Abstract
To date, there have been conflicting reports concerning the clinical significance of nitric oxide (NO) in Plasmodium falciparum malaria. Some authors have proposed that NO contributes to the development of severe and complicated malaria, while others have argued that NO has a protective role. To investigate these apparently contradictory reports, reverse transcription-coupled PCR was used to study inducible NO synthase (iNOS) in whole-blood RNA samples from patients with severe and complicated malaria or uncomplicated malaria and from healthy donors. This work produced three principal findings. First, samples of patients with severe and complicated malaria were variably positive, with weak to moderate intensity. Markedly higher iNOS RNA levels were observed in samples of patients with uncomplicated malaria than in patients with severe and complicated malaria. Samples of healthy donors were uniformly negative. Second, since we initially demonstrated iNOS expression in whole-blood RNA samples, we extended our investigations to individual blood cells such as monocytes, lymphocytes, neutrophils, and platelets to identify the cellular source of iNOS. We found that iNOS was expressed predominantly in monocytes. Third, retrospective statistical analysis of monocyte counts clearly demonstrated that patients with uncomplicated malaria had higher monocyte counts at the time of presentation than patients with severe and complicated malaria. Taken together, our findings give room to the interpretation that NO may have a beneficial rather than a deleterious role in falciparum malaria.
Inducible nitric oxide synthase (iNOS) is one of the three distinct isoenzymes responsible for the catalytic oxidation of the terminal guanidino nitrogen atom of l-arginine, yielding citrulline and the short-lived reactive free radical NO in various nucleated cells. iNOS, which was originally identified in macrophages, is a cytosolic Ca2+-independent enzyme whose transcription is induced by microbial products or inflammatory cytokines (23, 24, 38). NO produced from monocytes/macrophages by the enzymatic action of iNOS has been implicated in mediating cytotoxicity during host defense reactions. A unique feature of iNOS is that when it is triggered by an immunologic or inflammatory stimulus, a sustained production of NO results. Since the initial observation by James and Glaven (20) that NO has schistosomicidal activity, its antimicrobial functions in a variety of microorganisms have been confirmed with both humans and animal models (11, 18, 19, 33).
The past decade has witnessed an explosion of interest in the role of NO in falciparum malaria; however, there is currently no consensus on the clinical significance of NO in falciparum malaria. Some authors have associated NO with severe and complicated malaria, particularly cerebral malaria (1, 3, 6, 9, 10, 12, 13, 29), whereas other authors have argued that NO has a protective role (2, 4, 21, 22, 26, 34–37). An interesting contradiction concerning the role of NO in malaria emerged from two recent studies. Kun et al. (22) demonstrated an association between polymorphism in the promoter region of the iNOS gene and protection from severe malaria in a Gabonese population. However, observations by Burgner et al. (6) of a Gambian population are somewhat different. Data from this study suggest that similar regions of iNOS genes encode a susceptibility determinant for fatal cerebral malaria.
To investigate these apparently contradictory reports, we studied the expression of iNOS in whole-blood RNA samples of patients with falciparum malaria and attempted to relate the transcription of this gene to the outcome of the disease. Since NO is a short-lived free radical, its measurement is difficult (5). To circumvent this obstacle, we indirectly assessed its role in malaria by measuring iNOS, an enzyme responsible for the sustained synthesis of NO in large amounts.
Characteristics of the study subjects.
