SUMMARY
Introduction
Monkeypox, historically a zoonotic disease caused by monkeypox virus, is a new global health emergency. Since May 2022, dozens of non-endemic countries have seen new cases with rapid spread. Generally a self-limited disease, there are vulnerable populations, in which severe or deadly illness can occur. There is limited data on immunocompromised patients in this outbreak, particularly on people living with HIV, who are disproportionately affected.
Methods
We reported seven cases of monkeypox in people living with HIV in South Florida, USA. Relevant demographic, epidemiologic and clinical data were described.
Results
All the patients were men, identified as gay or bisexual, and were on combination antiretroviral therapy (cART) for HIV. Six of the seven had CD4 counts more than 200 cells/mm3 (one unknown level), and one of the seven had detectable HIV viral load. Six had sexual or intimate contact with asymptomatic partners prior to development of symptoms. Two were hospitalized, one for proctitis and one for an increasing number of lesions. Six had disseminated lesions and one had localized perianal lesions and all had 5–25 total number of lesions. Five received tecovirimat with resolution of lesions in 2–14 days and all were doing well at the time of the present report. Close contacts received the Jynneos vaccine which was well tolerated.
Conclusions
Our case series described monkeypox in people living with HIV and have noted atypical symptoms (lack of fever and more notable anogenital lesions) and relatively mild course as described in HIV seronegative patients. We stress the importance of early detection and isolation as well as vaccination to contacts, which has been well tolerated. In our case series, we are unable to estimate the effectiveness of tecovirimat given the limited number of patients, but all our patients had lesions that resolved within two weeks of rash onset and had no side effects reported.
Keywords: Monkeypox, HIV, South Florida, rash, sexual contact
INTRODUCTION
Monkeypox, a zoonotic disease caused by the monkeypox virus, results in a skin rash similar to smallpox, which is endemic in several Central and West African countries [1]. There are two clades of Monkeypox virus, the West African, and Central African/Congo Basin, with the latter causing more severe and contagious illness [1, 2]. Monkeypox is rarely exported from the endemic areas of Africa. In 2003, there was a zoonotic outbreak in the United States of America (USA) causing 47 confirmed or suspected cases [3]. This outbreak was linked to the importation of Gambian giant rats, squirrels and dormice which had transmitted the virus to prairie dogs that were then sold as pets. There were no confirmed cases of person-to-person transmission. Imported monkeypox infections in humans following travel have been sporadically reported in the United Kingdom (UK), Europe, Israel, Singapore and most recently in the USA in 2021 [2, 4–8].
Since May 2022, a global outbreak of monkeypox has been occurring in many non-endemic countries. In mid May 2022, the Massachusetts Department of Public Health (MDPH) and the Centers for Disease Control and Prevention (CDC), confirmed the presence of the West African clade of Monkeypox virus from a lesion swab on a Massachusetts resident [9]. Since then, confirmed cases have been reported in 47 states in the USA, as well as 72 other countries, none of which had endemic monkeypox with the highest number in the USA and Spain. However, these numbers are likely to be underreported given lack of familiarity with this entity, poor access to testing and generally self-limited course [10, 11]. Cases have largely been in males, especially concentrated amongst men who have sex with men (MSM) and although initially non-endemic monkeypox was found in Europe, now it has spread to the Americas, Asia, and Australia [11]. Recent data from a large case series of 528 patients, noted 98% of which were men who identified as gay or bisexual. Cases were thought to be largely related to sexual contact (95%), only 26% had known monkeypox contact and most (72%) had no foreign travel in the month prior to diagnosis [12].
For most individuals, monkeypox is a self-limited disease with symptoms lasting from two to four weeks. Severe cases of monkeypox occur more commonly among children and are related to the extent of virus exposure, patient health status and nature of complications. The incubation period from time of exposure to clinical illness is usually 5–13 days (range 5–21). The patients present with a prodromal phase that includes fevers, chills, and myalgias followed by a characteristic rash, which typically begins as macules and evolves to papules, vesicles and then pustules. The lesions eventually crust over, which then dry up and fall off. However, during this outbreak, some patients have presented with only genital, rectal, and/or oral lesions without the initial prodrome. Additionally, other atypical symptoms include few or single lesions, skin lesions in different stages of development, and isolated anal pain and bleeding [10].
