Abstract
Background:
Studies suggest that non-steroidal anti-inflammatory drugs (NSAID) may contribute to inflammatory bowel disease (IBD) exacerbations. We examined whether variation in the likelihood of IBD exacerbations is attributable to NSAIDs.
Methods:
In a cohort of patients with IBD (2004–2015), we used three analytic methods to examine the likelihood of an exacerbation following NSAID exposure. First, we matched patients by propensity for NSAID use and examined the association between NSAID exposure and IBD exacerbation using an adjusted cox-proportional hazards model. To assess for residual confounding, we estimated a prior event rate ratio, and used a self-controlled case series analysis to further explore the relationship between NSAIDs and IBD exacerbations.
Results:
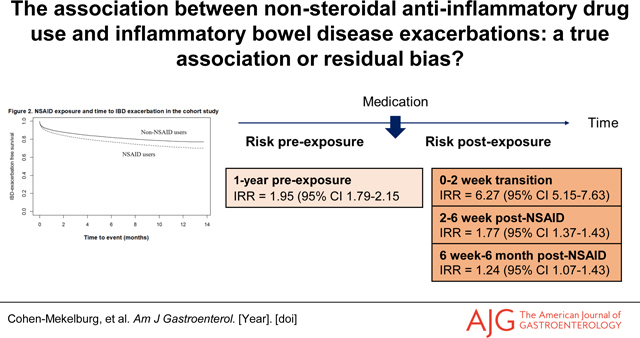
We identified 15,705 (44.8%) and 19,326 (55.2%) IBD patients with and without an NSAID exposure, respectively. Findings from a Cox proportional hazards model suggest an association between NSAIDs and IBD exacerbation (HR 1.24; 95%CI 1.16–1.33). However, the likelihood of an IBD exacerbation in the NSAID exposed arm preceding NSAID exposure was similar (HR 1.30; 95%CI 1.21–1.39). A self-controlled case series analysis of 3,968 patients who had both an NSAID exposure and IBD exacerbation demonstrated similar exacerbation rates in the 1-year preceding exposure, 2–6 weeks post-exposure, and 6-weeks to 6-months post-exposure, but higher incidence 0–2 weeks post-exposure, suggesting potential confounding by reverse causality.
Conclusion:
While we see an association between NSAIDs and IBD exacerbations using traditional methods, further analysis suggests this may be secondary to residual bias. These findings may reassure patients and clinicians considering NSAIDs as a non-opioid pain management option.
Graphical Abstract
Background:
Patients with inflammatory bowel disease (IBD) are prone to both inflammatory and non-inflammatory pain. Given opioid-associated morbidity and mortality, consideration of non-opioid medication options is essential.1–3 Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most commonly prescribed analgesics worldwide.4,5 However, NSAIDs are known to cause gastrointestinal toxicity in the form of mucosal injury which can lead to erosions, ulcers, bleeding, strictures, and bowel perforation.6 In IBD, there has been a long-standing concern that NSAIDs may play a role in disease exacerbations.7,8 Single center prospective data has demonstrated a higher likelihood of IBD flares among patients with quiescent IBD after exposure to the non-selective NSAIDs as compared to acetaminophen.8 On the other hand, a recent systematic review of eighteen studies found no consistent association between NSAIDs and IBD exacerbations.9 However, the included studies had small sample sizes and were single center, and the definition of IBD exacerbation varied. Ultimately, the current literature is inconclusive, highlighting the need for additional studies.
Given that the relationship between NSAIDS and IBD exacerbations is unlikely to be studied in randomized controlled studies, rigorous methodologies need to be applied to observational data to adjust for residual confounding. For example, residual confounding in the form of reverse causality (also known as protopathic bias) can occur when a medication initiated to treat a symptom or disease outcome appears to cause the outcome.10 11 For example, NSAIDs may be prescribed for the treatment of abdominal pain or arthralgias related to an early IBD exacerbation, and consequently overestimate the risk of IBD exacerbation associated with NSAID use. We aimed to examine whether variation in the likelihood of IBD exacerbation is attributable to differences in patients’ NSAID exposure, in context of the potential for residual confounding.
Methods:
Data source
We used the Veterans Affairs (VA) Corporate Data Warehouse (CDW) to identify patients with IBD who had an outpatient encounter between January 1, 2004, and September 30, 2015. IBD patients were identified using a validated algorithm based on a combination of inpatient and outpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for Crohn’s disease (555.x), and ulcerative colitis (556.x).12 Patients were selected for inclusion if they had two or more of these codes during at least two clinical encounters, with at least one of these encounters being an outpatient visit.12,13 Patients with codes for both Crohn’s disease and ulcerative colitis were labeled as having indeterminate colitis.
