Abstract
Infection of cultured HeLa epithelial cells with enteropathogenic Escherichia coli (EPEC) or enterohemorrhagic E. coli (EHEC) O157:H7 results in accumulation of cortactin under the adherent bacteria. Tyrosine phosphorylation of cortactin is not induced following HeLa cell infection with EHEC or EPEC, contrary to what has been reported to occur with Shigella flexneri.
Enteropathogenic Escherichia coli (EPEC) is an organism able to cause severe diarrhea in infants, especially in developing parts of the world, where the infection is frequently fatal (12). Enterohemorrhagic E. coli (EHEC), particularly serotype O157:H7 and serogroups O26 and O111, often causes infections ranging from asymptomatic infection or mild diarrhea to hemorrhagic colitis and, in some cases, hemolytic-uremic syndrome, sometimes leading to death (9). Both EPEC and EHEC are able to cause attaching and effacing (A/E) lesions characterized by the loss of the epithelial microvilli and strong adherence, leading to the formation of a pedestal-like structure beneath the bacterial adherence site. A/E lesions are observed in biopsy specimens from patients and can be reproduced in infected animals, as well as in cultured colonic or epithelial cells (14). In EPEC, the genes necessary for A/E lesion formation are encoded in a 35-kb chromosomal segment called the LEE (locus of enterocyte effacement) locus (3). This locus is also present in EHEC and other A/E lesion-causing organisms but absent in normal bacterial flora (8). A model for EPEC adherence to epithelial cells has been proposed that comprises an initial adherence stage followed by a signal transduction stage during which a complex secretion apparatus, called the type III secretion system, is involved in injecting bacterial virulence factors directly into host cells (2). Intimate adherence is mediated by the binding of intimin, a bacterial outer membrane protein, to Tir (translocated intimin receptor), a bacterial protein that is injected and modified inside the host mammalian cell (10). During the intimate stage of adherence, myosin light chain (13), actin, α-actinin, ezrin, and talin (5) rearrange and accumulate beneath the adherent bacteria.
We found that another mammalian cell protein, an actin-binding protein called cortactin, is also recruited to the EPEC and EHEC attachment site. Recruitment of cortactin has, so far, been reported only for Shigella flexneri and Chlamydia trachomatis (1, 4). In S. flexneri infection, cortactin was described to be tyrosine phosphorylated by pp60c-src and to accumulate around the invading bacteria during HeLa cell infection, while in C. trachomatis infection, no tyrosine phosphorylation of cortactin has been observed.
The bacterial strains used in this study were obtained from the Research Institute for Microbial Diseases (RIMD) bacterial culture collection. RIMD 0509829 is a typical EPEC strain belonging to serotype O142:H2. RIMD 0509952 is a serotype O157:H7 EHEC strain isolated in Osaka, Japan, and confirmed to secrete verotoxins (VT1 and VT2). S. flexneri RIMD 3102002 was used as a positive control for cortactin mobilization and tyrosine phosphorylation experiments. Subconfluent HeLa cell (Riken) monolayers were grown on glass coverslips and infected for 4 h or, as indicated elsewhere in the text, fixed and permeabilized as described by Rosenshine et al. (16). In some experiments, the inhibitors used, i.e., the F actin-depolymerizing agent cytochalasin D (Cyt-D; Sigma Chemical Co., St. Louis, Mo.) (2 μM) and the tyrosine protein kinase (TPK) inhibitor staurosporine (Sigma) (1 μM), were both added to HeLa cells 30 min prior to infection. PP1 (Alexis), a potent Src family tyrosine kinase inhibitor (6), was added to the HeLa cells at 10 μM and maintained overnight, and cells were infected the next day. All of the drugs were maintained during the infection period. Reorganization of cortactin was detected with anticortactin monoclonal antibodies (anti-p80/85; UBI); this was followed by incubation with a proper fluorescein isothiocyanate-labeled second antibody. Micrographs were obtained by confocal microscopy, and all images were processed in exactly the same manner. Protein extraction for immunoprecipitation and Western blotting was performed essentially as detailed by Rosenshine et al. (16). Vanadate-treated HeLa cells were used as a positive control for cortactin tyrosine phosphorylation (17).
