Abstract
Background
To evaluate the prognostic impact of lymph node dissection (LND) in patients who underwent radical nephroureterectomy (RNU) with bladder cuff excision (BCE) for clinically node-negative (cN0) upper urinary tract urothelial carcinoma (UTUC).
Methods
We retrospectively enrolled 520 patients with cN0 UTUC in a single tertiary referral center from 2000 to 2015. The patients were divided into three groups: patients with and without pathologically proved lymph node metastasis (pN1–3 and pN0, respectively) and patients without LND (pNx). We analyzed associations between overall survival (OS)/ disease-free survival (DFS)/ cancer-specific survival (CSS) and clinical characteristics.
Results
The patients were divided into three groups (pN1–3, pN0 and pNx with 20, 303, and 197 patients, respectively). OS/DFS/CSS in the pN1–3 group were significantly worse (all p<0.001) compared with the pN0 group. However, there were no significant differences between the pNx and pN0 groups. In the multivariate analyses, CSS was only affected by age [(hazard ratio (HR) = 1.03, p = 0.008]), positive surgical margin (HR = 3.38, p<0.001) and pathological T3–4 stages (HR = 4.07, p<0.001). In the subgroup analyses for patients with LND, locally advanced disease (pT3 and pT4) had significantly more metastases [T3–4: 13.91% (16/115) vs. T0–2: 1.92% (4/208), p<0.001].
Conclusions
In the pN0 group, LND for cN0 UTUC did not show therapeutic benefits in terms of DFS, CSS, and OS. However, LND with RNU allowed optimal tumor staging, through patients still had a poor prognosis. Clinically occult LN metastases were found in 6.2% of our patients.
Introduction
Upper urinary tract urothelial carcinoma (UTUC), which originates from the pyelocaliceal cavities and ureter, is an infrequent malignancy, only accounting for 5–10% of urothelial carcinomas (UCs) [1–3].
Taiwan is the one of the endemic area where UTUC accounts for approximately a third of all urothelial tumors. According to the Taiwan Cancer Registry Annual Report, the age-standardized incidence rate of UTUC is 3.71 and 3.99 per 100,000 population in men and women, respectively [4, 5].
Radical nephroureterectomy (RNU) and ipsilateral bladder cuff excision (BCE) with or without lymph node dissection (LND) is the standard surgical intervention for localized UTUC [1, 6]. However, due to the poor prognostic nature with a high risk of lymphatic spread and disease progression [7–9], the five year survival for patients with UTUC is <50% and <10% for stage pT2/3 and pT4 disease, respectively [3, 10–12].
Regarding the management of UC of the urinary bladder (UCUB), the National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology has suggested the standard therapy of neo-adjuvant chemotherapy (NAC) followed by radical RNU with LND for stages ≥cT2 [7, 13]. Recent systematic reviews have also reported on the survival benefit of NAC in patients with locally advanced UTUC [14–17].
In addition, adjuvant chemotherapy (AC) should be considered for patients with pT3–4 or nodal-positive disease. A phase III POUT trial has demonstrated the benefit of platinum-based AC for patients with locally advanced UTUC [18]. A meta-analysis has reported that platinum-based AC is associated with improved disease-free survival (DFS) for locally advanced UCs [19]. Alternatively, immune checkpoint inhibitors (ICIs) that had been investigated as an adjuvant treatment of UTUC in the CheckMate-274 trial (nivolumab) may be considered [20]. Alessandro et al. has suggested receiving ICIs have survival benefits in programmed cell death ligand 1 (PD–L1) for patients with positive metastatic UC (mUC) [21].
Regarding LND at the time of RNU, therapeutic benefits have been reported for patients with UTUC, particularly those with muscle-invasive or locally advanced disease [22, 23]. According to the NCCN guidelines, LND should be performed in patients with high-grade disease, large tumors, and tumors invading the renal parenchyma [13]. However, the benefits of LND for patients with cN0 disease remain debatable, and the procedure is not standardized.
In this study, we aimed to evaluate the prognostic impact of LND on overall survival (OS), DFS, and cancer-specific survival (CSS) in patients undergoing RNU with BCE for cN0 UTUC.
Materials and methods
We reviewed 728 patients with UTUC who received RNU with ipsilateral BCE between 2001 and 2015 in a retrospectively built UTUC database at the Taichung Veterans General Hospital, a single tertiary referral center in central Taiwan. We excluded patients who had NAC, previous or concurrent cystectomy, incomplete clinical data (without clinical status), distant metastasis, clinical lymph node involvement (≥cN1), and short follow-up duration (<one year). Finally, we identified 520 patients, whom we then divided into three groups: those with pathologically confirmed lymph node metastases (pN1–3), pN0, and without LND (pNx).
Surgeries were performed by seven well-experienced urological surgeons in our hospital. Until 2007, we performed hilar and regionalLND only in patients with clinically/surgically suspicious lymph node metastasis. Beginning in 2008, at least hilar LND with or without regional LND was routinely performed with RNU. Hilar LND was performed over the renal vein root at the right side and renal artery root at the left side. The templates of regional LND depended on the tumor location, in that para-aortic and peri-caval LND were performed for renal pelvic or proximal ureteral tumors and pelvic LND, for middle or distal ureter tumors. AC was considered for patients with advanced tumor features. The regimens of chemotherapy were based on cisplatin or carboplatin and depended on renal function. However, the indications of performing AC or regional LND were based on the patient’s clinical stage and the surgeons’ preference.
The Institutional Review Board (IRB) of Taichung Veteran General Hospital approved the current study, and informed written consent was obtained from all of the participants (IRB No. CE13240A-3). The procedures performed were in accordance with the Declaration of Helsinki guidelines.
The endpoints of this study after RNU were OS, DFS, and CSS. DFS was defined as local recurrence and lymph node and/or distant metastasis, not including recurrences at the contralateral upper urinary tract or bladder. The time duration from the date of treatment for UTUC was defined as OS. CSS was the time duration from the date of diagnosis to death solely due to UTUC. In addition, we performed a subgroup analysis on patients with LND (pN0 and pN+), with 278 patients in the hilar-only and 45 patients in the regional LND groups.
Correlations between the three groups and other clinic-pathological characteristics were tested using the chi-square or Kruskal-Wallis test. The survival curve for the presence of LND (patients with/without lymph node metastasis) was estimated via the Kaplan-Meier method, and differences were assessed using the log-rank statistic (Mantel-Cox). Univariate and multivariate analyses were performed with Cox proportional hazards regression models to determine the impacts of LND on OS and CSS. Results were showed with hazard ratios (HRs) to reflect relative risks at 95% confidence intervals (CIs). All reported p-values were two-sided, and statistical significance was set at p≤0.05.
