S., Olson I.R., Murty V.P., Smith D.V. (2022). Distinct alterations in cerebellar connectivity with substantia nigra and ventral tegmental area in Parkinson’s Disease. Scientific Reports, 12, 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan N., Szczepanowski R., El-Deredy W., Mason L., Bentall R.P. (2011). FMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia, 49(10), 2825–35. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. (2013). The neural bases of emotion and emotion regulation: a valuation perspective. In: Gross, J.J.Handbook of Emotion Regulation, 2nd edn. Guilford Publications; New York City, NY, 23–37. [Google Scholar]
- Opal P., Ashizawa T. (1998). Spinocerebellar Ataxia Type 1. In: Adam, M., Ardinger, H., Pagon, R., et al. (editors). GeneReviews ® [Internet]. Seattle: University of Washington, Seattle. [Google Scholar]
- Orsi L., D’Agata F., Caroppo P., et al. (2011). Neuropsychological picture of 33 spinocerebellar ataxia cases. Journal of Clinical and Experimental Neuropsychology, 33(3), 315–25. [DOI] [PubMed] [Google Scholar]
- Parvizi J., Anderson S., Coleman M., Damasio H., Damasio A. (2001). Pathological laughter and crying: a link to the cerebellum. Brain, 124(9), 1708–19. [DOI] [PubMed] [Google Scholar]
- Parvizi J., Schiffer R. (2007). Exaggerated crying and tremor with a cerebellar cyst. The Journal of Neuropsychiatry and Clinical Neurosciences, 19(2), 187–90. [DOI] [PubMed] [Google Scholar]
- Patronas N.J., Di Chiro G., Smith B.H., et al. (1984). Depressed cerebellar glucose metabolism in supratentorial tumors. Brain Research, 291(1), 93–101. [DOI] [PubMed] [Google Scholar]
- Paulus K.S., Magnano M., Conti P., et al. (2004). Pure post–stroke cerebellar cognitive affective syndrome: a case report. Neurological Sciences, 25(4), 220–4. [DOI] [PubMed] [Google Scholar]
- Paulus M.P. (2015). Cognitive control in depression and anxiety: out of control? Current Opinion in Behavioral Sciences, 1, 113–20. [Google Scholar]
- Peng J., Liu J., Nie B., et al. (2011). Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. European Journal of Radiology, 80(2), 395–9. [DOI] [PubMed] [Google Scholar]
- Pessiglione M., Seymour B., Flandin G., Dolan R.J., Frith C.D. (2006). Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature, 442(7106), 1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J.E., Péron J. (2020). The basal ganglia and the cerebellum in human emotion. Social Cognitive and Affective Neuroscience, 15(5), 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty B.K., Gulve N., Govind S., et al. (2018). Active HHV-6 infection of cerebellar pukinje cells in mood disorders. Frontiers in Microbiology, 9, 4–5.doi: 10.3389/fmicb.2018.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder R.O., Mann L.S., Weinberger D.R., van Kammen D.P., Post R.M. (1983). Computed tomographic scans in patients with schizophrenia, schizoaffective, and bipolar affective disorder. Archives of General Psychiatry, 40(7), 735–9. [DOI] [PubMed] [Google Scholar]
- Riva D., Giorgi C. (2000). The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain, 123(Pt 5), 1051–61. [DOI] [PubMed] [Google Scholar]
- Rizvi S.J., Lambert C., Kennedy S. (2018). Presentation and neurobiology of anhedonia in mood disorders: commonalities and distinctions. Current Psychiatry Reports, 20(2), 13. [DOI] [PubMed] [Google Scholar]
- Rolfs A., Koeppen A.H., Bauer I., et al. (2003). Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17). Annals of Neurology, 54(3), 367–75. [DOI] [PubMed] [Google Scholar]
- Rowland T.A., Marwaha S. (2018). Epidemiology and risk factors for bipolar disorder. Therapeutic Advances in Psychopharmacology, 8(9), 251–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B., Baldi E., Lorenzini C.A., Bucherelli C. (2002). Cerebellar role in fear-conditioning consolidation. Proceedings of the National Academy of Sciences of the United States of America, 99(12), 8406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D., Nummenmaa L. (2021). Reward and emotion: an affective neuroscience approach. Current Opinion in Behavioral Sciences, 39, 161–7. [Google Scholar]
- Schlerf J., Wiestler T., Verstynen T., Diedrichsen J. (2014). Big challenges from the little brain-imaging the cerebellum. In: Papageorgiou, T.D., Christopoulos, G.I., Smirnakis, S.M., (editors). IntechOpen, London, UK, 2. Advanced Brain Neuroimaging Topics in Health and Disease- Methods and Applications. [Google Scholar]
- Schmahmann J.D. (1996). From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping, 4(3), 174–98. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. (1998). Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences, 2(9), 362–71. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. (2000). The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics, 13(2–3), 189–214. [Google Scholar]
- Schmahmann J.D. (2010). The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review, 20(3), 236–60. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. (1998). The cerebellar cognitive affective syndrome. Brain: A Journal of Neurology, 121(4), 561–79. [DOI] [PubMed] [Google Scholar]
- Schore A.N. (2016). Affect Regulation and the Origin of the Self: The Neurobiology of Emotional Development. New York, NY: Psychology Press. [Google Scholar]
