INTRODUCTION
Death rates from opioid overdose continue to rise at an alarming pace. Many overdose victims die with untreated opioid use disorder (OUD), despite the availability of effective and lifesaving medications for opioid use disorder (MOUD). Significant barriers to treatment access include transportation, competing responsibilities, stigma, and related costs1. While millions of individuals receive buprenorphine in a given year2, the majority discontinue treatment within a few weeks or months3,4 forfeiting ongoing protective effects5.
OUD treatment via virtual care platforms may help overcome these common barriers to retention in care3,4. Until recently, the 2008 Ryan Haight Act required an in-person assessment before prescribing any controlled substance, greatly limiting telehealth for OUD1. However, COVID-19-related reforms largely waived such requirements and hold promise for improving access to care, especially in underserved rural areas. These regulatory changes have attracted innovation among startups as well as incumbent health systems1. Given long-standing challenges in retaining patients in traditional office-based buprenorphine treatment3,4, research is needed to determine how virtual care outcomes can optimize retention.
METHODS
We analyzed data from a cohort of individuals with OUD treated at Ophelia, a virtual-first telehealth OUD treatment platform. Individuals in New York and Pennsylvania were recruited directly online (e.g., Facebook and Google ads). Medical visits and clinically indicated urine drug screens, organizational structure, custom EHR, and care coordination services were all built explicitly for remote care without requiring any in-person visits. In-network patients, predominantly Medicaid beneficiaries, used insurance. Patients out of network or uninsured paid $195 monthly to receive unlimited real-time, video-based clinical visits. Eligible patients (i.e., those with OUD not requiring a higher level of care) were prescribed buprenorphine at intake, and seen weekly during the stabilization phase, and then stepped down to monthly visits under a nurse care manager model.
To investigate 180-day treatment retention, a minimum duration of pharmacotherapy for OUD endorsed by the National Quality Forum6, we analyzed a sample of consecutive new intakes from July 1, 2020, to April 15, 2021. Kaplan-Meier survival analyses determined retention, with discontinuation being defined as a 60+ day gap between clinical visits. Consistent with prior studies, care episodes of ≤ 7 days (9% of total episodes) were excluded3 as they often reflected patients ineligible or unwilling to initiate treatment. Geographic heat maps compared distribution of patients to the SAMHSA locator for buprenorphine x-waivered prescribers at the zip code level with eSpatial mapping technology. Patients’ home addresses were categorized under USDA Rural-Urban Commuting Area Codes, RUCA codes, with 1–3 denoting urban and 4–10 denoting rural locations.7 Secondary outcomes measuring adherence included the proportion of days covered (PDC) and medication possession ratio (MPR) of buprenorphine. The Western (WCG) IRB approved a waiver of consent for the study conducted under STROBE guidelines.
RESULTS
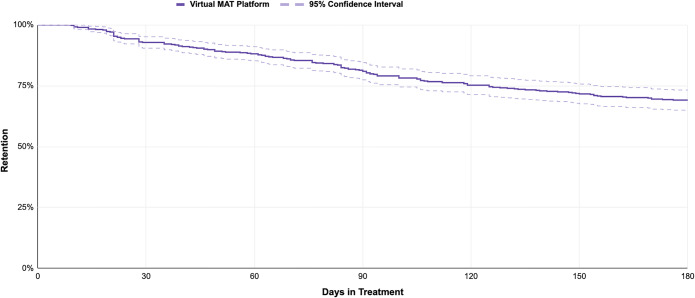
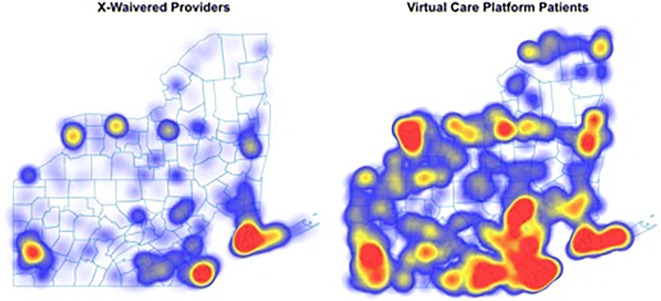
A total of 475 patients were included, 60.3% male, mean age 36.3 years (SD=7.1 years). Two-thirds (66.5%) self-reported race/ethnicity: 88.3% were white. The majority reported Medicaid coverage, consistent with prior studies.3,4 The 180-day retention was 69.1% (95%CI: 65.0–73.2%) (Fig. 1); 21.9% of patients resided in rural/small town areas reflecting much greater geographic variation than that of x-waivered prescribers (Fig. 2). 28% of urban patients were in a zip code without an x-waivered provider as were 31.96% of rural patients. Patients had a proportion of days covered of 0.96 and a medication possession ratio of 1.05 reflecting high adherence without evidence of stockpiling medication.
Figure 1.
The 180-day retention among patients with opioid use disorder in buprenorphine maintenance treatment provided by telehealth.
Figure 2.
Density distribution of x-waivered buprenorphine providers (per SAMHSA locator) vs patients enrolled via a virtual care platform in Pennsylvania and New York (n=475), 2020–2021 data. *Among 475 patients, 43.4% (206) resided in New York and 56.6% (269) in Pennsylvania.
DISCUSSION
The observed 180-day retention rate of 69.1% is superior to prior observational studies analyzing multi-state Medicaid (27.0%)3 and commercial insurance prescription claims (31.0%)4. Unlike requirements for in-person care, telehealth enables patients to access care that may not be available locally and attend visits with less interruption and more discretion, decreasing stigma.
Expanding access to medication-based care and improving treatment retention are vital to address the worsening opioid overdose crisis. Technology-enabled telehealth platforms may be important tools for increasing access, retention, and patient satisfaction with evidence-based OUD care. Telehealth is not a single entity and further research is needed to determine best practices, especially for improving patient retention in OUD treatment with buprenorphine. Further research is also necessary to determine how telehealth interventions can best reach racially and ethnically diverse populations who are underserved by OUD treatment generally and for whom fatal overdose rates are currently rising the fastest.
Funding
Financial support for this work was provided by Ophelia Health, Inc.
Declarations
Conflict of interest
The authors receive compensation in the form of equity, salary, consulting fees, and/or travel expenses from Ophelia Health, Inc., a telehealth provider for opioid use disorder. ARW also receives consulting fees from the National Quality Forum for work on measure development for the treatment of opioid use disorder.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verma, S. Early impact of CMS expansion of Medicare telehealth during COVID-19. Health Affairs blog, 2020.
- 2.2019 National Survey of Drug Use and Health (NSDUH) Releases | CBHSQ Data. https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases. Last accessed October 2021.
- 3.Samples H, Williams AR, Olfson M, Crystal S. Risk factors for premature discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. Journal Subst Abus Treat. 2018;95:9–17. doi: 10.1016/j.jsat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan JR, Schachkman BR, Leff JA, Linas BP, Walley A. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abus Treat. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AR, Samples H, Crystal S, Olfson M. Retention on buprenorphine beyond six months and risk of acute care service utilization, opioid prescription use, and overdose. Am J Psych. 2019;177(2):117–124. doi: 10.1176/appi.ajp.2019.19060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Quality Forum. Behavioral Health 2016-2017 Final Report. 2017. Accessed 3/6/2022 at https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx
- 7.Economic Research Service, U.S. Department of Agriculture. Rural-Urban Commuting Area Codes: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx last accessed August 20, 2022.