ABSTRACT
Objectives:
Excessive use of antibiotics has increased antimicrobial resistance (AMR) worldwide, which is a major public concern among the countries. To control this threat proper monitoring of the antimicrobial usage with increasing rate of AMR is required. Moreover, alternatives for antibiotics are surveyed and are being researched for quick use in the future. Thus, multisector intervention is highly encouraged for better outcomes. In this research article, six different European countries are discussed in terms of antimicrobial usage and AMR in human and livestock sectors with the help of literature study and various reports published by different organizations.
Methods:
Data study has been conducted to collect data for comparison study. Data sources of AMR and antimicrobial usage are analyzed and both antimicrobial use and AMR are compared.
Results:
This article provides surveillance systems that are formed to keep a track on the upcoming situation of AMR and the consumption of antimicrobials by humans as well as animals. The article firmly allows the readers to get broad information about the AMR across six countries of Europe. These annual reports have hugely helped the government to decide for alternatives and have focused in many training activities to combat the AMR situation globally.
Conclusion:
As antibiotic resistance genes persist on an interface between environment and animal and animal health, an approach is required in all three areas that stress the concept of “One Approach to Health.”
Keywords: Alternative antibiotics, AMR, Comparative medicine, One Health Approach, Phage therapy, Surveillance
Introduction
In the last few decades extensive use of antibiotics has resulted in rising cases of antimicrobial resistance (AMR) against various organisms. From narrow-spectrum antibiotics, people shifted to broad-spectrum antibiotics, which eventually increased the high resistance rates. Multidrug-resistant (MDR) bacterial infections are rapidly emerging and spreading over the world, posing a severe threat to global healthcare. Carbapenem-resistant Enterobacteriaceae (CRE), a type of gram-negative bacteria that has resisted all or virtually all current antibiotics, is one cause for concern. Likewise, various other antibiotics are also becoming resistant against the microorganisms causing immense threat among the population. This global threat comprises of both commensal and pathogenic bacteria. The similarities between human and animal diseases, as well as the interactions between animals and humans who come into contact with them, have long been recognized. Human and veterinary medicine diverged in the twentieth century. During the same time span, our understanding of infectious diseases and antibiotics grew dramatically. The necessity for partnerships between human health and veterinary sectors to prevent and control zoonotic illnesses and antibiotic resistance grew in the second half of the twentieth century. The notion of ecosystem health developed toward the end of the twentieth century, extending the integration and collaboration of human and animal medicine to the environment. Later on, the phrase “One Health” was coined to describe a holistic approach to improving human, animal, and environmental health through multidisciplinary cooperation and communication. Several global plans have been established to combat the AMR epidemic, including the World Health Organization’s (WHO) Global Action Plan (GAP), the new European One Health Action Plan against AMR, and the Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR) network (1). Surveillance and monitoring systems for Antimicrobial Usage (AMU) and AMR in humans and animals are critical for assessing and controlling global trends in antimicrobial use and antimicrobial susceptibility patterns of bacteria in various populations. In the context of a One Health strategy, zoonotic and indicator microorganisms are especially important. A strategic framework for reducing infectious disease risks at the animal-human ecosystem interface was published in 2008, adopting and promoting the One Health concept. The One Health Approach has been supported and implemented by a wide number of national and international institutes since 2008. Research on the human-animal environment interaction is critical to supporting the call for a One Health Approach to AMR and infectious illnesses. Furthermore, training and extension initiatives are critical for promoting the One Health idea and facilitating its application among various stakeholders (2,3). Several governments and international organizations have now included a One Health Approach in the AMR action plans. Improvements in antimicrobial use, better regulation and policy, improved surveillance, stewardship, infection control, sanitation, animal husbandry, and identifying antimicrobial alternatives are all necessary efforts. This report summarizes research and educational activity in the field of One Health in Western Europe, with an emphasis on infectious diseases. It might act as a springboard for future collaborations and projects.
Materials and methods
Data sources
We conducted a database study for collecting major characteristics of surveillance and monitoring systems on antimicrobial use and AMR in cattle and people, as well as AMR systems in food, in this publication. Countries such as Spain, Germany, France, the Netherlands, Norway, and the United Kingdom were considered for this project. The literature searches in recent times have been carried out to understand and collect the data from different gray reports and other AMR databases. The database study has been conducted by searching the terms “Antimicrobial Resistance,” “Antibiotic Usage,” “One Health Approach” on PubMed and desired research papers or data sheets annually published by different agencies are studied for collective data required for this research article. Additionally, information about One Health Approach in these countries was also investigated for obtaining the results. One Health policy publications issued by international organizations and countries also provided background information on the One Health program and European One Health projects. Moreover, Google research on One Health with its associated activities and trainings among these countries was conducted for acquiring more relevant outcomes. Alternative antibiotics for resolving the issue of AMR were also researched which focuses on better solution for AMR and antimicrobial usage. One of the renowned projects also known as ARDIG (Antimicrobial Resistance Dynamics the Influence of Geographic origin) together collects and gathers data related to AMR and usage of antimicrobials from both human and veterinary sectors. The stipulated graph depicts the predicted deaths that will be increasing for the usage of antibiotics globally by the year 2050 (Fig. 1) (4). This helps us to portray the comparison study of all the data collected for different countries along with the Asian countries. In addition, all the surveillance systems for monitoring AMR in different countries of Europe have been explained thoroughly in this article. The primary reason for tracking the reports of European countries is because One Health has gained a lot of traction throughout Europe. The One Health strategy is currently being promoted in Europe mostly in regard to AMR. Many nations have adopted the One Health concept in their anti-AMR policies, and funding opportunities for AMR research have considerably increased. In the areas of zoonotic diseases and One Health, the number of national and international multidisciplinary research networks is growing (1,5,6).
Fig. 1 -.
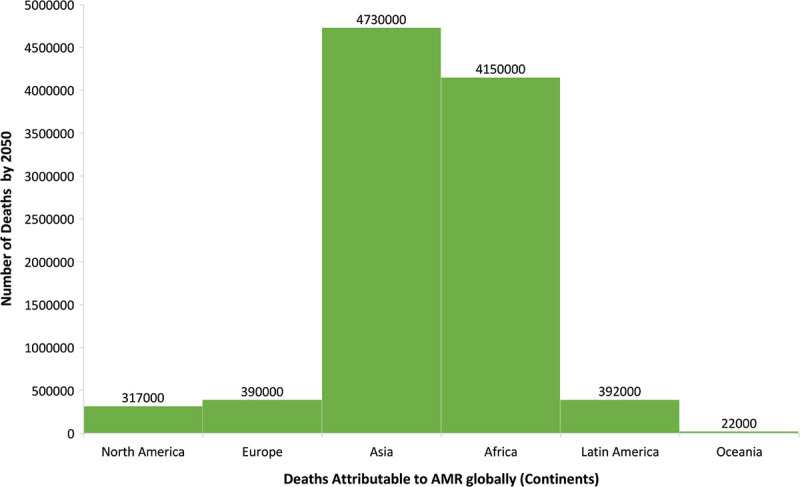
The stipulated graph depicts the predicted increase in the number of deaths for using antibiotics globally by the year 2050. X-axis denotes the continents and Y-axis denotes the number of deaths by the year 2050. (from: https://www.publichealthpost.org/databyte/antibiotic-resistant-bacteria/)
Surveillance strategies of AMR and monitoring system
Data collection and data analysis
Different European countries have various surveillance strategies and monitoring systems for controlling the rising threat of AMR. Additionally, multiple organizations are coming together for joint efforts that require combating this situation. A complete summary of the data collected is provided in a tabular form for better understanding of the data gathered.
