Abstract
Objective:
The current study applies a precision medicine approach to Trigeminal Nerve Simulation (TNS), an FDA approved, neuromodulation treatment for attention-deficit/hyperactivity disorder (ADHD) by testing secondary outcomes of cognitive and electrophysiological (EEG) predictors of treatment response among subjects from the original randomized controlled trial.
Method:
Children aged 8–12 years with ADHD, were randomized to four weeks of active or sham TNS treatment; after which, the sham group crossed over into four weeks of open-label treatment. TNS treatment responders (RESP) had an ADHD Rating Scale (ADHD-RS) Total score reduction of ≥25%, while non-responders (NR) had <25% reduction post-treatment. Assessments included weekly behavioral ratings and pre-/post-treatment cognitive and EEG measures.
Results:
The final sample was 25 RESP and 26 NR (34 male children, mean age 10.3 (1.4) years). Baseline measures that significantly differentiated RESP from NR include: lower working memory, lower spelling and math achievement, deficits on behavioral ratings of executive function (BRIEF), and lower resting state EEG power in the right frontal (F4) region (all p’s <.05). Compared to NRs, responders showed significantly increased right frontal EEG power with TNS treatment, which was predictive of improved executive functions and ADHD symptomatology (β=.65, p<.001). When EEG and behavior were modeled together, the area under the curve (AUC) for BRIEF Working Memory scale was .83 (p=.003), indicating moderate prediction of treatment response.
Conclusion:
Children with ADHD who have executive dysfunction are more likely to be TNS responders and show modulation of right frontal brain activity, improved/normalized executive functions, and ADHD symptom reduction.
Keywords: neuromodulation, electroencephalography, executive functions, BRIEF
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is highly prevalent (5–11% of children 4–17 years1, bears significant cost to the economy (e.g., $143 to $266 billion per year)2 and negatively impacts the quality of life of affected individuals.3, 4 The consequences of ADHD are lifelong with ADHD-related impairment persisting in 65% or more, regardless of whether formal diagnostic criteria for the disorder are met.5 As many as 50% of individuals with ADHD have deficits in higher order problem solving and self-regulation skills6–8, also known as ‘executive functions’ (EFs), which underlie functional impairments in academic and occupational settings.9
Although psychostimulant medications are the gold standard of treatment for ADHD, there has been increasing interest in non-medication approaches to symptom management due to noncompliance, negative side effects, and non-response in a significant minority of patients. Trigeminal nerve stimulation (TNS) is a non-pharmacological, non-invasive, minimal risk neuromodulation treatment that has demonstrated efficacy for reducing ADHD symptoms in open-label10 and double-blind, sham-controlled studies, with an estimated effect size (Cohen’s d) of 0.511, comparable to that of non-stimulant medications.12 In the blinded randomized controlled trial (RCT), approx. 52% participants in the active group showed clinically meaningful improvement, as determined by the Clinical Global Impression—Improvement (CGI-I) scale, compared to 14% with sham by the end of the 4-week trial. Importantly, study analyses confirmed the fidelity of study blinding, further strengthening the integrity of study results. Based on this clinical trial, the U.S. Food and Drug Administration (FDA) issued its first approval of a non-pharmacological, device-based treatment for ADHD among children ages 8–12 years in April 2019.
TNS stimulates the V1 branch of the trigeminal nerve and activates several brain regions implicated in ADHD and executive function, including the anterior cingulate cortex, inferior and middle frontal gyri.13 In the double-blind study11, the active treatment group displayed significantly increased electroencephalogram (EEG) power in mid- and right-frontal electrodes compared to sham, which was consistent with “bottom-up” effects of subcortical trigeminal nerve activation rather than direct stimulation of frontal cortices by electrodes placed on the forehead14. EEG changes were associated with lower ADHD-RS scores, particularly hyperactive-impulsive and total scores at trial end.
Previous EEG studies have reported higher power in right frontal electrodes with successful stopping within a stop signal task15, suggesting an association between the right frontal cortex and inhibitory control. The right inferior frontal cortex, pre-supplemental motor area, and subthalamic nuclei are believed to be part of a fronto-basal ganglia network used in suppression of motor behavior.16 Thus, it is hypothesized that TNS treatment should also be associated with improvement in executive functions such as inhibitory control. In the open-label TNS study, a significant decrease in flanker task incongruent reaction time was reported after 8 weeks of treatment10, whereas secondary outcomes such as cognitive measures have not yet been reported for the blinded trial.11 The current paper will now provide analyses of these secondary outcomes to address potential mechanisms of TNS-associated ADHD treatment response.
While the ~50% response rate of TNS is promising, principles based in precision medicine suggest that higher response rates might result from targeting the treatment to particular pathophysiological mechanisms underlying an individual patient’s symptomatic presentation.17 Consistent with this approach, this study tests whether baseline cognitive or EEG characteristics are predictors of positive TNS response and associated with ADHD symptom reduction within the original TNS RCT sample11. Given prior findings of frontal EEG power modulation with treatment, we hypothesized that 1) lower right frontal EEG power and poor executive functions at baseline would be predictive of positive TNS treatment response; 2) improvements in these measures would be associated with lower ADHD symptoms among responders. Successful prediction of positive response would aid clinicians and families in identification of more personalized treatment interventions and economic allocation of treatment costs.