Underlying immune deficiencies may lead to worse monkeypox outcomes. Although data in immunocompromised patients with monkeypox are very limited, severe complications have been seen in immunocompromised patients who have had smallpox or have received smallpox vaccination with a replication-competent vaccinia virus [3]. The literature in people living with HIV is scant, with some reports suggesting a more severe disease, and others describing a benign course, very similar to that observed in immunocompetent patients [12, 13]. One notable complication is superimposed bacterial cellulitis, which may require hospitalization [12, 14]. Given that people living with HIV have been disproportionately affected during the present outbreak, there is a clear need for studies aiming at evaluating the clinical characteristics and outcomes of monkeypox in this population. There is currently one medication, tecovirimat, which is under investigational use, recommended routinely for treatment in severe disease or those at high risk for disease, including immunocompromised patients. Brincidofovir and cidofovir are alternatives that have been used in the treatment of smallpox but have adverse effects of elevated liver enzymes, nephrotoxicity and interactions with antiretroviral therapy which precludes their use. Prevention includes the use of the Jynneos, ACAM2000 and LC16 smallpox/monkeypox vaccines but data on effectiveness for all are limited [15, 16].
We report seven cases of people living with HIV who were diagnosed with monkeypox at University of Miami Hospital, Jackson Memorial Hospital, and AIDS Healthcare Foundation (AHF) in South Florida, USA during the period of June 13 to July 11, 2022. We described relevant demographic, epidemiologic and clinical data. A table that summarizes the clinical characteristics of the patients is also presented (Table 1).
Table 1.
Clinical characteristics of people living with HIV diagnosed with monkeypox in the case series.
| Characteristic | All patients (N=7) |
|---|---|
|
| |
| Median age (range) - years | 41 (33–53) |
|
| |
| Median Abs CD4/% (range) - cells/mm3 | 529/29% (262–668/14–42%) |
|
| |
| Median HIV Viral load (range) - copies/ml | <20 (<20–236,988) |
|
| |
| Known to be taking antiretroviral therapy (ART) - no. (%) | 7 (100%) |
|
| |
| Prodrome symptoms | |
| Fatigue - no. (%) | 6 (86%) |
| Fever - no. (%) | 1 (13%) |
|
| |
| Site of lesions | |
| Face - no. (%) | 5 (71%) |
| Anogenital - no. (%) | 6 (86%) |
| Extremities - no. (%) | 6 (86%) |
|
| |
| Extracutaneous manifestations | |
| Rectal/perianal pain - no. (%) | 3 (43%) |
| Lymphadenopathy - no. (%) | 3 (43%) |
|
| |
| Received monkeypox specific treatment (tecovirimat) - no. (%) | 6 (86%) |
|
| |
| Outcome | |
| Resolved | 6 (86%) |
| Lost to follow up | 1 (13%) |
CASE PRESENTATIONS
Case 1
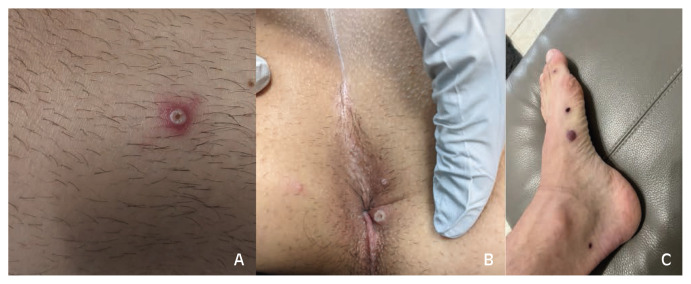