The patients included in this study were admitted to the Medical Department of the Bernhard Nocht Institute for Tropical Medicine with a confirmed diagnosis of falciparum malaria. This diagnosis was based on identification of asexual forms of the parasite in peripheral blood smears subjected to thin or thick staining. The percentage of parasitemia was calculated from the number of infected erythrocytes per 1,000 erythrocytes in thinly stained film. When parasites were found only in the thickly stained film, parasitemia was defined as below 1% (less than 50 parasites per thickly stained film field). The study population was comprised of 24 patients (age, 40 ± 18 years). After written informed consent was obtained from the patients and/or their immediate relatives, peripheral blood samples were drawn before treatment from (i) 12 patients with severe and complicated falciparum malaria, (ii) 12 patients with uncomplicated falciparum malaria, and (iii) 12 healthy medication-free donors (age, 32 ± 12 years) with no previous history of malaria, who were evaluated as controls. All patients who had severe and complicated falciparum malaria were of European origin. Of the 12 patients with uncomplicated falciparum malaria, 4 were of European origin and 8 were African immigrants from either western or eastern Africa who had been living in Germany for at least 2 years. All patients presented with fever lasting between 1 and 5 days and had contracted their malarial infection in Africa. All patients included in this study had not spent more than 4 weeks in Africa when they contracted their malarial infection and had no evidence of concomitant infections. None of the patients had received antimalarial treatment before our administration. Patients were defined as semi-immune if they were raised in a malarious area and had lived in a nonmalarious area for a period not exceeding 5 years. Nonimmune patients were patients who were raised in a nonmalarious area and had lived in a malarious area for a period not exceeding 2 years. Nonimmune patients included in this study had no previous history of malaria. Patients were described as having severe and complicated malaria if they met one of the following criteria: (i) impaired cerebral function (disorientation, drowsiness, unconsciousness), (ii) pathological global clotting tests (prothrombin time activity, <50%, and partial thromboplastin time, >45 s), (iii) impaired renal function (creatinine in serum, >2.0 mg/dl), (iv) respiratory insufficiency (partial pressure of oxygen, <60 mm Hg), (v) hepatic damage (alanine aminotransferase and aspartate aminotransferase levels, ≥100 U/liter), and (vi) parasitemia (≥5%) (7).
Antiparasitic therapy.
Patients with severe and complicated malaria received the standard regimen of quinine (20 mg/kg of body weight/day) and doxycyline (100 mg/day) for 10 days. Patients with uncomplicated falciparum malaria received mefloquine (20 mg/kg/day) in three doses taken 6 h apart). Therapy was initiated immediately after confirming the diagnosis of malaria with blood smears on admission.
RNA isolation from whole blood.
Heparinized blood was drawn with minimum venostasis, and RNA was isolated with TRI Reagent BD (Molecular Research Center, Inc., Cincinnati, Ohio) according to the manufacturer's specifications.
Isolation of monocytes, lymphocytes, neutrophils, and platelets.
Peripheral blood mononuclear cells were isolated by Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation. Monocytes were isolated by adherence to 75-ml plastic culture flasks (Costar). The nonadherent lymphocytes were removed by aspiration. Neutrophils were isolated by the method of Ross and Densen (30) as modified by Estabrook et al. (16). Briefly, heparinized blood from patients was sedimented in an equal volume of 3% dextran T-500 (Pharmacia) in 0.85% NaCl at room temperature for 25 min. The leukocyte-rich supernatant was removed by centrifugation at 200 × g for 10 min at 4°C. The supernatant was layered on Ficoll-Paque (Pharmacia) and centrifuged at 250 × g for 20 min. Heparinized blood drawn with minimum venostasis at times of admission was collected into a 1/10 volume of 3.8% sodium citrate and then centrifuged at 400 × g for 20 min at room temperature. Platelet-rich plasma was collected and centrifuged for 20 min at 1,000 × g to sediment platelets. The resulting pellet was resuspended in modified Tyrode's buffer (140 mM NaCl, 5 mM KCl, 10 mM glucose, 15 mM HEPES, 0.38 g of bovine serum albumin [pH 7.4] per 100 ml), and the platelets were subsequently washed three times with the buffer. The levels of purity of cells were 96% for monocytes, 96% for platelets, 98% for neutrophils, and 98% for lymphocytes as determined by differential cell counting.
RNA isolation from monocytes, lymphocytes, and neutrophils.
Total cellular RNA was prepared with TRIZol reagent (Life Technologies) based on the acid guanidinium thiocynate-phenol-chloroform method of Chomcyznski and Sacchi (8) as modified by the manufacturers.
RT-PCR, slot blot analysis, and cloning and sequencing of RT-PCR products.