METHOD
Participants
Children ages 8 to 12 years with clinically diagnosed DSM-5 ADHD, based on semi-structured diagnostic (Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL)18 and clinical interview, clinician-administered ADHD-IV Rating Scale (ADHD-RS) ≥ 2419, baseline CGI - Severity (CGI-S) score ≥ 420, estimated full scale IQ ≥ 85 based on Wechsler Abbreviated Scales of Intelligence (WASI)21 subtests, and able to cooperate with EEG and other study procedures were enrolled. Exclusion criteria were current major depression, autism spectrum disorder, lifetime psychosis, mania, seizure disorder, head injury with loss of consciousness, or baseline suicidality. Participants were recruited through community advertisements and internet postings. Children were medication free for at least one month prior to participation and remained off medication throughout the trial. Prior to screening and initiation of any study procedures, parents and children received thorough verbal and written descriptions of study requirements and provided written permission/assent. The UCLA Institutional Review Board approved all study procedures.
Procedures
See McGough et al.11 for detailed methods and procedures for the randomized clinical trial. In brief, the study was a four-week, double-blind, sham-controlled investigation. Participants were randomized 1:1 to active TNS or sham, which was administered nightly during sleep for four weeks, after which treatment was discontinued. After one-week discontinuation, participants assigned to sham were given the option to cross-over into four weeks of open-label TNS treatment. Methods pertaining to the sham, including blinding and demonstrating the effectiveness of study blinding are described in detail in McGough et al (2019); a CONSORT diagram for the trial is available (see Figure S1, available online).
Outcomes.
In addition to the screening measures for study inclusion (ie, KSADS-PL, ADHD-RS, CGI-S and WASI), study participants were assessed with 1) additional parent-completed behavioral measures of executive function: Child Behavior Checklist [CBCL]22, Behavior Ratings of Individual Executive Functions [BRIEF]23; 2) cognitive tasks: Wechsler Intelligence Scale for Children (WISC-4)24 Digit Span subtest and Wide Range Achievement Test [WRAT-3]25 and 3) computerized tests of executive function: Spatial Working Memory [SWM] and Flanker Task26, 27, and 4) resting state EEG. Blinded clinician (ADHD-RS and CGI-I) and parent (BRIEF) ratings occurred weekly throughout the active phase and (unblinded) bi-weekly during the sham crossover phase of the trial. Cognitive and EEG measures were administered at baseline and the end of week four of the active blinded trial, but were not administered at the end of the sham-crossover trial.
TNS Intervention.
Stimulation was via a CE-mark approved neurostimulator, the Monarch eTNS System™ (NeuroSigma, Inc., Los Angeles CA). Parents applied self-adhesive patch electrodes centered on their child’s forehead, which were worn for 7–9 hours nightly and removed each morning. The active condition utilized a 120-Hz repetition frequency, with 250-µs pulse width, a duty cycle of 30 seconds on/30 seconds off, and stimulator current settings between 2 and 4 milli-amperes (mA) (range: 0–10 mA). Power was provided by 9-volt lithium medical-grade batteries (Energizer L522, Eveready Battery Co., St. Louis, MO), which were recharged and replaced every other day.
Electroencephalography.
EEG acquisition followed procedures used in previous studies28. Participants underwent EEG recording during a five minute, eyes open resting condition. EEG recording was carried out using Electrical Geodesics (EGI; Eugene, Oregon) GES300 system with 128-electrode sensor nets. Data were referenced to Cz, impedance threshold was set at 50 kOhms (per manufacturer standard), and sampling rate was 1000 Hertz (Hz). Eye movements were monitored by electrodes placed on the outer canthus of each eye for horizontal movements (REOG, LEOG) and by electrodes above the eyes for vertical eye movements.
Continuous EEG data were imported into the EEGLAB29 environment for processing. The EEG data were preprocessed (high pass filtered (>1 Hz), re-referenced to the common average, noisy electrodes excluded). and decomposed using independent components analysis (ICA), which separates brain from non-brain (e.g., muscle artifact) activities. ICs reflecting non-brain sources of signal (e.g., eye blinks, muscle, artifacts, etc…) were excluded from further analyses. Cleaned ICs were back-projected into channel space for resting state analyses. Fourier transform was used to estimate spectral power, which was averaged across all cleaned data and extracted for the following channels: F3/4, Fz, C3/4, Cz, P3/4, PZ in standard frequency bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), and beta (13–25 hz).
Responder Status.
Past studies of ADHD medication treatments have accepted score reductions ≥ 25% on the ADHD-RS in designating responder status 30, 31. TNS treatment response for the current study was determined by ADHD-RS Total score using a threshold of ≥25% reduction to identify responders (RESP), while participants with score reductions < 25% were considered non-responders (NR). To determine responder status, changes between baseline and week 4 ADHD-RS Total score was used for the active blinded trial and changes between week 4 and week 9 ADHD-RS Total score was used for the sham crossover group. Analyses to establish the equivalency of the RESP and NR groups for each phase of the trial were conducted before any subsequent analyses began.
Statistical analysis
All analyses were conducted in Statistical Package for the Social Sciences (SPSSv23). To determine if there is a baseline profile of treatment responders, group differences (RESP/NR) in baseline behavioral and cognitive measures of executive function and right frontal EEG measures were tested using analyses of variance (ANOVA). Prediction by baseline measures was tested in two ways: 1) linear regression analyses were used for prediction of post-treatment ADHD-RS Total scores; and 2) receiver operating characteristics (ROC) curve analysis to determine area under the curve (AUC) for prediction of responder status. The ROC analysis was conducted solely on active trial participants who were blinded during the trial.