Variables of interest
Our primary independent variable was any NSAID exposure regardless of dosage or duration. NSAIDs were identified using the CDW outpatient pharmacy file, based on definitions from prior studies (Supplemental Table 1).8,9,14 In our primary analysis, we considered exposure to non-selective NSAIDs based on NSAID dispense date. We excluded aspirin and acetaminophen from our NSAID exposure definition in our analysis to minimize heterogeneity of the exposure effect.9 Our primary outcome was IBD exacerbation, defined as any outpatient IBD-related encounter requiring steroids or IBD hospitalizations for an IBD flare, based on the need for corticosteroids. We defined IBD-related corticosteroid prescriptions as requiring at least a one-week supply, and absence of a non-IBD indication in the week preceding the prescription fill date. This definition of IBD exacerbation is well-established and has been utilized in prior studies.13,15 While this definition of IBD exacerbation may not capture IBD exacerbations that are not treated with corticosteroids, the definition is meant to allow for higher specificity at the cost of sensitivity, maximizing for precision in defining a flare.
Study design
We started with a traditional cohort study design to examine the association between NSAID exposure and IBD exacerbations. We then conducted a subgroup analysis using self-controlled case series design to further explore the relationship between NSAID exposure and IBD exacerbations based on a within-person analysis.
Cohort study:
We matched patients with at least one NSAID exposure during the study period to patients who were not exposed to NSAIDs. Patients were matched one-to-one by propensity for NSAID use based on age, IBD type, gender, and smoking status. Patients were included if data was available for at least 1 year preceding the index date, which was defined by first NSAID exposure or a matched date for the unexposed patients. Baseline patient characteristics were derived from data in this year preceding the index date. Patients were followed until a first IBD exacerbation, date of death, or study end date (December 31, 2015). We only examined the first occurrence of an IBD exacerbation to not overrepresent patients with multiple exacerbations, as patients with one exacerbation are at a higher likelihood for further exacerbations. We reported descriptive statistics by NSAID exposure status.
Statistical analysis:
First, we estimated hazard ratios using a Cox proportional hazards model to examine the association between NSAID exposure and time to IBD exacerbation in the matched cohort and adjusted for potential confounders. These confounders were selected a priori and included age, gender, race, Charlson comorbidity score, smoking status, IBD type, and use of an immunomodulator or biologic.16 Immunomodulators included 6-mercaptopurine, azathioprine, and methotrexate, and anti-tumor necrosis factor inhibitors included infliximab, adalimumab, and certolizumab. Golimumab, vedolizumab, and ustekinumab were not included as they were not readily available in the Veterans Affairs health system during the study period.
As a secondary analysis to assess for residual confounding, we estimated a prior event rate ratio, which is the ratio of the unadjusted hazards for IBD exacerbation between the NSAID exposed and unexposed patients following an NSAID dispense date as compared to the unadjusted hazards for IBD exacerbation in the 1 year preceding the NSAID dispense date. The prior event rate ratio adjusts for pre-exposure hazards for IBD exacerbation.17 95% confidence intervals (CI) were obtained by bootstrapping. This method assumes that the differences in hazards for IBD exacerbation in the exposed as compared to unexposed patients preceding NSAID exposure is independent of the effect of NSAIDs on IBD exacerbations.