France
AMR data related to agriculture, food, and the environment are monitored by the French Agency for Food, Environmental, and Occupational Health and Safety (ANSES). The French monitoring network for antibiotic resistance in pathogenic bacteria of animal origin (RESAPATH) and the Salmonella network are coordinated by this agency. The Salmonella network is a surveillance system designed to keep nonhuman Salmonella under control throughout the food chain. The Investigation and Surveillance of Nosocomial Infection Network (RAISIN) coordinates the nosocomial infection surveillance coordination centers across the country. BMR-RAISIN, a private RAISIN module for multidrug-resistant bacteria, reports on AMR data in the community. Healthy animals, food, and the environment are all sampled. The RESAPATH voluntary surveillance system compiles AMR data for primary bacterial species and general isolates from sick animals from each animal sector in the annual RESAPATH report (7).
Germany
Clinical AMR data from companion and food-producing animals is collected in Germany through the German veterinary monitoring system (GERM-VET). AMR testing in the Zoonosis-Monitoring System (ZOMO) report includes data on zoonotic and commensal bacteria in various food chains, as well as AMR data on Salmonella from national control programs, which are also reported to the European Food Safety Authority (EFSA). Antimicrobial Resistance Surveillance (ARS) is the human national AMR surveillance system. It gathers routine susceptibility data for all bacterial species from any sample site, including hospital and outpatient care facilities. The Hospital Infection Surveillance System (KISS) is a nosocomial infection surveillance system made up of multiple sub-systems that collect AMU and AMR data in hospitals. Surveillance of Antibiotic Use and Resistance in Intensive Care Units (SARI) gathered data on antimicrobial sensitivity for selected pathogenic microorganisms and the creation of AMU-AMR on a volunteer basis (1).
Spain
To keep track of AMR, the Spanish Veterinary Antimicrobial Resistance Surveillance Network (VAV) was formed. VAV provides nonclinical data to the EFSA, which is included in the agency’s annual reports. According to EU legislation, this report contains information on zoonotic infections and diseases in animals, humans, and food, as well as data on AMR in select zoonotic bacteria and indicator bacteria (1,6).
Norway
The three AMR surveillance programs in Norway are the Norwegian Surveillance System for Antimicrobial Drug Resistance (NORM), Norwegian Veterinary Antimicrobial Resistance Monitoring (NORM-VET), and the Norwegian Surveillance System for Communicable Diseases (MSIS). This annual report contains updated information on AMU and AMR prevalence and distribution in the human, animal, and food sectors (8,9).
The Netherlands
The “Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands” (MARAN), which brings together the Food and Consumer Product Safety Authority’s AMR food database, is the Netherlands’ AMR monitoring system for animals and food. It disseminates information on foodborne pathogen resistance as well as commensal indicators from animals and food. The Infectious Disease Surveillance Information System on Antibiotic Resistance (ISIS-AR) monitors AMR in key pathogens in the human sector (10). These surveillance systems are extremely helpful in tracking down the situation caused by antimicrobial use and AMR. The various features of different organizations built by the agencies have successfully helped the researchers in providing the necessary data for handling the threat worldwide. In addition to strengthening the AMR surveillance, numerous policies have been prepared by WHO and other agencies that apparently help in working with the solution of either decreasing or avoiding the AMR situation. For teaching and training, surveillance and risk assessment, and research, the AMR Coordinating Office emphasizes a One Health Approach. Political commitment, policy formation, sustainable finance, program creation, knowledge sharing, institutional collaboration, capacity enhancement, civil society involvement, and active community participation are all part of the framework for effective One Health implementation. One health is a straightforward and strong idea with complex processes. The national response to zoonoses must be revised, food safety improved, and environmental integrity guaranteed. The transformation must be driven by the senior leadership. Strong, ongoing lobbying by international development partners, in particular: the FAO, the OIE and the WHO, should be shared with the leading national leadership, disseminating the evidentiary results, predicted economic benefits, and best practice globally. The interconnected sustainable development goals offer a unique opportunity for advocacy and an integrated approach to development. The effectiveness of One Health implementation depends on the extent to which institutional cooperation, common planning and coordination thorough monitoring for early detection and prevention of zoonoses are achieved. The key planning, implementation and surveillance are data and science. Initial efforts for rapid tracking should be performed quickly in order to create multisectoral capacity across various organizations. The theory and practice of One Health should be fully integrated and visible in the educational curriculum as well as in the constant upgrading of skills for all subjects for long-term implementation.
United Kingdom
In the United Kingdom, the EU-Harmonized Surveillance System (a native UK system) collects mandatory AMR data on indicator commensal Escherichia coli and/or Campylobacter spp. from meat and fecal content of healthy animals (chicken, beef, turkey, and pigs). There are also Salmonella National Control Programs in the United Kingdom that are hosted in the EU-Harmonized Surveillance System. In Scotland, the Scotland’s Rural College Veterinary Services and Capital Diagnostics (SRUC) surveillance system collects clinical isolates from animals. In England, monitoring surveillance system Vet Pathogens APHA collects AMR data from infected animals that veterinarians proactively offer for diagnostic services, covering all relevant bacteria and animal species. On the human aspect, the British Society for Antimicrobial Chemotherapy’s (BSAC) Resistance Surveillance Program provides antibiotic resistance data from cooperating labs in the UK and Ireland for a variety of clinically relevant bacteria from community-acquired respiratory illnesses. AMR data are collected through the Electronic Communication of Surveillance in Scotland (ECOSS) network from participating National Health Service (NHS) and reference laboratories in Scotland (1,11).
Results
The accomplished research revealed that various surveillance systems are actively working to follow a trail of the upcoming situation of AMR and antimicrobial consumption by humans as well as animals. These surveillance systems of European countries are jointly contributing in statistically analyzing the rising situation of AMR and the prominent measures taken by different organizations for implementing One Health Approach. Moreover, various training institutes and alternative measures for preventing AMR are firmly encouraged in these six European countries along with Taiwan and India. Data of AMR solely do not arise from consuming antibiotics. There are multiple more aspects such as food habits of the humans, food chains maintained by the healthy animals, and the environment that together exhibit the importance of surveillance systems for AMR as these features put up the AMR issue topmost. The EFSA is in charge of communication on food chain concerns. Annually, the EFSA and the European Centre for Disease Prevention and Control (ECDC) collect AMR data on humans, food, and healthy animals from EU States and some affiliated countries (5). The European Union summary report on AMR in zoonotic and indicator bacteria from humans, animals, and food is prepared and published by the EFSA. Also, some nongovernmental organizations such as European Animal Health Study Centre (CEESA) are also contributing by researching about AMR and forming relevant systems to perform the activities efficiently (6). Precisely, the organization is working in monitoring the antimicrobial susceptibility of the bacterial pathogens that have the potential of causing diseases among the animals along with the foodborne pathogens in animal’s food. These organizations are not the only aide for this surveillance system; a prime system also called as the European Antimicrobial Resistance Surveillance Network (EARS-Net) mainly helps in the surveillance of AMR data. This is an AMR surveillance network established in compliance with European Union and European Economic Area legislation. The ECDC collects AMR data from EU States through EARS-Net and publishes the annual EARS-Net report. On that account it is extremely crucial to compare the percentages of antibiotic usage and AMR, which will help in displaying the numbers accurately acquired from different organizations and relevant measures will be implemented for better solution. Similarly, acknowledging this, two joint interagency reports for antibiotic consumption and the analysis of AMR were published that clearly demonstrate the effects of using extensive antibiotics on humans and animals and the data were compared to AMR reports for better understanding. This report is jointly published by the European Medicines Agency (EMA), the EFSA, and the ECDC. The ECDC and EARS-Net require other platforms to jointly work for this (Fig. 2). Every country from Europe has its own surveillance system for AMR that they follow, and prepared reports are further provided to EFSA. The AMR surveillance system is developed distinctly for humans and livestock (1).
