Abstract
Objective
African American older adults living in disadvantaged communities are disproportionately burdened by disabling pain. To address their needs, we tested the feasibility and potential effects of a cognitive-behavioral chronic pain self-management program delivered by community health workers.
Design
A single-group, pre-post evaluation of the STEPS-2 (Seniors using Technology to Engage in Pain Self-management) intervention, in which participants learned pain-management skills through web-based videos. They were also given wearable activity trackers to facilitate incremental increases in walking. In weekly telephone calls, community health workers helped participants apply skills and set goals.
Subjects/setting
Thirty-one adults in Detroit, Michigan (97% African American, 97% female, mean 68.7 years), with chronic musculoskeletal pain.
Methods
Participants completed telephone surveys at baseline and eight weeks. We measured changes in PROMIS pain interference and pain intensity, as well as Patient Global Impression of Change in pain and functioning. Feasibility indicators included participant engagement and satisfaction, and fidelity to session protocols by community health workers.
Results
Participants on average completed 6.6/7 sessions, and 100% agreed or strongly agreed that they improved their understanding of pain management. Average community health worker fidelity score was 1.79 (0 to 2 scale). Pain interference decreased from baseline to post-program (T-score 61.6 to 57.3, P=.000), as did pain intensity (0 to 10 scale, 6.3 to 5.1, P=.004). Approximately 90% of participants reported that pain and function were at least “a little better” since baseline.
Conclusions
An intervention combining mobile health tools with support from community health workers holds promise for improving pain outcomes among underserved older adults.
Keywords: Chronic Pain, African Americans, Older Adults, Community Health Workers, Cognitive-Behavioral Therapy
Introduction
Chronic pain is a major cause of diminished physical, psychological, cognitive, and social functioning among older adults [1–7]. Chronic pain self-management (CPSM) programs, rooted in principles from cognitive behavioral therapy for chronic pain [8], can reduce pain’s interference with daily functioning. Used alone or as an adjunct to other treatments including analgesic medications, CPSM programs teach cognitive (e.g., distraction) and behavioral strategies (e.g., relaxation), as well as progressive physical activity and goal-setting. These elements are all recommended for treatment of chronic pain in older adults [9, 10].
Nowhere is the need for effective CPSM support more acute than among economically disadvantaged African American older adults. This group faces greatly disproportionate pain-related disability and inferior pain care [11–15], including less access to safe non-pharmacological treatments, which generally are not covered by insurance [16]. Yet standard ways of providing CPSM support may not be broadly accessible to this population. Group-based classes such as the Stanford-developed Chronic Pain Self-Management Program [17] can be difficult to access in a population where transportation and mobility challenges are the norm [18]. Although web-based CPSM interventions have shown promising results [19–21] and can reach older adults in their homes, they pose challenges in underserved populations due to low technological literacy and access [22–24]. Moreover, while high engagement and adherence are critical for good outcomes in CPSM interventions [25], self-directed web-based programs have high drop-out rates [26, 27].
When it comes to marginalized populations such as African American older adults, another limitation of standard CPSM interventions is that they do not address the social determinants of health—including housing issues, transportation, and other unmet daily needs—that can exacerbate chronic pain and interfere with its management. In the United States, centuries of structural racism have given rise to residential segregation, severe economic and educational deprivation, toxic physical environments, and inferior medical care for African Americans [28, 29]. These adverse conditions lead to more severe pain in this population via their profound noxious impact on physical and mental health and also contribute to pain-related disability [14, 15, 30, 31]. Yet they are not typically accounted for in the design of CPSM programs.
One novel way to address modifiable social factors as part of an individual-level CPSM intervention is to engage community health workers (CHWs) in program delivery. CHWs are lay healthcare workers who have close connections to the communities they serve. This shared community and cultural identity fosters good communication and a strong therapeutic alliance (i.e., trusting relationship between provider and client), which may enhance outcomes from pain treatment [32, 33]. CHW programs provide structured training for specific tasks, such as connecting patients to health and social services to address social determinants of health and supporting behavior change [34, 35].
Building on the strengths of CHWs as a way to address the need for CPSM support among underserved older adults, we conducted a pilot study of the “STEPS-2” intervention (Seniors using Technology to Engage in Pain Self-management-2). STEPS-2 content was adapted from a previous web-based CPSM intervention [36]. It is based on the biopsychosocial model of chronic pain [37] and principles from cognitive behavioral therapy (CBT), the leading evidence-based psychosocial treatment for pain. CBT for pain has been successfully used in a wide variety of populations, including older adults and socioeconomically disadvantaged groups [38–40]. STEPS-2 teaches participants cognitive-behavioral pain management strategies via short, web-based videos featuring experts in each of the core skills presented. In weekly telephone sessions, CHWs guide and motivate participants to apply video content to their daily lives and to set behavioral goals, as goal-setting is a difficult skill to learn in a fully self-directed electronic format [20, 26]. To facilitate incremental increases in physical activity, participants use wearable activity trackers (FitbitTM Zip or Charge), which we previously demonstrated to be feasible in this population [41]. To address social determinants of health, CHWs help participants problem-solve and as needed make referrals to health and social services in their community. Our study setting is Detroit, Michigan—one of the most disadvantaged cities in the United States in terms of racial segregation, income, health, and social mobility [42–44].
In this pilot study, we assessed the feasibility and potential efficacy of STEPS-2. Our aims were as follows: 1) determine feasibility in terms of participant engagement, satisfaction, recruitment/retention and CHW fidelity to session protocols; 2) estimate pre-post change in PROMIS pain interference, and perceptions of change in pain and functioning; and 3) explore changes in pain intensity, self-efficacy, self-assessed change in pain medication use, and step counts.
Methods
Participants and Recruitment
The study had a single-group, pre-post design. It was approved by the University of Michigan’s Institutional Review Board (HUM00154949) and registered at clinicaltrials.gov (NCT04095650).
Eligibility criteria: Participants were 60+ years of age, community-living, and ambulatory with or without an assistive device. They reported pain in their muscles or joints for ≥3 months, a pain intensity rating ≥4 (on a scale from 0 = no pain to 10 = worst imaginable pain) over the last week, and ≥1 day in past month when pain made it difficult to do usual activities. In addition, they were required to have a cell or landline phone and internet access at home or elsewhere. Individuals were excluded if they had a serious acute illness or hospitalization in the last month; major surgery planned in next 3 months that would interfere with program participation (e.g., knee replacement); or a severe physical, cognitive, or psychiatric disorder judged to pose significant barrier to participation. Participants were asked if they had “significant memory issues that get in the way of your usual activities”; those answering yes were read a brief description of the intervention and asked if they thought they would be able to participate.
Recruitment sources: Participants were recruited via flyers and word of mouth at community locations in Detroit serving older adults (e.g., senior centers and senior housing facilities). Participants were also recruited from a research-volunteer registry maintained by the Healthier Black Elders Center at the Wayne State University Institute of Gerontology [45]. Participants were offered a total of $30 in incentives for the two research interviews and were invited to keep the electronic activity tracker.