Subgroup analysis using a self-controlled case series method:
The analysis above estimates the hazards ratio for IBD exacerbations between NSAID exposed and unexposed patients. However, within-person confounding may persist based on individual disease activity, medication regimens, and IBD history. Therefore, to validate or refute our initial findings, we used a second approach to estimate an individual’s risk of IBD exacerbation after NSAID exposure using a self-controlled case series method. The self-controlled case series method is a within-person research design that conditions on the presence of the event, thus requiring only patients with the outcome of interest—an IBD exacerbation. A self-controlled case series estimates a within-person incidence rate ratio (IRR) for relative risk, comparing time periods of hypothesized excess risk after exposure to all other times (e.g., baseline or non-exposed periods)18–20. Patients who do not experience an IBD exacerbation during the study period do not contribute information to the effect estimation and were excluded from further analysis. A key advantage of the self-controlled case series methodology is that patients serve as their own control and hence the analysis accounts for both measured (e.g., gender) and unmeasured (e.g., IBD phenotype) time-invariant patient-level factors.21 This methodology allowed us to adjust for factors that are difficult to characterize in large retrospective studies like IBD phenotype, disease severity, and prior IBD history, all of which may modify the risk of IBD exacerbation.21
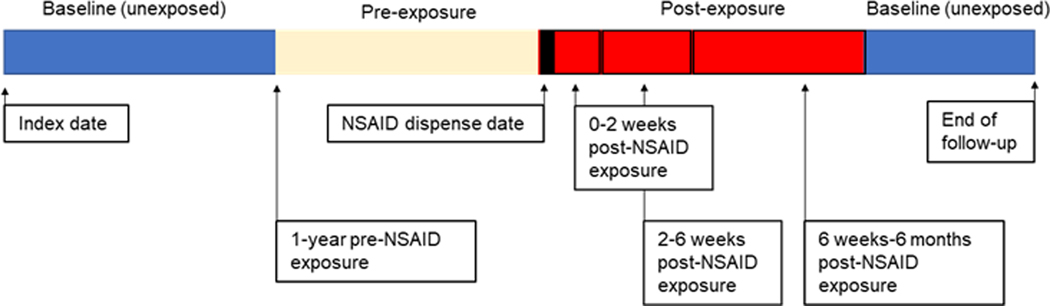
From the cohort of patients with IBD exposed to NSAIDs, we selected for patients who had also experienced an IBD exacerbation. To examine the relationship of the timing of NSAID exposure and IBD exacerbation, patients were followed from their first encounter for an IBD diagnosis to their last visit record (Figure 1). We examined the risk of IBD exacerbation in the first 6 months post NSAID dispense date. To avoid under-representation of flares occurring later during the post-exposure period, we excluded patients with less than 6 months of follow-up after initial NSAID exposure. We divided this observation time into individual risk windows: the 1 year preceding an NSAID dispense date (pre-exposure period), 0–2 weeks after NSAID dispense date (transition period), 2– 6 weeks after NSAID dispense date, and 6 weeks to 6 months after NSAID dispense date. The pre-exposure period allowed us to examine the baseline risk for an IBD exacerbation. The transition period allowed us to examine whether an IBD exacerbation might have precipitated an NSAID prescription. Both the pre-exposure and transition periods allow us to address the potential for capturing reverse causality at different time points, and wrongfully attributing IBD exacerbations to NSAIDs started in response to an IBD exacerbation The remaining observation time was considered the baseline or unexposed period. Incidence rate ratios are reported for all IBD types. In a sensitivity analysis, we also examined whether differences in observed risk occurred when using an alternative pre-exposure period defined by 1 month preceding an NSAID dispense date. The study was approved by VA Ann Arbor Healthcare System Institutional Review Board (IRB), #1597542–3.
Figure 1.
NSAID exposed and unexposed observation time in the self-controlled case series
Results:
Among a cohort of 42,999 patients with IBD, we identified 15,705 (36.5%) patients who were also exposed to NSAIDs between 2004 and 2015 and used propensity scores to match them with 19,326 patients with IBD who were not exposed to NSAIDs during the study period. Overall, among the 35,031 patients that comprised the analytic cohort, 15,705 (44.8%) with and 19,326 (55.2%) without an NSAID exposure, a majority (93.2%) of patients were male, white (88.8%) with a mean age of 60 years (standard deviation [sd] 15 years) and a mean Charlson comorbidity index of 0.89 (sd 1.2) (Table 1). Patients were followed for a mean 5.9 years (sd 3.9 years). Among the 15,705 patients who were prescribed and dispensed an NSAID, there were a total of 30,295 NSAID prescriptions during the study period; the most common NSAID medications used by patients were ibuprofen (n = 7649, 48.7%), naproxen (n = 6505, 41.4%), and etodolac (n = 3183, 20.3%).] Selective COX-2 inhibitors were uncommon (n=1103, 3.1%). Patients exposed to NSAIDS were younger (57.1 versus 61.9 years; p<0.001), more likely to be female (91.4% versus 94.6%; p<0.001) or white (85.7% versus 91.5%; p<0.001) and had more comorbidities (Charlson index of 0.96 versus 0.84; p<0.001) (Table 1). They were also more likely to be taking an immunomodulator or biologic medication (14.2% versus 13.1%; p=0.007). A total of 7,409 patients had an IBD exacerbation during follow-up, with 3,968 (53.6%) occurring in the NSAID exposed group and 3,441 (46.4%) occurring in the unexposed group.