A 37-year-old man with HIV on cART (CD4 262 cells/mm3, viral load undetectable) and Kaposi Sarcoma on partial remission was admitted to the hospital with severe rectal pain and skin pustules. Symptoms of fever, lymphadenopathy and fatigue started one week before hospitalization. He has had systemic symptoms intermittently for the last three years from his Kaposi Sarcoma but worsened in the week prior to his skin lesions. Three days later, he noticed a pustule-like facial lesion which spread to his head, torso, extremities, scrotum and anal lesions with significant rectal pain and scant blood in stools. He reported not having sex in the last two months. He did not travel outside of Florida in the last 12 months but went to a concert two weeks prior to symptoms. On exam, he had about 20 lesions, in various stages of development, including papules, vesicles and pustules with an umbilicated center (Figure 1A). He had bilateral increased size of inguinal lymphadenopathy (previous biopsy confirmed Kaposi sarcoma). He had two perianal lesions with pain on palpation, clear drainage from rectum and rectal bleeding (Figure 1B). Biopsy was negative for herpes simplex virus, varicella zoster virus, Treponema pallidum, bacterial or fungal organisms. Swabs from the pustules were positive for Orthopox PCR. He declined a rectal swab due to extreme rectal pain and was treated empirically for proctitis with ceftriaxone, doxycycline and valacyclovir, with significant improvement of symptoms. He developed more lesions through the first three days of his hospital course but then improved with lesions becoming crusted, all prior to tecovirimat (Figure 1C).
Figure 1.
Vesicular and umbilicated lesions in leg (A) and perianal region (B). Healing lesions in left foot (C).
Case 2
A 43-year-old man with HIV on cART (CD4 668 cells/mm3, viral load undetectable) and history of multiple sexually transmitted infections was seen in the outpatient clinic for generalized weakness and fatigue for two days followed by a skin rash and pain in his perianal and inguinal region. The skin rash consisted of a few papules that progressed into vesicles and umbilicated pustules. He reported having unprotected sex with a male about one month prior his symptoms started at which time he developed the weakness and fatigue. He did not travel outside of Florida within the past two months. On examination he had umbilicated pustules on his left perianus and eft anal verge with pustular non umbilicated lesions on the right wrist and right thigh (Figure 2). He had tender left sided inguinal lymphadenopathy. Testing from lesion’s swabs came back positive for Orthomyxoviridae. The patient was started on tecovirimat with rapid improvement of his lesions in the next 48 hours. At that time, he noted all his lesions had begun crusting. The lesions had completely resolved by day seven of antiviral therapy.
Figure 2.
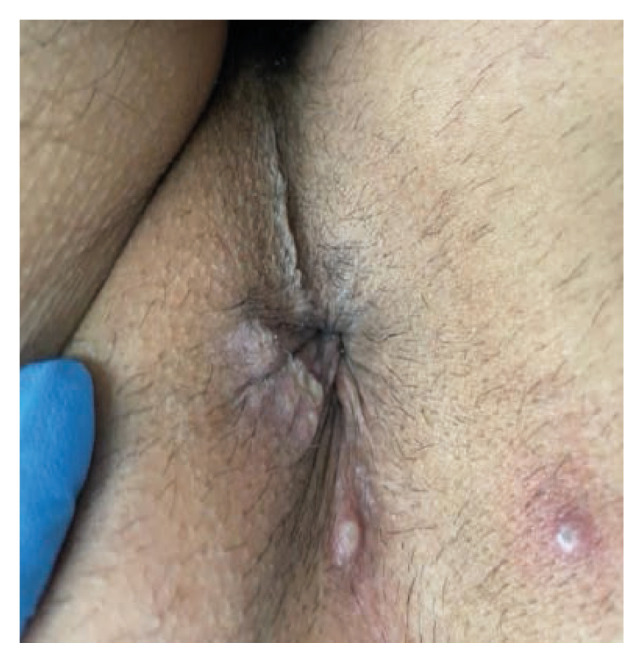
Perianal region with pustular lesions.