Results
A total of 520 patients were included in our study. They were divided into three groups based on histopathology: 303 (58.3%) with pN0 disease, 20 (3.8%) with pN+ disease (pN1-3), and 197 (37.9%) with no LND (pNx). The mean follow-up duration was 47.63 months [standard deviation (SD) = 28.96]. The respective median ages of the three groups at diagnosis were: (a) 68.3 years [interquartile range (IQR) = 62.1–76.6], (b) 70.2 years (IQR = 57.5–78.5), and (c) 67.5 years (IQR = 57.8–75.7). There was a significant difference, respectively, in the rate of advanced pathological stage (≥T2; 46.8%, 85.0%, and 43.8%, p<0.001), tumor grade 3 (68.6%, 100.0%, and 43.7%, p<0.001), positive lymphovascular invasion (17.5%, 75.0%, and 15.7%, p<0.001), positive surgical margin (8.6%, 40.0%, and 2.1%, p<0.001) and post-AC (18.8%, 70.0%, and 18.3%, p <0.001) for the three groups (Table 1).
Table 1. Association of LND status and clinic-pathological characteristics of patients undergoing RNU with BCE for cN0 UTUC.
| PN0 (n = 303) | PN1-N3 (n = 20) | PNx (n = 197) | P value | |
|---|---|---|---|---|
| Gender | 0.026 * | |||
| Male | 111 (36.6%) | 9 (45.0%) | 96 (48.7%) | |
| Female | 192 (63.4%) | 11 (55.0%) | 101 (51.3%) | |
| Age, years | 68.3 (62.1–76.6) | 70.6 (57.5–78.5) | 67.6 (57.8–75.7) | 0.448 |
| BMI | 23.6 (21.3–25.6) | 24.4 (22.3–26.5) | 24.0 (21.9–26.3) | 0.256 |
| Performance Status ECOG | <0.001 ** | |||
| 0 | 50 (16.5%) | 5 (25.0%) | 2 (1.0%) | |
| 1 | 187 (61.7%) | 11 (55.0%) | 173 (87.8%) | |
| 2 | 64 (21.1%) | 3 (15.0%) | 19 (9.6%) | |
| 3 | 2 (0.7%) | 1 (5.0%) | 2 (1.0%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | |
| Smoking status | 0.639 | |||
| Never | 215 (76.0%) | 15 (78.9%) | 137 (70.6%) | |
| Current | 39 (13.8%) | 2 (10.5%) | 29 (14.9%) | |
| Former | 29 (10.2%) | 2 (10.5%) | 28 (14.4%) | |
| Comorbidity | ||||
| CAD/HTN | 187 (61.7%) | 11 (55.0%) | 109 (55.3%) | 0.341 |
| DM | 65 (21.5%) | 5 (25.0%) | 37 (18.8%) | 0.680 |
| COPD/Asthema | 6 (2.0%) | 0 (0.0%) | 9 (4.6%) | 0.176 |
| CVA | 11 (3.6%) | 1 (5.0%) | 9 (4.6%) | 0.852 |
| CKD(Cr>1.5, non-uremic status) | 55 (18.2%) | 3 (15.0%) | 71 (36.0%) | <0.001 ** |
| Uremia at diagnosis | 44 (14.5%) | 2 (10.0%) | 23 (11.7%) | 0.596 |
| HBV or HCV carrier | 26 (8.6%) | 1 (5.0%) | 35 (17.8%) | 0.005 ** |
| Pathological T | <0.001 ** | |||
| T0 | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | |
| T1 | 160 (52.8%) | 3 (15.0%) | 111 (56.3%) | |
| T2 | 43 (14.2%) | 1 (5.0%) | 33 (16.8%) | |
| T3 | 91 (30.0%) | 8 (40.0%) | 46 (23.4%) | |
| T4 | 8 (2.6%) | 8 (40.0%) | 7 (3.6%) | |
| Multifocalty | 0.065 | |||
| No | 182 (60.1%) | 14 (70.0%) | 138 (70.1%) | |
| Yes | 121 (39.9%) | 6 (30.0%) | 59 (29.9%) | |
| Tumor cell differentiation | ||||
| CIS | 0.020 * | |||
| Negative | 246 (81.2%) | 17 (85.0%) | 178 (90.4%) | |
| Positive | 57 (18.8%) | 3 (15.0%) | 19 (9.6%) | |
| Tumor grading | <0.001 ** | |||
| G1 | 7 (2.3%) | 0 (0.0%) | 11 (5.6%) | |
| G2 | 88 (29.0%) | 0 (0.0%) | 100 (50.8%) | |
| G3 | 208 (68.6%) | 20 (100.0%) | 86 (43.7%) | |
| Lymphovascular invasion | <0.001** | |||
| Negative | 249 (82.5%) | 5 (25.0%) | 161 (84.3%) | |
| Positive | 53 (17.5%) | 15 (75.0%) | 30 (15.7%) | |
| Surgical margin | <0.001 ** | |||
| Negative | 276 (91.4%) | 12 (60.0%) | 187 (97.9%) | |
| Positive | 26 (8.6%) | 8 (40.0%) | 4 (2.1%) | |
| Adjuvant chemotherapy | <0.001 ** | |||
| No | 246 (81.2%) | 6 (30.0%) | 161 (81.7%) | |
| Yes | 57 (18.8%) | 14 (70.0%) | 36 (18.3%) |
Chi-square test. Kruskal-Wallis test, Median (IQR).
*P<0.05
**P<0.01.
ECOG = Eastern Cooperative Oncology Group. CAD/HTN = coronary artery disease / hypertension. DM = diabetes mellitus. COPD = chronic obstructive pulmonary disease. CVA = cerebrovascular accident. CKD = chronic kidney disease. HBV = hepatitis B virus. HCV = hepatitis C virus. CIS = carcinoma in situ. G = Grade.