Table 1:
Characteristics of NSAID exposed and non-NSAID exposed patients at baseline
| Overall | NSAID exposed | non-NSAID exposed | P-value | |
|---|---|---|---|---|
| Number of patients | 35,031 | 15,705 | 19,326 | |
| Follow-up in years (mean, SD) | 5.93 (3.90) | 6.22 (3.95) | 5.70 (3.83) | <0.001 |
| Age at IBD index (mean, SD) | 59.71 (15.49) | 57.05 (14.76) | 61.86 (15.73) | <0.001 |
| Male (n,%) | 32589 (93.2) | 14348 (91.4) | 18241 (94.6) | <0.001 |
| Race (n,%) | <0.001 | |||
| White | 24545 (88.8) | 11132 (85.7) | 13413 (91.5) | |
| Black | 2528 (9.1) | 1544 (11.9) | 984 (6.7) | |
| Other | 575 (2.1) | 313 (2.4) | 262 (1.8) | |
| IBD disease type (n, %) | < 0.001 | |||
| Crohn’s | 12450 (35.5) | 5429 (34.6) | 7021 (36.3) | |
| Ulcerative colitis | 18864 (53.8) | 8274 (52.7) | 10590 (54.8) | |
| Indeterminate | 3717 (10.6) | 2002 (12.7) | 1715 (8.9) | |
| Charlson comorbidity index (mean, SD) | 0.89 (1.18) | 0.96 (1.25) | 0.84 (1.12) | <0.001 |
| Immunomodulator or Biologic use (n, %) | 4774 (13.6) | 2242 (14.3) | 2532 (13.1) | 0.006 |
| Immunomodulator use (n, %) | 4763 (13.6) | 2236 (14.2) | 2527 (13.1) | 0.007 |
| Biologic use (n, %) | 642 (1.8) | 342 (2.2) | 300 (1.6) | < 0.001 |
| Infliximab | 267 (0.8) | 147 (0.9) | 120 (0.6) | |
| Adalimumab | 375 (1.1) | 198 (1.3) | 177 (0.9) | |
| Certolizumab | 19 (0.1) | 11 (0.1) | 8 (0.0) | |
| Region (n,%) | <0.001 | |||
| North Atlantic | 9348 (27.0) | 3984 (25.7) | 5364 (28.1) | |
| Southeast | 6446 (18.6) | 3142 (20.3) | 3304 (17.3) | |
| Continental | 4892 (14.1) | 2330 (15.0) | 2562 (13.4) | |
| Pacific | 5269 (15.2) | 2435 (15.7) | 2834 (14.8) | |
| Location of residence (n, %) | 0.926 | |||
| Urban | 32319 (93.4) | 14468 (93.2) | 17851 (93.4) | |
| Rural | 2290 (6.6) | 1042 (6.7) | 1248 (6.5) | |
| Facility complexity (n, %) | <0.001 | |||
| Highest | 14891 (43.0) | 6839 (44.1) | 8052 (42.1) | |
| High | 6861 (19.8) | 3005 (19.4) | 3856 (20.2) | |
| Mid-high | 6508 (18.8) | 2987 (19.3) | 3521 (18.4) | |
| Medium | 2855 (8.2) | 1219 (7.9) | 1636 (8.6) | |
| Low | 3494 (10.1) | 1460 (9.4) | 2034 (10.6) | |
| Missing | 11 (0) | 6 (0) | 5 (0) |
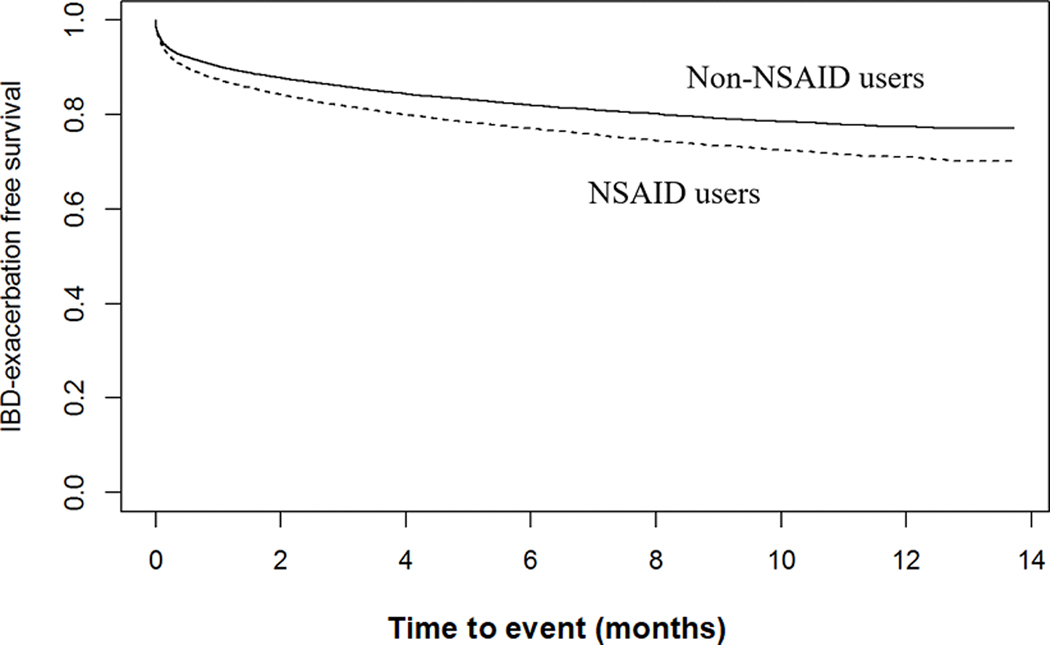
Cohort study using traditional methods:
In a propensity score matched Cox proportional hazards model, patients with IBD exposed to NSAIDs demonstrated a higher likelihood for IBD exacerbation than those patients not exposed to NSAIDs (HR 1.24; 95%CI 1.16–1.33), after adjusting for age, gender, race, Charlson comorbidity index, smoking status, IBD type, and use of immunomodulator or biologic medications (Figure 2 & Table 2). The likelihood of an IBD exacerbation in the pre-NSAID exposure period was 1.30 (95%CI 1.21–1.39) in NSAID exposed patients as compared to the patients not exposed to NSAIDs. Therefore, the prior event rate ratio for IBD exacerbation based on the adjusted hazards ratio post-NSAID exposure divided by the adjusted hazards ratio for pre-NSAID exposure was 0.95 (95%CI 0.89–1.01).
Figure 2.
NSAID exposure and time to IBD exacerbation in the cohort study
Table 2.
Findings from a Cox Proportional Hazards Model on Likelihood of IBD Exacerbation
| Patient characteristic | Adjusted HR | 95% CI |
|---|---|---|
| NSAID exposure | 1.24 | 1.16–1.33 |
| Age | 0.98 | 0.98–0.98 |
| Male sex | 1.39 | 1.24–1.55 |
| Charlson comorbidity index | 0.82 | 0.79–0.84 |
| Race | ||
| White | ref | ref |
| Black | 1.00 | 0.92–1.10 |
| Other | 0.97 | 0.81–1.16 |
| Immunomodulator or biologic use | 1.64 | 2.48–2.82 |
| Current tobacco use | 0.92 | 0.85–0.99 |
| IBD type | ||
| ulcerative colitis | 1.09 | 1.02–1.16 |
| Crohn’s disease | ref | ref |
| indeterminate colitis | 1.85 | 1.71–2.01 |
HR, hazards ratio; CI, confidence interval
Subgroup analysis using a self-controlled case series method:
We identified a subgroup of 3,968 (11.3%) patients in this cohort who had at least one IBD exacerbation for the self-control case series analysis (Supplemental Table 2). We examined the association between NSAID exposure and IBD exacerbation for each pre-defined pre- and post-exposure period separately, to assess for the potential for reverse causality. The incidence rate ratios (IRR) were 1.95 (95%CI 1.79–2.15) in the 1-year pre-NSAID exposure, 6.27 (95%CI 5.15–7.63) in the 0–2-week transition period, 1.77 (95%CI 1.37–1.43) in the 2–6 weeks post-NSAID dispense date, and 1.24 (95%CI 1.07–1.43), in the 6 weeks to 6 months post-NSAID dispense date, as compared to the risk for IBD exacerbation outside the exposure periods (Table 3). Similar findings are evidence when examining patients with Crohn’s disease and ulcerative colitis, separately (Table 3).
Table 3.