The expression of iNOS was measured by amplification by reverse transcription-PCR (RT-PCR). This approach was necessary, as the available amounts of RNA were too small for analysis by either Northern blotting or ribonuclease protection assays. Prior to RT, possible chromosomal DNA contaminants were degraded by treating the total RNA samples with 10 U of DNase I (GenHunter Corp., Nashville, Tenn.) for 30 min at 37°C, followed by phenol extraction, ethanol precipitation, and finally dilution in RNase-free water. In batched and parallel reactions, equal amounts of total cellular RNA from each control were subjected to RT with SuperScript II RNase H− reverse transcriptase (Gibco BRL Life Technologies) according to manufacturer's specifications. In a 12-μl reaction mixture, 1 μg of RNA and 1 μg of oligo(dT) (0.5 g/μl) were heated for 10 min at 70°C and immediately chilled on ice for 5 min. The reaction mixture was preincubated for 5 min at 42°C. The mixture contained 4 μl of 5× First Strand Buffer (250 mM Tris-HCl [pH 8.3], 375 mM KCl, 15 mM MgCl2), 1 μl of a deoxynucleoside triphosphate mix (10 mM), 2 μl of 0.1 M dithiothreitol, and 200 U of Superscript reverse transcriptase. After preincubation, the mixture was incubated at 42°C for 50 min and the reaction was terminated by heating the mixture at 95°C for 5 min and chilling it on ice. cDNAs were subjected to enzymatic amplification with primers specific for human iNOS (Clontech) and reagents from a GeneAmp kit (Perkin-Elmer). The 35 cycles of PCR were performed under the following conditions as recommended by the manufacturer: denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min; the last cycle extension was performed at 72°C for 7 min in a Biometra UNO-Thermoblock. To assess the integrity of the starting RNA as well as to control for variability in RNA or cDNA handling during the RT-PCR method and as the standard for semiquantitative comparisons, the levels of β-actin RNA transcripts were determined simultaneously for every PCR sample with primers specific for β-actin (Clontech) according to the manufacturer's instructions. After completion of the PCR, the amplified DNA was recovered with QIAquick (Qiagen, Hilden, Germany). Eight microliters of each of the recovered iNOSs and 8 μl of the respective coamplified β-actin PCR products were denatured by heating at 95°C for 4 min in 190 μl of 0.4 M NaOH containing 25 mM EDTA. Samples were rapidly cooled on ice and then blotted directly onto a Nylon membrane (Hybond-N+) (Amersham, Braunschweig, Germany) presoaked in 20× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) in a slot blot apparatus (Minifold II; Schleicher & Schuell, Dassel, Germany). Each well was washed with 400 μl of 20× SSPE. After transfer, the DNA was covalently linked to the nylon membrane by exposure to UV light. Human iNOS and β-actin cDNA probes obtained by PCR were radiolabeled by random primer extension with [α-32P]dCTP. The blots were prehybridized for 4 h in hybridization solution at 42°C, hybridized overnight with labeled cDNA, and washed as described previously (31). The level of hybridization for 32P-labeled cDNA probes was visualized by autoradiography with Kodak XAR film and quantitated with a PhosphorImager and the ImageQuant program (Molecular Dynamics, Sunnyvale, Calif.). To confirm that the amplified PCR product represented the iNOS mRNA, we eluted the PCR product and cloned it using a TA cloning kit (Invitrogen) according to the manufacturer's instructions and determined the nucleotide sequence by the dideoxynucleotide chain-termination method (32) using a Sequenase kit (version 2.0; United States Biochemicals). Analysis of the sequence of the PCR-amplified DNA fragment revealed that bases 568 through 3459 were identical to the previously described sequence (17).
Statistical analysis.
Statistical analysis was performed by Student's t test with the STAT software package from SAS Institute Inc., Cary, N.C. All test results were judged to be significant at a P of <0.05.
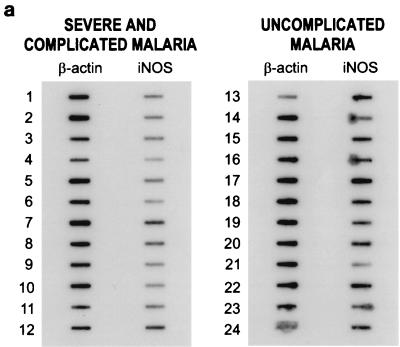
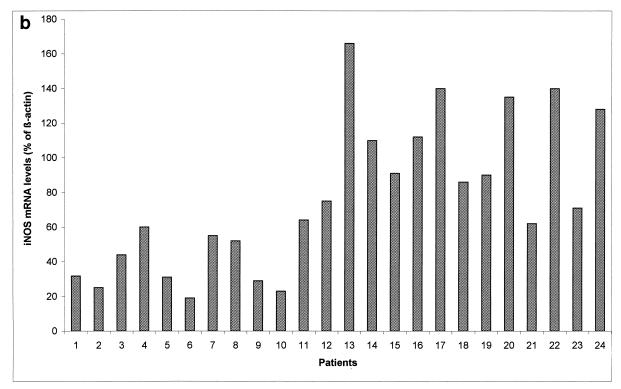
RT-PCR analyses of iNOS mRNAs prepared from whole blood from patients with severe and complicated malaria and patients with uncomplicated malaria are shown in Fig. 1a. In this semiquantitative RT-PCR assay, the signals of iNOs and β-actin PCR products were quantitated by densitometry (Fig. 1b). RNA samples from patients with severe and complicated malaria were variably positive, with weak to moderate intensity (slots 1 to 12). iNOS mRNAs were strongly expressed in 10 samples from patients with uncomplicated malaria (slots 13 to 20, 22, and 24). Moderate expression of iNOS mRNA was observed in the samples from two patients with uncomplicated malaria (slots 21 and 23). Densitometric values were determined from the slot blot shown in Fig. 1a and are presented as iNOS mRNA levels (percentages of β-actin) (Fig. 1b). Samples are shown in the same order as in Fig. 1a. Results of RT-PCR and slot blot analysis of RNA samples from healthy donors were, as predicted, all positive for expression of the housekeeping gene β-actin but uniformly negative for iNOS (data not shown).