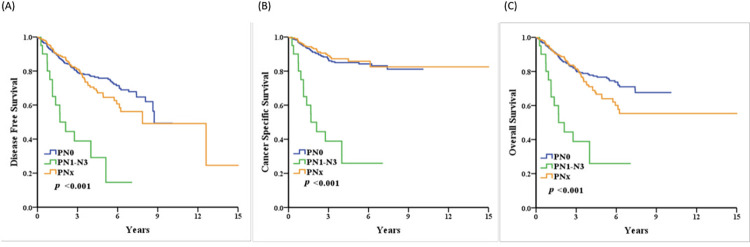
During the following-up, 152 patients (29.2%) experienced disease recurrence, 79 (15.2%) died of UTUC, and 61 (11.7%) died of other causes. The two-year OSs were 85.9%, 50.0%, and 88.6%, respectively, in the PN0, PN+, and PNx groups. We found that the pN+ group, compared with the pN0 group has a significantly worse OS (five years, 76.6% vs. 25.9%, p<0.001), DFS (75.8% vs. 29.2%, p<0.001), and CSS (85.0% vs. 25.9%, p<0.001). On the other hand, no significant difference was found in the pNx group compared with the pN0 group in terms of DFS, CSS and OS, through there may have been a worse trend in the pNx group compared to the pN0 group in the five-year OS and DFS (64.0% vs. 76.6%, P = 0.101 and 64.5% vs. 75.8%, p = 0.204) (Fig 1) (S1–S3Tables).
Fig 1.
Kaplan-Meier curves of DFS (A), CSS (B) and OS (C) for 520 patients with pathologically proved lymph node status (pN1-3 and pN0) or without lymph node dissection (pNx)in clinical node-negative upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy.
In the univariate analysis, worse CSS was found to be correlated with age (HR = 1.03, p = 0.011), smoking status, patients with pN1–3 (HR = 6.93, p<0.001), pathological T3–4 stage (HR = 6.17, p<0.001), positive lymphovascular invasion (HR = 4.46, p<0.001), positive surgical margin (HR = 8.49, p<0.001), and post-AC (HR = 2.09, p = 0.002). However, in the multivariate analysis, only age (HR = 1.03, p = 0.017), patients with pN1–3 (HR = 2.10, p = 0.049), pathological T3–4 stage (HR = 4.42, p< 0.001), and positive surgical margin (HR = 3.37, p<0.001) significantly affected CSS (Table 2).
Table 2. Univariate and multivariate Cox regression analysis predicting CSS, OS, and DFS for 520 cN0 UTUC patients with pathologically proved lymph node status (pN1–3 and pN0) or without LND (pNx) undergoing RNU with BCE.
| CSS | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | ||||||
| Hazard ratio | 95%CI | pvalue | Hazard ratio | 95%CI | Pvalue | ||
| Gender | |||||||
| Male | Reference | Reference | |||||
| Female | 0.64 | (0.41–1.00) | 0.050 | ||||
| Age, years | 1.03 | (1.01–1.05) | 0.011 * | 1.03 | (1.01–1.05) | 0.017 ** | |
| BMI | 0.94 | (0.88–1.01) | 0.081 | ||||
| Group | |||||||
| PN0 | Reference | Reference | Reference | Reference | |||
| PN1–3 | 6.93 | (3.71–12.97) | <0.001 ** | 2.01 | (1.00–4.39) | 0.049* | |
| PNx | 0.88 | (0.53–1.47) | 0.636 | 0.97 | (0.56–1.69) | 0.915 | |
| Performance Status ECOG | |||||||
| 0 | Reference | Reference | |||||
| 1 | 0.50 | (0.28–0.88) | 0.017 | ||||
| 2 | 0.81 | (0.40–1.62) | 0.549 | ||||
| 3 | 0.96 | (0.13–7.29) | 0.972 | ||||
| 4 | -- | ||||||
| Smoking status | |||||||
| Never | Reference | Reference | Reference | Reference | |||
| Current | 1.95 | (1.10–3.46) | 0.021 * | 1.27 | (0.69–2.35) | 0.438 | |
| Former | 2.28 | (1.27–4.10) | 0.006 ** | 1.45 | (0.76–2.75) | 0.261 | |
| Comorbidity | |||||||
| CAD/HTN | 0.90 | (0.57–1.40) | 0.625 | ||||
| DM | 1.54 | (0.94–2.54) | 0.089 | ||||
| COPD/Asthema | 1.11 | (0.27–4.50) | 0.889 | ||||
| CVA | 1.45 | (0.53–3.97) | 0.468 | ||||
| CKD(Cr>1.5) | 1.35 | (0.83–2.20) | 0.223 | ||||
| Uremia at diagnosis | 1.30 | (0.70–2.41) | 0.399 | ||||
| HBV or HCVcarrier | 0.91 | (0.45–1.81) | 0.780 | ||||
| Pathological T | |||||||
| T0–1 | Reference | Reference | Reference | Reference | |||
| T2 | 2.17 | (0.99–4.74) | 0.052 | 2.07 | (0.89–4.81) | 0.091 | |
| T3–4 | 6.17 | (3.57–10.68) | <0.001 ** | 4.42 | (2.20–8.88) | <0.001 ** | |
| Multifocalty | |||||||
| No | Reference | Reference | |||||
| Yes | 1.27 | (0.81–1.99) | 0.298 | ||||
| Tumor cell differentiation | |||||||
| CIS | |||||||
| Negative | Reference | Reference | |||||
| Positive | 1.25 | (0.70–2.23) | 0.447 | ||||
| Tumor grading | |||||||
| G1 | Reference | Reference | |||||
| G2–3 | 0.05 | (0.00–7.18) | 0.233 | ||||
| Lymphovascular invasion | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 4.46 | (2.83–7.03) | <0.001 ** | 1.40 | (0.78–2.52) | 0.261 | |
| Surgical margin | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 8.49 | (5.19–13.92) | <0.001 ** | 3.37 | (1.81–6.28) | <0.001 ** | |
| Adjuvant chemotherapy | |||||||
| No | Reference | Reference | Reference | Reference | |||
| Yes | 2.09 | (1.30–3.34) | 0.002 ** | 0.82 | (0.47–1.44) | 0.484 | |
| OS | |||||||
| Univariate | Multivariable | ||||||
| Hazard ratio | 95%CI | Pvalue | Hazard ratio | 95%CI | Pvalue | ||
| Gender | |||||||
| Male | Reference | Reference | |||||
| Female | 0.69 | (0.49–0.96) | 0.027 * | 0.90 | (0.54–1.49) | 0.679 | |
| Age, years | 1.04 | (1.02–1.06) | <0.001 ** | 1.04 | (1.02–1.06) | <0.001 ** | |
| BMI | 0.97 | (0.92–1.01) | 0.163 | ||||
| Group | |||||||