Significant baseline predictors of ADHD symptoms were then tested for TNS treatment-related change by responder status and time (pre-/post-TNS) using repeated measures ANOVAs. Here, the group x time interaction was of most interest as this would indicate different trajectories of change according to TNS treatment response. For sham cross-over subjects, the baseline observation was carried forward so that the Week 4 rating was used as pre-treatment and the Week 9 rating was the post-treatment measurement (see Figure 1). This was done to control for any placebo symptom improvement that occurred due to being in the sham condition for the first 4 weeks of the study. Finally, Pearson correlations between baseline predictors and ADHD symptoms were used to characterize degree of change occurring in both variables with TNS treatment. Due to the strong age effects age was used as a covariate of no interest in all EEG analyses. Partial eta squared (partial η2) was used as the measure of effect size and was interpreted as follows: small: .01, medium: .06, large: .14 (Cohen, 1973). To balance hypothesis generation with type 1 error, we used two procedures: 1) to reduce the number of contrasts, only variables that were significant at p≤.05 in the baseline profile were further tested for prediction of treatment outcomes and treatment related change; and 2) a conservative p-value of p≤.01 was used as the threshold for significance in subsequent analyses.
Figure 1: Attention-Deficit/Hyperactivity Disorder (ADHD) Symptom Scores by Study Phase.
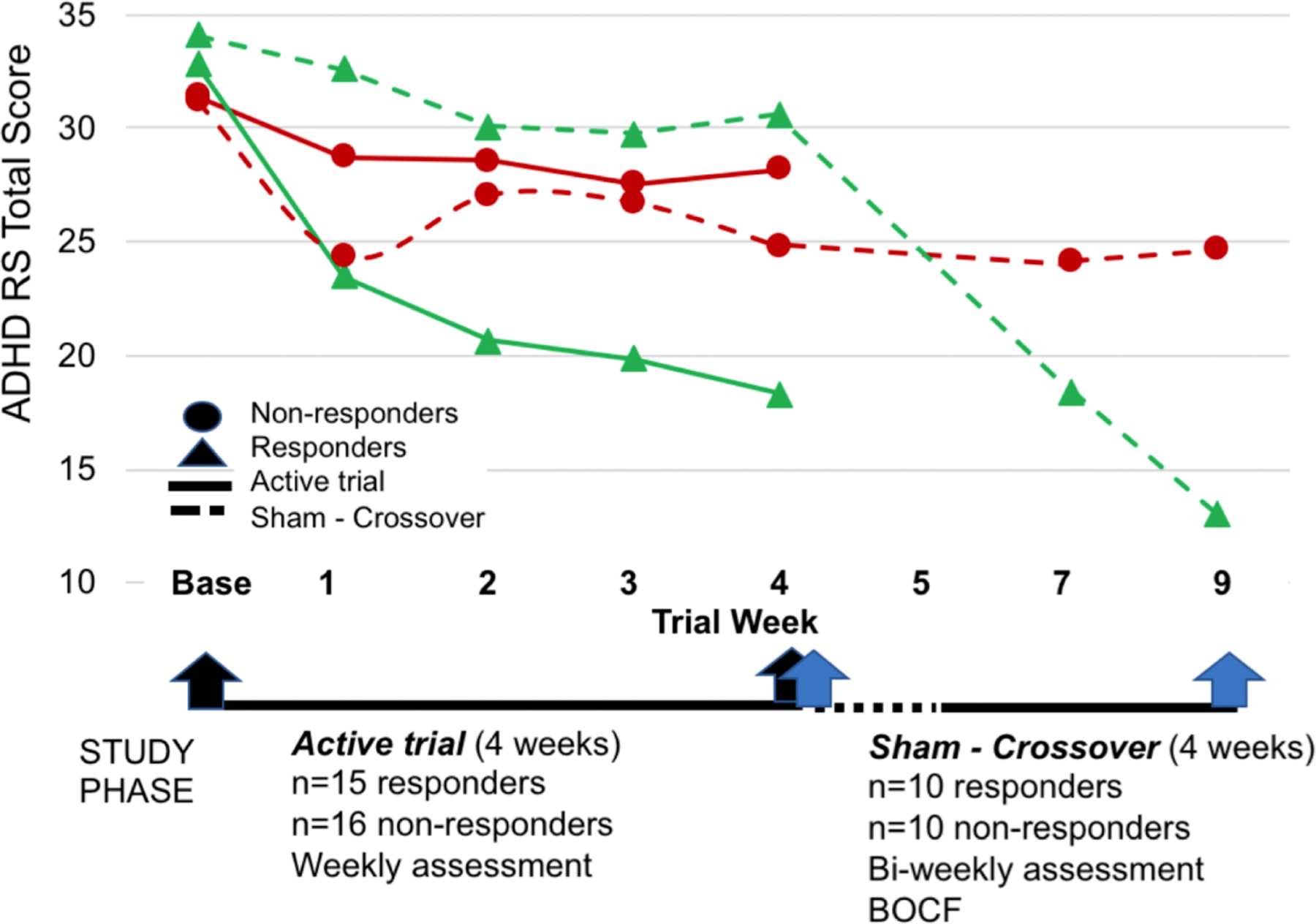
Note: Blinded treatment was discontinued between week 4 and 5 (indicated by dotted line). Sham crossover treatment began week 5. Black and blue arrows indicate pre- and post-treatment measurements for the Active Trial and Sham Crossover groups, respectively. BOCF=baseline observation carried forward; RS = rating scale.
