SUMMARY
Purpose
To assess clinical, laboratory and radiological differences between Delta and Alpha SARS-CoV-2 variants.
Materials and Methods
Twenty SARS-CoV-2 patients admitted from 30th of August to 30th of October 2021 (period with estimated highest prevalence of Delta variant circulation in Italy) were enrolled. Patients were matched in a 1:1 ratio with same gender and same age +/− 2 years controls admitted from 1st of September 2020 to 30th of January 2021 (predominant circulation of Alpha variant). Chest computed tomography (CT) were retrospectively evaluated. Main clinical parameters, radiological and laboratory findings were compared between two groups.
Results
Patients with probable Delta variant had significantly higher CT severity scores, lower PaO2/FiO2 ratio and higher C-reactive protein and lactate dehydrogenase levels at admission. On multivariate analysis, probable Delta variant infection was associated with higher CT severity score. Ground glass opacities and crazy paving patterns were more frequently noticed than consolidation, with the latter being more frequent in Delta cohort, even though not significantly. According to prevalent imaging pattern, the consolidation one was significantly associated with pregnancy (p=0.008).
Conclusions
Patients admitted during predominance of Delta variant circulation had a more severe lung involvement compared to patients in infected when Alpha variant was predominant. Despite imaging pattern seems to be not influenced by viral variant and other clinical variables, the consolidative pattern was observed more frequently in pregnancy.
Keywords: SARS-CoV-2, computed tomography, pneumonia, variant of concern
INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the pandemic coronavirus associated with Coronavirus Infectious Diseases 2019 (COVID-19), a clinical entity that may cause from pauci-symptomatic illness to severe bilateral interstitial pneumonia, also leading to acute respiratory distress syndrome [1–4]. Ground glass opacities, consolidation and crazy paving pattern are typical chest computed tomography (CT) findings of COVID-19 [3, 5–11]. Spontaneous mutations in viral genome, especially when they occur on the receptor binding domain, can be associated with the circulation of viral variant of concern (VOCs), leading to either increased transmissibility, disease severity, or reduction in antibody neutralization and response to vaccines and therapies [12–14].
Since the summer 2021, SARS-CoV-2 B.1.617.2 (Delta/Indian) became the prevalent circulating variant in Europe, replacing the B.1.1.7 (Alpha/UK) variant predominating until then [15].
Delta variant was associated with higher transmissibility and higher risk of hospitalization, often with rapid evolution of the respiratory impairment and needing for intensive care admission, especially in pregnant women, among which severe and critical cases significantly increased with Delta peak compared to previous pandemic waves, according to a prospective study by Adikhari et al. [16]. Nonetheless, data about clinical-radiological implications of such phenomenon are lacking in literature.
At our institution, the largest University Hospital in Southern Italy and the regional reference center for COVID-19 in pregnancy, we observed more than 1,000 cases of COVID-19 from the beginning of the pandemic, with around 30% of them that were pregnant women.
The aim of this this study was to assess clinical, laboratory and radiological differences between Delta and Alpha variants during the first 10 days of the disease.
MATERIALS AND METHODS
Study design and participants
We conducted a retrospective case-control study of patients admitted to our COVID facility from 30th of August to 30th of October 2021 (predominance of Delta variant circulation). In all patients, nasopharyngeal swabs were collected and processed for RT-PCR assay to confirm the diagnosis. Only patients having chest CT-scan within 10 days from symptoms onset were included. Patients were matched in a 1:1 ratio with same gender and same age +/− 2 years controls consecutively admitted from 1st of September 2020 to 30th of January 2021 (predominant circulation of Alpha variant). We excluded charts with missing data.
Data collection
An electronic search was conducted among patients admitted in the study period in Infectious Diseases Unit of our facility. We collected data about: age; sex; date of admission and discharge; days from admission to CT scan; date of COVID-19 symptoms onset; clinical severity according to the World Health Organization (WHO) Ordinal Scale for Clinical Improvement (OSCI); Charlson Co-morbidity Index; pregnancy status; partial oxygen pressure/oxygen fraction (PaO2/FiO2) ratio, lactate dehydrogenase (LDH), C-reactive protein (CRP), white blood cell, absolute lymphocytes and neutrophils count [17, 18]. All laboratory data were collected on admission.