FIG. 1.
(a) Slot blot semiquantitative analysis. RNAs isolated from peripheral whole blood from patients with falciparum malaria were reverse transcribed and subjected to 45 PCR cycles. Eight microliters from each reaction mixture was slot blotted as described in Materials and Methods. cDNA probes obtained from the PCRs were hybridized to PCR products, which were applied to Hybond-N+ membranes with a slot blot manifold. Slots 1 to 12 contained PCR products from patients with severe and complicated malaria, and slots 13 to 24 contained PCR products from patients with uncomplicated malaria. Samples of patients with severe and complicated falciparum malaria (slots 1 to 12) were variably positive, with weak to moderate intensity. iNOS levels were markedly higher in patients with uncomplicated malaria than in those with complicated malaria. (b) Ratios of iNOs gene expression to β-actin gene expression. Densitometric values were determined from the slot blot shown in panel a and are presented as iNOS mRNA levels (percentages of β-actin). Samples are shown in the same order as in panel a.
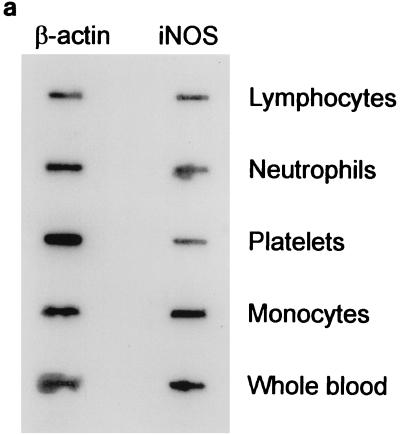
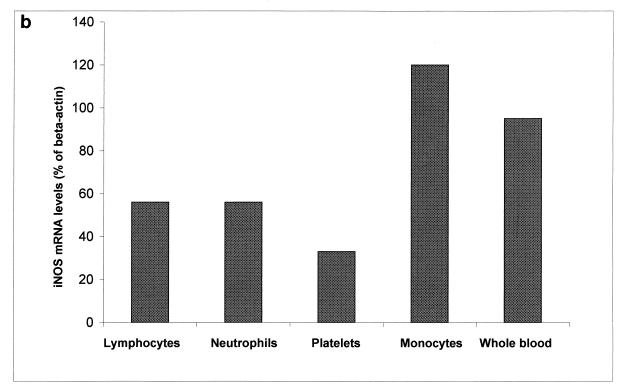
Since iNOS was initially detected in whole-blood RNA samples, we expanded our experiments to ascertain the cellular origin of iNOS. To this effect we studied iNOS expression in individual blood cells such as platelets, monocytes, neutrophils, and lymphocytes. We found that iNOS was predominantly expressed in monocytes. A representative autoradiogram from these experiments is shown in Fig. 2a. A diagramatic representation of the densitometric values is shown in Fig. 2b.
FIG. 2.
(a) Comparison of levels of expression of iNOS mRNAs in different peripheral blood cells. Total RNA (1 μg) from lymphocytes, neutrophils, platelets, monocytes, and whole blood was reverse transcribed with an oligo(dT) primer. Five percent of the product was amplified for 35 cycles with primer pairs specific for human iNOS or β-actin iNOS. Eight microliters of each PCR product was slot blotted, hybridized, and visualized by autoradiography. (b) Ratios of iNOS gene expression to β-actin gene expression. Densitometric values were determined from the blot shown in panel a and are presented as iNOS mRNA levels (percentages of β-actin). Samples are shown in the same order as in panel a.