Findings from the Self-Controlled Case Series on the Risks of IBD Exacerbation
| Overall IRR | Crohn’s disease IRR | Ulcerative colitis IRR | |
|---|---|---|---|
| 1-year pre-NSAID dispense date | 1.95 (95% CI 1.79–2.15) | 1.99 (95% CI 1.71–2.35) | 1.91 (95% CI 1.66–2.19) |
| 0–2 weeks post-NSAID dispense date | 6.27 (95% CI 5.15–7.63) | 7.12 (95% CI 5.16–9.78) | 6.16 (95% CI 4.59–8.25) |
| 2–6 weeks post-NSAID dispense date | 1.77 (95% CI 1.37–1.43) | 1.86 (95% CI 1.21–2.88) | 1.54 (95% CI 1.03–2.31) |
| 6 weeks-6 months post-NSAID dispense date | 1.24 (95% CI 1.07–1.43) | 1.40 (95% CI 1.11–1.77) | 1.22 (95% CI .98–1.51) |
IRR, incidence rate ratio; CI, confidence interval
Sensitivity analysis:
Given the possibility that our pre-defined risk periods could influence our findings, we examined whether differences in observed risk occurred when using an alternative pre-exposure period defined by 1 month preceding an NSAID dispense date. The findings were not substantially different, with IRRs of 1.50 (95%CI 1.15–1.95) in the pre-NSAID exposure period, 5.56 (95%CI 4.58–6.76) in the 0–2-week transition period, 1.57 (95%CI 1.22–2.04) in the 2–6 weeks post-NSAID exposure period, and 1.10 (95%CI 0.95–1.27) in the 6 weeks-6 months post-NSAID exposure period. We also separately examined the relationship between IBD exacerbation and the most common NSAID types. 1,131 patients were exposed to ibuprofen and had an IBD exacerbation. The IRRs for IBD exacerbation over the distinct risk windows were 2.31 for the 1-year pre-exposure, 6.46 for the 0–2 week transition, 1.61 for the 2–6 week post-ibuprofen period, and 1.04 for the 6 weeks to 6 months post- ibuprofen period. Similarly, 753 patients were exposed to naproxen and had an IBD exacerbation. The IRRs for IBD exacerbation over the distinct risk windows were 1.64 for the 1-year pre-exposure, 4.85 for the 0–2 week transition, 1.15 for the 2–6 week post-naproxen period, and 1.13 for the 6 weeks to 6 months post-naproxen period.
Discussion:
We conducted a multimethod analysis of the relationship between NSAID exposure and IBD exacerbation in the context of residual confounding. We saw an association between NSAID exposure and IBD exacerbation in a cohort of 35,031 patients. However, the estimated prior event rate ratio of 0.95 (95%CI 0.89–1.01) suggests that this may be independent of NSAID exposure and related to an underlying increased likelihood of IBD exacerbation in NSAID exposed patients preceding NSAID exposure. Further, using a self-controlled case series approach to verify our findings and adjust for other immeasurable patient-level confounders, the risk of IBD exacerbation did not increase in the 2 weeks to 6 months after NSAID exposure.
While most studies examining the effect of medications on disease-specific outcomes adjust for patient characteristics that may confound the outcome of interest, residual confounding may persist. When examining IRRs by risk window, we observe a high IRR for IBD exacerbation in the 0–2-week transition period after the NSAID dispense date. It is difficult to differentiate the pre-existence of IBD exacerbation symptoms at the time of NSAID dispensing given the timeframe between the NSAID dispense date and IBD exacerbation requiring corticosteroids. Given the drop in IRR after the transition period, it is possible that this reported association may be secondary to residual confounding related to reverse causality. The potential for reverse causality occurs when a medication is prescribed as a response to early signs or symptoms of an undetected disease outcome. This concept has been identified in studies examining the effects of proton pump inhibitors (PPI), antimicrobials, and other medications on other short-term clinical outcomes.22 A recent analysis examining the relationship between PPIs and mortality among Medicare beneficiaries demonstrated this effect to address the discrepancy in findings between observational studies where a significant association had been reported and negative randomized controlled trials. PPIs appeared to have a small but significant association with a higher likelihood of mortality on initial examination. However, this association was no longer significant after excluding the 90-day perimortem period, since PPIs are often prescribed as a result of critical illness or end of life care rather than contributing to mortality.10
Analgesic selection in IBD patients remains a global concern for clinicians despite pain representing a dominant feature of IBD owing to the sparsity of available safe analgesic options and unfavorable side effect profiles. While NSAIDs represent the most common first-line analgesic, their use in IBD patients is variable, in part due to the suspected risk of IBD exacerbation despite inconclusive evidence of harm to date. 23–28 In our study population, 36.5% of patients with IBD received at least one NSAID prescription, demonstrating that NSAID use is common in this population. Furthermore, 75% of patients with IBD who were prescribed NSAIDs did not have an IBD exacerbation during a mean of 5.9 years of follow-up.