Intervention Development
We developed a multicomponent CPSM intervention (calling it “STEPS-2” to distinguish it from “STEPS,” our earlier study that tested the feasibility and potential effects of wearable activity trackers as a pain self-management tool [41]). STEPS-2 is based on a successful web-based CBT program for fibromyalgia [36, 46] adapted to be responsive to the needs and cultural preferences of our priority population and compatible with support provided by CHWs. Adaptation of the program was informed by the following: 1) two focus groups with members of the priority population, to learn about their pain self-management practices, preferences, and challenges [47]; 2) a series of meetings with three CHWs, who had many years’ combined experience working in the Detroit community, to obtain input and iterative feedback on program design and materials; and 3) usability testing of the STEPS-2 website with three older adults from the priority population, after which substantial revisions were made to make it easier to navigate. Finally, the program design incorporated practical learnings from our prior study about needs and preferences for technology use in our priority population (e.g., a preference for simple, written instructions) [41].
Ultimately, the following intervention materials were developed: 1) a simple, mobile-friendly program website that housed videos for each weekly session with additional optional links for more information on a given topic. Each video was a brief, didactic presentation by a University of Michigan expert explaining a particular skill, such as engaging in pleasant activities. The video format increased the standardization of the STEPS-2 intervention and meant that CHWs, who are not specialized CBT providers, did not have to introduce new concepts. Rather, CHWs focused on guiding and motivating participants in skills practice, goal-setting, and problem-solving. Videos were put on YouTube so that participants who had difficulty navigating a web browser could simply click a link in a text message from their CHW to easily view the video on their phone; 2) a participant workbook that reinforced key points from the videos and provided worksheets (e.g., for goal-setting) as well as resource lists; and 3) a CHW manual with detailed scripts for each telephone session, along with additional information needed for successful program delivery; e.g., background information on cognitive-behavioral chronic pain management, a guide for interpreting and using weekly step-count data from participants, updated lists of local resources, “how-to” guides for using REDCap for tracking key session information, and how to identify and report adverse events.
Materials for both participants and CHWs were written in plain language so that they would be appropriate for a range of health literacy levels [48]. Materials used culturally familiar examples and language throughout, as well as images that reflected our priority population of African American older adults.
Cognitive-behavioral content: The cognitive-behavioral skills taught in STEPS-2 are shown in Table1. These included: goal-setting, progressive exercise, sleep hygiene, pleasant activity scheduling, relaxation, and problem solving. While these modules were all included in the original web-based intervention from which STEPS-2 was derived, we added a session on patient-provider communication and making the most of health care appointments, based on focus group findings that dissatisfaction with pain care was common.
Table 1.
STEPS-2 intervention content by session
| Session topics |
|---|
| Note: All sessions include reviewing and setting two goals: 1) Chronic pain self-management goal; 2) Weekly step count goal and new goal for 10% increase. |
|
| Week 2: Staying Active: Why physical activity is effective for managing pain and fatigue; strategies for incorporating physical activity into daily routine. Using step counts to increase/maintain activity. Introduce NIA’s Go4Life booklet. Discuss resources available to participants in Detroit; e.g., senior centers and transportation. |
|
| Week 4: Partnering With Your Provider: Communicating with health care providers. Preparing for medical visits to make sure concerns are addressed. |
| Week 5: Relaxing and Reducing Stress: The relationship between pain and stress. Training your body to produce the relaxation response; how relaxation helps symptoms. Simple problem-solving process to address stressors. |
| Week 6: Getting a Good Night’s Sleep: Impact of poor sleep on pain; sleep hygiene. |
| Week 7: Moving Forward: Review of key skills and planning for long-term goals. |
Intervention flow: In the first session, participants met in a small group led by a CHW and study staff member. Participants were shown how to access the web-based videos and how to use their activity trackers and report step counts by replying to an automated text each evening. Two videos were shown during the session and discussed as a group: one provided basic pain psychoeducation—that is, how symptoms interfere with function, and how feelings and behaviors affect pain—and the other presented a process for goal-setting. With coaching from the CHW, each participant identified and set a chronic pain self-management goal (any behavioral goal related to pain management or functioning that was personally important). Finally, participants were asked to engage in their usual amount of physical activity over the next week so that a baseline weekly average step count could be established, and to watch the Session 2 video prior to their first telephone session with their CHW.
Telephone sessions 2 through 7 were conducted weekly by CHWs. All were one-on-one and followed the same general structure: 1) recap key points from that week’s video (in more detail if the participant had not yet watched it), engage in structured discussion about the video topic, and make a “Try it Out” plan to try new skill; 2) debrief about the “Try It Out” activity for last week’s skill; 3) review pain self-management goal progress and modify or continue goal; 4) review step counts from the prior week and set a step-count goal (last week’s average plus 10%, chosen to be a steady and safe increase and following a protocol successfully used in another chronic pain study [49]; 5) closing and reminder to watch next week’s videos and to reply to the text each evening to log daily step count. As issues related to social determinants of health came up during sessions (e.g., utility payments, transportation issues), CHWs referred participants to appropriate local resources. All intervention sessions were audio-recorded, with participants’ permission.
CHW training: Three community health workers were trained to deliver STEPS-2 in three half-day training sessions that included role-plays with feedback. CHWs were selected because they had prior experience working with older adults and with delivery of evidence-based interventions. All three CHWs were already certified by the Michigan Community Health Worker Alliance training program [50], which teaches core CHW competencies including communication skills, components of healthy lifestyles, and legal and ethical responsibilities. Following STEPS-2 training, we began enrolling participants into the study on a rolling basis.
Data Collection and Measures
Telephone interviews were conducted by trained research assistants at baseline and 2 months from baseline (immediately post-program). The survey included health, psychosocial, and demographic measures. The follow-up interview included a series of closed and open-ended questions about participants’ experience in the intervention.
Aim 1: Feasibility Measures
Participant Engagement: was indicated by the number of completed sessions (out of 7) and reported frequency of use of web-based modules.
Participant Satisfaction: Likert-scale questions elicited ratings of satisfaction with the overall program (“Participating in STEPS-2 increased my understanding of pain management” and “helped me reach my pain management goals”) as well as specific program components, including the videos, electronic activity tracker, and workbook. Open-ended items elicited details, both overall and per component, about what participants liked/didn’t like and why, along with other feedback about the program experience.
Recruitment and retention were indicated by the average number of participants enrolled per month and the number of sessions completed out of 7.
CHW Fidelity to Session Protocols: To determine fidelity (the degree to which CHWs delivered the intervention per protocol), investigators and staff listened to a selected subset of audio-recordings of telephone sessions while the study was still in progress, to inform coaching sessions with CHWs. A structured fidelity rating form was used to rate the extent to which CHWs completed each required element. Fidelity scores per element were assigned as follows: 0 = did not cover; 1 = partially covered, and 2 = completely covered.
Aim 2: Pain-Related Functioning Outcomes
Pain Interference: PROMIS-43 Adult Profile, 6-item subscale. Items ask how much pain (not at all to very much) in the last week has interfered with daily activities such as household chores and social activities [51]. A difference of 2 to 3 T-score points is considered the minimally important clinical difference [51]. All PROMIS measures have undergone extensive psychometric testing and the Adult Profile subscales have demonstrated high reliability and construct validity [52].
Patient Global Perception of Change: These are single-item measures that ask participants to rate their improvement “since you completed the first interview for this study” on a 7-point “much worse” to “much better” scale. This measure is recommended as a core outcome measure for pain research with strong psychometric properties of reliability, validity, and sensitivity [53–54]. We asked separate items about improvement in pain and functioning.