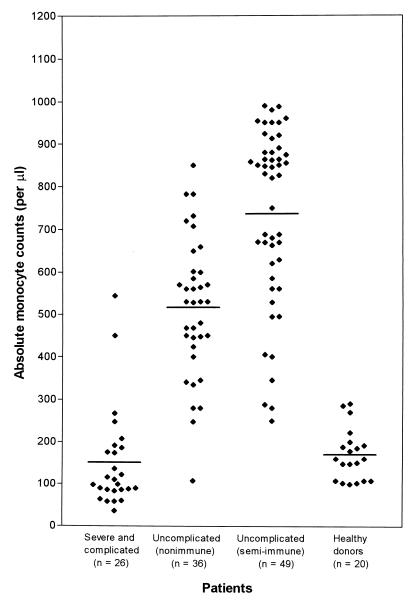
In light of the observation that the different blood cells differ so markedly in their expression of iNOS and that monocytes were the predominant source of iNOS in malaria patients, we conducted a retrospective analysis of monocyte counts. In this retrospective study, we reviewed the medical charts of 101 patients who were admitted to the Medical Department of the Bernhard Nocht Institute for Tropical Medicine during a 6-month period from January to June 1997 with severe and complicated malaria (n = 26) and with uncomplicated malaria (nonimmune [n = 36] and semi-immune [n = 49]). All 26 patients with severe and complicated malaria and all 36 nonimmune patients with uncomplicated malaria were of European origin. They had all contracted their malarial infection while travelling in Africa. All semi-immune patients were African immigrants (39 West Africans, 6 East Africans, and 4 Central Africans) who had lived in Europe for not more than 2 years. Of particular interest in this study was the observation that patients with uncomplicated malaria had higher absolute monocyte counts at the time of presentation than patients with severe and complicated malaria (Fig. 3). The mean absolute monocyte counts were 151 per μl of blood for patients with severe and complicated malaria, 516 per μl of blood for nonimmune patients with uncomplicated falciparum malaria, and 736 per μl of blood for semi-immune patients with uncomplicated malaria. The mean absolute monocyte count for healthy donors was 170 per μl of blood. Statistical analysis demonstrated significant differences in absolute monocyte counts between patients with severe and complicated malaria and patients with uncomplicated malaria (P < 0.001). Thus, the difference between levels of iNOS expression in patients with severe and complicated malaria and patients with uncomplicated malaria is at least in part due to the difference in monocyte counts. Compared with levels in healthy controls, iNOS is upregulated in patients with both severe and uncomplicated malaria.
FIG. 3.
Comparison of absolute monocyte counts from patients with severe and complicated malaria, nonimmune patients with uncomplicated malaria, semi-immune patients with uncomplicated malaria, and healthy donors. Solid lines indicate the median absolute monocyte count for each group. The mean absolute monocyte count for patients with uncomplicated malaria reflected a P of <0.001.
Patients with severe and complicated malaria showed a protracted clinical course and parasite clearance. Improvement was observed within 5 to 7 days of admission to the hospital. In contrast, patients with uncomplicated malaria showed a rapid clinical improvement and parasite clearance within 3 to 4 days of admission to the hospital. A dramatic improvement was observed in semi-immune patients with uncomplicated malaria. In this group defervescence and parasite clearance were observed within 2 days of admission to the hospital.