| PN0 | Reference | Reference | Reference | Reference | |||
| PN1–3 | 4.20 | (2.36–7.47) | <0.001 * | 1.55 | (0.79–3.02) | 0.200 | |
| PNx | 1.33 | (0.93–1.90) | 0.116 | 1.32 | (0.90–1.93) | 0.154 | |
| Performance Status ECOG | |||||||
| 0 | Reference | Reference | |||||
| 1 | 0.98 | (0.58–1.67) | 0.946 | ||||
| 2 | 1.72 | (0.95–3.12) | 0.074 | ||||
| 3 | 1.79 | (0.41–7.76) | 0.437 | ||||
| 4 | 3.46 | (0.46–26.12) | 0.229 | ||||
| Smoking status | |||||||
| Never | Reference | Reference | Reference | Reference | |||
| Current | 1.43 | (0.90–2.28) | 0.0134 | 0.98 | (0.53–1.81) | 0.947 | |
| Former | 2.09 | (1.35–3.25) | 0.001 ** | 1.59 | (0.87–2.90) | 0.135 | |
| Comorbidity | |||||||
| CAD/HTN | 1.15 | (0.82–1.61) | 0.434 | ||||
| DM | 1.70 | (1.17–2.46) | 0.005 * | 1.57 | (1.06–2.34) | 0.025 * | |
| COPD/Asthema | 1.29 | (0.48–3.49) | 0.618 | ||||
| CVA | 1.92 | (0.98–3.78) | 0.058 | ||||
| CKD(Cr>1.5, non-uremic status) | 1.38 | (0.96–2.00) | 0.084 | ||||
| Uremia at diagnosis | 1.62 | (1.05–2.49) | 0.030 * | 2.87 | (1.79–4.49) | <0.001 ** | |
| HBV or HCVcarrier | 1.06 | (0.65–1.74) | 0.820 | ||||
| Pathological T | |||||||
| T0–1 | Reference | Reference | Reference | Reference | |||
| T2 | 1.56 | (0.91–2.67) | 0.104 | ||||
| T3–4 | 3.49 | (2.41–5.05) | <0.001 ** | 2.82 | (1.76–4.49) | <0.001 ** | |
| Multifocalty | |||||||
| No | Reference | Reference | |||||
| Yes | 1.31 | (0.93–1.84) | 0.117 | ||||
| Tumor cell differentiation | |||||||
| CIS | |||||||
| Negative | Reference | Reference | |||||
| Positive | 1.05 | (0.66–1.67) | 0.834 | ||||
| Tumor grading | |||||||
| G1 | Reference | Reference | |||||
| G2–3 | 0.05 | (0.00–1.89) | 0.105 | ||||
| Lymphovascular invasion | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 3.20 | (2.25–4.57) | <0.001 ** | 1.31 | (0.82–2.10) | 0.256 | |
| Surgical margin | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 4.77 | (3.05–7.45) | <0.001 ** | 2.75 | (1.61–4.72) | <0.001 ** | |
| Adjuvant chemotherapy | |||||||
| No | Reference | Reference | Reference | Reference | |||
| Yes | 1.61 | (1.11–2.34) | 0.012 ** | 1.04 | (0.66–1.65) | 0.866 | |
| DFS | |||||||
| Univariate | Multivariable | ||||||
| Hazard ratio | 95%CI | Pvalue | Hazard ratio | 95%CI | Pvalue | ||
| Gender | |||||||
| Male | Reference | Reference | |||||
| Female | 0.71 | (0.52–0.97) | 0.034 * | 0.90 | (0.55–1.46) | 0.665 | |
| Age, years | 1.03 | (1.02–1.05) | <0.001 ** | 1.04 | (1.02–1.06) | <0.001 ** | |
| BMI | 0.98 | (0.93–1.03) | 0.367 | ||||
| Group | |||||||
| PN0 | Reference | Reference | Reference | Reference | |||
| PN1–3 | 4.54 | (2.56–8.05) | <0.001 ** | 1.68 | (0.86–3.28) | 0.126 | |
| PNx | 1.25 | (0.89–1.76) | 0.206 | 1.21 | (0.84–1.76) | 0.307 | |
| Performance Status ECOG | |||||||
| 0 | Reference | Reference | |||||
| 1 | 1.08 | (0.65–1.79) | 0.764 | ||||
| 2 | 1.71 | (0.96–3.06) | 0.068 | ||||
| 3 | 1.77 | (0.41–7.63) | 0.444 | ||||
| 4 | 3.58 | (0.48–26.92) | 0.215 | ||||
| Smoking status | |||||||
| Never | Reference | Reference | Reference | Reference | |||
| Current | 1.45 | (0.93–2.25) | 0.099 | 0.99 | (0.55–1.78) | 0.965 | |
| Former | 1.89 | (1.23–2.92) | 0.004 ** | 1.39 | (0.77–2.51) | 0.276 | |
| Comorbidity | |||||||
| CAD/HTN | 1.08 | (0.78–1.50) | 0.627 | ||||
| DM | 1.66 | (1.16–2.38) | 0.006 ** | 1.52 | (1.03–2.23) | 0.036 * | |
| COPD/Asthema | 1.23 | (0.45–3.31) | 0.689 | ||||
| CVA | 1.99 | (1.05–3.78) | 0.036 * | 1.01 | (0.47–2.17) | 0.973 | |
| CKD(Cr>1.5, non-uremic status) | 1.28 | (0.89–1.84) | 0.186 | ||||
| Uremia at diagnosis | 1.57 | (1.03–2.38) | 0.035 * | 2.60 | (1.62–4.17) | <0.001 ** | |
| HBV or HCVcarrier | 1.06 | (0.66–1.73) | 0.798 | ||||
| Pathological T | |||||||
| T0–1 | Reference | Reference | Reference | Reference | |||
| T2 | 1.60 | (0.97–2.64) | 0.066 | ||||
| T3–4 | 3.18 | (2.24–4.53) | <0.001 ** | 2.37 | (1.46–3.85) | <0.001 ** | |
| Multifocalty | |||||||
| No | Reference | Reference | |||||
| Yes | 1.34 | (0.97–1.86) | 0.074 | ||||
| Tumor cell differentiation | |||||||
| CIS | |||||||
| Negative | Reference | Reference | |||||
| Positive | 1.14 | (0.74–1.76) | 0.556 | ||||
| Tumor grading | |||||||
| G1 | Reference | Reference | |||||
| G2–3 | 0.15 | (0.02–1.04) | 0.055 | ||||
| Lymphovascular invasion | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 3.18 | (2.26–4.47) | <0.001 ** | 1.41 | (0.89–2.22) | 0.144 | |
| Surgical margin | |||||||
| Negative | Reference | Reference | Reference | Reference | |||
| Positive | 4.77 | (3.08–7.38) | <0.001 ** | 2.68 | (1.57–4.59) | <0.001 ** | |
| Adjuvant chemotherapy | |||||||
| No | Reference | Reference | Reference | Reference | |||
| Yes | 1.67 | (1.17–2.39) | 0.005 ** | 1.08 | (0.68–1.70) | 0.749 | |
Cox proportional hazard regression.