Aim 3: Exploratory Outcomes
Pain Intensity: Participants rated their average pain in the last week from 0 (no pain at all) to 10 (worst pain you can imagine). This item is included in the PROMIS-43 and is also a recommended core outcome measure in chronic pain clinical trials [53].
Pain Self-Efficacy Scale: 10 items assessing confidence (from 0 = not at all confident to 6 = completely confident) to participate in various life activities despite pain. This measure has good internal consistency reliability (Cronbach’s alpha >0.90) and stability across time as well as strong construct validity [55].
Perception of Change in Pain Medication Use: We created a single item with the same structure as the Patient Global Perception of Change measures: “How does the amount of pain medication you are taking now compare to what you were taking at the time of the first interview?” on a 7-point scale from “taking much more” to “taking muchless.”
Step Count Data: Step counts were reported by participants each evening for 6 weeks (between Sessions 1 and 7). They were given a choice of syncing their tracker with an app (n = 12), replying to an automated text with the step count number on their device (n = 16), or recording them on paper and sharing with the CHW (n = 3).
Other Measures
Health and demographic variables: Other variables used to describe the sample were: gender, race/ethnicity, educational attainment, employment status, health literacy [56], pain treatments including use of opioids and/or other medications, difficulty paying bills, and health insurance status.
Data analysis: We calculated descriptive statistics of health and demographic variables to characterize the sample at baseline. We converted the 6-item PROMIS Pain Interference subscale to T-scores (a standardized score with a mean of 50 and standard deviation of 10), by summing the scales and using the raw score to T-score conversion tables provided at HealthMeasures.net [57]. The standardized T-scores facilitate comparison to the general population and to other study samples. We used paired t-tests to assess changes in mean pain interference, pain intensity, and pain self-efficacy from baseline to follow-up.
For step count data, we treated values less than 100 (which suggested that the tracker was not worn that day) as missing and calculated the weekly mean step count per person for 6 weeks. Mean counts were log-transformed and a mixed-effect model nested at the individual level was used to determine differences in counts over time.
Qualitative (textual) data on satisfaction, barriers, and facilitators were compiled from open-ended survey responses recorded in REDCap. These were reviewed by the lead author and a research assistant. A coding scheme was developed by the research assistant based on the data, and modifications were made by the lead author. Codes were then applied to all responses for each program element and the program as a whole. Ultimately, certain codes were combined into themes to facilitate interpretation and, where applicable, labeled as a “pro” or a “con” of a given intervention element.
Results
As shown in Table 2, our analytic sample of 31 participants was 97% female and 97% African American, with an average age of 68.7 years (SD = 5.4; range 60–80 years). Slightly fewer than one-third (29%; n = 9) of participants had a bachelor’s degree or higher. Only 13% (n = 4) were working full or part-time. About one-fifth (19%; n = 6) were married/partnered. Just under half (48%; n = 15) reported at least some trouble paying bills each month. Participants had an average of 5.0 (SD = 2.3) chronic health conditions, and the most common self-rated health category was “good” (45%; n = 14) with 32% (n = 10) reporting their health was “fair” and 23% (n = 7) “very good.”
Table 2.
Sample characteristics (analytic sample; n = 31)
| Variable | Mean (SD) or % (n) |
|---|---|
| Demographics | |
| Age in years (range 60–80) | 68.7 (5.4) |
| Black/African American | 97% (n = 30) |
| Female | 97% (n = 30) |
| Bachelor’s degree or higher | 29% (n = 9) |
| Married or partnered | 19% (n = 6) |
| Working full or part time | 13% (n = 4) |
| Trouble paying monthly bills (moderate or greater) | 48.3% (n = 15) |
| Enrolled in Medicaid | 45.2% (n = 14) |
| Low health literacy (somewhat or less confident filling out forms) | 16.2% (n = 5) |
| Pain and other health variables | |
| High-impact chronic pain* | 45% (n = 14) |
| Chronic health conditions | |
| Arthritis† | 84.0% (n = 26) |
| Low back pain† | 71.0% (n = 22) |
| Migraine | 26.0% (n = 8) |
| High blood pressure | 71.0% (n = 22) |
| Diabetes | 19.4% (n = 6) |
| Depression | 29.0% (n = 9) |
| Asthma | 26.0% (n = 8) |
| GERD/heartburn | 36.0% (n = 11) |
| Number of chronic conditions (range 1–10) | 5.0 (SD = 2.3) |
| Self-rated health | |
| Very good | 23.0% (n = 7) |
| Good | 45% (n = 14) |
| Fair | 32% (n = 10) |
| Pain medication use | |
| Take OTC oral pain medications | 68% (n = 21) |
| Take OTC topical medications | 55% (n = 17) |
| Take opioid-based medication | 32% (n = 10) |
| Use medical cannabis | 7% (n = 2) |
| Other pain medication‡ | 48.3% (n = 15) |
| Technology access | |
| Computer at home with internet access | 68% (n = 21) |
| Have own smartphone | 84% (n = 26) |
Met National Pain Strategy criteria for high-impact chronic pain, i.e., “usually” having interference due to pain over the last six months in work, social/recreational activities, and/or daily self-care activities [58].
N = 19 participants reported both arthritis and low back pain.
Other medications include prescription topical, gabapentin, Lyrica, others.
The most common pain conditions were arthritis (84%) and low back pain (71%); 61% (n = 19) of participants reported both of these. PROMIS Pain Interference T-score at baseline was 61.6; representing over one standard deviation greater interference than the population average of 50 [57]. Participants’ average pain intensity in the last week was 6.3 (0 = no pain to 10 = worst imaginable pain). Nearly half the sample (45%; n = 14) met criteria for high-impact chronic pain, defined as pain that substantially interferes with daily life for more than 6 months. This type of disabling pain is considered a national priority for research and treatment [58]. The majority of participants reported use of over-the-counter oral (68%; n = 21) and topical (55%; n = 17) pain medications, and one-third (32%; n = 10) reported use of opioid medications.
Aim 1: Feasibility
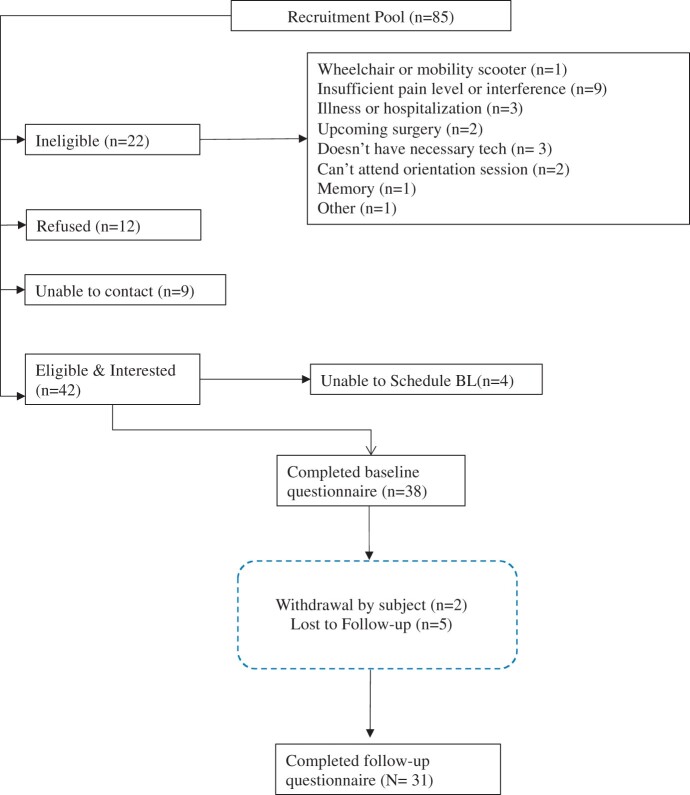
Recruitment and retention: Figure 1 depicts the study flow. We recruited 42 participants to this study between September 2019 through January 2020 out of an initial pool of 85 people screened. The most common reason for ineligibility was insufficient pain intensity or pain interference (n = 9). A total of 38 people completed the baseline questionnaire (i.e., 4 were lost to follow-up before completing baseline), for a recruitment rate of approximately 8 individuals per month. After completing the baseline questionnaire, another 7 individuals left the study, for a retention rate of 82%. Primary reasons for non-completion were lack of time and inability to attend the first (in-person) intervention session. Two adverse events were reported; both were determined to be unrelated to the intervention. Follow-up surveys were conducted with 31 participants.