We present the first comparative analysis of iNOS gene expression in healthy volunteers and adults (age range, 22 to 68 years) living in a nonendemic setting with severe and complicated malaria or uncomplicated malaria. In this study, iNOS gene expression in patients with falciparum malaria and healthy volunteers was studied in an attempt to improve our understanding of the role of iNOS in falciparum malaria. The increased levels of iNOS observed in patients with uncomplicated malaria may lead to an increased production of NO, which in turn may confer a protective advantage in falciparum malaria. On the other hand, the slightly increased expression of iNOS observed in patients with severe and complicated malaria may not produce sufficient NO to mediate an effective host defense against falciparum malaria. The absence of iNOS expression in healthy donors underscores the necessity of a stimulus for the expression of this gene. An important and novel observation from our investigations was that the patients with uncomplicated malaria had not only increased iNOS gene expression but also higher monocyte counts than the patients with severe and complicated malaria at the time of presentation. Our data also demonstrated that semi-immune patients can mount a more vigorous monocyte response in their peripheral blood during an acute malarial infection than nonimmune patients. This may explain why severe and complicated malaria was not observed in this group. From these findings we can infer that increased monocyte counts in samples of patients with uncomplicated malaria contribute to elevated expression of iNOS, which in turn leads to sustained production of NO. The produced NO may consequently effect parasite killing, thereby controlling the malarial infection. Alternatively, lower peripheral blood monocyte counts in patients with severe and complicated malaria might reflect monocyte sequestration or adherence to the activated vascular endothelium. Monocytes are activated in patients with malaria, and occasional case reports document sequestration of monocytes to the capillary or venular vessel wall (15, 28). However, larger post mortem studies of human falciparum malaria have failed to demonstrate massive adherence of monocytes or macrophages, which would explain drastically lower peripheral monocyte counts in patients with severe and complicated disease than in patients with uncomplicated disease (25, 27). Our findings are in agreement with previous findings from both human and animal studies which favor the protective role of NO in falciparum malaria (4, 21, 22, 26, 34–37).
In summary, this work was carried out to resolve the controversy surrounding the role of NO in falciparum malaria. We report the following findings: first, that high iNOS levels circulate in patients with uncomplicated malaria, second, that iNOS is predominantly expressed in monocytes, and, third, that patients with uncomplicated malaria have higher monocyte counts. Based on evidence cited here and from our observations, we conclude that NO has a beneficial rather than a deleterious role in falciparum malaria.
REFERENCES
- 1.Agbenyega T, Angus B, Bedu-Addo G, Baffoe-Bonnie B, Griffin G, Vallance P, Krishna S. Plasma nitrogen oxides and blood lactate concentrations in Ghanaian children with malaria. Trans R Soc Trop Med Hyg. 1997;91:298–302. doi: 10.1016/s0035-9203(97)90083-3. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yaman F M, Genton B, Clark I A. The ratio of reactive nitrogen intermediates to tumor necrosis factor and clinical outcome of falciparum malaria disease. Trans R Soc Trop Med Hyg. 1998;92:417–420. doi: 10.1016/s0035-9203(98)91073-2. [DOI] [PubMed] [Google Scholar]
- 3.Al Yaman F M, Mokela D, Genton B, Rockett K A, Alpers M P, Clark I A. Association between serum levels of reactive nitrogen intermediates and coma in children with cerebral malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1996;90:270–273. doi: 10.1016/s0035-9203(96)90243-6. [DOI] [PubMed] [Google Scholar]
- 4.Anstey N M, Weinberg J B, Hassanali M Y, Mwaikambo E D, Manyenga D, Misukonis M, Arnelle D R, Hollis D, McDonald M I, Granger D L. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993;7:349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- 6.Burgner D, Xu W, Rockett K, Gravenor M, Charles I G, Hill A V, Kwiatkowski D. Inducible nitric oxide synthase polymorphism and fatal cerebral malaria. Lancet. 1998;352:1193–1194. doi: 10.1016/S0140-6736(05)60531-4. [DOI] [PubMed] [Google Scholar]
- 7.Chiwakata C B, Hort G, Hemmer C J, Dietrich M. Sera from patients with falciparum malaria induce substance P gene expression in cultured human brain microvascular endothelial cells. Infect Immun. 1996;64:5106–5110. doi: 10.1128/iai.64.12.5106-5110.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomcyznski P, Sacchi N. Single step RNA isolation by acid guanidinium thiocyante-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Clark I A, Cowden W B, Rockett K A. Nitric oxide and cerebral malaria. Lancet. 1993;341:632–633. doi: 10.1016/0140-6736(93)90393-u. [DOI] [PubMed] [Google Scholar]