Fig. 2 -.
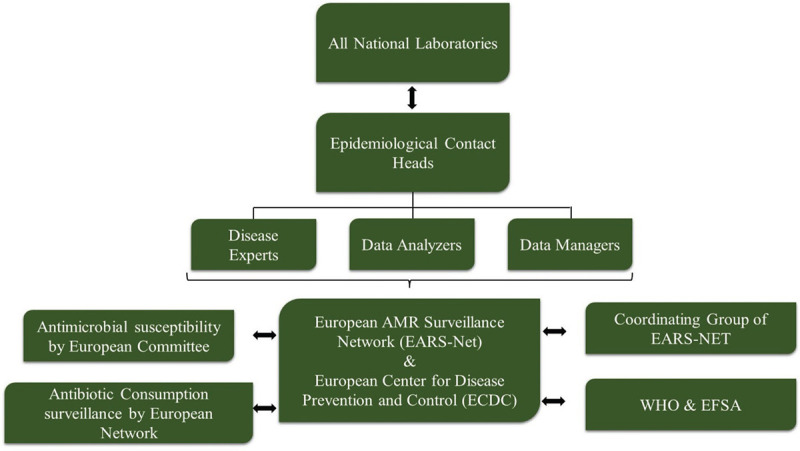
Different organizations jointly working together to provide data to EARS-Net and ECDC.
Different systems of the country contribute in forming the reports, which are eventually published by EFSA or ECDC (Fig. 3).
Fig. 3 -.
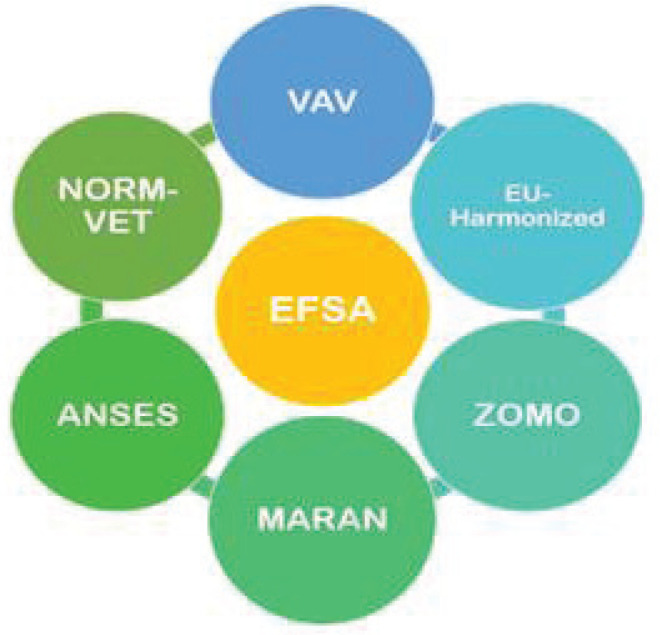
AMR surveillance systems for livestock of different countries reporting the data to EFSA. ANSES = The French Agency for Food, Environmental and Occupational Health & Safety; EU-Harmonized = The EU-harmonized Surveillance System; MARAN = Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands; NORM-VET = Norwegian Veterinary Antimicrobial Resistance Monitoring system; VAV = The Spanish Veterinary Antimicrobial Resistance Surveillance Network (VAV); ZOMO = Zoonosis-Monitoring System.
Similarly, AMR surveillance system for humans is also analyzed by different organizations formed in these six European countries. Figure 4 depicts the organizations that are being established for keeping the record of the AMR surveillance (6).
Fig. 4 -.
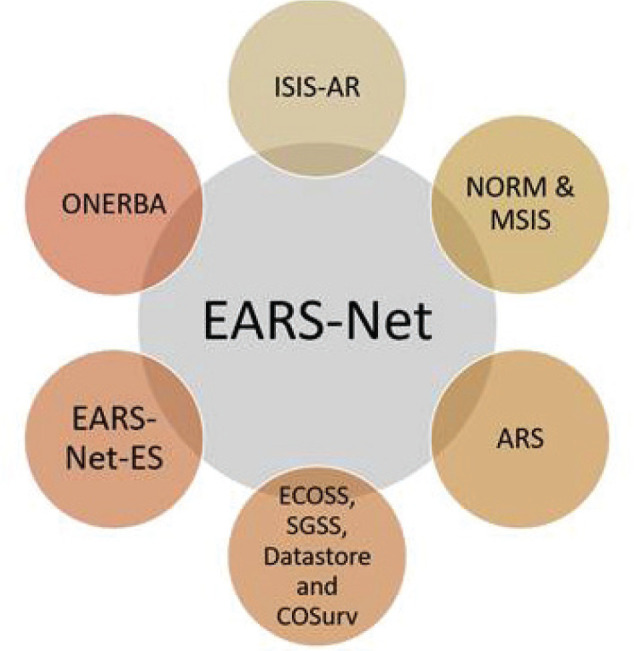
AMR surveillance systems for humans of different countries reporting the data to EFSA. ARS = antimicrobial resistance surveillance; EARS-Net-ES = European Antimicrobial Resistance Surveillance Network; ECOSS, SGSS, Datastore and COSurv = The Electronic Communication of Surveillance in Scotland, Second Generation Surveillance System; ISIS-AR = Infectious Disease Surveillance Information System on Antibiotic Resistance; NORM & MSIS = Norwegian Veterinary Antimicrobial Resistance Monitoring; ONERBA = National Observatory of the Epidemiology of Bacterial Antibiotic Resistance.
Tables I and II give details about all the aforementioned surveillance system followed by the distinct countries along with the features and roles they perform (1,7).
TABLE I -.
AMR surveillance system conducted in different countries for humans
| Surveillance system | Country | Roles of surveillance system |
|---|---|---|
| ISIS-AR | The Netherlands | This aims at monitoring AMR in major pathogens. |
| NORM and MSIS | Norway | It is an AMR surveillance program in Norway. This annual report provides updated information on AMU and AMR occurrence and distribution in human beings. |
| ARS | Germany | It is the national human medicine AMR surveillance system. Established by the Robert Koch Institute, it collects routine sensitivity data from any sample site in the hospital and from ambulatory care institutions for all bacterial species. |
| EARS-Net-ES | Spain | Maintains the records of AMR surveillance across Spain. |
| ONERBA | France | AMU and AMR as well as a leading AMR network that collects data from a complex subsystem network is an annual French report, ONERBA. |
| ECOSS, SGSS, Datastore, and COSurv | United Kingdom | The ECOSS database gathers AMR data from participating NHS laboratories and reference laboratories in Scotland. Electronic Communication of Surveillance in Scotland (ECOSS) (SGSS) captures 98% of the National Health Service (NHS) laboratories across England, from routine laboratory surveillance data on infectious diseases and antimicrobial resistance. |
AMR = antimicrobial resistance.