*p<0.05
**p<0.01.
CSS = cancer-specific survival. OS = overall survival. DFS = disease-free survival. CI = confidence interval. ECOG = Eastern Cooperative Oncology Group. CAD/HTN = coronary artery disease / hypertension. DM = diabetes mellitus. COPD = chronic obstructive pulmonary disease. CVA = cerebrovascular accident. CKD = chronic kidney disease. HBV = hepatitis B virus. HCV = hepatitis C virus. CIS = carcinoma in situ. G = Grade.
In the subgroup analysis for patients with LND (pN0 and pN+), 20 patients were LN-positive (6.2% of 323 patients). In the N+ group, locally advanced disease (pT3 and pT4) had a significantly higher rate of node metastasis [T3–4 vs. T0–2: 13.91% (16/115) and 1.92% (4/208), p<0.001]. We also found a trend in the N+ group had more grade 3 tumors [68.6% (208/303) and 100.0% (20/20)], more instances of lymphovascular invasion [17.5% (53/303) and 75.0% (15/20)], and a higher margin positive rate [8.65 (26/303) and 40.0% (8/20)]. In addition, 278 receiving RNU had hilar-only LND, and 45 had regional LND. The average number of removed lymph nodes were 1.0 (range 0.0 to 5.0) in hilar-only LND and 11.0 (range 6.0 to 41.0) in regional LND. Between these two groups of patients, the regional LND group had more dissected nodes [1.0 (0.0–5.0) vs. 11.0 (6.0–41.0), p<0.001], and more node metastases [9 (3.2%) vs. 11 (24.4%), p<0.001]. The regional LND group also had more locally advanced diseases (≥T2, 46.4% vs. 66.7%), p = 0.039) (Table 3).
Table 3. Association between types of LND and clinic-pathological characteristics of cN0 UTUC patients treated with RNU and BCE.
| Hilar-only LND | Regional LND | P value | |
|---|---|---|---|
| Total case | 278(86.1%) | 45(13.9%) | |
| number of dissected nodes † | 1.0(0.0–5.0) | 11.0(6.0–41.0) | <0.001 ** |
| Positive node | 9(3.2%) | 11(24.4%) | <0.001 ** |
| Pathological T | 0.039 * | ||
| T0–1 | 149(53.6%) | 15(33.3%) | |
| T2 | 35(12.6%) | 9(20.0%) | |
| T3–4 | 94(33.8%) | 21(46.7%) | |
| Tumor grading | 0.145 | ||
| G1 | 7(2.5%) | 0(0.0%) | |
| G2 | 80(28.8%) | 8(17.8%) | |
| G3 | 191(68.7%) | 37(82.2%) | |
| Hydronephrosis | 22(7.9%) | 5(11.1%) | 0.559 |
| Multifocal disease | 107(38.5%) | 20(44.4%) | 0.552 |
Chi-square test.
†Mann-Whitney U test, Median (Range)
*P<0.05
**P<0.01
G = Grade.
Discussion
This is a retrospective study to distinguish the prognostic impact of LND on patients treated with RNU with cN0 UTUC. As a result, LND for cN0 UTUC did not show therapeutic benefits in terms of DFS, CSS, and OS in the pN0 group. However, LND with RNU was observed to still lead a poor prognosis and allowed optimal tumor staging for further treatment if needed. Based on our study, the clinical T stage may indicate the need for LND. The regional LND should be performed on patients with cT3–4, or patients suspected to have sT3–4 disease during the operation, even though they had been considered cN0 at first. Moreover, for patients with characteristics of high grade tumors, positive lymphovascular invasion, or positive surgical margin, additional AC should be considered because of the risk of lymph node metastasis as implicated in our study.
Upper UTUC is a relatively rare disease with a prognosis poorer than that of bladder cancers [7–9]. The “gold standard” therapy for localized UTUC is RNU with ipsilateral BCE, but the role of LND in patients who are cN0 remains controversial [7, 13]. The presence of LN metastasis is associated with a poor prognosis [6, 24–26]. The reported incidence of LN metastasis was 37% for ≥pT3 disease, but only 3% for ≤pT2 disease [27]. The incidence of pN+ in patients with cN0 and ≥pT2 ranges from 14.3% to 40% [7]. Some studies have suggested performing LND at the time of RNU for patients with UTUC mainly due to the staging and therapeutic benefits of LND [22, 23]. In our present sample, 20 (3.8%) patients with pN+ disease had a significantly worse prognosis in terms of DFS, CSS, and OS compared with patients who were pN0. Our findings are consistent with the current literature.
According to the NCCN guidelines, patients with pT2–4 and pN+ UTUC should consider postoperative AC [13]. Although some observational studies have reported inconsistent results regarding the effectiveness of AC [28–32], a recent systematic review showed that AC is associated with better metastasis-free survival (HR = 0.65, p<0.001) and CSS (HR = 0.66, p<0.001). The association between AC and OS is significant in patients with locally advanced UTUC [33]. Seisen et al. found OS benefits of AC after RNU for patients with pT3/T4 and/or pN+ UTUC [34]. The phase III POUT trial has demonstrated the benefit of adjuvant platinum-based chemotherapy for patients with locally advanced UTUC, in that AC significantly improved DFS (HR = 0.45, 95% CI = 0.30–0.68; p = 0·0001) [18]. The post-operative nodal status allows the selection of those patients (pN+) who may benefit from adjuvant systemic therapy. In our study, 70.0% of the patients in the PN+ group received AC, compared with 18.8% and 18.3% in the PN0 and PNx groups, respectively. Due to poor performance status, the others (30%) in the PN+ group did not receive AC. The PN+ group had a large proportion of a more aggressive tumor grade (G3, 100%), lymphovascular invasion (75%), and pathological T stage (>T2, 80%). However, there was no significant difference in terms of CSS, OS, and DFS when these were correlated with AC after balancing the confounders in the multivariate analysis for the entire population (HR = 0.82, CI = 0.47–1.44, p = 0.484, HR = 1.04, CI = 0.66–1.65, p = 0.866 and HR = 1.08, CI = 0.68–1.70, p = 0.749, respectively).