RESULTS
TNS responders demographics and clinical characteristics.
Using ADHD-RS Total Score reduction ≥25% as the criteria for response, the active trial had 15 responders and 16 non-responders. Of the 30 originally randomized to the sham group, 20 participants crossed over and completed 4 weeks of active treatment, of which 10 (50%) were responders (see Fig 1). There were no serious adverse events and no participant withdrew due to adverse events. As seen in Table 1, the RESP and NR groups in active trial and sham cross-over phases did not differ on any demographic or baseline clinical variables, including age, gender, IQ, or SES. The degree of treatment change on the ADHD-RS Total Score was not significantly different between Active Trial and Sham Crossover groups for responders (F(1,23)=1.3, p=.26) and non-responders (F(1,19)=2.4, p=.14); therefore they were combined together across phases.
Table 1:
Demographic Information for Trigeminal Nerve Stimulation Treatment Responders and Non-responders
| Combined |
Active Trial |
Sham Cross-over |
||||
|---|---|---|---|---|---|---|
| Resp 25 |
NR 26 |
Resp 15 |
NR 16 |
Resp 10 |
NR 10 |
|
| N= | ||||||
| Age (years) | 10.5 (1.5) | 10.1 (1.3) | 10.4 (1.4) | 10 (1.5) | 10.7 (1.8) | 10.2 (1.2) |
| Sex, males (n, %) | 11 (44%) | 23 (88%) | 5 (33%) | 14 (88%) | 6 (60%) | 9 (90%) |
| SES rank | 2 (.7) | 1.8 (.8) | 1.9 (.9) | 1.9 (.8) | 2.1 (.6) | 1.7 (.5) |
| IQ | 108.1 (13.6) | 112.5 (13.3) | 108 (12) | 113.3 (14) | 109.6 (14) | 110.6 (18) |
| CGIS | 4.7 (.5) | 4.6 (.5) | 4.7 (.5) | 4.7 (.5) | 4.7 (.5) | 4.6 (.5) |
| Baseline ADHDRS Total | 31.9 (6.7) | 28.9 (7.2) | 32.8 (6.4) | 31.4 (6.5) | 30.6 (7.2) | 24.8 (6.5) |
| ADHDRS tx chg | 15.8 (6.6) | 2.2 (4.1) | 14.5 (6) | 3.3 (4.2) | 17.6 (7.2) | .3 (3.3) |
Note: Responders and non-responders within active trial and sham crossover study phases did not differ significantly from each other in demographic or ADHD symptom treatment change. Combined includes both active trial, which was double-blind, and sham crossover, which was open label. All data presented as means and standard deviations unless noted.
ADHDRS = ADHD Rating Scale; CGIS = Clinical Global Impression, Severity; IQ = estimated intelligence; NR = non-responders; Resp = responders; SES = socioeconomic status; tx chg = treatment change after 4 weeks of TNS.
TNS treatment responder baseline profile.
Responders were lower on baseline WRAT Spelling and (F(1,49)=4.6, p=.04) Math (F(1,49)=4.1, p=.05), with trends towards lower WRAT Reading (F(1, 49)=3.8, p=.06) and WISC Digit Span (F(1,49)=3.3, p=.08) than non-responders, but there were no significant differences on Flanker task performance (Accuracy, RT, RTSD, all p-values >.2) or SWM accuracy (p > .3). On the behaviorally rated measures of executive function, the RESP group had significantly worse cognitive functioning (ie, higher t-scores) relative to the NR group on the parent-completed the CBCL Sluggish Cognitive Tempo index (F(1, 49)=7.3, p=.009) and BRIEF Initiate (F(1, 49)=7.2, p=.01), Working Memory (F(1, 49)=20.7, p<.001), Planning (F(1, 49)=17.8, p<.001), Organization (F(1, 49)=5.9, p=.02), Metacognition (F(1, 49)=14.9, p<.001) scores and General Executive Composite (GEC; [F(1,49)=5.8, p=.02]). On EEG measures, right frontal (F4 electrode) spectral power in the theta (4–7 Hertz [Hz] [F(1,45)=9.2, p=.004]) and alpha (8–12 Hz; [F(1,45)=9.2, p=.004]) bands was significantly lower among treatment responders relative to non-responders (see Table 2).