Case 3
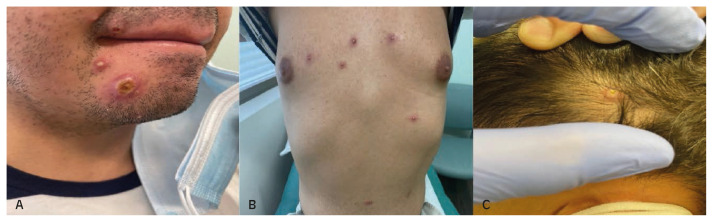
A 44-year-old man with HIV on cART (CD4 296 cells/mm3, viral load undetectable) and alcohol abuse presented to the STI (sexually transmitted infection) clinic for evaluation and treatment of monkeypox. One week prior, he developed fatigue and a rash, which started as a vesicle on his chin. The following day, he went to a pool party and found more lesions on his chest and legs. He went to the emergency room where samples from the lesions were taken and were positive for Orthomyxoviridae. He denied any travel outside of Florida within the past two months. The month before the onset of symptoms, he reported intimate contact with another man at a bar, where there was naked hugging and kissing but no sexual intercourse or oral sex. He did not recall any skin rash on that person. He lived with his ex-husband who was asymptomatic and denied any sexual or close physical contact with him. On exam, he had multiple umbilicated pustular lesions with erythematous borders scattered on his scalp, face, chest all in the same stage of development except for one 2·0 cm ulcerated lesion below his right lower lip that was crusted (Figure 3 A, B, C). He had tender left inguinal lymphadenopathy. No perineal or anal lesions were seen. The patient received tecovirimat with resolution of lesions after two weeks.
Figure 3.
Pustular and crusted lesions in chin (A). Pustular lesions with erythematous border in chest (B) and scalp (C).
Case 4
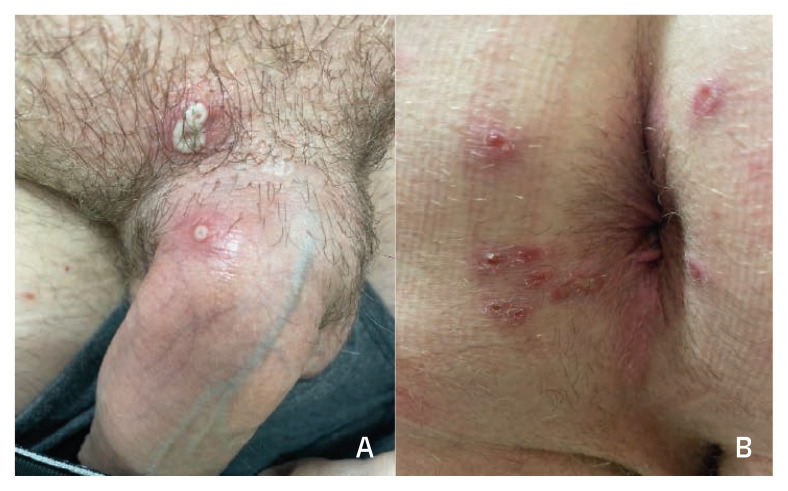
A 38-year-old man with HIV on cART (CD4 553 cells/mm3, viral load undetectable) presented to the clinic for follow up after a diagnosis of monkeypox at a different facility. The patient reported three days of extreme fatigue followed by scattered pustular lesions on his arms and chin followed by lesions on his penis and inguinal region. He was seen approximately one week after symptoms onset. He denied fever or other constitutional symptoms. He reported attending a pool party three weeks before with multiple sexual encounters that day. He lived with a partner who did not have any symptoms at the time of these encounters. On examination, the lesions on his face were dried and crusted. He had four other umbilicated lesions, one on the distal aspect of the dorsal penile shaft and the other three at the base of the penis (Figure 4A). He received tecovirimat and noted that all lesions had formed scabs by day eight of treatment.
Figure 4.
Umbilicated and vesicular lesions in the dorsal penile shaft and base of the penis from Case 4 (A). Perianal lesions in scabbing/healing process from Case 5 (B).
