SUMMARY
The presence of co-morbidities is associated with a poor outcome in patients with COVID-19. The aim of the present study was to investigate the outcomes of patients with SARS-CoV-2 infection and chronic kidney disease (CKD) in order to assess its impact on mortality and severity of disease. We performed a multicenter, observational, 1:2 matched case-control study involving seventeen COVID-19 Units in southern Italy. All the adults hospitalized for SARS-CoV-2 infection and with pre-existing CKD were included (Cases). For each Case, two patients without CKD pair matched for gender, age (+5 years), and number of co-morbidities (excluding CKD) were enrolled (Controls). Of the 2,005 patients with SARS-CoV-2 infection followed during the study period, 146 patients with CKD and 292 patients without were enrolled in the case and control groups, respectively. Between the Case and Control groups, there were no statistically significant differences in the prevalence of moderate (17.1% vs 17.8%, p=0.27) or severe (18.8% and 13.7%, p=0.27) clinical presentation of COVID-19 or deaths (20.9% vs 28.1%, p=0.27).
In the Case group, the patients dead during hospitalization were statistically higher in the 89 patients with CKD stage 4–5 compared to 45 patients with stages 1–3 CKD (30.3% vs 13.3%, p=0.03). Our data suggests that only CKD stage 4–5 on admission was associated with an increased risk of in-hospital death.
Keywords: Chronic kidney disease, COVID-19, SARS-CoV-2 infection, severity of disease, mortality
INTRODUCTION
Since December 2019 the current outbreak of pneumonia due to SARS-CoV-2 rapidly spread from China to all over the world, assuming the characteristics of a global pandemic responsible for over 6,252,316 deaths (last update 5 May 2022) [1–3].
Although the data suggest that 80% of infections are mild or asymptomatic, certain underlying medical conditions, e.g. hypertension, active cancer, diabetes, and chronic kidney disease (CKD), carry an increased risk of severe illness [4, 5].
The kidney is a target of SARS-CoV-2 due to the marked expression of angiotensin-converting enzyme 2 (ACE2) receptors that mediate virus internalization [6, 7]. However, the data on the role of CKD on the outcome of COVID-19 is not conclusive: several studies showed that the patients with CKD had a worse prognosis and in-hospital death compared with patients without, especially if they presented with a dialysis-dependent CKD [6–9]. Nevertheless, the studies were often retrospective, on a small-size population and frequently without the evaluation of co-factors associated with a poor prognosis of COVID-19.
The aim of this pair-matched case-control study was to investigate the outcomes of patients with SARS-CoV-2 infection and CKD in order to assess its impact on the mortality and severity of the disease.
PATIENTS AND METHODS
Study design and setting
We performed a multicenter, observational, 1:2 matched case-control study involving seventeen COVID-19 Units in eight cities in the Campania region in southern Italy: Naples, Caserta, Salerno, Avellino, Benevento, Pozzuoli, Eboli and Vallo della Lucania.
The patients were adults (≥18 years), hospitalized with a diagnosis of SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) on a naso-oropharyngeal swab. The study period was from February 28th 2020 to November 1st 2021. All the patients with CKD were enrolled as Cases (Case group). For each Case, 2 patients without CKD, pair matched for gender, age (+5 years), number of co-morbidities (excluding CKD), were chosen from the Campania COVID-19 cohort (CoviCamp cohort) (Control group) [10–12]. Exclusion criteria included minority age, and lack of clinical data and/or of informed consent. No study protocol or guidelines regarding the criteria of hospitalization were shared among the centres involved in this study and the patients were hospitalized according to the decision of physicians of each centre.
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (n° 10877/2020). All procedures performed in this study were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards. Informed consent was obtained from all participants included in the study.
Data collection and variables
All demographic, clinical and laboratory details of both Cases and Controls were collected in a database. From this database we extrapolated the data. The microbiological diagnosis of SARS-CoV-2 infection was defined as a positive RT-PCR test on a naso-oropharyngeal swab. All the sites included used the same RT-PCR kit, Bosphore V3 (Anatolia Genework, Turkey).
