Abstract
Evidence from in vitro studies suggests that gamma interferon (IFN-γ) and nitric oxide (NO) are important in host defense against the protozoan parasite Entamoeba histolytica. We used SCID mice with targeted disruption of the IFN-γ receptor gene and mice with targeted disruption of the gene encoding inducible NO synthase to show that IFN-γ plays a role in the innate immunity to amebic liver abscess seen in SCID mice while NO is required for control of amebic liver abscess in immunocompetent mice.
Infection by Entamoeba histolytica, the cause of amebic dysentery and amebic liver abscess, remains a significant public health problem in much of the world. The mechanisms by which E. histolytica damages intestinal and liver cells are being elucidated, and these studies have been greatly facilitated by the development of new in vivo models of amebiasis (2, 13). The study of E. histolytica infection in SCID mice has provided a model system for assessment of the role of innate immunity in the control of amebic liver abscess. The importance of neutrophils in the limition of tissue damage in amebic liver abscess was recently shown in the SCID mouse model of amebic liver abscess, where depletion of neutrophils from SCID mice resulted in significantly larger liver abscesses early in infection (15). E. histolytica trophozoites lyse resting macrophages and neutrophils in vitro, but macrophages or neutrophils activated by treatment with gamma interferon (IFN-γ) or tumor necrosis factor alpha and the addition of lipopolysaccharide or amebic antigens become amebicidal (3–6, 11). In vitro, the killing of E. histolytica trophozoites by activated macrophages is mediated by nitric oxide (NO) (8). To determine whether IFN-γ plays a role in the host defense against amebic liver abscess in vivo, we have examined amebic liver abscess formation in mice genetically engineered for the absence of the IFN-γ receptor α chain. In addition, we have assessed the role of NO in host defense against amebic liver abscess by studying the susceptibility to amebic liver abscess of mice with targeted disruption of the gene encoding inducible NO synthase (iNOS).
In initial studies, we compared the susceptibility to amebic liver abscess formation in IFN-γ receptor knockout mice (129/Sv/Ev × C57BL/6 background) and heterozygote controls. Under our previously described protocol, mice 8 to 10 weeks of age underwent direct hepatic inoculation of 106 E. histolytica trophozoites of the virulent HM1:IMSS strain (2). This strain has been passaged multiple times through SCID mice and is capable of causing amebic liver abscess in immunocompetent mice (14). Mice were sacrificed 2 or 4 days later, and the livers were examined for the presence of an amebic liver abscess. Abscesses were confirmed by histologic examination and by culture of E. histolytica trophozoites from abscess tissue. The entire liver, including the region of abscessed tissue, was weighed to calculate the percentage of the liver occupied by the abscess. The results of these initial studies are shown in Table 1. While the mean abscess size was larger in IFN-γ receptor knockout mice than in wild-type mice, the difference did not reach statistical significance in a trial of 10 animals in each group. We also noted no differences in histological appearance between liver abscesses in IFN-γ receptor knockout mice and those in wild-type mice. These data suggest that in otherwise immunocompetent mice, IFN-γ does not play a critical role in the mediation of protection against amebic liver abscess.
TABLE 1.
Susceptibility of IFN-γ receptor knockout mice and iNOS knockout mice to amebic liver abscess
| Mouse strain | No. of mice with liver abscess/no. challenged | Mean % of liver abscessed ± SD |
|---|---|---|
| 129/Sv/Ev × C57BL/6 | 10/10 | 8.5 ± 11 |
| 129/Sv/Ev × C57BL/6 (IFN-γ receptor α chain−/−) | 10/10 | 18.6 ± 14.4a |
| C.B-17 SCID | 10/10 | 19 ± 9.4 |
| C.B-17 SCID (IFN-γ receptor α chain−/−) | 10/10 | 41 ± 26b |
| 129/Sv/Ev × C57BL/6 (iNOS+/−) | 11/11 | 5 ± 4 |
| 129/Sv/Ev × C57BL/6 (iNOS−/−) | 11/11 | 33 ± 25c |
Different from 129/Sv/Ev × C57BL/6 mice at P = 0.12 (two-tailed t test).