TABLE II -.
AMR surveillance system conducted in different countries for livestock
| Surveillance system | Country | Roles of surveillance system |
|---|---|---|
| VAV | Spain | VAV monitors the AMR status throughout the country and is also responsible for monitoring animals and food.
In addition, VAV supplies EFSA with nonclinical data. |
| ANSES | France | ANSES generally monitors AMR data related to food and livestock. |
| ZOMO | Germany | This report also provides data on zoonotic and commensal bacteria of the different food chains reported to EFSA. |
| MARAN | The Netherlands | Data on foodborne pathogens and commensal indicators from cattle and food are published in the annual report of the Netherlands. |
| NORM-VET | Norway | Facilitate updated incidence and distribution information on animal AMU and AMR. |
| EU-Harmonized | United Kingdom | Mandatory AMR data for meat and feces in healthy animals, using the appropriate indicator Escherichia coli and/or Campylobacter spp. are collected under the European harmonized supervisory system. |
AMR = antimicrobial resistance; EFSA = European Food Safety Authority.
Humans and animals are exposed to AMR from their food habits. Thus, a surveillance system was set up especially for the food that is being consumed by both animals and humans. A thorough monitoring of the food consumed has the possibility of getting exposed to new pathogenic organisms, which could be a probable reason for pandemic, endemic, and epidemic. Again, some of the organizations similar to humans and livestock are formed for keeping the track of rising AMR cases from food habits (Fig. 5) (1).
Fig. 5 -.
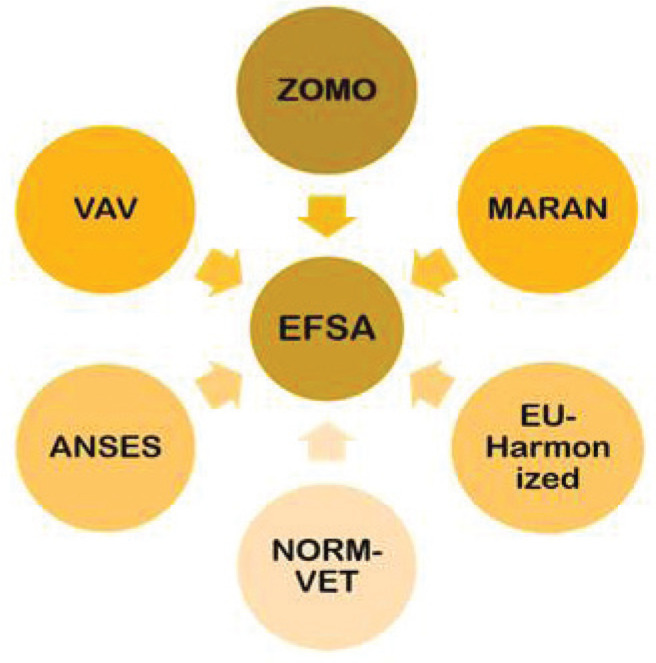
AMR surveillance systems for foods of different countries reporting the data to EFSA. ANSES = The French Agency for Food, Environmental and Occupational Health & Safety; EU-Harmonized = The EU-harmonized Surveillance System; MARAN = Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands; NORM-VET = Norwegian Veterinary Antimicrobial Resistance Monitoring system; VAV = The Spanish Veterinary Antimicrobial Resistance Surveillance Network (VAV); ZOMO = Zoonosis-Monitoring system.
All the aforementioned the organizations report their AMR data to EFSA but on the other hand, there are some organizations that do not report their AMR data to EFSA (Tab. III). The details of all these organizations contributing to different countries are described further (1).
TABLE III -.
Country-wise AMR surveillance systems not reporting data to EFSA
| Surveillance system | Country | Hosts |
|---|---|---|
| GERM-VET | Germany | Livestock |
| RESAPATH | France | Animals |
| APHA-VET PATHOGENS | United Kingdom | Diseased animals |
| SRUC | United Kingdom | Animals |
| PEG | Germany | Human pathogens |
| ARMIN | Germany | Humans |
| BARDa | Germany | Humans |
| ICU-KISS, OP-KISS, SARI-KISS, MRSA-KISS | Germany | Human pathogens |
| BSAC | United Kingdom | Humans |
| BMR-RAISINS | France | Human pathogens |
Reported microorganisms accountable for AMR in Europe
Discussing about the surveillance systems available to control the AMR and antimicrobial usage will not help the population be aware about the pathogenic disease-causing microorganisms accurately. Therefore, it is very important to understand the pathogens responsible for causing AMR also with the antimicrobials that are extensively used. EARS-Net received data from 29 countries for all eight bacterial species under observation (E. coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter species, Streptococcus pneumoniae, Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium). E. coli was the most commonly reported bacterial species (44.2%), followed by S. aureus (20.6%), K. pneumoniae (11.3%), E. faecalis (6.8%), P. aeruginosa (5.6%), S. pneumoniae (5.3%), E. faecium (4.5%), and Acinetobacter species (4.5%) (Fig. 6) (12). In 2019, more than half of E. coli isolates reported to EARS-Net were resistant to at least one antimicrobial group under surveillance, and more than a third of K. pneumoniae isolates were resistant to multiple antimicrobial groups. In general, resistance percentages in K. pneumoniae were higher than in E. coli. While carbapenem resistance was uncommon in E. coli, carbapenem resistance rates in K. pneumoniae were reported to be more than 10% in numerous countries. Carbapenem resistance was also found in larger percentages in P. aeruginosa and Acinetobacter species than in K. pneumoniae. The increase in the percentage of vancomycin-resistant E. faecium isolates in the EU/EEA from 10.5% in 2015 to 18.3% in 2019 is a cause for concern. The results of antimicrobial susceptibility testing (AST) from invasive (blood or cerebrospinal fluid) isolates of eight bacterial species are provided in this article. E. coli, K. pneumoniae, P. aeruginosa, Acinetobacter species, S. pneumoniae, S. aureus, E. faecalis, and E. faecium are all important bacteria for public health in Europe. In 2019, the estimated national population coverage of data provided to EARS-Net ranged from 11% to 100%, with more than a third of the nations reporting a population coverage of 80% or above (12).
Fig. 6 -.
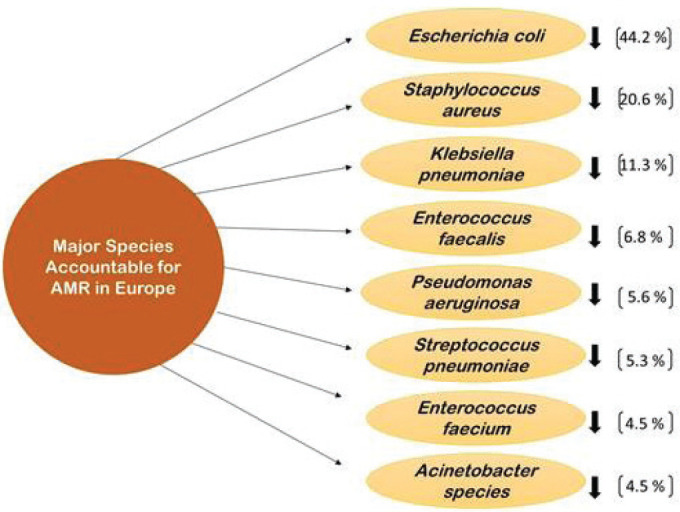
Major species responsible for AMR in Europe.