The estimated glomerular filtration rate (eGFR) was calculated using the Modified Diet and Renal Disease equation (MDRD) [13]. Chronic Kidney Disease was defined following the guidelines [12]. The CKD stage was assigned according to the Kidney Disease Improving Global Outcome (KDIGO) stage, a widely in use guideline for definition, classification and management of individuals with CKD supported by evidence [15]. The presence of underlying chronic disease and severity was assessed according to the Charlson age-comorbidity index (CCI).
We defined patients with a mild, moderate or severe disease according to the clinical presentation of COVID-19. Precisely, patients with a mild infection did not need oxygen (O2) therapy and/or had a MEWS score below 3 points. Patients with a moderate infection were hospitalized and required non-invasive O2 therapy and/or had a MEWS score equal to or above 3 points (≥3) [16]. Lastly, patients with a severe infection needed management in an intensive care unit (ICU) and/or mechanical ventilation (invasive or not invasive); in this definition we also included patients who died. Patients were followed until SARS-CoV-2-RNA negativity at naso-oropharyngeal swab or discharge from hospital.
Statistical analysis
For the descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies. Continuous variables were summarized as mean and standard deviation if normally distributed or as median and interquartile range (IQR) if not normally distributed. We performed a comparison of patients with CKD and without CKD using Pearson chi-square or Fisher exact test for categorical variables and Student’s t- or Mann-Whitney tests for continuous variables.
A p-value below 0.05 was considered statistically significant. Analyses were performed by STATA.
RESULTS
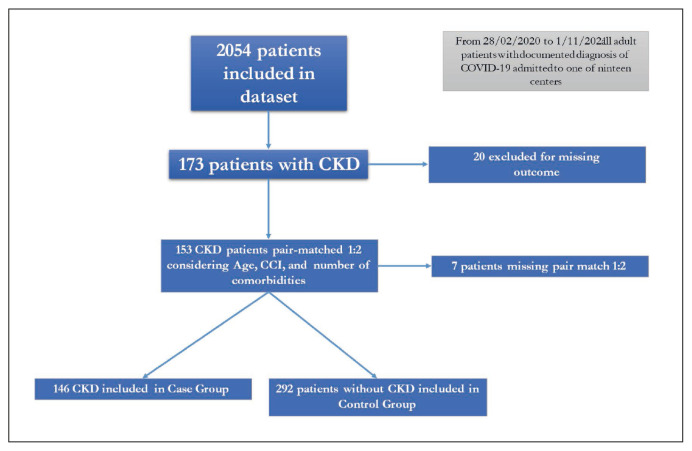
During the study period, 2,054 patients were included in the dataset (Figure 1), and 173 patients (8.4%) were affected by CKD. Of this, 20 missed the outcome data and were excluded (Figure 1). We pair matched every patient with CKD with 2 patients without CKD considering age (+5), number of co-morbidities (excluding CKD) and CCI (+1) (Figure 1). During this phase we didn’t find an opportune match for 7 patients with CKD. Thus, in the present study 146 patients with CKD were enrolled as Cases and 292 without CKD as Controls. Of the 146 with pre-existing CKD, 45 had a 1–3 stage CKD and 89 a 4–5 stage (Figure 1). The demographic, clinical characteristics and laboratory parameters of the Cases and Controls are shown in Table 1. In this study only 22 (5.1%) of patients were vaccinated for SARS-CoV-2. Considering laboratory parameters, excluding data related to CKD that showed statistical difference between groups (creatinine at admission, at nadir and Δ blood creatinine during hospitalization), higher serum values in international normalized ratio (INR), alanine amino-transferase (ALT), and bilirubin were observed in patients in Control group (Table 2). Moreover, CCI was higher in patients with CKD than that observed in those without (median 5, Q1–Q3 4–7 vs 5, Q1–Q3 4–7, p=0.006)
Figure 1.