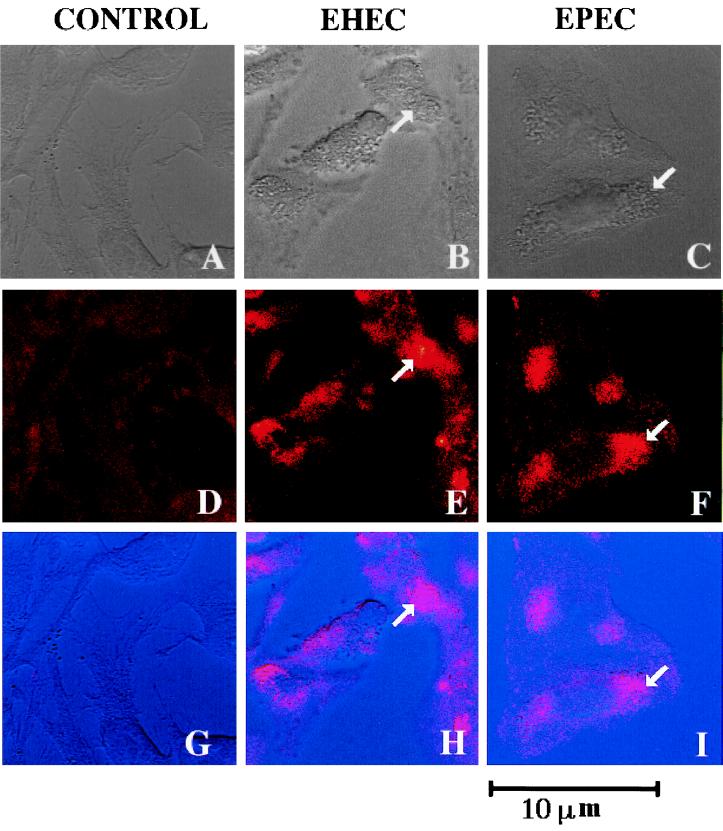
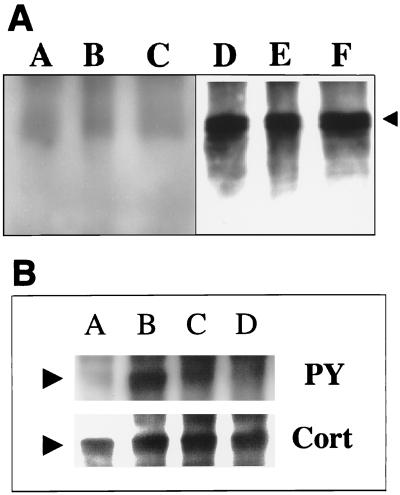
We investigated whether cortactin is recruited to the adherence site of EPEC and EHEC, two organisms known to accumulate F actin beneath the bacterial attachment site (11). By confocal microscopy, perfect colocalization of cortactin and the attaching bacteria could be observed, showing that these organisms are able to cause rearrangement of this cellular protein (Fig. 1). Next, we attempted to detect whether cortactin is tyrosine phosphorylated in response to EPEC or EHEC infection. Cortactin was immunoprecipitated from HeLa cell extracts obtained after infection with EPEC or EHEC organisms, as well as noninfected cells for comparison. Protein samples were analyzed after being transferred to polyvinylidene difluoride membranes (Millipore) and probed with antiphosphotyrosine antibodies (PY20; Transduction Laboratories). No differences in the tyrosine phosphorylation pattern were observed between infected and noninfected cells. Figure 2A shows the lack of tyrosine phosphorylation of cortactin in HeLa cells after 3 h of infection with EPEC and EHEC. A time course experiment in which cortactin tyrosine phosphorylation was analyzed 0.5, 1, and 2 h after HeLa cell infection with EPEC or EHEC revealed no changes in tyrosine phosphorylation compared to the control cells (data not shown). Tyrosine phosphorylation of cortactin is an event unlikely to occur earlier than 30 min postinfection, since by that time the attachment of EPEC or EHEC to the cells is still poor, and cortactin accumulation was not detected within the first hour after infection (data not shown). Our results suggest that the cortactin accumulation beneath adherent EPEC and EHEC occurs independently of tyrosine phosphorylation. On the other hand, S. flexneri was observed to induce tyrosine phosphorylation of cortactin 30 min after HeLa cell infection; after that, cortactin gradually became diffusely distributed over the entire cell cytoplasm, and by 1 h after infection, cortactin accumulation or tyrosine phosphorylation could no longer be observed (1) (Fig. 2B and 3B).