Table 2:
Trigeminal Nerve Stimulation Treatment Responder Status: Baseline Differences and Prediction of Treatment Response
| Baseline measures |
Prediction |
|||||
|---|---|---|---|---|---|---|
| Responder | Non-Responder | ADHD sxs | Resp status | |||
|
|
||||||
| mean (SD) | mean (SD) | F | β [CI] | t | AUC (SE) | |
| Child Behavior Checklist | ||||||
| Sluggish Cognitive Tempo | 66.0 (8.2) | 60.3 (8.9) | 7.3** | −0.41 [−.7, −.1] | −3.1*** | 0.69 (0.1) |
| BRIEF Rating Scale | ||||||
| Initiate | 69.8 (10.6) | 62.6 (8.7) | 7.2** | −0.13 [−.3, .1] | −0.9 | |
| Working Memory | 76.4 (6.5) | 68.0 (6.5) | 20.7*** | −0.41 [−.7, −.1] | −2.9*** | 0.83 (.1)** |
| Planning | 73.1 (9.0) | 62.7 (8.5) | 17.8*** | −0.36 [−.5, −.1] | −2.6** | 0.75 (.1)* |
| Organization | 61.2 (9.6) | 55.5 (6.7) | 5.9* | −0.21 [−.5, .1] | −1.4 | |
| Metacognition | 73.2 (8.6) | 64.9 (6.5) | 14.9*** | −0.32 [−.6, −.03] | −2.2* | 0.74 (.1)* |
| General Executive Composite | 72.2 (10.3) | 66.0 (7.4) | 5.8* | −0.79 [−.3, .18] | −0.6 | |
| Wide Range Achievement Test | ||||||
| Spelling | 102.5 (17) | 112 (14.1) | 4.6* | 0.24 [−.03, .3] | 1.7**** | |
| Reading | 102.5 (16.3) | 111.1 (14.4) | 3.9**** | |||
| Math | 98.9 (12.4) | 108.1 (18.7) | 4.1* | 0.21 [−.04, .24] | 1.5 | |
| Flanker Task | ||||||
| Accuracy | 0.66 (0.2) | 0.62 (0.2) | <1 | |||
| Reaction time | 658 (121) | 621 (106) | 1.2 | |||
| Reation time Variability | 191 (54) | 190 (68) | <1 | |||
| Working Memory | ||||||
| WISC Digit Span | 9.6 (2.3) | 10.8 (2.6) | 3.3**** | |||
| SWM Accuracy | 0.73 (0.1) | 0.71 (0.1) | <1 | |||
| EEG | ||||||
| F4 theta (4–7 Hz) power | 51.7 (6) | 54.7 (4.3) | 7.7** | 0.43 [.2 1.1] | 3.0*** | 0.23 (.1) |
| F4 alpha (8–12 Hz) power | 49.6 (5.8) | 52.9 (3.9) | 8.3** | 0.44 [.3, 1.2] | 3.2*** | 0.21 (.1) |
Note. Responders had poorer cognitive performance and lower EEG power at baseline. BRIEF Working Memory score was the strongest predictor of treatment response and post treatment ADHD symptoms treatment. ADHD sxs = Attention-deficit/hyperactivity symptoms post TNS treatment; AUC=area under curve; β=standardized Beta coefficient; BRIEF=Behavioral ratings of individual executive functions; CI= 95% confidence interval; EEG=electroencephalogram; Hz=hertz; SWM=spatial working memory; SE=standard error; WISC=Wechsler Intelligence Scale for Children.
p <.05
p < .01
p <.005
p <.1
Prediction of treatment response
The measures that differed significantly at baseline were then tested for whether the baseline score was predictive of end of treatment ADHD-RS Total Score. Several behavioral measures of cognitive dysfunction, such as the CBCL Sluggish Cognitive Tempo (β=−.40, 95% confidence intervals [CIs]=−.68, −.14], p=.004), BRIEF Working Memory (β=−.40, 95% CI=−.70, −.14], p=.004), Planning (β=−.36, 95% CI=−.51, −.08], p=.01) and Metacognition (β=−.32, 95% CIs=−.57,−.04], p=.02) subscales were significantly predictive of post-TNS treatment ADHD scores (see Table 2). In addition, the EEG right-frontal theta (β=.43, 95% CIs=.2, 1.1], p=.005) and alpha band power (β=.45, 95% CI=.3, 1.2], p=.003) measures significantly predicted ADHD symptoms after treatment. In contrast, WRAT Spelling (β=.24, 95% CI=−.02, .3], p=.09) and Math (β=−.21, 95% CI=−.04, .24], p=.15) and BRIEF Initiate (β=−.14, 95% CI=−.35, .11], p=.32), Organization (β=−.22, 95% CI=−.48, .06], p=.13), and GEC (β=−.07, 95% CIs=−.3, .2], p=.58) were not predictive of ADHD symptoms post-treatment.
TNS treatment-related change in cognitive function and EEG power.
EEG and BRIEF measures that significantly predicted post-treatment ADHD symptoms were examined for TNS treatment related change. EEG was collected at baseline and week 4 of the active trial and not for sham crossover participants, therefore EEG treatment changes were limited to the active trial responders. EEG data were missing (technical difficulties at baseline or Week 4) for three participants, one from the responder group and two from the non-responder group, leaving 14 participants in the RESP and NR groups for pre-post treatment analyses. Among RESP, TNS treatment resulted in right frontal theta- and alpha-band power increase whereas the NR group did not (F4 theta: F(1, 25)=4.4, p=.05, F4 alpha: F(1, 25) =4.1, p=.06, partial η2 =.18) (see Fig 3). Finally, treatment related change in F4 theta was moderately correlated with ADHD symptom change (r=.3, p=.14), however it did not reach statistical significance.
The BRIEF was collected during the crossover period, thus significant treatment responder effects were tested in the combined active trial and sham crossover groups. To account for placebo effects that may have occurred during the active trial, pre-treatment observations for the sham cross-over group were moved forward from baseline to week 4 and post-treatment measurement was at week 9. Several BRIEF scales, such as Working Memory, Metacognition, Initiate, Planning, and Organization, demonstrated significant treatment related change from pre- to post-TNS measurements (Fig. 2). Significant group by time interactions indicated that TNS responders showed significant treatment related improvement in BRIEF Metacognition (F(1,45)=38.6, p<.001, partial η2 =.47), Working Memory (F(1,45)=41.1, p<.001, partial η2 =.48), Initiate (F(1,45)=18.3, p<.001, partial η2 =.29), Planning (F(1,45)=36.7, p=.001, partial η2 =.51), and Organization (F(1,45)=19, p=.001, partial η2 =.30), while NR did not change. Finally, treatment related change in these BRIEF variables and ADHDRS Total scores were very strongly correlated, with Pearson r’s ranging from .65 (Planning, p=6.5E-7) to .79 (Working memory, p =3.0E-11), indicating the BRIEF score changes are commensurate with ADHD symptoms during TNS treatment.