Flow chart of patients enrolled in the study.
Table 1.
Demographic and clinical characteristics of the patients according to the presence or absence of chronic kidney disease.
|
Cases Patients with CKD
N=146 |
Controls Patients without CKD
N=292 |
P value | |
|---|---|---|---|
| Males, N. (%) | 95 (65.1) | 193 (66.1) | 0.831a |
| Age, years, median (Q1–Q3) | 73 (64–82) | 70 (62–81) | 0.236b |
| Charlson co-morbidity index, median (Q1–Q3) | 5 (4–7) | 5 (3–6) | 0.006 b |
| Days of nasopharyngeal swab for SARS-CoV-2, median (Q1–Q3) | 19 (11–28) | 18 (12–25) | 0.404c |
| Days from symptoms onset to admission, median (Q1–Q3) | 4 (1–8) | 5 (2–9) | 0.322c |
| Median (Q1–Q3) of co-morbidity (excluding CKD): | 2 (1–3) | 2 (1–3) | 0.642b |
| 1. With hypertension | 92 (64.3) | 170 (58.2) | 0.221a |
| 2. With cardio-vascular disease | 81 (55.9) | 139 (47.6) | 0.104a |
| 3. With diabetes | 65 (45.1) | 119 (40.8) | 0.383a |
| 4. With chronic obstructive pulmonary disease | 27 (18.6) | 67 (22.9) | 0.300a |
| 5. With hepatopathy | 10 (7) | 22 (7.6) | 0.824a |
| 6. With malignancy | 15 (10.5) | 39 (13.4) | 0.368a |
| 7. With obesity | 16 (15) | 26 (12.4) | 0.523a |
| 8. With dementia | 8 (5.9) | 33 (11.5) | 0.071a |
| 9. With HIV | 2 (1.4) | 1 (0.3) | 0.212a |
| median (Q1–Q3) white blood cells (WBC) at admission | 7390 (4900–11270) | 8000(5525–10375) | 0.565b |
| median (Q1–Q3) International Normalized Ratio (INR) at admission | 1.05 (1–1.17) | 1.13 (1.04–1.26) | 0.001 c |
| median (Q1–Q3) Blood creatinine at admission | 3.69 (1.6–6.5) | 0.9 (0.74–1.19) | <0.001 c |
| median (Q1–Q3) Blood creatinine at nadir | 4.1 (1.9–7.3) | 1 (0.8–1.3) | <0.001 c |
| median (Q1–Q3) Δ blood creatinine during hospitalization (nadir/admission) | 0 (0–0.6) | 0 (0–0.1) | 0.001 c |
| median (Q1–Q3) creatine-phosphokinase (CPK) at admission | 105 (59–207) | 81 (55–157) | 0.248c |
| median (Q1–Q3) lactico-dehydrogenase (LDH) at admission | 291.5 (217.5–448.5) | 316.5 (233–450.5) | 0.567c |
| median (Q1–Q3) PaO2/FiO2 Ratio (P/F) at admission | 193 (118–328) | 220 (134–310) | 0.783b |
| median (Q1–Q3) AST at admission | 28 (18.5–41) | 31 (21–47) | 0.082c |
| median (Q1–Q3) ALT at admission | 21 (13–32) | 28 (20–48) | <0.001 c |
| median (Q1–Q3) Bilirubin at admission | 0.4 (0.3–0.58) | 0.6 (0.45–0.85) | <0.001 c |
| N. (%) of patients with mild clinical outcome | 59 (40.4) | 126 (43.2) | 0.277a |
| N. (%) of patients with moderate clinical outcome | 26 (17.8) | 50 (17.1) | |
| N. (%) of patients with severe clinical outcome | 20 (13.7) | 55 (18.8) | |
| N. of deaths (%) | 41 (28.1) | 61 (20.9) | |
| Days from admission to discharge*, median (Q1–Q3) | 13 (7–21) | 14 (9–22) | 0.629c |
| Days from admission to death, median (Q1–Q3) | 7 (4–14) | 8.5 (5–19.5) | 0.280c |
Notes:
subjects alive at the end of hospitalization;
chi-square test;
Student-t test;
Mann-Whitney U test.