Case 5
A 53-year-old man with HIV on cART (CD4 634 cells/mm3, viral load undetectable) was seen in the clinic for management of monkeypox. The patient is the partner of case 4 and was seen at the same time. They both went to the same pool party and had multiple sexual encounters that day. As in case 4, the patient reported extreme fatigue at the same time as his partner and for three days preceding the skin rash. In his case, he had perianal lesions with no other lesions in other parts of his body. On examination, all lesions were either crusting or already in the process of scabbing/healing (Figure 4B). He had no inguinal lymphadenopathy despite the location of his lesions. He received tecovirimat with resolution of lesions after day eight of therapy.
Case 6
A 41-year-old man with HIV on cART (CD4 505 cells/mm3, viral load 236,988 copies/ml) and history of syphilis presented to the Emergency Department (ED) for rash. Three days prior, he went to a party and the following day he began to experience a slight itching sensation over his face. The next day he developed non-tender, fluid filled vesicles on his face. Over the next three days the lesions began to enlarge and spread down to his arms, back and genitals with umbilication. He returned to the ED, where he was admitted due to the increasing number of lesions. He denied systemic symptoms and had only skin involvement. He traveled to the Bahamas about six weeks before. He had sexual intercourse with both male and female partners, with oral, anal (penetrative and receptive) and genital intercourse. His last sexual encounter was on the day of the party. He also had intercourse without condoms about two weeks prior to the rash. Superficial swab of lesion was positive for monkeypox virus PCR. He had negative testing for gonorrhea, chlamydia and change in RPR titer. He was admitted for one day and was discharged home feeling well without treatment. He was subsequently lost to follow up.
Case 7
A 33-year-old man with HIV on cART (unknown CD4, viral load undetectable), history of syphilis and rectal gonorrhea presented to the clinic for headache, fatigue, chills, pruritic rash, and rectal pain for four days. His exam was notable for pustules on his forearm, penis, upper lip and on anal verge. He had sex with a male partner one week prior. He did not travel outside of the country for the past three months but had contact with his mother who is from Peru. Skin swab was positive for Orthopox, received tecovirimat and at his three weeks follow up, he had no further signs or symptoms of monkeypox.
DISCUSSION
During the 2022 monkeypox outbreak, 22141 confirmed cases have been reported worldwide in locations that have not historically reported monkeypox. As of July 29, 2022, there were 5189 cases confirmed in the United States, with 373 of those cases from Florida [17, 18]. Although Florida has 67 political subdivisions (counties), cases are reported in only 16 of them and most of the cases are spread in two areas (184 in Broward County and 96 in Dade County). Our seven cases live in those two areas (four in Broward County and three in Miami-Dade) and they represent the early cases identified in our state. We witnessed the rapid spread of cases locally with 10 times increase in cases in only a three-week period.
In the current outbreak in the USA, patients with monkeypox generally report having close, sustained physical contact with other people who have monkeypox. In a recent update given by the CDC, most infected patients were male, with a median age of 36 years (range 20–76), and 99% of them reported sexual contact with other men. Most cases present with a skin rash, with small and firm lesions that are circumscribed, sometimes itchy, and umbilicated in different phases of development that rapidly progressed to pustules with crusting. Prodromal symptoms were mild or absent and lymphadenopathies and fever were less common. Anorectal pain with visualization of proctitis by exam was another finding [19].