FIG. 1.
EPEC and EHEC induce cortactin accumulation in cultured HeLa cells. Noninfected HeLa cells (A, D, and G) and HeLa cells infected for 4 h with EHEC O157:H7 (B, E, and H) or EPEC (C, F, and I) were visualized with anticortactin antibodies labeled with rhodamine (D, E and F) by confocal laser scanning microscopy. Panels A, B, and C are phase-contrast images. Panels G, H, and I are superimposed images A and D, B and E, and C and F, respectively. The arrows indicate a bacterial cluster (phase-contrast images) and the corresponding accumulation of cortactin at the adherence site (confocal images).
FIG. 2.
(A) EPEC or EHEC does not induce tyrosine phosphorylation of cortactin during HeLa cell infection. Cortactin was immunoprecipitated from noninfected HeLa cell extracts (A, D) or from extracts of HeLa cells infected with EPEC (B, E) or EHEC O157:H7 (C, F), blotted, and probed with antiphosphotyrosine serum (A, B, and C). The same membrane was reprobed with anticortactin serum (D, E, and F). The arrowhead indicates the cortactin bands. (B) Induction of tyrosine phosphorylation of cortactin during HeLa cell infection with S. flexneri. Cortactin was immunoprecipitated from HeLa cells infected with S. flexneri (C, D), vanadate-treated HeLa cells (B), or control cells (A). A Western blot membrane was probed with antiphosphotyrosine antibodies (PY) and reprobed with anticortactin antibodies (Cort). The arrowheads indicate the cortactin bands.
FIG. 3.
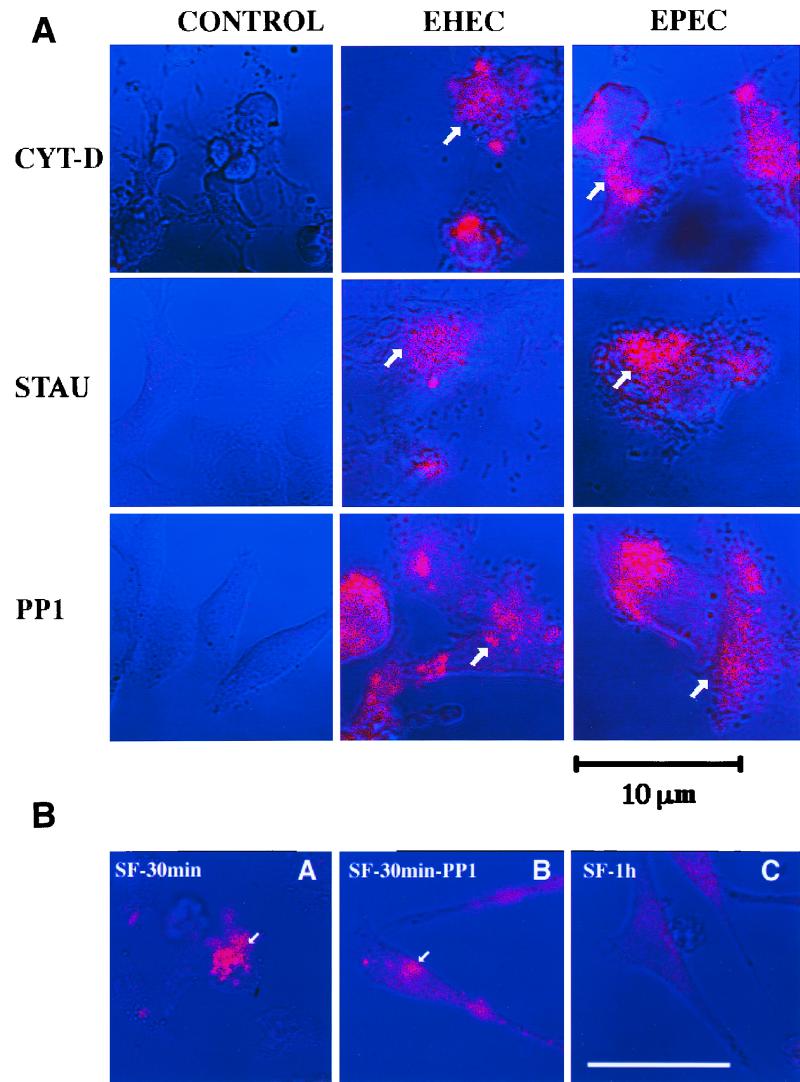
(A) Effects of inhibitors on EHEC- or EPEC-induced cortactin accumulation observed by confocal laser scanning microscopy. HeLa cells were treated with Cyt-D, staurosporine (STAU), or the drug PP1 (see the text for details) and were either not infected (CONTROL) or infected with EHEC O157:H7 or EPEC organisms. Cortactin (pink) was stained with anticortactin serum labeled with appropriate rhodamine-conjugated goat anti-mouse antibodies. The arrows indicate a bacterial cluster and the corresponding accumulation of cortactin at the bacterial adherence site. (B) Confocal