Escherichia coli
In Europe, E. coli is a common cause of bloodstream infection. Infections caused by antimicrobial-resistant E. coli account for the majority of AMR cases in the EU. The percentages of AMR reported in 2019 were substantially higher than in 2002, underlining the need for more antimicrobial stewardship and infection prevention and control activities. According to the latest data from the European Surveillance of Antimicrobial Consumption Network (ESAC-Net), there are large inter-country variations in the use of broad-spectrum antimicrobials, indicating a need for increased antimicrobial stewardship and the potential for further antimicrobial consumption reductions (13).
Pseudomonas aeruginosa
Although P. aeruginosa is naturally resistant to a wide range of antimicrobials, acquired resistance complicates the treatment of P. aeruginosa infections. Because P. aeruginosa is still one of the most common causes of healthcare-associated illness in Europe, the public health consequences of AMR in P. aeruginosa should not be overlooked (12).
Klebsiella pneumoniae
Due to K. pneumoniae’s great resistance, the European Union is currently dealing with a significant issue. Although carbapenem resistance has increased more than seven-fold since 2006, it has been more moderate in the last 5 years than in earlier eras. The WHO believes that novel drugs targeting third-generation cephalosporin- and carbapenem-resistant Enterobacterales, such as K. pneumoniae and E. coli, are urgently needed.
Staphylococcus aureus
Many nations have created and implemented national methicillin-resistant Staphylococcus aureus (MRSA) prevention recommendations and guidance documents, emphasizing on enhanced infection prevention and control as well as sensible antibiotic usage. Despite this progress, MRSA remains a significant pathogen in Europe. S. aureus is one of the most frequent bacteria that causes bloodstream infections, with a significant morbidity and fatality rate. MRSA surveillance in animals and food is currently voluntary and only carried out in a few countries. This monitoring, however, reveals an ever-changing situation, including the detection of livestock-associated MRSA (LA-MRSA), healthcare-associated MRSA, and community-associated MRSA from companion animals and/or livestock. LA-MRSA has recently received increased attention as a zoonotic risk, particularly for those who work in close proximity to livestock.
Acinetobacter species
Acinetobacter species have the widest inter-country range in resistance percentages of any bacterial species under EARS-Net surveillance. Depending on the reporting country, the percentage of isolates resistant to at least one of the antimicrobial groups under surveillance (fluoroquinolones, aminoglycosides, or carbapenems) ranged from 0% to 95.8% in 2019. Because Acinetobacter species is naturally resistant to many antimicrobial agents, acquired resistance complicates treatment of Acinetobacter species infections. MDR Acinetobacter species are a problem in the healthcare environment because they can survive for long periods of time in the environment and are notoriously difficult to eradicate once established.
Streptococcus pneumoniae
In addition to EARS-Net, the enhanced surveillance program for invasive pneumococcal disease (IPD), which is also supervised by ECDC, collects additional data on IPD cases from reference laboratories across the EU/EEA. The frequency of resistance to penicillin and erythromycin grew somewhat in all countries that consistently supplied antimicrobial susceptibility data, according to data from this surveillance project (13).
Enterococcus faecalis and Enterococcus faecium
There are grounds for concern that E. faecium is fast and constantly increasing in the percentage of vancomycin resistance in the EU. The ECDC study on AMR’s health burden estimated that vancomycin-resistant enterococci (VRE) infections and fatalities virtually doubled. A large issue for infection prevention and an important cause for dietary-related illnesses remain high levels of antimicrobial-resistant enterococci. In addition to being difficult to cure infections caused by resistant strains, enterococci are easily spread in medical settings (12).
Overview of the reported microorganisms resistant against the antimicrobials
The above-mentioned subsequent organisms have been tried to be treated with multiple antimicrobials, which has not benefited healthcare. The initial treatment method implemented against these species was applying a single antimicrobial. Later on due to nonobservance of the former antimicrobials, the healthcare sector switched to provide double antimicrobial treatment to the patients for more efficient results but to our surprise, the species were found to be successfully resistant against them. Recently, a combination of antimicrobials is being applied to fight against the resistance that is acquired by the organisms but eventually extensive use of multiple antimicrobials has not only triggered AMR globally but has also shown significant amounts of increase in MDR cases worldwide (Tab. IV) (12-14). The number of deaths attributed to bacterial AMR in 2019 has been estimated at 4.95 million based on previous research and several statistical methods. E. coli, S. aureus, K. pneumoniae, S. pneumoniae, Acinetobacter baumannii, and P. aeruginosa are the top infections for mortality associated to resistance in 2019 (15). Understanding the exact cost of resistance is a difficult task when trying to combat AMR, especially in areas with little surveillance and scant data. High percentages of third-generation cephalosporin and carbapenem resistance in K. pneumoniae, as well as high percentages of carbapenem-resistant Acinetobacter in various countries are of concern, according to a WHO/ECDC report from the year 2022. Resistance to last-resort antibiotics like vancomycin and members of the carbapenem family is also strongly triggered. There are very few treatment choices available if these antibiotics stop working, and some of them may even be lethal if they don’t. The effectiveness of life-saving medical measures like cancer treatment and organ transplantation is likewise threatened by resistance to last-line antibiotics (16). The prevalence of MDR and XDR tuberculosis as well as resistance in gram-negative bacteria are India’s biggest worries. The community’s Enterobacterales are producing extended-spectrum beta-lactamases at an alarming rate (17).
TABLE IV -.
| Bacterial species | Resistant against antimicrobial groups | Geographical location |
|---|---|---|
| Escherichia coli | Resistant to beta-lactam antibiotics | India, Europe, USA, and Taiwan |
| Staphylococcus aureus | MRSA (methicillin-resistant Staphylococcus aureus) | India, Europe, and USA |
| Klebsiella pneumoniae | Third-generation cephalosporin resistance, carbapenem resistance, aminoglycoside resistance, fluoroquinolone resistance | India, Europe |
| Pseudomonas aeruginosa | Carbapenem resistance, fluoroquinolone resistance, aminoglycoside resistance | India, Europe |
| Streptococcus pneumoniae | Resistant to macrolides | India, Europe |
| Acinetobacter species | Carbapenem resistance, aminoglycoside resistance, fluoroquinolone | India, Europe |
One Health Approach and training programs regulating AMR
One Health largely emphasizes the collaboration between human and animal health issues today, but also other disciplines should be merged, such as the environmental and social sciences. These One Health training agreements are notably integrated more into veterinary schools than into medical training, as the review of One University Training projects in Western Europe shows. Moreover, multidisciplinary and global health research and training activities must be undertaken, as zoonotic illnesses and AMR do not stop at national borders. Increasing emergent human infectious diseases of zoonotic origin and microorganism resistance to antimicrobial medicinal products have demonstrated that there is a need for cooperation between the human, animal, and environmental sectors. Increasingly, the One Health concept is recognized by politicians and scientists all across the world. In this overview, research and training efforts have been assembled with the aim of focusing on infectious diseases in One Health in Western Europe, particularly in France, Spain, the Netherlands, UK, Germany, and Norway. It can serve as a basis for future projects and partnerships. This summary indicates that One Health in Europe is widely recognized, as most recent educational activities are. In Europe, the One Health strategy in respect to AMR is now being pushed. Many nations have included the One Health strategy in their anti-AMR policy and there have been considerable increases in funding options for AMR research. The number of multidisciplinary national and international research networks on zoonotic diseases and One Health has grown. European institutes have researched on the topic of One Health Approach and many minor projects and training activities are being conducted in the countries of Europe for spreading the awareness of the importance of One Health Approach to fight against AMR and figure out a solution for it. Tables 5 and 6 depict the information related to One Health Approach conducted or training activities performed in European countries (3,20).