Figure 1.
Study flow.
Participants completed a mean of 6.6 out of 7 sessions. This average includes 12 “combined” sessions that included content from 2 different weeks, to make up for missed sessions. More than 90% of participants agreed or strongly agreed that STEPS-2 increased their understanding of pain management and helped them reach their pain management goals. The majority of participants (68%; n = 21) used the website (where the videos were housed) more often than the minimum suggested one time per week. Details on ratings of specific program elements are found in Table 3.
Table 3.
Participant satisfaction with STEPS-2 (n = 31)
| Follow-up survey item | Responses | n (%) |
|---|---|---|
| Participating in STEPS-2 increased my understanding of pain management. | Strongly agree | 21 (67.7%) |
| Agree | 10 (32.3%) | |
| Neither agree nor disagree | — | |
| Disagree | — | |
| Strongly disagree | — | |
| The information in the workbook helped me reach my pain management goals | Strongly agree | 14 (45.2%) |
| Agree | 15 (48.4%) | |
| Neither agree nor disagree | 1 (3.2%) | |
| Disagree | 1 (3.2%) | |
| Strongly disagree | — | |
| My community health worker explained information during the sessions in a way that was easy to understand. | Strongly agree | 23 (74.2%) |
| Agree | 7 (22.6%) | |
| Neither agree nor disagree | 1 (3.2%) | |
| Disagree | — | |
| Strongly disagree | — | |
| What did you think about the length of the calls with the Community Health Worker? | Far too short | — |
| Too short | — | |
| About the right length | 27 (87.1%) | |
| Too long | 3 (9.7%) | |
| Far too long | 1 (3.2%) | |
| The videos helped me better understand pain and pain management techniques. | Strongly agree | 17 (54.8%) |
| Agree | 14 (45.2%) | |
| Neither agree nor disagree | — | |
| Disagree | — | |
| Strongly disagree | — | |
| How often did you use the STEPS-2 website? | Once per week | 8 (25.8%) |
| Few times per week | 17 (54.8%) | |
| Once per day | 2 (6.5%) | |
| Several times per day | 2 (6.5%) | |
| Don’t know | 2 (6.5%) | |
| I will continue to track my steps with the activity tracker. | Strongly agree | 14 (45.2%) |
| Agree | 12 (38.7%) | |
| Neither agree nor disagree | 2 (6.5%) | |
| Disagree | 1 (3.2%) | |
| Strongly disagree | 1 (3.2%) |
Qualitative participant reactions to the program were overwhelmingly positive (see Table 4). Participants reported benefits including reduced pain, encouragement to adopt a healthier lifestyle, and an improved understanding of what can be done to cope with pain. Comments indicating dissatisfaction or suggestions for improvement were few and primarily had to do with program length—that it was either too long or not long enough—and that there should be a way to connect with fellow participants.
Table 4.
Summary of qualitative feedback
| Program element | Themes | Example quotes |
|---|---|---|
| Overall program experience |
Pros: Pain reduction, encouragement/motivation, healthy habits, well-being, empowerment Cons: Too long, not long enough |
“It is a very doable program. You learn a lot about yourself and about pain and the things you can do to help it.” “It helps people reach goals they had just given up on. I used to take a lot more medicine than what I’m taking now. It has helped me control my pain.” “This program has helped me to stay focused on something other than my pain. If you can focus on something other than the pain, you can experience less pain. I don't stop, I keep moving to help minimize my pain.” |
| Wearable activity tracker |
Pros: Motivating, user-friendly, used multiple features, allowed to track activity Cons: Remembering to put it on, technical challenges |
“I liked keeping track of my activity. It challenged me. I was surprised because I never thought I could find much use in that piece of technology, but I was pleasantly surprised. It made me challenge myself.” “Remembering to put it on [was challenging]. Somedays I thought I had it on and I was so excited because I was going out to run errands where I knew I would get a lot of steps but then I didn't have it on and I would be so upset.” |
| Community health worker sessions |
Pros: Motivation, encouragement, patience, connected to resources, calming Cons: In a couple cases, participants wanted the opportunity to talk more |
“If it had not been for [CHW] I would not have tried to reach any goals. I had some with exercise but I never accomplished them before. Talking to her helped me set small goals with small times and I had not been doing that. I could accomplish them by taking baby steps instead of big steps.” “She helped me by encouraging me to walk more and exercise, she really encouraged me to do a lot of things I thought I couldn’t do, and I could do them well.” |
| Program website and videos |
Pros: Easy to use, informative, liked being able to rewatch videos, liked additional links Cons: Did not use additional links |
“Some websites are confusing or overwhelming and I did not think [this one was], I didn't have to call anyone to ask how to use it.” “I liked that you could go back and watch videos over again if you want. I liked the doctors that gave the videos and explained everything.” |
Table 5.
Pain-Related Outcomes: Pre-post change (baseline to 2 months) and participant perceptions of change since baseline (n = 31)
| Baseline mean (SD) | Post-program (2 months) mean (SD) or n (%) | P-value for pre-post change* | |
|---|---|---|---|
| PROMIS-43 Pain Interference (6-item subscale): T-score (higher=worse; population mean = 50, SD = 10) | 61.6 (5.5) | 57.3 (7.2) | P =.000 |
| Pain Intensity (0 = no pain to 10 = worst you can imagine) | 6.3 (2.4) | 5.1 (2.4) | P =.004 |
| Pain Self-Efficacy (0 = least confidence to 6 = most confidence) | 4.2 (1.3) | 4.5 (1.1) | P =.160 |
| Global Impression of Change –Pain | N/A | N/A | |
| Much worse | — | — | |
| Worse | — | 1 (3.2%) | |
| A little worse | — | — | |
| No change | — | 3 (9.7%) | |
| A little better | — | 13 (42.0%) | |
| Better | — | 9 (29.0%) | |
| Much better | — | 5 (16.1%) | |
| Global Impression of Change –Functioning | N/A | N/A | |
| Much worse | — | — | |
| Worse | — | — | |
| A little worse | — | — | |
| No change | — | 4 (13.0%) | |
| A little better | — | 9 (29.0%) | |
| Better | — | 10 (32.3%) | |
| Much better | — | 9 (29.0%) | |
| Global Impression of Change—Pain medication use | N/A | ||
| Taking much more | — | ||
| Taking more | — | ||
| Taking a little more | 1 (3.2%) | ||
| No change | 15 (48.4%) | ||
| Taking a little less | 3 (9.7 %) | ||
| Taking less | 8 (25.8%) | ||
| Taking much less | 4 (12.9%) |
Based on paired sample t-test.