Figure 2: Treatment Change in Right Frontal Electroencephalogram Power and Executive Function Scores.
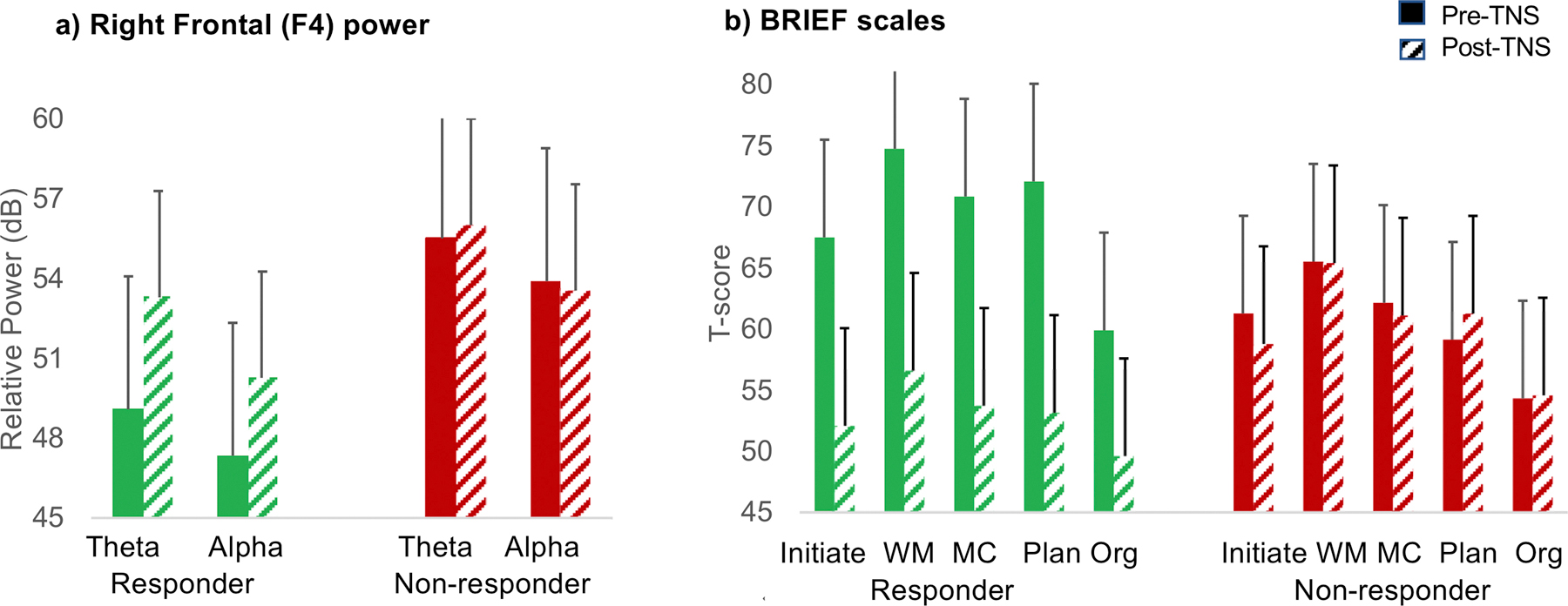
Note: In panel A (left), responders exhibited increased power in right frontal theta (4–7 Hertz [Hz]) and alpha (8–12 Hz) power, whereas non-responders showed no change. In panel B (right), Responders generally had pre-treatment scores in the clinically impaired range (T-score ≥65) on the Behavioral Rating of Individual Executive Function (BRIEF) scales, which improved and normalized (T-score <60) with treatment, whereas non-responders show no changes and generally had T-score >60. Solid bars indicate pre-Trigeminal Nerve Stimulation (TNS) treatment. Diagonal bars indicate post-TNS treatment. dB=decibel; MC=Metacognition; Org=Organization; Plan=Planning; WM=Working Memory.
Using the measures that predicted post-treatment ADHD symptoms and the treatment related change in right frontal EEG power, a ROC curve analysis to predict post-treatment responder status indicated that baseline BRIEF WM score was the strongest predictor (AUC=.83, p=.003), followed by treatment-related change in F4 theta power (AUC=.81, p=.03), BRIEF Planning (AUC= .75, p=.02), and BRIEF Metacognition (AUC=.74, p=.03); baseline F4 theta and alpha EEG power measures were not significant predictors of responder status (AUC=.2, p=.13) (see Table 2). Collectively, these results indicate that the BRIEF WM score was the strongest predictor of TNS responder status and modulation of right frontal EEG power was the neural mechanism underlying TNS response.