images of cortactin accumulation by S. flexneri. HeLa cells were infected with S. flexneri for 30 min (A and B) or 1 h (C). Cortactin accumulation was observed in the absence (A and C) or presence (B) of PP1. The arrows show cortactin accumulation at the bacterial adherence site. See the text for details. Bar, 10 μm.
S. flexneri is able to recruit actin and tyrosine-phosphorylated cortactin around the invading bacteria, a process mediated by the proto-oncoprotein pp60c-src (1). Infection with EPEC, EHEC, or S. flexneri was performed in the presence of PP1. HeLa cells were stained for cortactin and analyzed by confocal microscopy. Figure 3A shows that rearrangement of cortactin by EPEC and EHEC does occur despite the presence of Src family inhibitor PP1. Moreover, indirect immunofluorescence experiments using anti-pp60c-src antibodies were unable to demonstrate any accumulation of this protein kinase beneath the EPEC and EHEC attachment site (data not shown). Infection of PP1-treated HeLa cells with S. flexneri resulted in a visible decrease in cortactin accumulation around invading bacteria, further suggesting that mobilization of cortactin by S. flexneri is pp60c-src dependent (Fig. 3B).
Cyt-D was used to evaluate whether cortactin can be rearranged independently of F actin accumulation. HeLa cells were treated with Cyt-D for 30 min prior to infection with EPEC or EHEC organisms and maintained throughout the 4-h infection period. Accumulation of cortactin beneath adherent bacteria occurred irrespective of Cyt-D treatment (Fig. 3A). Immunofluorescence microscopy using phalloidin-rhodamine revealed no accumulation of F actin under the bacteria attached to Cyt-D-treated HeLa cells (data not shown).
Staurosporine, a TPK inhibitor, has been reported to significantly inhibit the induction of tyrosine phosphorylation of proteins in HeLa cells infected with EPEC (15). Cortactin rearrangement by EPEC or EHEC is not affected by staurosporine treatment, suggesting that its redistribution is not related to the TPK activity of HeLa cells (Fig. 3A).