- Schraa-Tam C.K., Rietdijk W.J., Verbeke W.J., et al. (2012). fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum, 11(1), 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2016). Dopamine reward prediction-error signalling: a two-component response. Nature Reviews Neuroscience, 17(3), 183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J., Koolschijn P.C., Peper J.S., Crone E.A. (2012). The cerebellum link to neuroticism: a volumetric MRI association study in healthy volunteers. PLoS One, 7(5), e37252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.A., Doraiswamy P.M., Husain M.M., et al. (1992). Posterior fossa abnormalities in major depression: a controlled magnetic resonance imaging study. Acta Psychiatrica Scandinavica, 85(6), 474–9. [DOI] [PubMed] [Google Scholar]
- Slattery C., Agius M., Zaman R. (2010). Bipolar disorder associated with paraneoplastic cerebellar degeneration: a case report. Psychiatria Danubina, 22, S137–8. [PubMed] [Google Scholar]
- Stoessel M.B., Majewska A.K. (2021). Little cells of the little brain: microglia in cerebellar development and function. Trends in Neurosciences, 44(7), 564–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Smith L., Watt K., Barron L., Zeman A. (2001). Incoordinated thought and emotion in spinocerebellar ataxia type 8. Journal of Neurology, 248(3), 229. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Limperopoulos C. (2016). Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Seminars in Fetal & Neonatal Medicine, 21(5), 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46(7), 831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strata P. (2015). The emotional cerebellum. Cerebellum, 14(5), 570–7. [DOI] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. (2009). Cerebellum and nonmotor function. Annual Review of Neuroscience, 32, 413–34. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Rosenbloom M.J., Deshmukh A., Desmond J.E., Pfefferbaum A. (1995). Alcohol and the cerebellum: effects on balance, motor coordination, and cognition. Alcohol Health and Research World, 19(2), 138–41. [PMC free article] [PubMed] [Google Scholar]
- Supple W.F., Leaton R.N., Fanselow M.S. (1987). Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Physiology & Behavior, 39(5), 579–86. [DOI] [PubMed] [Google Scholar]
- The National Institute of Mental Health, T. N. I. o. M. (2018). Depression. Available: https://www.nimh.nih.gov/health/topics/depression/index.shtml [December 9, 2020].
- Trautmann S.A., Fehr T., Herrmann M. (2009). Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Research, 1284, 100–15. [DOI] [PubMed] [Google Scholar]
- Tsai P.T., Hull C., Chu Y., et al. (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature, 488(7413), 647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan T., Beşirli A., Asdemir A., Özsoy S., Eşel E. (2010). Manic episode associated with mega cisterna magna. Psychiatry Investigation, 7(4), 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.M., Paradiso S., Marvel C.L., et al. (2007). The cerebellum and emotional experience. Neuropsychologia, 45(6), 1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaga C.E., Brown S.T., Raman I.M. (2020). Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. Elife, 9, 11–19.doi: 10.7554/eLife.54302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Pu M., Ma Q., et al. (2021). The involvement of the posterior cerebellum in reconstructing and predicting social action sequences. Cerebellum, 5–6.doi: 10.1007/s12311-021-01333-9. [DOI] [PubMed] [Google Scholar]
- Victor M. (1990). MR in the diagnosis of Wernicke-Korsakoff syndrome. American Journal of Roentgenology, 155(6), 1315–6. [DOI] [PubMed] [Google Scholar]
- Weinberger D.R., Wagner R.L., Wyatt R.J. (1983). Neuropathological studies of schizophrenia: a selective review. Schizophrenia Bulletin, 9(2), 193–212. [DOI] [PubMed] [Google Scholar]
- Westbrook M.T., Viney L.L. (1982). Psychological reactions to the onset of chronic illness. Social Science & Medicine, 16(8), 899–905. [DOI] [PubMed] [Google Scholar]
- Wu C., Ren C., Teng Z., et al. (2021). Cerebral glucose metabolism in bipolar disorder: a voxel-based meta-analysis of positron emission tomography studies. Brain and Behavior, 11(5), e02117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadalam K.G., Jain A.K., Simpson G.M. (1985). Mania in two sisters with similar cerebellar disturbance. The American Journal of Psychiatry, 142(9), 1067–9. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kim M., Imai H., Itakura Y., Ohtsuki G. (2019). Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Reports, 28(11), 2923–38.e2928. [DOI] [PubMed] [Google Scholar]
- Yeganeh-Doost P., Gruber O., Falkai P., Schmitt A. (2011). The role of the cerebellum in schizophrenia: from cognition to molecular pathways. Clinics (Sao Paulo), 66(Suppl 1), 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel K., Nazarov A., Taylor V.H., Macdonald K., Hall G.B., Macqueen G.M. (2013). Cerebellar vermis volume in major depressive disorder. Brain Structure & Function, 218(4), 851–8. [DOI] [PubMed] [Google Scholar]
- Zhu L., Sacco T., Strata P., Sacchetti B. (2011). Basolateral amygdala inactivation impairs learning-induced long-term potentiation in the cerebellar cortex. PLoS One, 6(1), e16673. [DOI] [PMC free article] [PubMed] [Google Scholar]