In terms of specific program components, CHWs were perceived as encouraging, helpful, and motivating. Participants reported benefits from the activity trackers, including motivation to be more active. Some participants also experienced drawbacks; for example, difficulty remembering to wear the tracker or technical problems. The STEPS-2 website was described as easy to navigate and the videos easy to understand and informative. Some participants mentioned going back to the website frequently to re-watch videos or use the other resources linked there.
CHW fidelity: We assessed CHW adherence to session protocols, including core cognitive-behavioral components, for 88 of 206 sessions. This was a purposive sample of sessions selected to represent all CHWs and session numbers. The mean overall fidelity score was 1.79 (range 0 to 2, low to high). Elements with the lowest fidelity scores were Video Recap (1.66) and Step Count Goal (1.65). Fidelity scores improved over time with regular feedback for CHWs (avg. 1.78 before initiation of feedback sessions and 1.93 after). In session logs, CHWs recorded referrals to resources to help participants with issues related to utility payments, legal services, consumer protection, free produce, and COVID-19.
Aim 2: Pain-Related Functioning
Our primary outcome of PROMIS Pain Interference decreased significantly from baseline (mean T-score 61.6, SD 5.5) to follow-up (mean 57.3, SD = 7.2) (t = 4.8, d.f.=30, P = .000). For Patient Global Impression of Change in pain and functioning, 14 participants (45%) rated their pain as “better” or “much better” from baseline, and another 13 (42%) “a little better.” Functioning was rated as “better” or “much better” by 19 people (61%) and “a little better” by 9 (29%).
Aim 3: Exploratory Outcomes
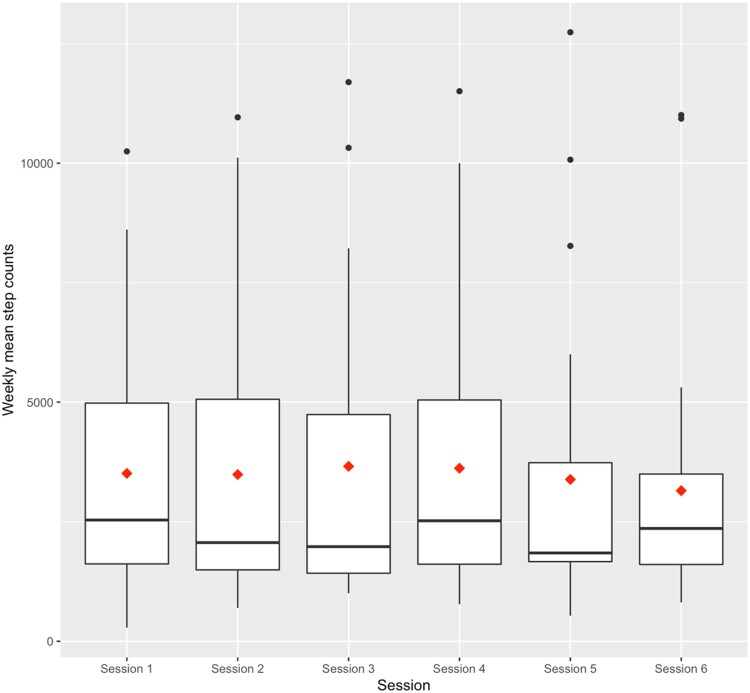
Exploratory outcomes (see Table 5) included pain self-efficacy, which increased from a mean of 4.2 (SD = 1.3) at baseline to 4.5 (SD = 1.1) at follow-up, a nonsignificant increase. An equal number of participants (n = 15) reported no change in their medication use vs taking “a little less” to “much less.” Only one participant reported taking “a little more” pain medication at follow-up, and no one reported taking “more” or “much more.” Pain intensity also decreased from baseline (mean 6.3, SD = 2.4) to follow-up (mean 5.1, SD = 2.4) (t = 3.11, d.f.=30, P = .004). As shown in the boxplots in Figure 2, there were increases in mean steps from week 1 (baseline average 3232 steps/day) to week 2, and week 2 to week 3. However, starting from week 4, average steps decreased over time. None of the changes were statistically significant. Median step counts similarly did not suggest a clear trend over time.
Figure 2.
Weekly step counts. Notes: Diamond indicates mean step count; horizonal line indicates median. Step count by week (mean, median): Week 1 (3232, 2227), Week 2 (3393, 2139), Week 3 (3426, 1978), Week 4 (3318, 2471), Week 5 (3105, 1794), Week 6 (3045, 2189).
Discussion
To address the disproportionate burden of pain-related disability among African American older adults, we developed a chronic pain self-management (CPSM) intervention, STEPS-2, which combined culturally congruent telephone support from community health workers (CHWs) with web-based video content and the use of wearable activity trackers. PROMIS pain interference and pain intensity scores decreased significantly from baseline to post-program, and more than four-fifths of the sample reported improved pain and functioning after the intervention. Open-ended and closed program satisfaction items indicated that STEPS-2 was well-received by participants. The motivation and encouragement provided by CHWs and the activity trackers were described as particularly beneficial. Fidelity by CHWs to telephone session protocols as well as participant completion of sessions was high. Thus, this pilot study demonstrated the intervention’s feasibility and potential efficacy.
Pre-post changes strongly suggested that participants experienced improved pain and functioning over the study period. The magnitude of change in PROMIS pain interference—a T-score difference of 4.3—is in the range of what is considered clinically meaningful for this measure [51]. The present study lacked a control group; however, in a previous pilot trial in the same population and setting [41], we found that pain interference was essentially unchanged in the control group over the same 8-week time period, bolstering the case that the improvement in the present study was attributable to the STEPS-2 intervention. We also saw promising results in the Patient Global Impression of Change (PGIC) measures for both pain and functioning. The two highest categories of self-assessed improvement on PGIC measures are thought to reflect meaningful change [54]; in our study the proportion of participants in these two categories was 45% for pain and 61% for functioning. Average pain intensity (0–10 scale) in the last week decreased by 1.2 points in our sample, which does not reach the suggested approximately 2-point threshold for clinically meaningful change in this measure [54].
The observed increase in pain self-efficacy, an exploratory outcome, was small and did not reach statistical significance. Self-efficacy—that is, confidence in one’s ability to manage pain—is thought to play a critical role in leveraging analgesic placebo effects [59] and has been shown in a meta-analysis to be strongly and inversely associated with impairment, distress, and severity [60]. Future iterations of STEPS-2 may be enhanced with additional efficacy-building components, such as increased opportunities to observe modeling of successful pain management from peers [61].