Cortactin is a versatile molecule which can avidly bind actin through several tandem 37-amino-acid repeats located in the N-terminal half of the molecule (18). Cortactin also contains a Src homology 3 domain in the C-terminal portion of the molecule, suggesting the ability to bind to other cytoskeletal proteins, as well as the potential to transduce signals from the cell surface to the cytoskeleton. The experiments in our study revealed that although cortactin is efficiently recruited to the EPEC or EHEC adherence site, in contrast to S. flexneri, EPEC or EHEC is able neither to induce tyrosine phosphorylation of cortactin nor to recruit pp60c-src to the bacterial attachment site. These results suggest that the mechanism of cortactin mobilization following HeLa cell infection by EHEC or EPEC differs from that of S. flexneri. Cortactin seems to be able to bind other cytoskeleton proteins and is also able to cross-link F actin, a process that was reported to be more efficient with the dephosphorylated form of cortactin (7). In the case of S. flexneri, the actin network that forms around the invading bacteria may be constantly reorganized in order to facilitate bacterial access to the host cell (1). Reduction of the actin-binding capacity of cortactin through tyrosine phosphorylation by pp60c-src may be important for S. flexneri to escape from the phagosome vacuole, facilitating bacterial access to the host cell cytoplasm. In the case of EPEC or EHEC, it is possible that cortactin plays an important role during the formation of the characteristic pedestal that appears under the attached bacteria, through the efficient F actin-binding and cross-linking activities of the tyrosine-dephosphorylated form of cortactin. The results of this study also demonstrated that cortactin can be recruited to the proximity of attached EPEC or EHEC even after treatment with the F actin-depolymerizing agent Cyt-D, suggesting that it is mobilized through some still unknown bacterial signal following infection and not simply translocated while bound to F actin. The hypothesis that cortactin is involved in the bridging of F actin filaments to others cytoskeletal or bacterial proteins is under investigation in our laboratory.
Acknowledgments
We thank I. Yanagihara and T. Kodama for helpful discussions. V.V.C. thanks Monaliza, Raquel, and Bianca for continuous encouragement.
V.V.C. was partially supported by Laboratorio Weinmann. This study was supported by a grant-in-aid from the Research for the Future program of the Japan Society for the Promotion of Science (JSPS-RFJF 97L00704).
REFERENCES
- 1.Dehio C, Prévost M-C, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signaling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnenberg M S, Kaper J B, Finlay B B. Interaction between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 3.Elliott S J, Wainwright L A, MacDaniel T K, Jarvis K G, Deng Y, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 4.Fawaz F S, van Ooij C, Homola E, Mutka S C, Engel J N. Infection with Chlamydia trachomatis alters the tyrosine phosphorylation and/or localization of several host cell proteins including cortactin. Infect Immun. 1997;65:5301–5308. doi: 10.1128/iai.65.12.5301-5308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Ni Y, Wang T, Gao Y, Haudenschild C C, Zhan X. Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:13911–13915. doi: 10.1074/jbc.272.21.13911. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis K G, Giron J A, Jerse A E, MacDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmali M A. Infection by verotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 11.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M M, Bergquist E J, Nalin D R, Waterman D H, Hornick R B, Young C R, Scotman S, Rowe B. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxin and are noninvasive. Lancet. 1978;i:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
- 13.Manjarrez-Hernandez H A, Baldwin T J, Aitken A, Knutton S, Williams P H. Intestinal epithelial cell protein phosphorylation in enteropathogenic Escherichia coli diarrhoea. Lancet. 1992;339:521–523. doi: 10.1016/0140-6736(92)90340-9. [DOI] [PubMed] [Google Scholar]
- 14.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenshine I, Donnenberg M, Kaper S, J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 17.Van Damme H, Brok H, Schuuring-Scholtes E, Schuuring E. The redistribution of cortactin into cell-matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and posttranslational modification. J Biol Chem. 1997;272:7374–7380. doi: 10.1074/jbc.272.11.7374. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Parsons T. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]