Table 2.
Demographic and clinical characteristics of the patients according to the stage of chronic kidney disease.
|
Patients with CKD stage 1–3
N=45 |
Patients with CKD stage 4–5
N=89 |
P value | |
|---|---|---|---|
| Males, N. (%) | 30 (66.7) | 55 (61.8) | 0.580a |
| Age, years, median (Q1–Q3) | 75 (66–82) | 72 (63–81) | 0.352b |
| Charlson co-morbidity index, median (Q1–Q3) | 5 (3–6.5) | 6 (4–7) | 0.657b |
| Days of nasopharyngeal swab for SARS-CoV-2, median (Q1–Q3) | 19.5 (15–26) | 19 (9–32) | 0.432c |
| Days from symptoms onset to admission, median (Q1–Q3) | 4 (1–7) | 3 (1–8) | 0.97c |
| Median (Q1–Q3) of co-morbidity (excluding CKD): | 2 (1–3) | 2 (1–3) | 0.847b |
| 1. With hypertension | 30 (68.2) | 56 (64.4) | 0.664a |
| 2. With cardio-vascular disease | 27 (60) | 49 (55.7) | 0.634a |
| 3. With diabetes | 17 (38.6) | 45 (51.1) | 0.175a |
| 4. With Chronic obstructive pulmonary disease | 6 (13.3) | 19 (21.6) | 0.249a |
| 5. With liver hepatopathy | 4 (9.1) | 6 (6.9) | 0.655a |
| 6. With malignancy | 7 (15.6) | 5 (5.7) | 0.060a |
| 7. With obesity | 7 (20) | 9 (12.9) | 0.337a |
| 8. With dementia | 3 (7) | 5 (5.7) | 0.784a |
| 9. With HIV | 0 (0) | 2 (2.3) | 0.314a |
| N. (%) of patients with mild clinical outcome | 23 (51.1) | 34 (38.2) | 0.15a |
| N. (%) of patients with moderate clinical outcome | 8 (17.8) | 18 (20.2) | 0.73a |
| N. (%) of patients with severe clinical outcome | 8 (17.8) | 10 (11.2) | 0.29a |
| N. of deaths (%) | 6 (13.3) | 27 (30.3) | 0.03 a |
| Days from admission to death, median (Q1–Q3) | 10.5 (5–15) | 7 (4–14) | 0.575c |
| median (Q1–Q3) PaO2/FiO2 Ratio (P/F) at admission | 191 (145–300) | 204 (110–336.5) | 0.994b |
| Days from admission to discharge, median (Q1–Q3) | 17 (13–24) | 11 (6–20) | 0.004c |
| median (Q1–Q3) Blood creatinine at admission | 1.4 (1.2–1.6) | 5.68 (3.77–7.6) | <0.001 c |
| median (Q1–Q3) Blood creatinine at nadir | 1.57 (1.28–1.9) | 6.21 (4–9.55) | <0.001 c |
| median (Q1–Q3) Δ blood creatinine during hospitalization (nadir/admission) | 0.1 (0–0.35) | 0 (0–0.8) | 0.552c |
| eGFR (MDRD) at admission, median (Q1–Q3) | 42.8 (38.1–59.2) | 9 (6.2–13.5) | <0.001 c |
| eGFR (MDRD) nadir during hospitalization, median (Q1–Q3) | 39.6 (31.6–52) | 8.28 (5.4–12.2) | <0.001 c |
| Δ eGFR (MDRD) during hospitalization (nadir/admission), median (Q1–Q3) | −2.54 (−12.3–0) | 0 (−1–0) | <0.001 c |
Notes:
subjects alive at the end of hospitalization;
chi-square test;
Student-t test;
Mann-Whitney U test.