There is limited information about monkeypox in people living with HIV. A study from Nigeria in 2020, prior to this current outbreak, included 40 patients, of which nine had HIV (four newly diagnosed, five on ART, CD4 range 20–357 cells/mm3). Compared to patients without HIV, they found those with HIV were more likely to have larger skin rashes (≥2 cm), secondary bacterial skin infection, genital ulcers, and illness duration ≥ 28 days [13]. An earlier study from Nigeria in 2017–18 noted that of 122 confirmed or probable cases of monkeypox, there were seven deaths (6% fatality rate), four of which had HIV infection. However, there was no mention of the total number of patients with HIV or other baseline comorbidities [20]. A recent study conducted by Thornhill et al. reported 528 cases of monkeypox in 16 countries between April and June of 2022. A total of 218 cases were in people living with HIV, of whom 99% were MSM. The median CD4 count in this group was 680 cells/mm3. In the vast majority of these persons, HIV infection was well controlled; 96% of patients were taking ART, and the HIV viral load was less than 50 copies/mL in 95% of individuals. They found a benign course in individuals with HIV, similar to those without HIV, and no deaths were reported [12]. However, following this study, there have been reports of the first five monkeypox deaths outside of Africa. One was in an HIV infected person in Peru, another with lymphoma on treatment and three with unknown comorbidities. Historically, immunocompromised patients, pregnant women, children, especially under 8 years, and patients with compromising skin integrity conditions are considered at high risk for severe disease; for this reason, continued vigilance of these subpopulations is required [18, 21].
According to the last WHO situation report in the current outbreak, among cases with reported sexual orientation, 60% (1214/2025) identified as gay, bisexual, and other men who have sex with men and 41% (335/827) of cases with known HIV status were positive for HIV [22]. In England, the UK Health Security Agency revealed that of a total of 445 patients who underwent surveillance questionnaires, 29.5% reported HIV infection, of whom 99.2% were on HIV treatment [23].
In this paper, we reported seven cases of monkeypox in people living with HIV in the United States (Table 1). All were male and receiving antiretroviral treatment with CD4 counts >200 cells/mm3 except for one unknown CD4 count but undetectable HIV viral load. One patient had a detectable HIV viral load. There was one patient with Kaposi sarcoma (AIDS-defining illness) who was the only patient that required admission to the hospital for proctitis. A second patient was admitted for an increasing number of lesions but was discharged the following day. All cases had mild prodromal symptoms with the main presentation of extreme fatigue days before the skin rash, and only one with fever. None of the seven patients had traveled outside Florida within the prior one month, suggesting the local spread of the infection. Five of the seven patients reported multiple unprotected sexual encounters with other men weeks before the skin rash. One patient reported close intimate contact with another male with kissing and naked contact but no sexual intercourse. Only one patient reported no close sexual or intimate contact nor known exposures. Of interest, the patients who had sexual and intimate skin contact did not recall seeing any skin lesions on their partner, suggesting acquisition of monkeypox from asymptomatic carriers with viral shed in body fluids or from oligosymptomatic individuals whose lesions were overlooked [24]. In a recent study conducted in Spain, the authors tested various body fluids of 12 patients with monkeypox and noted positive virus in not only skin pustules but also rectal swabs (11/12), nasopharyngeal swabs (10/12), semen (7/9), urine (9/12) and feces (8/12), suggesting multiple potential sources of transmission [25]. This outbreak’s monkeypox virus genome has noted mutations from endemic virus, therefore further studies regarding the infectivity of oral, rectal, and vaginal mucosa are necessary [26].
All the patients in the present series had skin lesions, starting as small papules, rapidly evolving into pustules. According to the WHO rash burden classification (benign, 5–25 lesions; moderate, 26–100 lesions; grave, 101–250 lesions; and plus grave, >250 lesions), all our patients had benign burden (5–25 lesions) [3]. Six cases had disseminated lesions most starting on their face and then spreading to the extremities followed by the genitals. Only one case had localized lesions in the perianal area. Despite the benign rash burden in our patients, one of them experienced extreme rectal pain and required hospitalization for three days. Overall, the manifestations and disease severity in our case series seemed to be similar to those described in non-HIV affected individuals during the current outbreak.
In terms of concomitant STIs, Girometti et al. reported a case series of 54 patients in the UK, 24% living with HIV (all of them on ART and undetectable) and found a high rate of concurrent STIs. Of the 51 patients who had sexual health screening, 13 (25%) patients were positive for gonorrhea and chlamydia, with a higher prevalence in individuals with HIV (7/13, 54%) than in those HIV-negative (6/41, 15%) [27]. In our study, we had one patient with proctitis. Although his urine gonorrhea and chlamydia PCR were negative, we could not exclude a concomitant STI completely because he declined rectal testing. However, he improved on ceftriaxone and doxycycline rapidly, suggesting an STI coinfection.