With respect to pregnancy, CT scan was performed after cesarean section in all patients.
CT protocol
All exams were performed on a single CT scanner (Toshiba Astelion 16 Slices, Tokyo, Japan) dedicated to COVID-19 patients. The scanning range was from the apex to lung base. The detailed parameters were the following: tube voltage, 120 kVp, mAs modulation, 80–120 mAs, slice thickness, 1.0 mm, reconstruction interval, 0.8mm. The CT dose index volume (CTDI vol; in mGy), a standardized measure of the output radiation dose of a CT scanner, was collected.
Image analysis
CT images were reviewed by two radiologists with 6 and 25 years of experience in thoracic imaging, respectively. According to the standard glossary reported by the Fleischner Society prevalent radiological patterns were identified as ground glass opacity (GGO), crazy-paving pattern, and pulmonary consolidation [19]. In all patients a semi-quantitative CT severity score proposed by Chung et al. was calculated per each of the 5 lobes considering the extent of anatomic involvement, as follows: 0, no involvement; 1, 5–25% involvement; 2, 26–50% involvement; 3, 51–75% involvement; and 4, >75% involvement [20]. An overall lung total severity score was reached by summing the five lobe scores (range of possible scores, 0–20). The presence of extra-pulmonary findings such as lymphadenopathies and pleural effusion was also collected.
Statistical analysis
The statistical analysis was performed using SPSS version 27 (SPSS Inc. Chicago, IL). Continuous variables were reported as median and interquartile range and categorical variables as frequency and percentages. Categorical variables were confronted with Chi-squared test and Fisher’s exact test when appropriate. Continuous variables were confronted with logistic regression. A significance level of 0.05 was set for the interpretation of the results. A multivariate logistic analysis was used to confront the variables that resulted significant at the univariate analysis.
RESULTS
Among 60 patients admitted to our facility during the observational period for cases (Delta period), only 37 were admitted during the first 10 days of the disease. Out of 37 screened patients with probable Delta variant infection, 10 patients did not undergo a CT-scan and 7 had no radiological images available on hospital online consultation platform at the time of the analysis. Therefore, we included 20 cases (Table 1). The median CT dose index volume was respectively 11.4 mGy (IQR,4) for patients with probable Delta variant infection and 11 mGy (IQR,5.5) for the control group.
Table 1.
Demographic and clinical characteristics of cases and controls.
| Cases (N=20) | Controls (N=20) | Total (N=40) | Chi 2 | OR (95%CI) | aOR (95%CI) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Females, n (%) | 11 (55) | 8 (40) | 19 (47.5%) | Chi2=0.9, p=0.26 | ||
|
| ||||||
| Age (years), median (IQR) | 51.5 (33–63.25) | 52 (33.25–65.25) | 52 (33.25–64.25) | 0.9 (0.97–1.037), p=0.766 | ||
|
| ||||||
| Charlson Index, median (IQR) | 1 (1–3.75) | 1 (0–2.75) | 1 (0–3) | 1.03 (0.74–1.2), p=0.83 | ||