This study’s strengths include a national cohort with a large sample size and sufficient follow-up. The multimethod approach, which considers measurable and non-measurable patient-level factors greatly reduces the risk of bias by confounding. This study has its inherent limitations. First, there is a potential limitation to generalizability given a predominantly male population of Veterans. However, we see similar rates of NSAID use as compared to the general IBD population making these results more robust. Second, this dataset has limited availability of details regarding IBD history, including phenotype and duration of disease, though likely this does not impact the relationship between NSAIDs and IBD exacerbations. Third, it is possible that patients at higher risk of an exacerbation are less likely to be prescribed NSAIDs, which would falsely minimize the magnitude of association between NSAID exposure and IBD exacerbation. However, our bivariate analysis demonstrates that patients receiving immune targeted therapy (i.e., with moderate-severe disease as compared to mild disease) are more likely to receive NSAIDs. In addition, we adjust for several covariates associated with higher risk of IBD exacerbation in our analyses (i.e., smoking) and in our self-controlled case series analysis we control for patient characteristics such as IBD severity. However, it is important to also note that disease activity varies over time and may not be adequately captured in this analysis. It is possible that disease activity moderates the relationship between NSAID exposure and IBD exacerbation and should be studied in future work. Fourth, as an over-the-counter medication, NSAIDs are often difficult to track using electronic medical record data. Therefore, the potential for a misclassification bias and not capturing NSAID exposures exists in the unexposed arm, which would bias our findings towards no association. However, our primary analysis using traditional methods still demonstrates an association between NSAIDs and IBD exacerbations, which subsequently is better differentiated in risk windows in our secondary analysis using prior event rate ratios and subgroup analysis using a self-controlled case series design. In addition, data for the indication of each NSAID prescription is unavailable.
In addition, NSAID dose effect was not examined given that NSAID dosage varies by NSAID type and there is no standard method to determine dose equivalences across medications. We also did not evaluate NSAID duration of use, as this measure is difficult to reliably capture. To reduce heterogeneity in our results, we focus on initial NSAID exposure, and cannot account for the role of multiple NSAID exposures on IBD exacerbations. We did not explore the relationship between acetaminophen and IBD exacerbations as this would require a separate cohort for propensity score matching. In addition, a disproportionate amount of acetaminophen is prescribed in combination with opioids. It is therefore difficult to separate out the role of acetaminophen from the role of opioids which is known to complicate IBD management. Also, it is difficult to separate out opioid use which may be prescribed for IBD related chronic pain/exacerbations from acetaminophen preceding an exacerbation. We also did not explore the relationship between selective COX-2 inhibitors and IBD exacerbations as this would require a separate cohort for propensity score matching, and the overall use of selective COX-2 inhibitors was low (3.1%). Finally, there may be several reasons to potentially avoid NSAIDs in patients with IBD, which are not examined in this study, such as gastric ulcers or kidney injury.
In conclusion, this study did not find convincing evidence to suggest a causal relationship between NSAID exposure and IBD exacerbations. Our findings suggest that observed associations between IBD exacerbation and NSAID exposure may be explained by existing underlying risks for IBD exacerbations, residual confounding, and reverse causality. The potential for residual confounding by reverse causality should be considered as the interest in using large administrative data for conducting observational studies to evaluate the safety of medications continues to grow. While the study design is not tailored to evaluate the safety of NSAIDs, it provides support against the previously described potential causal relationship between NSAIDs and IBD exacerbations. We hope these study findings will aid providers in better directing IBD patients on their risk for IBD exacerbation with NSAID use. This may guide therapy for both IBD and non-IBD related pain management, and the comfort of patients with IBD and the clinicians who treat them when considering NSAIDs as a non-opioid treatment option. We also hope this work will bring attention to the role of protopathic bias in large association studies.
Supplementary Material
WHAT IS KNOWN
Patients with inflammatory bowel disease are prone to both inflammatory and non-inflammatory pain.
In IBD, there has been a long-standing concern that NSAIDs may play a role in disease exacerbations.
WHAT IS NEW HERE
The observed higher likelihood of IBD exacerbation among patients with IBD who are exposed to NSAIDs may be influenced by residual bias, particularly the bias of reverse causality.
These findings may help guide therapy for non-opioid pain management for patients with IBD.