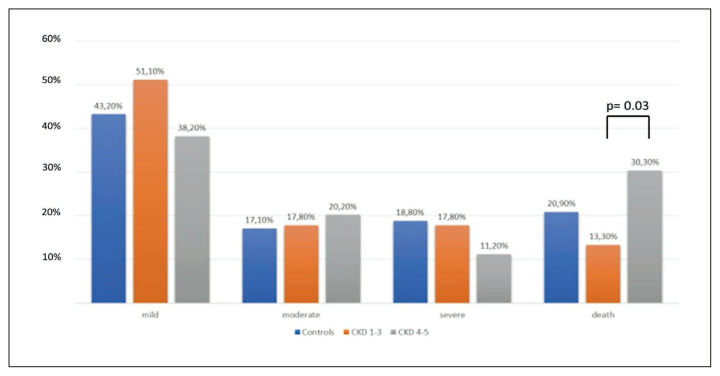
As regard clinical outcome of COVID-19, no differences were observed in the prevalence of patients with a moderate and severe clinical outcome between the Cases and Controls (17.1% vs. 17.8%, p=0.27, and 18.8% and 13.7%, p=0.27, respectively) (Table 2). Similarly, no statistical difference was reported in the prevalence of deaths between the two groups (20.9% vs. 28.1%, p=0.27) (Table 2).
The patients with CKD were divided in two group, the first including the 45 with CKD stage 1–3, the latter the 89 with CKD stage 4–5. No statistically significant difference was observed in the two groups in the age, the presence of comorbidities and other variables. However, considering clinical outcome, the prevalence of patients dead during hospitalization was statistically higher in patients with CKD stage 4–5 than those with CKD stage 1–3 (30.3% vs 13.3%, p=0.03) (Figure 2).
Figure 2.
Clinical outcomes in different groups of patients.
DISCUSSION
In the present study performed in seventeen COVID-19 Units in southern Italy we found a rate of pre-existing CKD of 8.4% in patients hospitalized for COVID-19. Moreover, in a 1:2 case-control study we found that the severity of COVID-19 was similar in patients with pre-existing CKD and in those without. Moreover, similar mortality rates were reported among the two groups.
In our cohort the percentage of CKD in COVID-19 is about 8%, in line with the literature data, in fact from the results of a recent meta-analysis and according to a systematic search recently published, the prevalence of CKD in COVID-19 patients was about 2% [17, 18].
Some studies have evaluated the severity of COVID-19 in patients with pre-existing CKD showing, in contrast with the present study, an association between a pre-existing CKD and severe clinical presentation of COVID-19 was observed [6, 19, 20]. For example, in a retrospective study on 836 patients with COVID-19 (39 with CKD), a pre-existing CKD was identified as an independent risk factor for in-hospital death and poor prognosis [6]. Moreover, Flythe et al. analyzed 4,264 COVID-19 critically ill adults admitted in 68 U.S. intensive care units and found that a pre-existing kidney disease was associated with higher in-hospital mortality rates, also according to the degree of baseline kidney dysfunction [20]. Different meta-analyses found as people with CKD were more likely to develop severe COVID-19 symptoms and may have a higher incidence of death than people with CKD without COVID-19 [21–24]. However, these meta-analyses enrolled often observational and retrospective studies and in most studies the role of other potential confounder factors for severe COVID-19 were not analyzed. In fact, compared to the general population, the patients with CKD were generally older and had more and serious co-morbidities, especially cardiovascular diseases and diabetes, other factors associated with a more severe clinical presentation of COVID-19 [25–28]. To make these variables irrelevant, we designed a pair-matched Case-Control study, and for each patient with CKD we chose 2 patients without CKD pair matched for sex, age (+5 years) and the number of other co-morbidities except CKD. In our study we did not find any association between CKD and poor outcome in COVID-19 patients. Only, when evaluating the different degree of kidney dysfunction (CKD stage 1–3 and 4–5), we found differences in terms of death in patients with COVID-19 with CKD stage 4 or 5. This is in line with what some studies have reported that dialysis patients had higher risk for in-hospital death, compared to patients without pre-existing CKD [18, 29]. For example, in a