|
| ||||||
| Pregnant (post C-section), n (%) | 4 (20) | 4 (20) | 8 (20) | Chi2=1, p=0.653 | ||
|
| ||||||
| Days from symptoms to CT scan, median (IQR) | 7 (3–8.5) | 5 (1–7) | 6 (2.25–8) | 1.2 (1–1.44), p=0.05 | ||
|
| ||||||
| Days from admission to CT-scan, median (IQR) | 0 (0–1.75) | 1 (1–2.75) | 0.5 (0–2) | 0.95 (0.7–1.3), p=0.76 | ||
|
| ||||||
| WHO severity scale on admission (OSCI), median (IQR) | 4 (4–4) | 4 (3–4) | 4 (4–4) | 1.95 (0.9–5), p=0.15 | ||
|
| ||||||
| CT severity score, median (IQR) | 8 (6.25–11.75) | 5 (5–9.75) | 7 (5–10.75) | 1.225 (1.008–1.48), p=0.04 | 1.132 (1.002 1.453) p=0.035 | |
|
| ||||||
| WBC (x 10^3/uL), median (IQR) | 7.38 (4.9–9.6) | 7.36 (5.44–9.38) | 7.38 (5.2–9.38) | 0.954 (0.755–1.2), p=0.7 | ||
|
| ||||||
| Neutrophils (x 10^3/uL), median (IQR) | 6 (3.9–8.2) | 5.63 (4–8) | 5.9 (4–8.2) | 1.011 (0.795–1.285), p=0.98 | ||
|
| ||||||
| Lymphocytes (x 10^3/uL), median (IQR) | 0.69 (0.55–0.93) | 1.02 (0.61–1.75) | 0.790 (0.590–1.29) | 0.284 (0.073–1.115), p=0.7 | ||
|
| ||||||
| PaO2/FiO2, median (IQR) | 200 (150–277) | 314 (242–334) | 266 (150–330) | 0.992 (0.985–0.999), p=0.036 | 0.996 (0.99 1.02) p=0.12 | |
|
| ||||||
| CRP (mg/dL), median (IQR) | 7.6 (3.8–14.75) | 3.6 (1.4–5.44) | 4.8 (2.5–9.3) | 1.173 (1.009–1.363), p=0.038 | 1.060 (0.8 1.15) p=0.42 | |
|
| ||||||
| LDH, median (IQR) | 377 (324–460) | 256 (207–363) | 344 (237–414) | 1.010 (1.002–1.018), p=0.01 | 1.004 (0.97 1.005) p=0.21 | |
|
| ||||||
| Chest CT prevalent pattern, n (%) | ||||||
| - Consolidative | 6 (30) | 3 (15) | 9 (22) | Chi2= 1.29, p=0.22 | ||
| - Non consolidative (GGO or CP) | 14 (70) | 17 (85) | 31 (77) | |||
|
| ||||||
| Chest CT extra-pulmonary findings, n (%) | ||||||
| - Lymphadenopathy | 3 (15) | 2 (10) | 5 (12.5) | Chi2=0.3, p=0.5 | ||
| - Pleural effusion | 1 (5) | 1 (5) | 2 (5) | Chi2= 0, p=1 | ||
|
| ||||||
| ICU admission, n (%) | 6 (30) | 2 (10) | 8 (20) | Chi2=2.5, p=0.118 | ||
|
| ||||||
| Death, n (%) | 3 (15) | 0 (0) | 3 (7.5) | Chi2=3.24, p=0.115 | ||
C-section: cesarean section; CT: computed tomography; WHO: World Health Organization; OSCI: Ordinal Scale for Clinical Improvement; WBC: white blood count; PaO2/FiO2: partial oxygen pressure/oxygen fraction; CRP: C-reactive protein; LDH: lactate dehydrogenase; GGO: ground glass opacity; CP: crazy paving; ICU: Intensive Care Unit.
Patients with probable Delta variant infection had significantly higher CT severity score (p=0.04), lower PaO2/FiO2 ratio (p=0.036) and higher CRP (p=0.038) and LDH (p=0.01) levels at admission. At multivariate analysis, probable Delta variant infection was only associated with higher CT severity score (aOR 0.56; 95%CI 0.32–0.961; p=0.035). In the whole sample, ground glass opacities and crazy paving patterns were more frequent than consolidation (77% vs 22%), with the latter being more frequent in the Delta cohort than in the Alpha cohort (30% vs 15%), even though not significantly (Chi2=1.29, p=0.22). No differences among cases and controls were found in terms of frequency of lymphadenopathies and pleural effusion.