TABLE V -.
European institutes which researched on One Health Approach and also published article on this topic (3)
| Country | Research institute | Topic |
|---|---|---|
| France | OIE | Advocating the One Health Approach in general and in relation to rabies and Rift Valley fever |
| Germany | Freie Universitate Berlin | Publication on AMR and zoonoses in the food chain such as Vibrio and Campylobacter |
| United Kingdom | The Royal Veterinary College
London School of Hygiene and Tropical Medicine University of Cambridge University of Liverpool University of Edinburgh |
Research of AMR advocating the One Health concept and research of zoonoses and AMR research on zoonotic diseases such as emerging zoonosis and neglected research on zoonosis such as Japanese encephalitis virus and rabies |
| Norway | Norwegian Veterinary Institute | EU’s Horizon 2020 One Health Project |
| The Netherlands | Netherland Centre for One Health | Netherland Centre for One Health Project |
| Spain | Center for Veterinary Health Surveillance (VISAVET) | Project on One Health |
TABLE VI -.
Training activities conducted in the European counties on One Health
| Country | Institute | Type of training |
|---|---|---|
| Spain | Veterinary School of the Universitat Autonoma de Barcelona | Masters on zoonoses and One Health |
| The Netherlands | Utrecht University | Honours Program One Health, One Health Track |
| United Kingdom | Royal Veterinary College (RVC)/London School of Hygiene & Tropical Medicine (LSHTM), London Royal (Dick) School of Veterinary Sciences, Edinburgh, University of Bristol | Masters in One Health
Honours Program population medicine and One Health |
| France | Nantes-Atlantic National College of Veterinary Medicine, Food Science and Engineering, in partnership with the University of Nantes’ Department of Medicine and the University of Angers’ Department of Medicine | Master in Animals in training |
Various training activities for One Health are conducted in the universities of Europe. Students have also paticipated n One Health in recent years. Some countries have One Health student associations or public health veterinary organizations and networks, such as Holland and the United Kingdom. Extension activities are part of several European research projects. An annual One Health Workshop and One Health for Next Generation project is organized for instance in Anticipating a Global Onset of Novous Epidemics (ANTIGONE) (3,21).
One Health Approach regulating AMR in India
A national plan for controlling AMR has been formed in India. The plan suggests targeting a number of critical components of AMR in both the human sector and the nonhuman one, including agriculture, fishing, animal husbandry, and environment. The strategy addresses all the five main GAP goals and provides a further goal of boosting India’s AMR leadership. There are certain priorities that are being maintained to address all the issues. Below are the main objectives of the plan:
enhance awareness of AMR through effective communication, training and education;
enhance surveillance knowledge and evidence;
reduce infection incidence by efficient infection, prevention, and control;
optimize the use of antibiotics in all industries;
promote AMR investment, research, and innovation activities;
enhance India’s AMR leadership through international, national and sub-national collaborations on AMR.
The Indian NAP for AMR is a well-designed global plan that incorporates all of the key GAP goals and pledges to address critical antibiotic policy and regulatory problems within the “One Health Approach.” India’s National Action Plan (NAP) for AMR was released in April 2017 by the Union Ministry of Health and Family Welfare. Implementation was delayed but all parties needed a major push. Failure to achieve separate funding remains the major hurdle to implement NAPs and/or state action plans, not just in India (21,22). The mapping of the surveillance system set up for AMR in India is described in Figure 7.
Fig. 7 -.
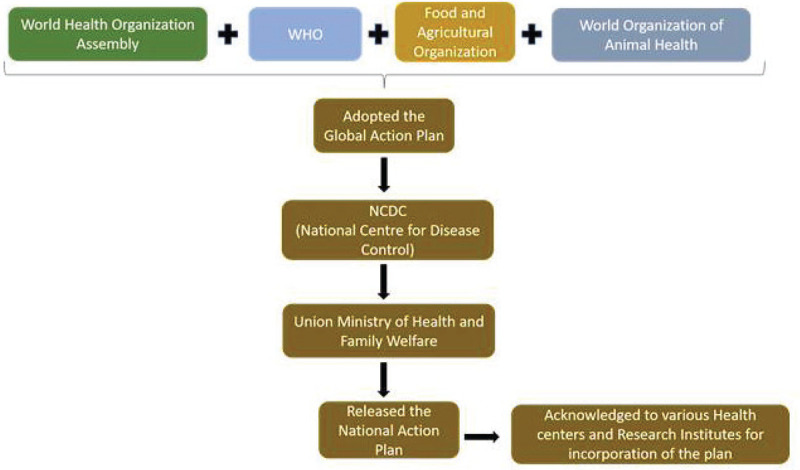
Mapping of the surveillance system set up in India for controlling antimicrobial resistance (21,22)
One Health Approach regulating AMR in Taiwan
Taiwan’s Centers for Disease Control (CDC) implemented the National Antimicrobial Stewardship Program; established multi-channel monitoring of MDR organisms, hospital accreditation, and hospital infection control inspections related to antimicrobial stewardship; coordinated infection control interventions; and carried out antimicrobial control interventions in response to the growing threat posed by AMR. Taiwan CDC also proactively establishes relevant guidelines, e-learning materials, manual hygiene, and antimicrobial awareness campaigns to encourage everyone to reduce this condition. Main objectives are:
Enhanced surveillance and control of carbapenem-resistant Enterobacteriaceae of antimicrobial-resistant pathogens;
Accredit and control hospital infections;
Hospital inspections, the antimicrobial stewardship of all hospitals, are necessary or encouraged;
Offer a number of e-learning courses to improve the understanding and consciousness of health workers on antimicrobial stewardship;
Conduct national public and health awareness-raising campaigns;
Cooperate on the fight against AMR with human and animal health sectors (23).
Thus, a comparative study has highlighted the fact that European countries as well as Asian countries such as India and Taiwan are equally contributing in building various agencies and organizations for combating AMR by implementing various policies and many other surveillance systems, which has actively increased the implementation of One Health Approach. In addition, after European countries, Taiwan has successfully accomplished many of their objectives which have helped the country in fighting against the AMR. Some of the above-mentioned strategies to prevent AMR are broadly explained (Figs. 8 and 9) (24). The mapping of the surveillance system set up for AMR in Taiwan is described further (Fig. 8) (25).
Fig. 8 -.
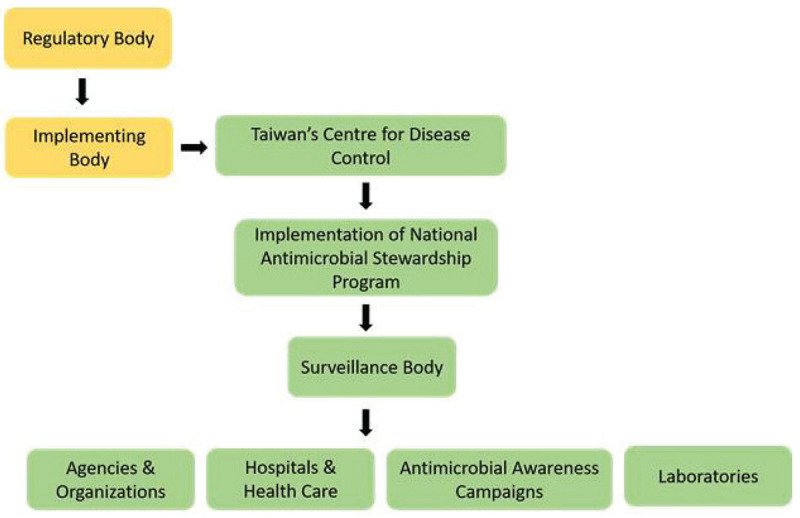
Mapping of the surveillance system set up in Taiwan for controlling antimicrobial resistance.