In contrast to the role of LND for UTUC staging, the therapeutic benefit of LND for UTUC remains controversial. Several studies have indicated that the prognosis of patients with pNx disease is poorer than that of those with pN0 disease, further demonstrating the therapeutic benefits of LND [35–37]. Abe et al. found significant differences in CSS among pN0, pNx, and pN+ patient groups. Notably, the survival difference between the pN0 and pNx groups remained significant in the multivariate analysis (HR = 3.36, 95% CI = 1.90–5.93, p<0.001) [35]. Similar results were found by Roscigno et al., in that pNx is significantly associated with a poorer prognosis (five-year CSS) than is pN0 in ≥pT2 populations (70% vs. 58% vs. 33%; p = 0.017 and p<0.01, respectively) [36, 37]. In our study, no difference was found between the pNx and pN0 groups in terms of DFS, CSS, and OS. However there might be a worse trend in the pNx group than in the pN0 group in terms of five-year DFS and OS, indicating that the LND might have a potential survival benefit for the patient’s survival. However, pN1–3 disease was also not an independent prognostic factor of CCS, OS, and DFS in our multivariate analysis (HR = 2.10, p = 0.049; HR = 1.67, p = 0.149 and HR = 1.68, p = 0.126, respectively), a finding that is consistent with other scales. These discrepancies in our results may have been due to few patient numbers (3.8%, 20/520) and the large proportion of advanced disease (T3–4, 80%, 16/20) in our pN+ group.
Most large scale studies found no therapeutic benefit of LND in the overall population [36–44]. In those studies, no statistically significant difference existed between the two groups in the overall population, but a clear benefit of LND was revealed when the focus was on patients with muscle-invasive or locally advanced UTUC. These patients had significantly better survival rates when compared with those in the pNx group [36–39]. LND benefits are less clear in patients who are cN0, just as how no benefit was found in our present study. This could have been due to selection bias, as LND was performed on those with more severe diseases.
In our subgroup analysis, advanced disease (pT3 and pT4) showed significantly more node metastases [T3–4 vs. T0–2: 13.91% (16/115), 1.92% (4/208), p<0.001]. The number of dissected nodes [1.0 (0.0–5.0) vs. 11.0 (6.0–41.0), p<0.001] and the rate of positive node metastasis [9 (3.2%) vs. 11 (24.4%), p<0.001] were significantly higher in the regional LND group. These patients with regional LND also had more locally advanced diseases (≥T2, 46.4% vs. 66.7%, p = 0.039) and showed a trend of having higher grade tumors and more advanced pathological T stages. A reasonable explanation is related to the personal preferences of our surgeons.
The limitations of the present study were its retrospective design and setting in a single center. The incidence of UTUC in Taiwan at the time of writing is higher than in other regions, which might affect the results of the analysis [45]. Due to the different strategy of LND that resulted from clinical circumstances, surgeons’ personal preferences and evolution of surgical techniques, there may have been potential selection biases. In addition, there were 86 patients with muscle-invasive UTUC who did not undergo LND in the PNx group (86/197, 43.65%), and who were considered to be at a high potential risk for LN metastasis, which may have affected their survival. Only 20 patients were found to have pathologically confirmed lymph node metastases, which was a small sample size. Furthermore, there may have been additional unmeasured factors that we did not consider which may have affected the results, although multiple clinical variables were included in our study. Despite these limitations, the strengths of our study were its setting in a tertiary referral center and a large number of UTUC cases, most of which underwent lymphadenectomy (62.1%).
Conclusions
LNDs for cN0 UTUC showed no therapeutic benefits in terms of DFS, CSS, and OS in the pN0 group. However, LND with RNU allowed optimal tumor staging. We found that 6.2% (20 pN+/323 with LND) of our patients had clinically proven occult LN metastasis. Furthermore, the clinical T stage may indicate the need for LND. Regional LND should be performed on patients with cT3–4 or patients suspected of sT3–4 disease during the surgical operation. Further prospective and well‐controlled clinical trials should be done to better establish the impact of LND on patients with cN0 UTUC.
Supporting information
Kaplan-Meier analysis of DFS for 520 patients with pathologically proved lymph node status (pN1–3 and pN0) or without LND (pNx) in cN0 UTUC undergoing RNU with BCE.
(TIF)
Kaplan-Meier analysis of CSS for 520 patients with pathologically proved lymph node status (pN1–3 and pN0) or without LND (pNx) in cN0 UTUC undergoing RNU with BCE.
(TIF)
Kaplan-Meier analysis of OS for 520 patients with pathologically proved lymph node status (pN1–3 and pN0) or without LND (pNx) in cN0 UTUC undergoing RNU with BCE.