All our patients were given/offered treatment with tecovirimat given their HIV status. Five had received and taken treatment with three seeing improvement and two that had already begun to improve prior to treatment. Delays in treatment were due to need for confirmatory testing and limited availability of medications, which at the time were stockpiled at the CDC. The medication was well tolerated with no reported side effects in any of the five cases (two have not yet received treatment). In the USA, tecovirimat (TPOXX®) is only approved for the treatment of smallpox and is offered under an investigational new drug/compassionate use protocol to patients with monkeypox at high risk of complications or presenting with severe disease. The effectiveness of this medication for monkeypox has not been tested in prospective trials. The clinical data is based on observational studies and anecdotal cases that suggest a shorter duration of symptoms and faster healing of pox lesions [16, 28]. It is not possible to comment about the benefit of tecovirimat in our case series. Although the patients improved while taking it, we are uncertain if this can be attributed to the medication or the natural course of the disease.
The vaccine deployed for monkeypox in the USA during this current outbreak is the Jynneos vaccine, a live attenuated non-replicating vaccine, which is FDA approved for the prevention of smallpox and monkeypox [29]. According to the current policy in the State of Florida, the vaccine is recommended for immunocompromised MSM with HIV (CD4 count <200 cells/mm3) and other MSM with a recent history of sexually transmitted infections. It is also indicated as post-exposure prophylaxis in close contacts to monkeypox cases within 14 days of exposure [30]. Our patients’ spouses and partners were evaluated, and all asymptomatic partners received the vaccine with no side effects. Mild arm pain was reported by others who were vaccinated.
CONCLUSION
Monkeypox has had a resurgence of cases in the past couple of decades in endemic countries in West and Central Africa but now there is a significant and rapid rise of cases in non-endemic countries. We noted cases of monkeypox in persons living with HIV in the United States, in whom the majority had anogenital lesions and a relatively mild disease course, very similar to people without HIV. Transmission appeared to have been through sexual or intimate contact with asymptomatic or oligosymptomatic partners. Information about the burden of monkeypox infection in high-risk asymptomatic persons is in need, to determine if screening in persons without skin lesions is warranted. We stress the importance of early detection and isolation as well as vaccines for contacts, which has been well tolerated. We are unable to comment on the clinical benefit of tecovirimat given the limited number of patients in this case series, but all had lesions that resolved within two weeks of rash onset and had no side effects reported. More data on manifestations and outcomes of monkeypox in patients with uncontrolled HIV is needed.
Footnotes
Conflict of interest
The authors declare no competing conflict of interest.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors’ contributions
Conceptualization, PL; literature review, LW, PL, and JAGZ; writing-original draft preparation, LW, PL, and JAGZ; writing-review and editing, LB, ZH, LW, PL, and JAGZ. All authors have read and agreed to the published version of the manuscript.
Informed consent statement
In order to publish the pictures, written informed consent was obtained from the patients.
Institutional review board
IRB approval not required per University of Miami, Florida, USA.
REFERENCES
- 1. McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mauldin MR, McCollum AM, Nakazawa YJ, et al. Exportation of monkeypox virus from the African continent. J Infect Dis. 2022;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huhn G, Bauer A, Yorita K, et al. Clinical characteristics of Human Monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 4. Eteng WE, Mandra A, Doty J, et al. Notes from the field: responding to an outbreak of monkeypox using the one health approach-Nigeria, 2017–2018. MMWR Morb Mortal Wkly Rep. 2018;67:1040–1041. doi: 10.15585/mmwr.mm6737a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom. Eurosurveil. 2018;23(38):1800509. doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erez N, Achdout H, Milrot E, et al. Diagnosis of imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ng OT, Lee V, Marimuthu K, et al. A case of imported Monkeypox in Singapore. Lancet Infect Dis. 2019;19(11):1166. doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cunha BE. Monkeypox in the United States: an occupational health look at the first cases. AAOHN J. 2004;52(4):164–168. [PubMed] [Google Scholar]
- 9. CDC. Monkeypox Outbreak-Nine States, May 2022. MMWR. 2022 June 10;71(23):764–769. doi: 10.15585/mmwr.mm7123e1. https://www.cdc.gov/mmwr/volumes/71/wr/mm7123e1.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Multi-country monkeypox outbreak in non-endemic countries. Jun 27, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 .