Another exploratory outcome, pain medication use, yielded intriguing findings. At baseline, about two-thirds of our sample reported at least some use of OTC pain medications and about one-third (10 of 31) use of opioids. At follow-up, we assessed perceived change in pain medication use (the item did not differentiate by medication type). Nearly 40% of the sample indicated they were taking less or much less medication than at baseline. Pain medication use was not specifically addressed in the program curriculum. CHWs were provided with information about current guidelines regarding opioid use and safety but were instructed to refer participants to their doctors for any specific questions. The self-management techniques taught in STEPS-2 were presented to participants as being potentially helpful “with or without medication use”. The finding that so many participants nonetheless reported taking less medication following the intervention means that it is possible that with the increased use of self-management skills, they felt less need for medication. Decreased use of pain medications, particularly opioid analgesics, has been noted as a self-identified goal of care for African American older adults with persistent pain [62]. This finding should be evaluated more rigorously in a larger trial.
While we do not have information on medication dose or frequency in our sample, regular use of opioid analgesics is of concern in older adults as it confers an increased risk of falls, fall injuries, and fractures [63]. African American older adults in economically disadvantaged areas are particularly vulnerable to clinical mismanagement of pain and adverse effects from polypharmacy including opioids [64]. Therefore, future iterations of STEPS might include more focused attention on screening, education, and appropriate referrals around opioid use.
Qualitative feedback from participants offers insight into why the program may have been effective at improving pain and function. Common themes about the overall program included that it was empowering, instilled healthy habits, and changed the way that participants thought about pain and how to manage it—including being more physically active, using distraction, and using relaxation techniques. The three main program elements—CHWs, website, and activity trackers—were each described by the majority of participants as beneficial. Participants said that CHWs were very encouraging, which helped them to reach personally important goals that they could not have reached otherwise. Participants generally felt that the website was easy to navigate and that the videos featuring experts teaching pain-management skills were clear and helpful.
Analysis did not reveal a steady increase in step counts throughout the program. This was despite the fact that participants were encouraged to increase steps by 10% per week, and that qualitative feedback indicated that they found tracking their steps motivated them to be more active, consistent with our previous research in this population [41]. Two external factors may help to explain why we did not observe a positive trend in step counts. First, physical distancing orders due to the COVID-19 pandemic began in March 2020 (when about two-thirds of the sample started the intervention), sharply limiting opportunities for physical activity outside of the home. An earlier cohort of participants began in October 2019 and ended in December, the time of year when inclement weather in Detroit begins to make getting out more difficult. Last, we note that some participants reported difficulty remembering to wear the activity tracker each day. Therefore, it is also possible that lower weekly average step counts in the last weeks of the intervention were due to participants increasingly forgetting to put them on right away in the morning.
While the majority of participants did not experience challenges with the technology used in STEPS-2, there were exceptions. The intervention was designed to be accessible to individuals with basic technology know-how and access (i.e., a smartphone or another way of accessing the internet). During orientation, participants were shown how to navigate the STEPS-2 website on their device. Staff assisted them in bookmarking the site. Nonetheless, some participants ended up needing support for using the website, which they obtained from a variety of sources: study staff, CHWs, and family members/friends. Only three participants were unable to sync or text a daily step count for tracking purposes and had to record their counts on paper instead. The activity trackers were an additional source of technical problems, often related to charging the device; in five instances the trackers were defective (we used “recycled” devices) and had to be replaced.
This pilot study sets the stage for advancing research on pain management in underserved populations. To our knowledge, STEPS-2 represents one of the first attempts to enlist CHWs as part of the care workforce in a culturally responsive pain intervention. Expanding the roles of non-specialist practitioners is promoted as a valuable research direction in cognitive-behavioral interventions for pain, as this could have the dual benefit of decreasing service delivery costs while increasing access [38]. Moreover, in the context of a guided internet intervention like STEPS-2, standardized electronic content may provide structure that helps nonspecialists adhere to protocols and deliver content in a consistent way.
Converging evidence indicates that CHWs can successfully deliver interventions for chronic conditions such as diabetes and asthma [35, 65], as well as for mental health disorders [34, 66]. However, we identified only one prior study in which a CHW provided education and support for chronic pain management—in this case, to rural, working-age, Hispanic adults [18]. CHWs have many untapped advantages for working to improve pain care in underserved populations; for example, they are able to serve as a bridge between community members and formal health and social services in addition to being able to help individuals address social needs that may be affecting pain and its management. Compared to peer leaders, who have long been utilized in self-management interventions for arthritis and other chronic diseases [67–69], CHWs have more formal training and experience yet share with peer leaders the quality of being relatable. Moreover, modest and null results in studies of peer-led programs for pain self-management have raised concerns that peer leaders may not be effective at improving pain-related outcomes [69]. CHWs are often integrated into clinical care teams to help address the unmet social needs of complex patients [70]; this model could be readily transferred to pain care.
Our study also contributes to the small but growing body of literature on psychosocial pain interventions specifically tailored for marginalized populations. For example, Allen et al. conducted a trial of a culturally-tailored pain coping skills training intervention for African Americans with osteoarthritis [71]. Thorn et al. [40] tested a psychologist-led cognitive behavioral pain intervention for “multiply disadvantaged” patients with low health literacy, offered in community sites. A trial is underway of a telephone-based coaching intervention, also employing pedometers, for pain self-management among African Americans in the VA health system [72]. These studies represent diverse modalities, which may be suitable for a variety of settings. In these interventions, cultural relevance was largely achieved by adapting intervention content, language, and examples. Booker et al. [73] recently suggested that dominant pain self-management frameworks may be inadequate when applied to African American older adults, as they lack key elements such as the integration of spiritual faith and culturally specific ways of accepting pain and communicating about it to others. This supports Robinson-Lane’s [74] findings, who identified that African American older adults were able to adapt to living with chronic pain by using coping strategies such as remaining positive, being engaged in their communities, using prayer or meditation, and maintaining positive support systems. Many of these strategies were incorporated into the current intervention design, which resulted in a strengths-based, culturally responsive intervention. CHWs, who are likely to share cultural traditions and values with participants, became an important part of the social networks and positive support systems of participants. This profound and natural form of cultural relevance contributes to a strong therapeutic alliance and ultimately can lead to enhanced pain-related outcomes.
Limitations: In addition to the inherent limitations of the single-group pre-post design used in this pilot study, several other limitations are worth noting. Almost all participants were women. Also, our recruitment sources meant that most participants were already engaged in community-based activities (e.g., senior centers) and thus may not represent more severely pain-affected and/or homebound older adults. Outcomes were measured over a relatively short period, and it would be important in a larger trial to track program impacts over a longer time period. Outcomes data were collected by research assistants via telephone; the presence of the interviewer may have introduced a respondent bias toward more favorable reports. Last, the final data collection point for over half of the sample took place not long after the lockdown related to COVID-19 was implemented; this unprecedented and stressful situation may have affected outcomes.
Conclusion
We have demonstrated the feasibility of a CHW-led, technology-assisted chronic pain self-management intervention for African American older adults, a group severely affected by pain-related disability with obstacles to traditional pain self-management. This work provides a strong foundation for a planned larger-scale efficacy trial, while also paving the way for additional research on ways to incorporate CHWs into improving pain care for some of the hardest to reach populations.
Acknowledgements
Our heartfelt gratitude to our community health workers: Philesha Gough, Felicia Lane, and Linda Reyes; student assistants Elizabeth Brines, Greta Cheng, Leah Fein, Sarah Knapp, Varick Shute, and Shuji Tsuda; and to the Henry Ford Health System, the staff of Village Center Apartments, Neighborhood Service Organization, and St. Patrick’s Senior Center. We would also like to thank RecycleHealth (https://www.recyclehealth.com/) for their generous donation of electronic activity trackers for use in this pilot study.