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Cell Carcinoma: 2015 Update. European urology. 2015;68(5):868–79. Epub 2015/07/21. doi: 10.1016/j.eururo.2015.06.044 . [DOI] [PubMed] [Google Scholar]
- 2.Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World journal of urology. 2013;31(1):141–5. Epub 2012/05/04. doi: 10.1007/s00345-012-0877-2 . [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. European urology. 2017;71(3):447–61. Epub 2016/06/22. doi: 10.1016/j.eururo.2016.05.041 . [DOI] [PubMed] [Google Scholar]
- 4.Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. 2002;59(5):681–7. Epub 2002/05/07. doi: 10.1016/s0090-4295(02)01529-7 . [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, Liao YM, Tsai WM, Kuo HC. Upper urinary tract urothelial carcinoma in eastern Taiwan: high proportion among all urothelial carcinomas and correlation with chronic kidney disease. J Formos Med Assoc. 2007;106(12):992–8. Epub 2008/01/16. doi: 10.1016/S0929-6646(08)60074-1 . [DOI] [PubMed] [Google Scholar]
- 6.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–33. Epub 2009/01/22. doi: 10.1002/cncr.24135 . [DOI] [PubMed] [Google Scholar]
- 7.Dominguez-Escrig JL, Peyronnet B, Seisen T, Bruins HM, Yuan CY, Babjuk M, et al. Potential Benefit of Lymph Node Dissection During Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the European Association of Urology Guidelines Panel on Non-muscle-invasive Bladder Cancer. Eur Urol Focus. 2019;5(2):224–41. Epub 2017/11/22. doi: 10.1016/j.euf.2017.09.015 . [DOI] [PubMed] [Google Scholar]
- 8.Grabstald H, Whitmore WF, Melamed MR. Renal pelvic tumors. Jama. 1971;218(6):845–54. Epub 1971/11/08. . [PubMed] [Google Scholar]
- 9.Batata MA, Whitmore WF, Hilaris BS, Tokita N, Grabstald H. Primary carcinoma of the ureter: a prognostic study. Cancer. 1975;35(6):1626–32. Epub 1975/06/01. doi: . [DOI] [PubMed] [Google Scholar]
- 10.Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. European urology. 2012;62(1):100–14. Epub 2012/03/03. doi: 10.1016/j.eururo.2012.02.030 . [DOI] [PubMed] [Google Scholar]
- 11.Abouassaly R, Alibhai SM, Shah N, Timilshina N, Fleshner N, Finelli A. Troubling outcomes from population-level analysis of surgery for upper tract urothelial carcinoma. Urology. 2010;76(4):895–901. Epub 2010/07/22. doi: 10.1016/j.urology.2010.04.020 . [DOI] [PubMed] [Google Scholar]
- 12.Jeldres C, Sun M, Isbarn H, Lughezzani G, Budäus L, Alasker A, et al. A population-based assessment of perioperative mortality after nephroureterectomy for upper-tract urothelial carcinoma. Urology. 2010;75(2):315–20. Epub 2009/12/08. doi: 10.1016/j.urology.2009.10.004 . [DOI] [PubMed] [Google Scholar]
- 13.Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. NCCN Guidelines Insights: Bladder Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(9):1041–53. Epub 2018/09/06. doi: 10.6004/jnccn.2018.0072 . [DOI] [PubMed] [Google Scholar]
- 14.Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and Adjuvant Chemotherapy for Upper Tract Urothelial Carcinoma: A 2020 Systematic Review and Meta-analysis, and Future Perspectives on Systemic Therapy. European urology. 2021;79(5):635–54. Epub 2020/08/17. doi: 10.1016/j.eururo.2020.07.003 . [DOI] [PubMed] [Google Scholar]
- 15.Kim DK, Lee JY, Kim JW, Hah YS, Cho KS. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2019;135:59–65. Epub 2019/03/02. doi: 10.1016/j.critrevonc.2019.01.019 . [DOI] [PubMed] [Google Scholar]
- 16.Qiu D, Hu J, He T, Li H, Hu J, Yi Z, et al. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: a pooled analysis. Transl Androl Urol. 2020;9(5):2094–106. Epub 2020/11/20. doi: 10.21037/tau-20-933 ; PubMed Central PMCID: PMC7658168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Ou Z, Wang R, Zhang M, He W, Zhang J, et al. Neoadjuvant Chemotherapy Benefits Survival in High-Grade Upper Tract Urothelial Carcinoma: A Propensity Score-Based Analysis. Annals of surgical oncology. 2020;27(4):1297–303. Epub 2019/12/20. doi: 10.1245/s10434-019-08128-7 . [DOI] [PubMed] [Google Scholar]
- 18.Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–77. Epub 2020/03/09. doi: 10.1016/S0140-6736(20)30415-3 ; PubMed Central PMCID: PMC7181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricci AD, Rizzo A, Mollica V, Schiavina R, Fiorentino M, Brunocilla E, et al. Platinum-based adjuvant chemotherapy for upper tract urothelial carcinoma: a change of paradigm? A meta-analysis of aggregate data. Anticancer Drugs. 2022;33(1):e61–e8. Epub 2021/08/14. doi: 10.1097/CAD.0000000000001200 . [DOI] [PubMed] [Google Scholar]
- 20.Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. The New England journal of medicine. 2021;384(22):2102–14. Epub 2021/06/03. doi: 10.1056/NEJMoa2034442 ; PubMed Central PMCID: PMC8215888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizzo A, Mollica V, Massari F. Expression of Programmed Cell Death Ligand 1 as a Predictive Biomarker in Metastatic Urothelial Carcinoma Patients Treated with First-line Immune Checkpoint Inhibitors Versus Chemotherapy: A Systematic Review and Meta-analysis. European Urology Focus. 2022;8(1):152–9. doi: 10.1016/j.euf.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Winer AG, Vertosick EA, Ghanaat M, Corradi RB, Carlsson S, Sjoberg DD, et al. Prognostic value of lymph node yield during nephroureterectomy for upper tract urothelial carcinoma. Urologic oncology. 2017;35(4):151.e9-.e15. Epub 2016/12/10. doi: 10.1016/j.urolonc.2016.11.002 ; PubMed Central PMCID: PMC5502780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seisen T, Shariat SF, Cussenot O, Peyronnet B, Renard-Penna R, Colin P, et al. Contemporary role of lymph node dissection at the time of radical nephroureterectomy for upper tract urothelial carcinoma. World journal of urology. 2017;35(4):535–48. Epub 2016/01/27. doi: 10.1007/s00345-016-1764-z . [DOI] [PubMed] [Google Scholar]
- 24.Rouprêt M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. The Journal of urology. 2013;189(5):1662–9. Epub 2012/10/30. doi: 10.1016/j.juro.2012.10.057 . [DOI] [PubMed] [Google Scholar]
- 25.Cha EK, Shariat SF, Kormaksson M, Novara G, Chromecki TF, Scherr DS, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. European urology. 2012;61(4):818–25. Epub 2012/01/31. doi: 10.1016/j.eururo.2012.01.021 . [DOI] [PubMed] [Google Scholar]