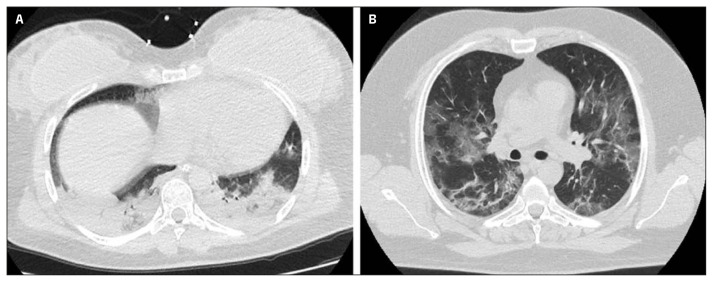
According to the prevalent imaging pattern (consolidated vs non-consolidated) in the overall population (cases and controls), patients with consolidated pattern were more frequently females, pregnant, younger, with lower LDH and Charlson Co-morbidity Score and higher WBC, neutrophils, lymphocytes, CRP and PaO2/FiO2 levels, compared to patients with non-consolidated pattern, although only female sex (Chi2=4.26, p=0.04), age (OR 0.94; 95%CI 0.902–0.999; p=0.046) and pregnancy (Chi2=9.17, p=0.008) resulted to be independently associated with consolidated pattern at admission (Table 2). Representative images of chest CT of two patients with probable Delta and Alpha variants infection are shown in Figure 1.
Table 2.
Demographic and clinical characteristics according to prevalent imaging pattern.
| Consolidated (N=9) | Non-consolidated (N=31) | Total (N=40) | Chi 2 | OR (95%CI) | |
|---|---|---|---|---|---|
| Females, n (%) | 7 (77.8) | 12 (38.7) | 19 (47.5) | Chi2=4.26, p=0.04 | |
| Age (years), median (IQR) | 33 (27.5–49) | 58 (41–65) | 52 (33.25–64.75) | 0.94 (0.902–0.999), p=0.046 | |
| Pregnant (post C-section), n (%) | 5 (55.6) | 3 (9.7) | 8 (20) | Chi2=9.17, p=0.008 | |
| Charlson Index, median (IQR) | 0 (0–1) | 2 (0–4) | 1 (0–3) | 0.11 (0.345–1.16), p=0.62 | |
| WHO severity scale on admission (OSCI), median (IQR) | 4 (3.5–4.5) | 4 (4) | 4 (4) | 1.118 (0.439–.215), p=0.735 | |
| WBC (x 10^3/uL), median (IQR) | 9.2 (6.5–9.7) | 7.2 (3.8–8.1) | 7.38 (5.23–9.38) | 1.081 (0.817–1.432), p=0.832 | |
| Neutrophils (x 10^3/uL), median (IQR) | 6 (4.9–8.3) | 5.6 (3.8–8.1) | 5.98 (4–8.2) | 1.053 (0.791–1.403), p=0.723 | |
| Lymphocytes (x 10^3/uL), median (IQR) | 0.86 (0.57–1.72) | 0.78 (0.59–1.29) | 0.79 (0.6–1.3) | 1.729 (0.433–6.9), p=0.438 | |
| PaO2/FiO2, median (IQR) | 200 (135–322) | 162 (280–330) | 266 (150–330) | 0.997 (0.989–1.004), p=0.1 | |
| CRP (mg/dL), median (IQR) | 5.7 (2–14.6) | 4.2 (2.6–9.3) | 4.8 (2.5–4.8) | 1.029 (0.916–1.157), p=0.62 | |
| LDH (U/L), median (IQR) | 327 (236–417) | 348 (237–418) | 344 (237–414) | 0.1 (0.993–1.006), p=0.8) | |
| ICU admission, n (%) | 4 (44) | 4 (13) | 8 (20) | Chi2=6.3, p=0.06 | |
| Death, n (%) | 0 (0) | 3 (10) | 3 (7.5) | Chi2=1, p=0.45 |
C-section: caesarean section; WHO: World Health Organization; OSCI: Ordinal Scale for Clinical Improvement; WBC: white blood count; PaO2/FiO2: partial oxygen pressure/oxygen fraction; CRP: C-reactive protein; LDH: lactate dehydrogenase; ICU: Intensive Care Unit.
Figure 1.
Chest CT findings of COVID-19 pneumonia on axial images of two patients respectively with probable Delta variant, showing predominant consolidative pattern with a CT severity score of 16 (A), and Alpha variant characterized by prevalent non-consolidative features with a CT severity score of 10 (B).