- 10.Clark I A, Cowden W B, Rockett K A. Nitric oxide in cerebral malaria. J Infect Dis. 1995;171:1068–1069. doi: 10.1093/infdis/171.4.1068. [DOI] [PubMed] [Google Scholar]
- 11.Clark I A, Rockett K A. Nitric oxide and parasitic diseases. Adv Parasitol. 1996;37:1–55. doi: 10.1016/s0065-308x(08)60218-3. [DOI] [PubMed] [Google Scholar]
- 12.Clark I A, Rockett K A, Cowden W B. Proposed link between cytokines, nitric oxide and human cerebral malaria. Parasitol Today. 1991;7:205–207. doi: 10.1016/0169-4758(91)90142-b. [DOI] [PubMed] [Google Scholar]
- 13.Clark I A, Rockett K A, Cowden W B. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet. 1992;340:894–896. doi: 10.1016/0140-6736(92)93295-x. [DOI] [PubMed] [Google Scholar]
- 14.Cot S, Ringwald P, Mulder B, Miailhes P, Yap-Yap J, Nussler A K, Eling W M C. Nitric oxide in cerebral malaria. J Infect Dis. 1994;69:1417–1418. doi: 10.1093/infdis/169.6.1417. [DOI] [PubMed] [Google Scholar]
- 15.Duarte M I, Corbett C E, Boulos M, Amato Neto V. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985;34:31–35. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- 16.Estabrook M M, Christopher N C, Groffiss J M, Baker C J, Mandrell R E. Sialylation and human neutrophil killing of group C Neisseria meningitidis. J Infect Dis. 1992;166:1079–1088. doi: 10.1093/infdis/166.5.1079. [DOI] [PubMed] [Google Scholar]
- 17.Geller D A, Lowenstein C J, Shapiro R A, Nussler A K, Di Silvio M, Wang S C, Nakayama D K, Simmons R L, Synder S H, Billiar T R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci USA. 1993;90:3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green S J, Nacy C A. Antimicrobial and immunopathologic effects of cytokine-induced nitric oxide synthesis. Curr Opin Infect Dis. 1993;6:384–396. [Google Scholar]
- 19.James S L. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James S L, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 21.Kremsner P G, Winkler S, Wildling E, et al. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90:44–47. doi: 10.1016/s0035-9203(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 22.Kun J F, Mordmüller B, Lell B, Lehman L G, Luckner D, Kremsner P G. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet. 1998;351:265–266. doi: 10.1016/S0140-6736(05)78273-8. [DOI] [PubMed] [Google Scholar]
- 23.Lowenstein C J, Glatt C S, Bredt D S, Synder S H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci USA. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons C R, Orloff G J, Cunningham J M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- 25.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 26.Mulder B, Cot S, Ringwald P, Miailhes P, Yap-Yap J, Nussler A, Eling W. The role of nitric oxide in cerebral malaria. Med Trop (Marseille) 1995;55:114–115. . (In French.) [PubMed] [Google Scholar]
- 27.Oo M M, Aikawa M, Than T, Aye T M, Myint P T, Igarashi I, Schoene W C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987;46:223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Porta J, Carota A, Pizzolato G P, Wildi E, Widmer M C, Margairaz C, Grau G E. Immunopathological changes in human cerebral malaria. Clin Neuropathol. 1993;12:142–146. [PubMed] [Google Scholar]
- 29.Rockett K A, Awburn M M, Aggarwal B B, Cowden W B, Clark I A. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin and interleukin-1: possible role in malaria. Infect Immun. 1992;60:3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross C R, Densen P. Opsonophagocytosis of Neisseria gonorrhoeae: interaction of local and dessiminated isolates with complement and neutrophils. J Infect Dis. 1985;151:33–41. doi: 10.1093/infdis/151.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seguin M C, Klotz F W, Schneider I, Weir J P, Goodbury M, Slayter M, Raney J J, Aniagolu J U, Green S J. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senaldi G, Kremsner P G, Grau G E. Nitric oxide and cerebral malaria. Lancet. 1992;340:8834–8835. doi: 10.1016/0140-6736(92)92812-t. [DOI] [PubMed] [Google Scholar]
- 35.Taylor-Robinson A W. Antimalarial activity of nitric oxide: cytostasis and cytotoxicity towards Plasmodium falciparum. Biochem Soc Trans. 1997;25:262S. doi: 10.1042/bst025262s. [DOI] [PubMed] [Google Scholar]
- 36.Taylor-Robinson A W, Looker M. Sensitivity of malaria parasites to nitric oxide at low oxygen tensions. Lancet. 1998;351:1630. doi: 10.1016/s0140-6736(05)77685-6. [DOI] [PubMed] [Google Scholar]
- 37.Weiss G, Thuma P E, Mabeza G, Werner E R, Herold M, Gordeuk V R. Modulatory potential of iron chelation therapy on nitric oxide formation. J Infect Dis. 1997;175:226–230. doi: 10.1093/infdis/175.1.226. [DOI] [PubMed] [Google Scholar]
- 38.Xie Q-W, Cho H J, Calaycay J, Mumford R A, Swiderek K M, Lee T D, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]