DISCUSSION
The current study tests secondary outcomes of cognitive and EEG predictors of treatment response from the first successful double-blind, sham-controlled investigation of external trigeminal nerve stimulation treatment, which provided the basis for the first FDA-approval of a non-pharmacological, device-based therapy for ADHD.10 Our current analyses provide analysis of secondary outcomes for a mechanistic basis in understanding TNS effects in ADHD and suggest baseline executive function and EEG treatment change might serve as biomarkers predictive of positive treatment outcomes, consistent with the aims of personalized medicine.17 If confirmed, these findings represent a successful application of precision medicine in ADHD and potentially provide a simple and cost-effective method to identify patients more likely to response to TNS therapy.
The data thus far indicate that the best candidates for TNS treatment are children with ADHD who have executive functioning weakness or deficits. Across studies, approximately 50% of children with ADHD show executive dysfunction6–8, which reflects difficulties with top down control of attention and response inhibition; this maps on well to the TNS treatment response rate of ~50%.11 The BRIEF WM scale was a significant predictor of treatment response (AUC = .83) and post-TNS ADHD symptoms. Additionally, responders were clinically impaired (t-score > 70) on several BRIEF subscales at baseline, which were subsequently normalized (t-score < 60) over the course of treatment. Non-responders, on the other hand showed virtually no change on BRIEF subscales after 4 weeks of TNS treatment.
Notably, performance on cognitive measures (SWM and Flanker task) were not predictive of treatment response and the pattern of scores differed significantly from the behaviorally rated measures of cognitive function. Low correlation between measures has been widely reported32, 33, suggesting they represent different aspects of cognitive functioning. While performance-based measures such as the SWM or Flanker task are thought to measure specific cognitive processes within the context of a controlled environment, behavioral ratings of executive functions encompass a broader set of cognitive skills that are utilized while functioning in everyday environments. Given our hypothesis of the fronto-basal ganglia network involvement, cognitive tasks that involve motor inhibition may show more treatment-related change in future studies. Pretreatment screening with the parent-rated BRIEF might prove to be a simple and cost-effective measure of identifying patients more likely to respond positively to TNS. This requires confirmation in prospective study.
The neural mechanism underlying TNS treatment effects was increased cortical activity in right frontal regions, which makes sense with the hypothesized TNS neural effects of activation of anterior cingulate, inferior frontal gyrus, and medial and middle frontal gyri including DLPFC13. These changes in frontal brain activity are predictive of treatment-related improvement in executive functioning, which in turn drove ADHD symptom reduction. Right frontal brain regions have been implicated by numerous studies in the pathophysiology of ADHD34–36 particularly during response inhibition tasks. The data presented here support our hypothesis regarding activation of the fronto-basal ganglia network, as reflected by TNS-related increase in EEG right frontal spectral power, resulting in normalization of executive dysfunction and improved top down control of ADHD symptoms.
Limitations of this first report of baseline predictors of TNS response include that the current study derives from the original RCT and all results should be considered exploratory until confirmed via independent replication. Both type 1 and type 2 error rate may be of concern due to limited sample size. Type 1 error rate was addressed using stepwise analyses and a conservative p-value (.01) threshold for significance. Several BRIEF subscales, particularly Working Memory, survived this correction; nonetheless, the possibility of false positive findings exist, which should be addressed via replication. Use of sham cross-over subjects in the analysis may be a limitation since they are technically in open label treatment. However, the baseline observation was carried forward for the sham cross-over group, which likely accounted for placebo effects that occurred during the active, double-blind trial. In addition, responders and non-responders in both the active double-blind trial and sham cross-over groups were not significantly different in terms of demographics, ADHD symptoms or amount of treatment-related change in ADHD symptoms, suggesting it was appropriate to combine the groups.
In conclusion, TNS is an FDA approved, non-pharmacological, minimal risk treatment that improves ADHD symptomatology and day-to-day executive functioning via increased cortical activity in right frontal regions for approximately half of children who received this therapy. The BRIEF rating scale is commonly used clinically and easily deployed in community settings, which should facilitate better screening of children who are most appropriate for TNS treatment. Future work on precision medicine approaches for treatment interventions, pharmacological and non-pharmacological alike, is highly feasible and should be utilized more commonly across psychiatric populations. More research is needed on TNS, including but not limited to durability of effects, efficacy for individuals outside of the 8–12 year old range, and additive or interaction effects with other empirically supported ADHD treatments.
Supplementary Material
Acknowledgments
This study was supported by a National Institute of Mental Health grant R34 MH10182 (to Drs. McGough and Loo, Co-PIs). Study devices and some materials were provided by NeuroSigma, Inc. in response to an investigator-initiated request.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the International Society for Child and Adolescent Psychopathology 19th Biennial Meeting; June 26–29, 2019; Los Angeles, California and the American Academy of Child and Adolescent Psychiatry 65th Annual Meeting; October 23–28, 2019; Chicago, Illinois.
Dr. Loo served as the statistical expert for this research.
Disclosure: Dr. McGough has provided expert witness testimony for Eli Lilly and Co. and Tris Pharma and has received DSMB honoraria from Sunovion. Drs. Loo, Ellis, Cowen, Dillon and Ms. Salgari have reported no biomedical financial interests or potential conflicts of interest.