- 26.Jeldres C, Sun M, Lughezzani G, Isbarn H, Shariat SF, Widmer H, et al. Highly predictive survival nomogram after upper urinary tract urothelial carcinoma. Cancer. 2010;116(16):3774–84. Epub 2010/06/22. doi: 10.1002/cncr.25122 . [DOI] [PubMed] [Google Scholar]
- 27.Kondo T, Nakazawa H, Ito F, Hashimoto Y, Toma H, Tanabe K. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology. 2007;69(2):265–9. Epub 2007/02/27. doi: 10.1016/j.urology.2006.10.014 . [DOI] [PubMed] [Google Scholar]
- 28.Shirotake S, Kikuchi E, Tanaka N, Matsumoto K, Miyazaki Y, Kobayashi H, et al. Impact of an adjuvant chemotherapeutic regimen on the clinical outcome in high risk patients with upper tract urothelial carcinoma: a Japanese multi-institution experience. The Journal of urology. 2015;193(4):1122–8. Epub 2014/12/03. doi: 10.1016/j.juro.2014.10.022 . [DOI] [PubMed] [Google Scholar]
- 29.Kang M, Yoo H, Kim K, Sung SH, Jeon HG, Park SH, et al. Role of Adjuvant Chemotherapy in Advanced Stage Upper Urinary Tract Urothelial Carcinoma after Radical Nephroureterectomy: Competing Risk Analysis after Propensity Score-Matching. Journal of Cancer. 2019;10(27):6896–902. Epub 2019/12/17. doi: 10.7150/jca.34103 ; PubMed Central PMCID: PMC6909941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassilakopoulou M, de la Motte Rouge T, Colin P, Ouzzane A, Khayat D, Dimopoulos MA, et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. 2011;117(24):5500–8. Epub 2011/06/04. doi: 10.1002/cncr.26172 . [DOI] [PubMed] [Google Scholar]
- 31.Hellenthal NJ, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Bolenz C, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. The Journal of urology. 2009;182(3):900–6. Epub 2009/07/21. doi: 10.1016/j.juro.2009.05.011 . [DOI] [PubMed] [Google Scholar]
- 32.Chen CS, Lin CY, Wang CL, Wang SS, Li JR, Yang CK, et al. Association between lymphovascular invasion and oncological outcome in node-negative upper tract urothelial carcinoma with different stage. Urologic oncology. 2020. Epub 2020/09/10. doi: 10.1016/j.urolonc.2020.08.008 . [DOI] [PubMed] [Google Scholar]
- 33.Quhal F, Mori K, Sari Motlagh R, Laukhtina E, Pradere B, Rouprêt M, et al. Efficacy of neoadjuvant and adjuvant chemotherapy for localized and locally advanced upper tract urothelial carcinoma: a systematic review and meta-analysis. International journal of clinical oncology. 2020;25(6):1037–54. Epub 2020/03/25. doi: 10.1007/s10147-020-01650-9 . [DOI] [PubMed] [Google Scholar]
- 34.Seisen T, Krasnow RE, Bellmunt J, Rouprêt M, Leow JJ, Lipsitz SR, et al. Effectiveness of Adjuvant Chemotherapy After Radical Nephroureterectomy for Locally Advanced and/or Positive Regional Lymph Node Upper Tract Urothelial Carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35(8):852–60. Epub 2017/01/04. doi: 10.1200/JCO.2016.69.4141 . [DOI] [PubMed] [Google Scholar]
- 35.Chung SD, Yu HJ, Chueh SC. The role of lymph-node dissection in the treatment of upper urinary tract cancer: a multi-institutional study. BJU international. 2008;102(5):639–40. Epub 2008/08/13. doi: 10.1111/j.1464-410X.2008.07944_2.x . [DOI] [PubMed] [Google Scholar]
- 36.Roscigno M, Cozzarini C, Bertini R, Scattoni V, Freschi M, Da Pozzo LF, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. European urology. 2008;53(4):794–802. Epub 2008/01/22. doi: 10.1016/j.eururo.2008.01.008 . [DOI] [PubMed] [Google Scholar]
- 37.Roscigno M, Shariat SF, Margulis V, Karakiewicz P, Remzi M, Kikuchi E, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. The Journal of urology. 2009;181(6):2482–9. Epub 2009/04/18. doi: 10.1016/j.juro.2009.02.021 . [DOI] [PubMed] [Google Scholar]
- 38.Burger M, Shariat SF, Fritsche HM, Martinez-Salamanca JI, Matsumoto K, Chromecki TF, et al. No overt influence of lymphadenectomy on cancer-specific survival in organ-confined versus locally advanced upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy: a retrospective international, multi-institutional study. World journal of urology. 2011;29(4):465–72. Epub 2011/06/02. doi: 10.1007/s00345-011-0705-0 . [DOI] [PubMed] [Google Scholar]
- 39.Ikeda M, Matsumoto K, Sakaguchi K, Ishii D, Tabata KI, Kurosawa K, et al. Effect of Lymphadenectomy During Radical Nephroureterectomy in Locally Advanced Upper Tract Urothelial Carcinoma. Clinical genitourinary cancer. 2017;15(5):556–62. Epub 2017/05/16. doi: 10.1016/j.clgc.2017.04.004 . [DOI] [PubMed] [Google Scholar]
- 40.Lughezzani G, Jeldres C, Isbarn H, Shariat SF, Sun M, Pharand D, et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology. 2010;75(1):118–24. Epub 2009/10/30. doi: 10.1016/j.urology.2009.07.1296 . [DOI] [PubMed] [Google Scholar]
- 41.Mason RJ, Kassouf W, Bell DG, Lacombe L, Kapoor A, Jacobsen N, et al. The contemporary role of lymph node dissection during nephroureterectomy in the management of upper urinary tract urothelial carcinoma: the Canadian experience. Urology. 2012;79(4):840–5. Epub 2012/03/01. doi: 10.1016/j.urology.2011.11.058 . [DOI] [PubMed] [Google Scholar]
- 42.Ouzzane A, Colin P, Ghoneim TP, Zerbib M, De La Taille A, Audenet F, et al. The impact of lymph node status and features on oncological outcomes in urothelial carcinoma of the upper urinary tract (UTUC) treated by nephroureterectomy. World journal of urology. 2013;31(1):189–97. Epub 2012/12/12. doi: 10.1007/s00345-012-0983-1 . [DOI] [PubMed] [Google Scholar]
- 43.Secin FP, Koppie TM, Salamanca JI, Bokhari S, Raj GV, Olgac S, et al. Evaluation of regional lymph node dissection in patients with upper urinary tract urothelial cancer. International journal of urology: official journal of the Japanese Urological Association. 2007;14(1):26–32. Epub 2007/01/04. doi: 10.1111/j.1442-2042.2006.01664.x . [DOI] [PubMed] [Google Scholar]
- 44.Yoo S, You D, Jeong IG, Hong B, Hong JH, Ahn H, et al. Does lymph node dissection during nephroureterectomy affect oncological outcomes in upper tract urothelial carcinoma patients without suspicious lymph node metastasis on preoperative imaging studies? World journal of urology. 2017;35(4):665–73. Epub 2016/08/10. doi: 10.1007/s00345-016-1918-z . [DOI] [PubMed] [Google Scholar]
- 45.Yang M-H, Chen K-K, Yen C-C, Wang W-S, Chang Y-H, Huang WJ-S, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. 2002;59(5):681–7. doi: 10.1016/s0090-4295(02)01529-7 . [DOI] [PubMed] [Google Scholar]