Fig. 9 -.
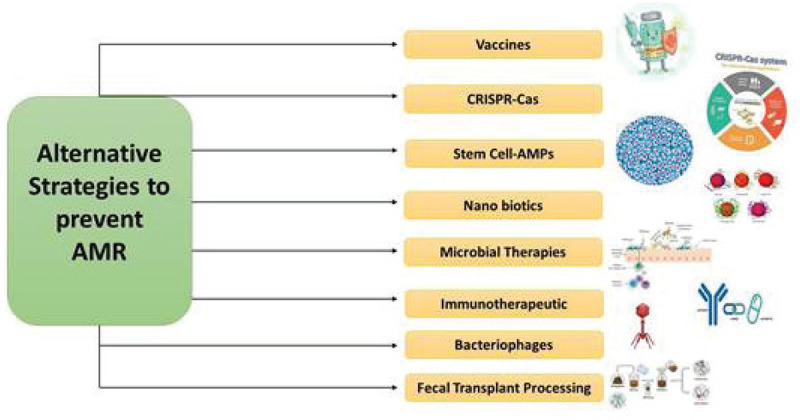
Possible alternative strategies to prevent AMR.
Discussion
This article provides an overview of various surveillance systems that are formed only to keep a track on the upcoming situation of AMR and the consumption of antimicrobials by humans as well as animals. The article does not provide all the details required to monitor the AMR issue but firmly allows the readers to get acknowledged with the broad information about the AMR across the six countries of Europe along with a comparative study between Taiwan and India. There are also a lot of debates on the themes of research covered by the term “One Health.” Any field of research, including anthropology, sociology, pedagogies, or comparative medicine, that may contribute to human, animal, or ecosystem health can be “One Health.” Failure to treat certain infections with currently available antibiotics is a concern for biomedicine. Phage therapy as an alternative therapy against bacterial infections has been extensively investigated. Although various challenges exist, bacteriophages treatment could be used in the future to replace antimicrobial agents with pathogens that are drug-resistant. The technique is now becoming popular as photographs are omnipresent, host-specific and harmless and can be administered with food orally. Antibiotic protein in target bacteria is developed for the delivery of recombinant phages. Topical treatment for open wounds or systemic infections may be performed intravenously. However, phage therapy gives rise to some serious concerns. The main thing about the host bacterium is its fine specificity. This prevents their use for acute infections as empirical therapy. The basis for their investigation was bacteriophageal lysins, the extremely specific peptidoglycan hydrolases, and was also referred to as enzybiotics. Incorporated lysins represent a new therapy form that is powerful and readily available to fight AMR as MDR diseases are becoming increasingly common threats (26,27). With the emerging crisis of AMR, vaccine treatments are seen as a possible solution by health authorities, healthcare providers, and drug developers. The biomolecules that boost the host immune system and give immunity against infectious agents are immunotherapeutic. Developments in the new technology of recombinant vaccines have been essential for reducing the use of different antibiotics for primary and secondary bacterial infection. One of the most important ways to prevent infections continues to be vaccines. Increasing the internal immune system is the advantage of immunotherapeutic agents (24). The CRISPR case is a distinguishing adaptive immune feature in archaea and bacteria, which offers protection against invasively invading bacteriophages and provides a regularly cross-sectional breast repeat. Short bacteriophages or plasmids known as spacers are inserted as a CRISPR array into the bacterial genome; the Cas proteins use guide RNAs from spacers to target the invading nucleic acid with the same sequence. Phagemids from CRISPR-Cas9 could kill certain in vivo bacteria. CRISPR Nanosized Compounds can target the Mec-A gene that is involved in the MRSA effectively (28). Mesenchymal stem cells (MSCs) have been intensively investigated for a variety of chronic diseases over several decades in order to develop a safe and promising therapeutical product. MSCs show promising skills in promoting immunomodulation, tissue cure and excessive inflammation control. Recently, human MSCs have been shown to synthesize antimicrobial peptide (AMP) factors that eradicate bacteria through several mechanisms including an inhibition of bacterial cell wall synthesis. Nonbacterial effects of MSCs (HUCMSCs) on drug-resistant clinical pathogens like E. coli, S. aureus, and K. pneumoniae have been detected (29). A number of names known as fecal microbiota transplantation are known as fecal bacteriotherapy. The Fecal Microbiota Transplantation (FMT) process involves the transplantation, using various routes, including enema, nasogastric, nasoduodenal and colonoscopy, of a fecal suspension of commensal bacteria by a healthy individual donor into the intestinal lumen of the recipients. Clinical trials have found an automotive FMT (aFMT) in antibiotic-disrupted human patients that is better than probiotic therapy and that has induced a fast and almost complete recovery of gastrointestinal microbiota (30). Nanoparticulate materials may be used for the supply or may contain antimicrobial materials. The nanoparticles and antibiotics based on metal and metal oxides are seen as promising therapeutic candidates for future applications of biomedical science, because they have lower toxicity and improved antibacterial, antiviral, and cancer efficacy. They are of unique size, such as an increased volume-to-surface ratio, making them efficient medicine carriers and improving their solubility, compatibility, and ease of delivery (31).
Advancing genetic engineering and next-generation sequence have enabled scientists to develop future strategies, such as bioengineered probiotics or pharmabiotics, that can become a bacterial infection biotherapy or prophylaxis. An option against antibiotics may be bioengineered probiotics with diverse immunogenic properties. Recombinant probiotics with high competence could provide a greater degree of site specificity than common drug administration regimes to produce drugs, therapeutic proteins, and gene therapy vectors (24).
Conclusion
The regular data collected by different organizations play a vital role in monitoring the status of AMR and antimicrobial usage by humans and livestock. These annual reports have highly helped the government to decide for alternatives and have focused in many training activities to combat the AMR situation globally. AMR prevention is linked to the One Health concept. As antibiotic resistance genes persist on an interface between environment and animal health, an approach is required in all three areas that stresses the concept of “One Approach to Health.” Finally, at any stage of life, antibiotic resistance can affect humans or animals. Alternative therapies should be developed to reduce dependency on chemical therapy. As antibiotics become part of modern medicine before many decades, antibiotic effectiveness is decreasing. Clinical research, microbiology, genetics and computer engineering, imaging and modeling experts should work together to develop strategies to deal with this problem and to develop new therapies. Patients with normal infections should avoid unnecessary prescription and over-prescription of antibiotics and patients should be advised to follow good hygiene such as hand washing and adequate infection management measures.