Contributor Information
Mary Janevic, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Sheria G Robinson-Lane, Department of Systems, Populations, and Leadership, University of Michigan School of Nursing, Ann Arbor, Michigan, USA.
Susan L Murphy, Physical Medicine and Rehabilitation, University of Michigan Medical School, Ann Arbor, Michigan, USA.
Rebecca Courser, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
John D Piette, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA; VA Center for Clinical Management Research, Ann Arbor, Michigan, USA.
Funding sources: This study was supported by grants from the National Institutes of Health, P30 AG015281 and the Michigan Center for Urban African American Aging Research, the National Institute on Aging [K01 AG050706-01A1 to MRJ]; and American Pain Society 2018 Future Leaders Pilot Grant (Janevic, PI). John Piette is a VA Senior Research Career Scientist.
Disclosure and conflicts of interest: None of the authors have any conflicts of interest to disclose.
References
- 1. Krein SL, Heisler M, Piette JD, Butchart A, Kerr EA.. Overcoming the influence of chronic pain on older patients' difficulty with recommended self-management activities. Gerontologist 2007;47(1):61–8. [DOI] [PubMed] [Google Scholar]
- 2. Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO.. Pain in aging community-dwelling adults in the United States: Non-Hispanic Whites, Non-Hispanic Blacks, and Hispanics. J Pain 2007;8(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Covinsky KE, Lindquist K, Dunlop DD, Yelin E.. Pain, functional limitations, and aging. J Am Geriatr Soc 2009;57(9):1556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fowler-Brown A, Wee CC, Marcantonio E, Ngo L, Leveille S.. The mediating effect of chronic pain on the relationship between obesity and physical function and disability in older adults. J Am Geriatr Soc 2013;61(12):2079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrews JS, Cenzer IS, Yelin E, Covinsky KE.. Pain as a risk factor for disability or death. J Am Geriatr Soc 2013;61(4):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilkie R, Tajar A, McBeth J.. The onset of widespread musculoskeletal pain is associated with a decrease in healthy ageing in older people: A population-based prospective study. PLoS One 2013;8(3):e59858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherry BJ, Zettel-Watson L, Shimizu R, Roberson I, Rutledge DN, Jones CJ.. Cognitive performance in women aged 50 years and older with and without fibromyalgia. J Gerontol B Psychol Sci Soc Sci 2014;69(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keefe FJ, Beaupre PM, Weiner DK, Siegler IC.. Pain in older adults: A cognitive-behavioral perspective. In: Ferrell BR, Ferrell BA, editors. Pain in the Elderly. Seattle: IASP Press; 1996: 11–9. [Google Scholar]
- 9. Reid MC, Eccleston C, Pillemer K.. Management of chronic pain in older adults. BMJ 2015;350(2):h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AGS Panel on Persistent Pain in Older Persons. The management of persistent pain in older persons. J Am Geriatr Soc 2002;50(Suppl 6):S205–24. [DOI] [PubMed] [Google Scholar]
- 11. Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain 2017;158(2):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, Piette JD.. Racial and socioeconomic disparities in disabling chronic pain: Findings from the health and retirement study. J Pain 2017;18(12):1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaughn IA, Terry EL, Bartley EJ, Schaefer N, Fillingim RB.. Racial-ethnic differences in osteoarthritis pain and disability: A meta-analysis. J Pain 2018;20(6):629–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med 2003;4(3):277–94. [DOI] [PubMed] [Google Scholar]
- 15. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM.. Advancing a national agenda to eliminate disparities in pain care: Directions for health policy, education, practice, and research. Pain Med 2012;13(1):5–28. [DOI] [PubMed] [Google Scholar]
- 16. Bonakdar R, Palanker D, Sweeney MM.. Analysis of state insurance coverage for nonpharmacologic treatment of low back pain as recommended by the American College of Physicians Guidelines. Glob Adv Health Med 2019;8:216495611985562.2164956119855629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LeFort SM, Gray-Donald K, Rowat KM, Jeans ME.. Randomized controlled trial of a community-based psychoeducation program for the self-management of chronic pain. Pain 1998;74(2–3):297–306. [DOI] [PubMed] [Google Scholar]
- 18. Turner BJ, Liang Y, Simmonds MJ, Rodriguez N, Bobadilla R, Yin Z.. Randomized trial of chronic pain self-management program in the community or clinic for low-income primary care patients. J Gen Intern Med 2018;33(5):668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruehlman LS, Karoly P, Enders C.. A randomized controlled evaluation of an online chronic pain self management program. Pain 2012;153(2):319–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rini C, Williams DA, Broderick JE, Keefe FJ.. Meeting them where they are: Using the internet to deliver behavioral medicine interventions for pain. Transl Behav Med 2012;2(1):82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heapy AA, Higgins DM, Cervone D, Wandner L, Fenton BT, Kerns RD.. A systematic review of technology-assisted self-management interventions for chronic pain: Looking across treatment modalities. Clin J Pain 2015;31(6):470–92. [DOI] [PubMed] [Google Scholar]
- 22. Parker SJ, Jessel S, Richardson JE, Reid MC.. Older adults are mobile too! Identifying the barriers and facilitators to older adults' use of mHealth for pain management. BMC Geriatr 2013;13(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi NG, Dinitto DM.. The digital divide among low-income homebound older adults: Internet use patterns, eHealth literacy, and attitudes toward computer/Internet use. J Med Internet Res 2013;15(5):e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Center PR. Older Adults and Technology Use. April 2014. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/.