Financial Support:
Dr. Cohen-Mekelburg and Dr. Wallace receive funding from the National Institute of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241). Dr. Dominitz received funding from Glaxo-Wellcome Institute for Digestive Health grant. Dr. Waljee and Dr. Hou receive fundings from a VA Health Services Research and Development Merit Award. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institute of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have no other conflicts of interest to disclose.
References
- 1.Burr NE, Smith C, West R, Hull MA, Subramanian V. Increasing Prescription of Opiates and Mortality in Patients With Inflammatory Bowel Diseases in England. Clin Gastroenterol Hepatol 2018;16:534–41 e6. [DOI] [PubMed] [Google Scholar]
- 2.Targownik LE, Nugent Z, Singh H, Bugden S, Bernstein CN. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol 2014;109:1613–20. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Mekelburg S, Rosenblatt R, Gold S, et al. The Impact of Opioid Epidemic Trends on Hospitalized Inflammatory Bowel Disease Patients. J Crohns Colitis 2018. [DOI] [PMC free article] [PubMed]
- 4.Nash DM, Markle-Reid M, Brimble KS, et al. Nonsteroidal anti-inflammatory drug use and risk of acute kidney injury and hyperkalemia in older adults: a population-based study. Nephrol Dial Transplant 2019;34:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trentalange M, Runels T, Bean A, et al. Analgesic prescribing trends in a national sample of older veterans with osteoarthritis: 2012–2017. Pain 2019;160:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis 2018;9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer AM, Ramzan NN, Heigh RI, Leighton JA. Relapse of inflammatory bowel disease associated with use of nonsteroidal anti-inflammatory drugs. Dig Dis Sci 2006;51:168–72. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:196–202. [DOI] [PubMed] [Google Scholar]
- 9.Moninuola OO, Milligan W, Lochhead P, Khalili H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment Pharmacol Ther 2018;47:1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baik SH, Fung KW, McDonald CJ. The Mortality Risk of Proton Pump Inhibitors in 1.9 Million US Seniors: An Extended Cox Survival Analysis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed]
- 11.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med 1980;68:255–8. [DOI] [PubMed] [Google Scholar]
- 12.Hou JK, Tan M, Stidham RW, et al. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn’s disease in the Veterans Affairs Health Care System. Dig Dis Sci 2014;59:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid Use and Complications in a US Inflammatory Bowel Disease Cohort. PLoS One 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominitz JA, Thomas D. Koepsell, and Edward J. Boyko. “Association between analgesic use and inflammatory bowel disease (IBD) flares: a retrospective cohort study.” Gastroenterology 118, no. 4 (2000): A581. [Google Scholar]
- 15.Waljee AK, Lipson R, Wiitala WL, et al. Predicting Hospitalization and Outpatient Corticosteroid Use in Inflammatory Bowel Disease Patients Using Machine Learning. Inflamm Bowel Dis 2017;24:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers LR, Dennis JM, Shields BM, et al. Prior event rate ratio adjustment produced estimates consistent with randomized trial: a diabetes case study. J Clin Epidemiol 2020;122:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace BI, Waljee AK. Burst Case Scenario: Why Shorter May Not Be Any Better When It Comes to Corticosteroids. Ann Intern Med 2020. [DOI] [PubMed]
- 20.Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association Between Oral Corticosteroid Bursts and Severe Adverse Events: A Nationwide Population-Based Cohort Study. Ann Intern Med 2020. [DOI] [PubMed]
- 21.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016;354:i4515. [DOI] [PubMed] [Google Scholar]
- 22.Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: population based study. BMJ 2016;355:i5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Role of Nonsteroidal Anti-Inflammatory Drugs in Exacerbations of Inflammatory Bowel Disease. J Clin Gastroenterol 2016;50:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 25.Bonner GF, Fakhri A, Vennamaneni SR. A long-term cohort study of nonsteroidal anti-inflammatory drug use and disease activity in outpatients with inflammatory bowel disease. Inflamm Bowel Dis 2004;10:751–7. [DOI] [PubMed] [Google Scholar]
- 26.Rampton DS, Sladen GE. Relapse of ulcerative proctocolitis during treatment with non-steroidal anti-inflammatory drugs. Postgrad Med J 1981;57:297–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufmann HJ, Taubin HL. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann Intern Med 1987;107:513–6. [DOI] [PubMed] [Google Scholar]
- 28.Evans JM, McMahon AD, Murray FE, McDevitt DG, MacDonald TM. Non-steroidal anti-inflammatory drugs are associated with emergency admission to hospital for colitis due to inflammatory bowel disease. Gut 1997;40:619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.