multicentre, retrospective, observational study, involving hospitalized adult patients with COVID-19 the authors compared the outcome of patients with CKD Stages 3–5, chronic haemodialysis (HD) and renal transplantation with patients without; the patients with stage 3–5 CKD had an in-hospital mortality rate as much as HD patients, which may be partly due to similar age and co-morbid burden [29]. Also, in a retrospective cohort study on critically ill patients with COVID-19, compared to patients without pre-existing CKD, those in dialysis had higher risk for 28-day in-hospital death (adjusted HR, 1.41 [95% CI, 1.09–1.81]), while patients with non-dialysis-dependent CKD had an intermediate risk (adjusted HR, 1.25 [95% CI, 1.08–1.44]) [17]. So, probably, neutralizing the confounding variables such as age and co-morbidity, the patients with end-stage renal disease are actually the ones most at risk for severe COVID-19 outcome. Our study shows several limits; first, the retrospective nature of the study; second, we evaluated only in-hospital mortality; third, the relatively small sample size of patients with CKD enrolled; fourth, despite the impact of vaccination and antivirals on the clinical presentation and clinical outcome of COVID-19 the data of antivirals were not available and the number of patients enrolled in the present study with vaccination was very low [30, 31]. The strengths of the study were the multicenter nature of the study and the case-control design, which make it possible to look at multiple risk factors at the same time.
In conclusion, in our study more severe CKD is associated with higher mortality so further research is warranted to assess the risk and well elucidate the prognosis and clinical evolution of CKD patients with COVID-19, especially in end-stage renal disease, in order to a better risk stratification of patients with poor prognosis.
Footnotes
Authors’ contribution
FC, AR, MaPi and NC were involved in study concept and design, drafting of the manuscript, PM, VE, VS, FGN, IG were involved in critical revision of the manuscript for important intellectual content; RoPu, RoPa, AP, AM, EM, RP, GC, ASM, MG, GDA, GR, AR were involved in acquisition of data, analysis and interpretation of data and in critical revision of the manuscript; Campania COVID-19 group was involve in the enrolment of the patients.
Funding
Funding: POR Campania FESR 2014-2020-Avviso per l’acquisizione di manifestazioni di interesse per la realizzazione di servizi di ricerca e sviluppo per la lotta contro il Covid-19 (DGR n. 140 del 17 marzo 2020), Regione Campania, Italy, Project: Identificazione dei fattori demografici, clinici, virologici, genetici, immunologici e sierologici associati ad outcome sfavorevole nei soggetti con COVID-19, and POR FESR Campania 2014 – 2020 - Avviso per l’acquisizione di manifestazioni di interesse da parte degli Organismi di Ricerca per la realizzazione di servizi di ricerca, sviluppo e innovazione per la lotta contro il COVID-19 (DGR n. 504 del 10.11.2021) - Regione Campania, Italy; Project: Impatto delle nuove varianti, l’uso di terapie antivirali precoci e stato vaccinale sulla presentazione clinica del COVID-19: studio restrospettivo/prospettico multicentrico.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (n° 10877/2020, May 11, 2020).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of interest
The authors declare no conflict of interest.
REFERENCES
- 1.European Centre for Disease Prevention and Control. COVID-19 situation update worldwide, as of week 17, updated 5 May 2022. [Internet] [(accessed on 05 May2022)]. Available from: https://www.ecdc.europa.eu/en/covid-19/situation-updates.