Different from C.B-17 SCID mice at P ≤ 0.02 (two-tailed t test).
Different from 129/Sv/Ev × C57BL/6 (iNOS+/−) mice at P < 0.01 (two-tailed t test).
Previous studies in our laboratory showed that SCID mice were more susceptible to amebic liver abscess than the congenic C.B-17 strain (2). We hypothesized that if IFN-γ plays a role in innate immunity against amebic liver abscess, it would be detectable in SCID mice. Therefore, we bred IFN-γ receptor knockout mice on the 129/Sv/Ev × C57BL/6 background with C.B-17 SCID animals and intercrossed the resulting double heterozygotes and screened them for homozygosity at both loci. These double-knockout animals (SCID and IFN-γ receptor negative) were subsequently backcrossed onto the C.B-17 SCID background for 6 generations, resulting in animals that theoretically should have >98% of the C.B-17 genome. In two separate experiments, groups of five C.B-17 SCID mice and groups of five C.B-17 SCID mice with targeted disruption of the IFN-γ receptor α-chain gene underwent intrahepatic challenge with 106 HM1:IMSS amebic trophozoites. The histologic appearance of liver abscesses in C.B-17 SCID mice homozygous for the disruption of the gene encoding the IFN-γ receptor α chain did not differ from that seen in C.B-17 SCID mice (2). However, we found that C.B-17 SCID mice homozygous for the disruption of the gene encoding the IFN-γ receptor α chain had developed significantly larger amebic liver abscesses at 48 h following infection than C.B-17 SCID mice (Table 1). These data suggest that IFN-γ plays a role in innate immunity to amebic liver abscess in SCID mice. We have previously shown that neutrophils are important for containment of amebic liver abscesses in SCID mice. IFN-γ may provide protection against amebic liver abscess in SCID mice by activating neutrophils and/or macrophages for amebicidal activity.
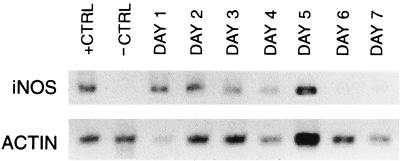
One of the important effector molecules for macrophage- and neutrophil-mediated killing is NO. In vitro studies indicate that NO plays a role in macrophage-mediated killing of E. histolytica trophozoites (8). To examine the role of NO in the host defense against amebic liver abscess, we first studied the induction of the gene encoding iNOS in SCID mice following intrahepatic inoculation of E. histolytica trophozoites. We used the reverse transcriptase (RT) PCR and primers specific for murine iNOS to assay iNOS mRNA production in the livers of (i) SCID mice after direct hepatic inoculation with amebic trophozoites and (ii) control SCID mice inoculated with medium alone (13). The primers used were 5′ ATGGCTTGCCCCTGGAAGTTTCTCTTCA and GTTGCCATTGTTGGTGGCATAAAG. We amplified the mRNA for murine actin by using RT-PCR to serve as a marker for mRNA levels in individual mice. As shown in Fig. 1, we found significant induction of iNOS mRNA in E. histolytica-infected SCID mouse livers. iNOS mRNA was detectable in RNA obtained from E. histolytica-infected livers within 24 h of infection, and the message remained detectable for the first 5 days of infection. Abscesses were still present at 6 and 7 days after infection, but an iNOS message was no longer detectable. Livers injected with medium alone showed no detectable induction of iNOS mRNA at any time point.
FIG. 1.
Infection of SCID mouse livers with E. histolytica induces expression of the gene encoding iNOS. Results of RT-PCR obtained with mRNA from liver samples taken 1 to 7 days following infection with E. histolytica are shown. Expression of iNOS appeared at day 1 (24 h following infection) and persisted until day 5. No induction of iNOS expression was seen in medium-inoculated mice at any time point. The positive control (+CTRL) is mRNA obtained from murine peritoneal macrophages stimulated with lipopolysaccharide and murine IFN-γ; the negative control (−CTRL) is mRNA obtained from unstimulated peritoneal macrophages. The RT-PCR results obtained with murine actin and each sample are also shown.