Clinical trial registration information: Developmental Pilot Study of External Trigeminal Nerve Stimulation for ADHD; http://clinicaltrials.gov; NCT02155608
References
- 1.Xu G, Strathearn L, Liu B, Yang B, Bao W. Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997–2016. JAMA Netw Open Aug 3 2018;1(4):e181471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry Oct 2012;51(10):990–1002 e1002. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry Apr 2006;163(4):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solanto MV, Pope-Boyd SA, Tryon WW, Stepak B. Social functioning in predominantly inattentive and combined subtypes of children with ADHD. J Atten Disord Jul 2009;13(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers Aug 6 2015;1:15020. [DOI] [PubMed] [Google Scholar]
- 6.Loo SK, Humphrey LA, Tapio T, et al. Executive functioning among Finnish adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry Dec 2007;46(12):1594–1604. [DOI] [PubMed] [Google Scholar]
- 7.Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. Jun 1 2005;57(11):1224–1230. [DOI] [PubMed] [Google Scholar]
- 8.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry Jun 1 2005;57(11):1336–1346. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Petty CR, Fried R, et al. Stability of executive function deficits into young adult years: a prospective longitudinal follow-up study of grown up males with ADHD. Acta Psychiatr Scand Aug 2007;116(2):129–136. [DOI] [PubMed] [Google Scholar]
- 10.McGough JJ, Loo SK, Sturm A, Cowen J, Leuchter AF, Cook IA. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain Stimul Mar-Apr 2015;8(2):299–304. [DOI] [PubMed] [Google Scholar]
- 11.McGough JJ, Sturm A, Cowen J, et al. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry Apr 2019;58(4):403–411 e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraone SV. Using Meta-analysis to Compare the Efficacy of Medications for Attention-Deficit/Hyperactivity Disorder in Youths. P T Dec 2009;34(12):678–694. [PMC free article] [PubMed] [Google Scholar]
- 13.Cook IA, Espinoza R, Leuchter AF. Neuromodulation for depression: invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg Clin N Am Jan 2014;25(1):103–116. [DOI] [PubMed] [Google Scholar]
- 14.McGough JJ, Loo SK, Cook IA. Reply to “Transcutaneous electric currents to target the peripheral and central nervous system in children with attention deficit hyperactivity disorder”. Clin Neurophysiol Oct 2019;130(10):2008–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner J, Wessel JR, Ghahremani A, Aron AR. Establishing a Right Frontal Beta Signature for Stopping Action in Scalp EEG: Implications for Testing Inhibitory Control in Other Task Contexts. J Cogn Neurosci Jan 2018;30(1):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessel JR, Aron AR. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron Jan 18 2017;93(2):259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan EB, Banaschewski T, Barker GJ, et al. Identifying biological markers for improved precision medicine in psychiatry. Mol Psychiatry Feb 2020;25(2):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry Jul 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 19.DuPaul G, T. P, Anastopoulos A, Reid R ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation New York: Guilford; 1998. [Google Scholar]
- 20.Guy W Assessment Manual for Psychopharmacology (Revised) Washington, DC: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 21.Weschler D Wechsler Abbreviated Scale of Intelligence: Manual San Antonio, Tx: Psychological Corporation; 1999. [Google Scholar]
- 22.Achenbach TM. Manual for the Revised Child Behavior Profile and Child Behavior Checklist Burlington, VT: Author; 2001. [Google Scholar]
- 23.Gioia GA, Isquith PK, Retzlaff PD, Espy KA. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol Dec 2002;8(4):249–257. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D The Wechsler Intelligence Scale for Children, 4th edition San Antonio, Texas: The Psychological Corporation; 2003. [Google Scholar]
- 25.Wide Range Achievement Test: Jastak Associates; 1978. [Google Scholar]
- 26.Glahn DC, Kim J, Cohen MS, et al. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage Sep 2002;17(1):201–213. [DOI] [PubMed] [Google Scholar]
- 27.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci Apr 1 2002;14(3):340–347. [DOI] [PubMed] [Google Scholar]
- 28.Loo SK, Bilder RM, Cho AL, et al. Effects of d-Methylphenidate, Guanfacine, and Their Combination on Electroencephalogram Resting State Spectral Power in Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry Aug 2016;55(8):674–682 e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makeig S, Bell AJ, T.P. J, Sejnowski TJ. Independent component analysis of electroencephalographic data. In: Touretzky D, Mozer M, Hasselmo M, eds. Advances in Neural Information Processing Systems 1996:145–151
- 30.Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry Nov 2002;159(11):1896–1901. [DOI] [PubMed] [Google Scholar]
- 31.Steele M, Jensen PS, Quinn DM. Remission versus response as the goal of therapy in ADHD: a new standard for the field? Clin Ther Nov 2006;28(11):1892–1908. [DOI] [PubMed] [Google Scholar]
- 32.Davidson F, Cherry K, Corkum P. Validating the Behavior Rating Inventory of Executive Functioning for Children With ADHD and Their Typically Developing Peers. Appl Neuropsychol Child 2016;5(2):127–137. [DOI] [PubMed] [Google Scholar]
- 33.Munoz M, Filippetti V. Confirmatory factor analysis of the BRIEF-2 parent and teacher form: Relationship to peformance-based measures of executive functions and academic achievement. Applied Neuropsychology: Child 2019. [DOI] [PubMed] [Google Scholar]
- 34.Bos DJ, Oranje B, Achterberg M, et al. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J Child Psychol Psychiatry Jul 2017;58(7):810–818. [DOI] [PubMed] [Google Scholar]
- 35.Cai W, Griffiths K, Korgaonkar MS, Williams LM, Menon V. Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Mol Psychiatry Oct 29 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry Feb 2013;70(2):185–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