DISCUSSION
In our study we specifically aimed to assess the clinical-radiologic characteristics of different circulating of SARS-CoV-2 VoCs during Alpha and Delta variant predominance. According to our results, probable infection with Delta variant is independently associated with higher severity score at CT scan performed in the early phase of COVID-19 compared to Alpha variant. The impact of such data on clinical course of the diseases and patient’s outcome is still to be demonstrated, although our cohort showed higher level of CRP and LDH and lower PaO2/FiO2 ratio among patients with probable Delta infection. Moreover, prevalent consolidative pattern resulted to be only associated with the demographic characteristics of the patients (young, female, pregnant), rather than clinical severity or prognostic markers such as high CRP or low lymphocyte count. Such data seems to support the evidence that parenchymal consolidation at CT scan of the disease should not be considered as a reliable marker of bacterial coinfection/superinfection in early stages of COVID-19, thus making empiric antibiotic prescription often inappropriate [21]. However, Granata et al. showed no radiological pattern differences among VoC in another retrospective study. Actually, they considered also vaccination as a confounding factor but they enrolled only ICU patients and they did not refer to the clinical history of COVID-19 [22]. In our opinion, this should be considered a bias as only severe cases were hospitalized in ICU.
Actually, also another larger retrospective Italian cohort showed no differences in radiological patterns between the different waves with a trend towards a more severity of lung involvement during the delta period [23]. However, even if they enrolled only patients that performed CT scan at admission, no data about COVID-19 clinical history and symptoms onset were available so that we cannot exclude that different radiological patterns could be due to different stages of the disease.
As we stated above, a consolidative pattern was more frequent in pregnant women. It is noteworthy a severe course of COVID-19 in pregnant women during delta variant surge. In detail, Seasly and colleagues have showed an increased proportions of severe–critical disease (13% vs 36%, aRR 2.76) and ICU admissions (8% vs 29%, aRR3.42) in the Delta cohort compared to the former waves [24]. Furthermore, they also demonstrated a significant increase in needs of respiratory support (13% vs 36%, aRR 2.76) and intubation (5% vs 23% aRR 4.18) [24]. Our data support this evidence, as we firstly demonstrate a different imaging pattern in pregnant women with Delta variant infection, with parenchymal consolidation even during the first days of COVID-19. However, in our study we believe that a significant different radiological presentation (consolidative vs non-consolidative pattern, p=0.22) was not demonstrated among cases and controls probably due to two main reasons: our small sample size and lack of certain VoC identification during the first period of Delta predominance. However, according to the European Centre for Disease Prevention and Control, within the time interval of cases inclusion, more than 99% of the newly reported SARS-CoV-2 infections were attributed to Delta variant [15].
Despite the limitations of the study, namely its retrospective nature, the small number of enrolled patients and the lack of a sequencing test to assess the variant, our data show that patients admitted during the highest peak of delta variant circulation had a more severe radiological picture within the first 10 days of symptoms onset compared to patients of the former wave. In the overall population, pregnancy, female sex and young age were the only variables that resulted independently associated with a prevalent consolidative pattern on CT scan performed at admission, while no relation seems to exist between imaging pattern and clinical and biochemical variable associated with worse prognosis and bacterial infection.
In conclusion, during this pandemic we have witnessed dramatic changes in clinical, laboratory and radiological findings as different VoCs emerged. Our study demonstrated higher CRP values and worse clinical condition in the first days of COVID-19 during Delta Variant surge compared to Alpha variant predominance, with consolidative pattern being observed more frequently in pregnant patients.
Finally, despite we showed results referring to an outdated VoC, our study save interest as new VoCs with similar characteristics may appear in the next future. Our data could help physicians to correctly manage COVID-19 for example avoiding inappropriate prescription of antibiotics only referring to CT scan pattern.