We next examined whether iNOS was required for host defense against E. histolytica infection in the murine model of amebic liver abscess. Mice with targeted disruption of the iNOS gene (provided by Carl Nathan of Cornell University) were challenged by intrahepatic inoculation of 106 E. histolytica HM1:IMSS trophozoites. We found that C57BL/6 × 129Sv/Ev iNOS−/− mice had significantly larger amebic liver abscesses than iNOS+/− control mice (Table 1). The size of the abscesses in iNOS−/− mice were among the largest detected to date in the murine disease model and were larger than those seen in SCID mice but were identical in histologic appearance. Recently, we found that much of the cell death occurring in amebic liver abscess comes from E. histolytica-induced apoptosis of hepatocytes (14). NO can inhibit apoptosis in cultured hepatocytes through at least two different mechanisms (7, 16). Thus, iNOS−/− mice could be more susceptible to E. histolytica-induced hepatocyte apoptosis. In addition, NO appears to play an important role in wound healing and liver regeneration in vivo (1). Therefore, the large abscesses seen in iNOS−/− mice may be attributable to at least three different factors: (i) impaired ability of neutrophils or macrophages to kill E. histolytica trophozoites, (ii) increased susceptibility to E. histolytica-induced apoptosis, and (iii) a defect in wound healing and hepatic regeneration in injured areas.
Protection against amebic liver abscess in the murine disease model appears to depend on several modalities. Passive immunization with antibodies against the serine-rich E. histolytica protein, monoclonal antibodies against the amebic lipophosphoglycan-like molecule, and polyclonal antibodies against specific regions of the 170-kDa subunit of the Gal/GalNAc lectin can protect SCID mice from developing amebic liver abscess (9, 10, 18). Antibodies derived from some patients with amebic liver abscess can also provide protection in the SCID mouse model, demonstrating that human antibodies have the ability to prevent disease (12). Innate immunity is also important in the murine model of disease, as neutrophils help limit amebic liver abscesses in SCID mice (15, 17). We have now shown that both IFN-γ and iNOS play a role in protection against amebic liver abscess in vivo. However, a requirement for IFN-γ in host protection against disease was most prominent in SCID mice, suggesting that IFN-γ is important when innate immunity is required for resistance to infection. IFN-γ has been shown to activate host neutrophils and macrophages to kill amebic trophozoites in vitro, and it may serve a similar function in the murine model of amebic liver abscess. In contrast, iNOS-deficient mice on the C57BL/6 × 129/Sv/Ev background developed large amebic liver abscesses, indicating that iNOS controls amebic liver damage in immunocompetent mice. It remains to be determined whether the primary role of NO in resistance to amebic liver abscess is based on its function in neutrophil and macrophage killing of amebic trophozoites, mediation of hepatocyte resistance to apoptotic stimuli, or stimulation of wound healing and hepatic regeneration.
Acknowledgments
This work was supported by grant AI 30084 and Research Career Development Award AI 01231 (to S.L.S.) from the National Institutes of Health and National Institutes of Health training grant 5T32AI-07172 (to K.B.S.). S. L. Stanley, Jr., is a Burroughs Wellcome Scholar in Molecular Parasitology.
We thank Lynne Foster for excellent technical assistance.