For the purpose of addressing the global epidemic of drug-resistant infections, an accurate evaluation of the existing and future burden of diseases caused by AMR is crucial. To effectively combat an apparent rise in resistance infections, detailed and dynamic information is required. This information enables policymakers and healthcare professionals to put global AMR action plans into place and allocate resources in an effective manner (32). The standard and accessibility of the supplied data affect how accurate AMR results will be. The current state of the global surveillance system is unconnected and unsatisfactory (33). Only 70 nations have reportedly signed up for the WHO’s Global Antimicrobial Resistance Surveillance System. The percentage is fewer than half of the AMR rates reported (34). Numerous restrictions, such as a lack of adequate global data, make it difficult to quantify AMR reports. Many countries lack the lab and data management capabilities needed to conduct efficient surveillance. Most significantly, surveillance data or analysis cannot remedy the problem right away; they can only estimate the burden that will arise. Only the creation of brand-new anti-pathogenic chemicals and herbal formulations can fix the problem. The exchange of data is also further constrained by many necessary and strict privacy concerns. More difficulties for the researchers are brought about by the lack of extensive and diverse datasets from various places, which is especially troubling in low-income countries where monitoring is essentially nonexistent.
Disclosures
Conflict of interest: The authors declare no conflict of interest.Financial support: This research was supported by two industry-academia collaboration projects. VtR Inc-CGU, R.O.C. project grant #SCRPD1L0221 and DOXABIO-CGU, R.O.C. project grant #SCRPD1K0131; it was additionally supported by CGU project grant #UZRPD1M0081.
Authors contribution: All authors contributed equally to this article.
References
- 1.Mesa Varona O, Chaintarli K, Muller-Pebody B et al. Monitoring antimicrobial resistance and drug usage in the human and livestock sector and foodborne antimicrobial resistance in six European countries. Infect Drug Resist. 2020;13:957–993. doi: 10.2147/IDR.S237038. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ionescu GF, Firoiu D, Pîrvu R et al. The potential for innovation and entrepreneurship in EU countries in the context of sustainable development. Sustainability (Basel). 2020;12(18):7250. doi: 10.3390/su12187250. [DOI] [Google Scholar]
- 3.Sikkema R, Koopmans M. One Health training and research activities in Western Europe. Infect Ecol Epidemiol. 2016;6(1):33703. doi: 10.3402/iee.v6.33703. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antibiotic use is rapidly increasing in developing countries. [Accessed August; 2022. ];The Lancet 2018; Online [Google Scholar]
- 5.The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. [Accessed August; 2022 ];2020 doi: 10.2903/j.efsa.2022.7209. Online [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrijver R, Stijntjes M, Rodríguez-Baño J, Tacconelli E, Babu Rajendran N, Voss A. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin Microbiol Infect. 2018;24(6):577–590. doi: 10.1016/j.cmi.2017.09.013. PubMed [DOI] [PubMed] [Google Scholar]
- 7.RESAPATH. French surveillance network for antimicrobial resistance in bacteria from diseased animals. [Accessed August; 2022 ];2017 Annual Report. 2019 March; Online [Google Scholar]
- 8.NIPH. Norwegian Institute of Public Health. [Accessed August; 2022 ];Antibiotic resistance in Norway. 2014 Online updated 2018. [Google Scholar]
- 9.NIPH. Norwegian Institute of Public Health. [Accessed August; 2022 ];NORM og NORM-VET: Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Online [Google Scholar]
- 10.National Institute for Public Health and the Environment. NethMap 2019. Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands. [Accessed July; 2019 ]; Online [Google Scholar]
- 11.Publication office of the European Union. 2013/652/EU: Commission Implementing Decision of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria (notified under document C(2013) 7145) Text with EEA relevance. [Accessed August; 2022 ]; Online [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net). [Accessed August; 2022 ];Annual Epidemiological Report for 2019. Online [Google Scholar]
- 13.Cassini A, Högberg LD, Plachouras D et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. 12th conference of HEPA Europe: “Implementing health-enhancing physical activity research: from science to policy and practice”. [Accessed August; 2022 ]; Online [Google Scholar]
- 15.Murray CJL, Ikuta KS, Sharara F et al. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. [Accessed August; 2022 ]. Online
- 17.Singhal T. Antimicrobial resistance: The “Other” Pandemic!: Based on 9th Dr. I. C. Verma Excellence Award for Young Pediatricians Delivered as Oration on 19th Sept. 2021. Indian J Pediatr. 2022;89(6):600–606. doi: 10.1007/s12098-021-04008-9. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu D, Ding Y, Yao K, Gao W, Wang Y. Antimicrobial resistance analysis of clinical Escherichia coli isolates in neonatal ward. Front Pediatr. 2021;9:670470. doi: 10.3389/fped.2021.670470. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang S-M, Chen J-W, Tsai C-S, Ko W-C, Scaria J, Wang J-L. Antimicrobial-resistant Escherichia coli distribution and whole-genome analysis of sequence Type 131 Escherichia coli isolates in public restrooms in Taiwan. Front Microbiol. 2022;13:864209. doi: 10.3389/fmicb.2022.864209. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health. IVSAISCO. [Accessed August; 2022 ]; Online [Google Scholar]
- 21.Singh S, Charani E, Devi S et al. A road-map for addressing antimicrobial resistance in low- and middle-income countries: lessons learnt from the public private participation and co-designed antimicrobial stewardship programme in the State of Kerala, India. Antimicrob Resist Infect Control. 2021;10(1):32. doi: 10.1186/s13756-020-00873-9. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjalkar J, Chandy SJ. India’s National Action Plan for antimicrobial resistance – an overview of the context, status, and way ahead. J Family Med Prim Care. 2019;8(6):1828–1834. doi: 10.4103/jfmpc.jfmpc_275_19. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Course. AOH. [Accessed August; 2022 ]; Online [Google Scholar]
- 24.Kumar M, Sarma DK, Shubham S et al. Futuristic non-antibiotic therapies to combat antibiotic resistance: a review. Front Microbiol. 2021;12:609459. doi: 10.3389/fmicb.2021.609459. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Online
- 26.Wright RCT, Friman V-P, Smith MCM, Brockhurst MA. Resistance evolution against phage combinations depends on the timing and order of exposure. MBio. 2019;10(5):1652–e19. doi: 10.1128/mBio.01652-19. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vázquez R, García E, García P. Phage lysins for fighting bacterial respiratory infections: a new generation of antimicrobials. Front Immunol. 2018;9:2252. doi: 10.3389/fimmu.2018.02252. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pursey E, Sünderhauf D, Gaze WH, Westra ER, van Houte S. CRISPR-Cas antimicrobials: challenges and future prospects. PLoS Pathog. 2018;14(6):e1006990. doi: 10.1371/journal.ppat.1006990. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx C, Gardner S, Harman RM, Van de Walle GR. The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. Stem Cells Transl Med. 2020;9(7):746–757. doi: 10.1002/sctm.19-0333. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello SP, Hughes PA, Waters O et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156–164. doi: 10.1001/jama.2018.20046. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Munoz R, Lopez FD, Lopez-Ribot JL. Bismuth nanoantibiotics display anticandidal activity and disrupt the biofilm and cell morphology of the emergent pathogenic yeast Candida auris. Antibiotics (Basel). 2020;9(8):E461. doi: 10.3390/antibiotics9080461. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnall J, Rajkhowa A, Ikuta K, Rao P, Moore CE. Surveillance and monitoring of antimicrobial resistance: limitations and lessons from the GRAM project. BMC Med. 2019;17(1):176. doi: 10.1186/s12916-019-1412-8. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay SI, Rao PC, Dolecek C et al. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018;16(1):78. doi: 10.1186/s12916-018-1073-z. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization (WHO) Global Antimicrobial Resistance Surveillance System. [Accessed August; 2022 ];(GLASS) report: early implementation 2017–2018. Online [Google Scholar]