- 25. Kerns RD, Burns JW, Shulman M, et al. Can we improve cognitive-behavioral therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychol 2014;33(9):938–47. [DOI] [PubMed] [Google Scholar]
- 26. Macea DD, Gajos K, Daglia Calil YA, Fregni F.. The efficacy of web-based cognitive behavioral interventions for chronic pain: A systematic review and meta-analysis. J Pain 2010;11(10):917–29. [DOI] [PubMed] [Google Scholar]
- 27. Rosser BA, Vowles KE, Keogh E, Eccleston C, Mountain GA.. Technologically-assisted behaviour change: A systematic review of studies of novel technologies for the management of chronic illness. J Telemed Telecare 2009;15(7):327–38. [DOI] [PubMed] [Google Scholar]
- 28. Krieger N. Measures of racism, sexism, heterosexism, and gender binarism for health equity research: From structural injustice to embodied harm—an ecosocial analysis. Annu. Rev Public Health 2020;41(1):37–62. [DOI] [PubMed] [Google Scholar]
- 29. Gee GC, Ford CL.. Structural racism and health inequities: Old issues, new directions. Du Bois Rev 2011;8(1):115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT.. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017;389(10077):1453–63. [DOI] [PubMed] [Google Scholar]
- 31. Fuentes M, Hart-Johnson T, Green CR.. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. J Natl Med Assoc 2007;99(10):1160–9. [PMC free article] [PubMed] [Google Scholar]
- 32. Ruben MA, Meterko M, Bokhour BG.. Do patient perceptions of provider communication relate to experiences of physical pain? Patient Educ Couns 2018;101(2):209–13. [DOI] [PubMed] [Google Scholar]
- 33. Makris UE, Abrams RC, Gurland B, Reid MC.. Management of persistent pain in the older patient: A clinical review. JAMA 2014;312(8):825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnett ML, Gonzalez A, Miranda J, Chavira DA, Lau AS.. Mobilizing community health workers to address mental health disparities for underserved populations: A systematic review. Adm Policy Ment Health 2018;45(2):195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perry HB, Zulliger R, Rogers MM.. Community health workers in low-, middle-, and high-income countries: An overview of their history, recent evolution, and current effectiveness. Annu. Rev Public Health 2014;35(1):399–421. [DOI] [PubMed] [Google Scholar]
- 36. Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ.. Internet-enhanced management of fibromyalgia: A randomized controlled trial. Pain 2010;151(3):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 38. Ehde DM, Dillworth TM, Turner JA.. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. Am Psychol 2014;69(2):153–66. [DOI] [PubMed] [Google Scholar]
- 39. Keefe FJ, Porter L, Somers T, Shelby R, Wren AV.. Psychosocial interventions for managing pain in older adults: Outcomes and clinical implications. Br J Anaesth 2013;111(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thorn BE, et al. Literacy-adapted cognitive behavioral therapy versus education for chronic pain at low-income clinics: A randomized controlled trial. Ann Intern Med 2018;168.7:471–80. [DOI] [PubMed] [Google Scholar]
- 41. Janevic MR, Shute V, Murphy SL, Piette JD.. Acceptability and effects of commercially available activity trackers for chronic pain management among older African American adults. Pain Med. 2020;21(2):e68–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulz AJ, Williams DR, Israel BA, et al. Racial and spatial relations as fundamental determinants of health in Detroit. Milbank Q 2002;80(4):677–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poverty Solutions at the University of Michigan. Multidimensional Index of Deep Disadvantage. 2020. Available at: https://tableau.dsc.umich.edu/t/UM-Public/views/IndexofDeepDisadvantage/CountiesCitiesMap?%3Aiid=4&%3AisGuestRedirectFromVizportal=y&%3Aembed=y.
- 44. Kallenbach LR, Smitherman HC.. Dying Before Their Time II: The Startling Truth About Senior Mortality in the Detroit Area and Urban Michigan. Detroit: Detroit Area Agency on Aging; June 2012. [Google Scholar]
- 45. Chadiha LA, Washington OG, Lichtenberg PA, Green CR, Daniels KL, Jackson JS.. Building a registry of research volunteers among older urban African Americans: Recruitment processes and outcomes from a community-based partnership. Gerontologist 2011;51(Suppl. 1):S106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murphy S, Janevic M, Lee P, Williams D.. Occupational therapist-delivered cognitive behavioral therapy for knee osteoarthritis: A randomized pilot study. Am J Occup Ther 2018;72(5):7205205040p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janevic MR, Robinson-Lane S, Murphy S, Piette J.. Chronic pain self-management practices and preferences among urban African American older adults. Innov Aging 2019;3(Supplement_1):S70–S70. [Google Scholar]
- 48. Ridpath JR. PRISM Readability Toolkit. Seattle: Group Health Research Institute; 2007. [Google Scholar]
- 49. Heapy AA, Higgins DM, LaChappelle KM, et al. Cooperative pain education and self-management (COPES): Study design and protocol of a randomized non-inferiority trial of an interactive voice response-based self-management intervention for chronic low back pain. BMC Musculoskelet Disord 2016;17(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michigan Community Health Worker Alliance. Training Opportunities. Available at: https://www.michwa.org/chw-training/.
- 51. Chen CX, Kroenke K, Stump TE, et al. Estimating minimally important differences for the PROMIS pain interference scales: Results from 3 randomized clinical trials. Pain 2018;159(4):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cella D, Choi SW, Condon DM, et al. PROMIS® adult health profiles: Efficient short-form measures of seven health domains. Value in Health 2019;22(5):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;1(113):9–19. [DOI] [PubMed] [Google Scholar]
- 54. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
- 55. Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain 2007;11(2):153–63. [DOI] [PubMed] [Google Scholar]
- 56. Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med 2008;23(5):561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Health Measures. Available at: http://www.healthmeasures.net/promis-scoring-manuals.
- 58. Interagency Pain Research Coordinating Committee, U.S. Department of Health and Human Services. Federal Pain Research Strategy. 2017.
- 59. Darnall BD, Colloca L.. Optimizing placebo and minimizing nocebo to reduce pain, catastrophizing, and opioid use: A review of the science and an evidence-informed clinical toolkit. Int Rev Neurobiol 2018;139:129–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jackson T, Wang Y, Wang Y, Fan H.. Self-efficacy and chronic pain outcomes: A meta-analytic review. J Pain 2014;15(8):800–14. [DOI] [PubMed] [Google Scholar]
- 61. Rosenstock IM, Strecher VJ, Becker MH.. Social learning theory and the health belief model. Health Educ Q 1988;15(2):175–83. [DOI] [PubMed] [Google Scholar]
- 62. Robinson-Lane SG, Vallerand AH.. Pain treatment practices of community-dwelling Black older adults. Pain Manage Nurs 2018;19(1):46–53. [DOI] [PubMed] [Google Scholar]
- 63. Yoshikawa A, Ramirez G, Smith ML, et al. Opioid use and the risk of falls, fall injuries and fractures among older adults: A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2020. [DOI] [PubMed] [Google Scholar]
- 64. Bazargan M, Cobb S, Wisseh C, Assari S.. Psychotropic and opioid-based medication use among economically disadvantaged African-American older adults. Pharmacy 2020;8(2):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kangovi S, Mitra N, Grande D, Huo H, Smith RA, Long JA.. Community health worker support for disadvantaged patients with multiple chronic diseases: A randomized clinical trial. Am J Public Health 2017;107(10):1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barnett ML, Lau AS, Miranda J.. Lay health worker involvement in evidence-based treatment delivery: A conceptual model to address disparities in care. Annu Rev Clin Psychol 2018;14(1):185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lorig KR, Mazonson PD, Holman HR.. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum 1993;36(4):439–46. [DOI] [PubMed] [Google Scholar]
- 68. Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39(11):1217–23. [DOI] [PubMed] [Google Scholar]
- 69. Mehlsen M, Hegaard L, Ørnbøl E, Jensen JS, Fink P, Frostholm L.. The effect of a lay-led, group-based self-management program for patients with chronic pain: A randomized controlled trial of the Danish version of the chronic pain self-management programme. Pain 2017;158(8):1437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kangovi S, Grande D, Trinh-Shevrin C.. From rhetoric to reality—community health workers in post-reform US health care. N Engl J Med 2015;372(24):2277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Allen KD, Somers TJ, Campbell LC, et al. Pain coping skills training for African Americans with osteoarthritis: Results of a randomized controlled trial. Pain 2019;160(6):1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhimani RH, Cross LJ, Taylor BC, et al. Taking ACTION to reduce pain: ACTION study rationale, design and protocol of a randomized trial of a proactive telephone-based coaching intervention for chronic musculoskeletal pain among African Americans. BMC Musculoskelet Disord 2017;18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Booker SQ, Tripp-Reimer T, Herr KA.. “Bearing the Pain”: The experience of aging African Americans with osteoarthritis pain. Glob Qual Nurs Res 2020;7:233339362092579.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Robinson-Lane SG. Adapting to chronic pain: A focused ethnography of Black older adults. Geriatr Nurs 2020;41(4):468–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]