REFERENCES
- 1.Calabrese F, Valente M, Pettenazzo E, Ferraresso M, Burra P, Cadrobbi R, Cardin R, Bacelle L, Parnigotto A, Rigotti P. The protective effects of l-arginine after liver ischaemia/reperfusion injury in a pig model. J Pathol. 1997;183:477–485. doi: 10.1002/(SICI)1096-9896(199712)183:4<477::AID-PATH955>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Cieslak P R, Virgin IV H W, Stanley S L., Jr A severe combined immunodeficient (SCID) mouse model for infection with Entamoeba histolytica. J Exp Med. 1992;176:1605–1609. doi: 10.1084/jem.176.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis M, Chadee K. Human neutrophils activated by interferon-gamma and tumor necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989;46:270–274. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- 4.Ghadirian E, Bout D T. In vitro killing of Entamoeba histolytica trophozoites by interferon-gamma-activated mouse macrophages. Immunobiology. 1988;176:341–353. doi: 10.1016/s0171-2985(88)80018-4. [DOI] [PubMed] [Google Scholar]
- 5.Ghadirian E, Denis M. Entamoeba histolytica extract and interferon-gamma activation of macrophage-mediated amoebicidal function. Immunobiology. 1992;185:1–10. doi: 10.1016/S0171-2985(11)80312-8. [DOI] [PubMed] [Google Scholar]
- 6.Guerrant R L, Brush J, Ravdin J I, Sullivan J A, Mandell G L. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981;143:83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y M, deVera M E, Watkins S C, Billiar T R. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 8.Lin J-Y, Seguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994;62:1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotter H, Zhang T, Seydel K B, Stanley S L, Jr, Tannich E. Identification of an epitope on the Entamoeba histolytica 170-kDa lectin conferring antibody mediated protection against invasive amebiasis. J Exp Med. 1997;185:1793–1801. doi: 10.1084/jem.185.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinets A, Zhang T, Guillen N, Gounon P, Bohle B, Vollmann U, Scheiner O, Wiedermann G, Stanley S L, Jr, Duchene M. Protection against invasive amebiasis by a single monoclonal antibody directed against a lipophosphoglycan antigen localized on the surface of Entamoeba histolytica. J Exp Med. 1997;186:1557–1565. doi: 10.1084/jem.186.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salata R A, Ravdin J I. The interaction of human neutrophils and Entamoeba histolytica increases cytopathogenicity for liver cell monolayers. J Infect Dis. 1986;154:19–26. doi: 10.1093/infdis/154.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Seydel K B, Braun K, Zhang T, Jackson T F H G, Stanley S L., Jr Human anti-amebic antibodies provide protection against amebic liver abscess formation in the SCID mouse. Am J Trop Med Hyg. 1996;55:330–332. doi: 10.4269/ajtmh.1996.55.330. [DOI] [PubMed] [Google Scholar]
- 13.Seydel K B, Li E, Swanson P E, Stanley S L., Jr Human intestinal epithelial cells produce pro-inflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seydel K B, Stanley S L., Jr Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect Immun. 1998;66:2980–2983. doi: 10.1128/iai.66.6.2980-2983.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seydel K B, Zhang T, Stanley S L., Jr Neutrophils play a critical role in early resistance to amebic liver abscesses in SCID mice. Infect Immun. 1997;65:3951–3953. doi: 10.1128/iai.65.9.3951-3953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzeng E, Billiar T R, Williams D L, Li J, Lizonova A, Kovesdi I, Kim Y M. Adenovirus-mediated inducible nitric oxide synthase gene transfer inhibits hepatocyte apoptosis. Surgery. 1998;124:278–283. [PubMed] [Google Scholar]
- 17.Velazquez C, Shibayama-Salas M, Aguirre-Garcia J, Tsutsumi V, Calderon J. Role of neutrophils in innate resistance to Entamoeba histolytica liver infection in mice. Parasite Immunol. 1998;20:255–262. doi: 10.1046/j.1365-3024.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang T, Cieslak P R, Foster L, Kunz-Jenkins C, Stanley S L., Jr Antibodies to the serine rich Entamoeba histolytica protein (SREHP) prevent amebic liver abscess in severe combined immunodeficient (SCID) mice. Parasite Immunol. 1994;16:225–230. doi: 10.1111/j.1365-3024.1994.tb00344.x. [DOI] [PubMed] [Google Scholar]