Acknowledgements
Federico II COVID team: Luigi Ametrano, Francesco Beguinot, Giuseppe Castaldo, Letizia Cattaneo, Maria Carmela Domenica Conte, Mariarosaria Cotugno, Alessia d’Agostino, Giovanni Di Filippo, Isabella Di Filippo, Antonio Di Fusco, Nunzia Esposito, Mariarosaria Faiella, Lidia Festa, Maria Foggia, Maria Elisabetta Forte, Ludovica Fusco, Antonella Gallicchio, Ivan Gentile, Agnese Giaccone, Anna Iervolino, Carmela Iervolino, Antonio Iuliano, Amedeo Lanzardo, Federica Licciardi, Matteo Lorito, Simona Mercinelli, Fulvio Minervini, Giuseppina Muto, Mariano Nobile, Biagio Pinchera, Giuseppe Portella, Laura Reynaud, Alessia Sardanelli, Marina Sarno, Nicola Schiano Moriello, Maria Michela Scirocco, Fabrizio Scordino, Riccardo Scotto, Stefano Mario Susini, Anastasia Tanzillo, Grazia Tosone, Maria Triassi, Emilia Trucillo, Annapaola Truono, Ilaria Vecchietti, Giulio Viceconte, Riccardo Villari, Emilia Anna Vozzella, Emanuela Zappulo, Irene Zotta, Giulia Zumbo.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Funding source
None
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional ethic committee approval was waived due to the nature of the study, an information about the study was sent to the Ethic Committee of Federico II University as acknowledgement, as per local ethic committee regulation.
Informed consent
Informed consent was obtained from all individual participants included in the study by signing informed consent form for publication of clinical data and radiologic pictures.
Authors’ contributions
Guarantor of integrity of the entire study: Ivan Gentile; conceptualization: Giulio Viceconte, Andrea Ponsiglione, Antonio Riccardo Buonomo, Luigi Camera, Ivan Gentile; literature research: Biagio Pinchera, Riccardo Villari, Maria Foggia, Gerardo Gerundo; data analysis: Marco De Giorgi, Lorenzo Pinto, Riccardo Scotto, Biagio Pinchera, Riccardo Villari, Maria Foggia; statistical analysis: Giulio Viceconte, Riccardo Scotto; manuscript preparation: Giulio Viceconte, Andrea Ponsiglione, Antonio Riccardo Buonomo, Marco De Giorgi, Lorenzo Pinto; manuscript editing: Luigi Camera, Riccardo Scotto, Biagio Pinchera, Riccardo Villari, Maria Foggia, Gerardo Gerundo, Pasquale Abete, Arturo Brunetti; supervision: Luigi Camera, Pasquale Abete, Arturo Brunetti, Ivan Gentile.
Data availability statement
Raw data were generated at Federico II University Hospital. Derived data supporting the findings of this study are available from the corresponding author (GV) on request.
REFERENCES
- 1.Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut [Internet] 2020. May 20, [cited 2020 Jun 26];gutjnl-2020-321666. Available from: https://gut.bmj.com/content/early/2020/06/02/gutjnl-2020-321666. [DOI] [PMC free article] [PubMed]
- 2. Nacoti M, Ciocca A, Giupponi A, et al. At the Epicenter of the Covid-19 pandemic and humanitarian crises in Italy: changing perspectives on preparation and mitigation. NEJM Catal. 2020:1–5. Figure 1. [Google Scholar]
- 3. Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020;30(9):4874–4882. doi: 10.1007/s00330-020-06827-4. Available from: https://link.springer.com/article/10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forchette L, Sebastian W, Liu T. A Comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr Med Sci. 2021;41(6):1037–1051. doi: 10.1007/s11596-021-2395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol [Internet] 2020 Aug 1;30(8):4381–4389. doi: 10.1007/s00330-020-06801-0. [cited 2022 Feb 14] Available from: https://link.springer.com/article/10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beitzke D, Salgado R, Francone M, et al. Cardiac imaging procedures and the COVID-19 pandemic: recommendations of the European Society of Cardiovascular Radiology (ESCR) Int J Cardiovasc Imaging. 2020;36(10):1801–1810. doi: 10.1007/s10554-020-01892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanzione A, Ponsiglione A, Cuocolo R, et al. Chest CT in COVID-19 patients: Structured vs conventional reporting. Eur J Radiol. 2021;138:109621. doi: 10.1016/j.ejrad.2021.109621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–687. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koch V, Gruenewald LD, Albrecht MH, et al. Lung opacity and coronary artery calcium score: a combined tool for risk stratification and outcome prediction in COVID-19 patients. Acad Radiol. 2022;29(6):861–870. doi: 10.1016/j.acra.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukuda A, Yanagawa N, Sekiya N, Ohyama K, Yomota M, Inui T, et al. An analysis of the radiological factors associated with respiratory failure in COVID-19 pneumonia and the CT features among different age categories. Jpn J Radiol. 2021;39(8):783–790. doi: 10.1007/s11604-021-01118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng M. The prevention and management of the coronavirus disease 2019 (COVID-19) outbreak in radiology departments in epidemic areas. Jpn J Radiol. 2020;38(6):483–488. doi: 10.1007/s11604-020-00974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ECDC. Assessment of the further spread and potential impact of the SARS-CoV-2 Omicron variant of concern in the EU/EEA. 19th update. [Google Scholar]
- 13. Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature. 2021;589(7841):177–178. doi: 10.1038/d41586-021-00031-0. [DOI] [PubMed] [Google Scholar]
- 14. Raman R, Patel KJ, Ranjan K. COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules. 2021;11(7):993. doi: 10.3390/biom11070993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.+ Assessing SARS-CoV-2 circulation, variants of concern, non-pharmaceutical interventions and vaccine rollout in the EU/EEA, 16th update. 2021. [cited 2022 Feb 7]; Available from: https://www.ecdc.europa.eu/en/covid-19/surveillance/weekly-surveillance-report.
- 16. Adhikari EH, SoRelle JA, McIntire DD, Spong CY. Increasing severity of COVID-19 in preg]nancy with Delta (B.1.617.2) variant surge. Am J Obstet Gynecol. 2022;226(1):149. doi: 10.1016/j.ajog.2021.09.008. Available from:/pmc/articles/PMC8437765/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020 [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis [Internet] 2017;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3558716. [DOI] [PubMed] [Google Scholar]
- 19.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: Glossary of Terms for Thoracic Imaging1. 2008 Mar 1;246(3):697–722. doi: 10.1148/radiol.2462070712. https://doi.org/101148/radiol2462070712[Internet] [cited 2022 Mar 7] Available from: https://pubs.rsna.org/doi/abs/10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 20. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-NCoV) Radiology. 2020;29(1):202–207. doi: 10.1148/radiol.2020200230. Available from: https://pubs.rsna.org/doi/abs/10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farrell JM, Zhao CY, Tarquinio KM, Brown SP. Causes and consequences of COVID-19-associated bacterial infections. Front Microbiol. 2021;12:682571. doi: 10.3389/fmicb.2021.682571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Granata V, Fusco R, Villanacci A, et al. Imaging Severity COVID-19 Assessment in vaccinated and unvaccinated patients: comparison of the different variants in a high volume italian reference center. J Pers Med. 2022;12:955. doi: 10.3390/jpm12060955. [Internet]. 2022 Jun 10 [cited 2022 Sep 21]; 12 (6), 955. Available from: https://www.mdpi.com/2075-4426/12/6/955/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maggialetti N, Villanova I, Castrì A, et al. COVID-19 in Italy: Comparison of CT findings from time zero to the Delta Variant. Microorg. 2022 2022 Apr 10 9;10(4):796. 796. doi: 10.3390/microorganisms10040796. [Internet] [cited 2022 Sep 21] Available from: https://www.mdpi.com/2076-2607/10/4/796/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seasely AR, Blanchard CT, Arora N, et al. Maternal and Perinatal outcomes associated with the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Delta (B.1.617.2) Variant. Obstet Gynecol [Internet] 2021 Dec 1;138(6):842–844. doi: 10.1097/AOG.0000000000004607. [cited 2022 Feb 26] Available from: https://journals.lww.com/greenjournal/Fulltext/2021/12000/Maternal_and_Perinatal_Outcomes_Associated_With.4.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]