- 11.Global Health Contributors. Monkeypox 2022 Global Epidemiology; report 2022–07-01. [Accessed on 2022, 07.02]. 2022. from https://www.monkeypox.global.health.
- 12. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries - April-June 2022 [published online ahead of print, 2022 Jul 21] N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 13. Ogoina D, Iroezindu M, James HI, et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–e214. doi: 10.1093/cid/ciaa143. 1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 14. Ortiz-Martínez Y, Rodríguez-Morales AJ, Franco-Paredes C, et al. Monkeypox - a description of the clinical progression of skin lesions: a case report from Colorado, USA. Ther Adv Infect Dis. 2022;9:20499361221117726. doi: 10.1177/20499361221117726. Published 2022 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farahat RA, Sah R, El-Sakka AA, et al. Human monkeypox disease (MPX) Infez Med. 2022;3:372–391. doi: 10.53854/liim-3003-6. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florida Department of Health. Reportable Diseases Frequency Report. [Accessed 14 July 2022]. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=FrequencyMerlinFrequency .
- 18.CDC. Monkeypox in the US. Centers for Disease Control and Prevention; www.cdc.gov, 14 July 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html. [Google Scholar]
- 19.Rao A, Mena L, Petersen B.Monkeypox: Updates about Clinical Diagnosis and Treatment. Jun 29, 2022. https://emergency.cdc.gov/coca/ppt/2022/062922_slides.pdf .
- 20. Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sah R, Mohanty A, Abdelaal A, Reda A, Rodriguez-Morales AJ, Henao-Martinez AF. First Monkeypox deaths outside Africa: no room for complacency. Ther Adv Infect Dis. 2022 Aug 26;9:20499361221124027. doi: 10.1177/20499361221124027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Multi-country outbreak of monkeypox, External situation report #1-6. Jul, 2022. [Accessed on July 14th, 2022]. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--1---6-july-2022 .
- 23.UK Health Security Agency. Investigation into monkeypox outbreak in England: technical briefing. [Accessed on July 19th, 2022]. p. 3. Available online: https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-3.
- 24. De Baetselier I, Van Dijck C, Kenyon C, et al. Asymptomatic Monkeypox virus infections among male sexual health clinic attendees in Belgium. SSRN Electronic Journal. doi: 10.2139/ssrn.4142074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28) doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amer FA, Hammad NM, Wegdan AA, et al. Growing shreds of evidence for monkeypox to be a sexually transmitted infection. Infez Med. 2022;3:323–327. doi: 10.53854/liim-3003-1. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Girometti N, Byrne R, Bracchi M, et al. Epidemiological characteristics and clinical features of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, United Kingdom. SSRN Electronic Journal. 2022 doi: 10.2139/ssrn.4125251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.STAT News. Monkeypox patients should not be left to suffer when an FDA-approved drug could help. [Accessed on July 18th, 2022]. Available online: https://www.statnews.com/2022/07/15/monkeypox-patients-should-not-be-left-to-suffer-when-an-fda-approved-drug-could-help/#:~:text=Even%20though%20the%20FDA%20approved,of%20both%20monkeypox%20and%20smallpox.
- 29.Jynneos FDA. [Accessed on July 18th, 2022]. Available online: https://www.fda.gov/vaccines-blood-biologics/jynneos.
- 30.Florida Department of Health. Monkeypox. [Accessed on July 18th, 2022]. Available online: https://www.floridahealth.gov/diseases-and-conditions/monkeypox/index.html.