- 2. Attena E, Albani S, Maraolo AE, et al. Remdesivir-Induced Bradycardia in COVID-19: A single center prospective study. Circ Arrhythm Electrophysiol. 2021;14(7):e009811. doi: 10.1161/CIRCEP.121.009811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pisapia R, Pisaturo M, Fusco FM, et al. Differences among confirmed and not-confirmed COVID-19 patients at D. Cotugno hospital, Naples (Italy): what we learned from first suspected cases? Infez Med. 2020;28(Suppl 1):84–88. [PubMed] [Google Scholar]
- 4. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis, and practical considerations. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang D, Xiao Y, Chen J, et al. COVID-19 and chronic renal disease: clinical characteristics and prognosis. QJM. 2020;113(11):799–805. doi: 10.1093/qjmed/hcaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo E, Esposito P, Taramasso L, et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2020;6:1–11. doi: 10.1007/s40620-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Portolés J, Marques M, López-Sánchez P, et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant. 2020;35(8):1353–1361. doi: 10.1093/ndt/gfaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russo A, Pisaturo M, Palladino R, et al. Prognostic value of transaminases and bilirubin levels at admission to hospital on disease progression and mortality in patients with COVID-19-an observational retrospective study. Pathogens. 2022;11(6):652. doi: 10.3390/pathogens11060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monari C, Sagnelli C, Maggi P, et al. More Severe COVID-19 in patients with active cancer: results of a multicenter cohort study. Front Oncol. 2021;11:662746. doi: 10.3389/fonc.2021.662746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pisaturo M, De Angelis G, Maggi P, et al. Clinical features of patients with home isolation Sars-Cov-2 Infection: a multicenter retrospective study in southern Italy. Life (Basel) 2021;11(4):347. doi: 10.3390/life11040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National-Kidney-Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 14. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 16. Barnett WR, Radhakrishnan M, Macko J, Hinch BT, Altorok N, Assaly R. Initial MEWS score to predict ICU admission or transfer of hospitalized patients with COVID-19: A retrospective study. J Infect. 2021;82(2):282–327. doi: 10.1016/j.jinf.2020.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of co-morbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control. 2021;49(2):238–246. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abrishami A, Khalili N, Dalili N, et al. Clinical and Radiologic Characteristics of COVID-19 in Patients With CKD. Iran J Kidney Dis. 2020;14(4):267–277. [PubMed] [Google Scholar]
- 20. Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190–203. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nandy K, Salunke A, Pathak SK, et al. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various co-morbidities on serious events. Diabetes Metab Syndr. 2020;14(5):1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou S, Xu J, Xue C, Yang B, Mao Z, Ong ACM. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: a meta-analysis and systematic review. Ren Fail. 2020;43(1):1–15. doi: 10.1080/0886022X.2020.1847724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung EYM, Palmer SC, Natale P, et al. Incidence and outcomes of COVID-19 in people with ckd: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78(6):804–815. doi: 10.1053/j.ajkd.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS One. 2021;16(5):e0250602. doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narayanan M. The many faces of infection in CKD: evolving paradigms, insights, and novel therapies. Adv Chronic Kidney Dis. 2019;26:5–7. doi: 10.1053/j.ackd.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 26. Syed-Ahmed M, Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 27. Macera M, De Angelis G, Sagnelli C, Coppola N. Clinical presentation of covid-19: Case series and review of the literature. Int J Environ Res Public Health. 2020;17(14):5062. doi: 10.3390/ijerph17145062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marfella R, Paolisso P, Sardu C, et al. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 2020;46(5):403–405. doi: 10.1016/j.diabet.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ozturk S, Turgutalp K, Arici M, et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35(12):2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angamo MT, Mohammed MA, Peterson GM. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 2022;50(1):27–41. doi: 10.1007/s15010-021-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baradaran HR, Dehghanbanadaki H, Moradpour F, et al. The effect of COVID-19 mRNA vaccines against post-vaccination laboratory-confirmed SARS-CoV-2 infection, symptomatic COVID-19 infection, hospitalization, and mortality rate: a systematic review and meta-analysis. Expert Rev Vaccines. 2022;13:1–10. doi: 10.1080/14760584.2022.2102001. [DOI] [PubMed] [Google Scholar]