Abstract
We have used human retinal pigment epithelial (HRPE) cultures to investigate the primary cellular responses of retinal resident cells to intracellular Toxoplasma gondii replication. At 4 days postinoculation, when all of the cells were infected, the secretion of interleukin 1β (IL-1β), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and intercellular adhesion molecule 1 (ICAM-1) was augmented by 23-, 10-, 8-, and 5-fold, respectively, over the control. Northern and reverse transcriptase PCR analyses showed significant upregulation of steady-state levels of mRNA for IL-1β, IL-6, GM-CSF, and ICAM-1. The secretion of these molecules by HRPE cells may play a critical immunoregulatory role in the pathophysiological processes associated with T. gondii-induced retinochoroiditis.
Retinochoroiditis caused by Toxoplasma gondii during ocular toxoplasmosis results in inflammation and disorganization of the retina, occasionally leading to severe loss of vision (11, 20, 23, 30). The intensity of damage to the retina and choroid depends on the severity of the infection and the associated inflammatory reaction (11, 13, 20). Inflammatory cells, predominantly macrophages and lymphocytes, infiltrate the retina, the subretinal space, and the vitreous (6, 11, 20). In the severe form of T. gondii-induced uveitis, destruction of large segments of the outer retina and pigment epithelium is observed (6, 9, 13, 24). Within the retina, lysosomal and other autolytic enzymes released by inflammatory cells are thought to contribute to the pathogenic mechanisms of retinal tissue damage (6, 11).
Retinal pigment epithelium (RPE), an integral part of the neuroretina in the posterior pole of the eye, acts as a barrier between the highly vascularized choroid and the retina with a complex architecture of neuronal cells (2, 31). In addition to its role in the transport of metabolites between retina and choroid, the RPE phagocytose the shed outer segments of retinal rods and cones (2, 31). Many of these activities of the RPE are essential for the structural and functional integrity of the retina and choroid. The RPE, because of its critical location and physiological activities, is constantly subjected to contact with various infectious agents and inflammatory mediators (12, 14).
Tachyzoites of T. gondii injected into the peritonial cavities of mice were thought to reach the retina via both choroidal and retinal circulation (8, 21, 26). In a rabbit model, injection of tachyzoites into the suprachoroidal space resulted in outer retinal lesions and localized foci of retinal pigment epitheliosis within 48 h. This suggests the crossing of the parasite through the RPE-Bruchs membrane barrier from the choroid to the retina (27). Histopathological examination of the eyes of patients with toxoplasma-induced retinochoroiditis revealed the presence of free tachyzoites and cysts in the RPE and the retina (9, 19, 24). Hence, studies of the mechanisms of T. gondii replication within retinal cells and the responses of the host cells to parasite invasion would be valuable in understanding the immunopathological basis of retinochoroiditis. Therefore, we have investigated the responses of human RPE (HRPE) cells in the secretion of immunologically relevant molecules upon parasite invasion and intracellular replication.
Infection of HRPE cultures with T. gondii.
Primary cell lines of HRPE were prepared from human donor eyes and propagated as described previously (15). HRPE cultures at passages 6 to 12 were used for the experiments reported in this study. Tachyzoites of T. gondii (RH strain), grown in HRPE cultures, were prepared for inoculation as previously described (17). The cultures were washed twice with serum-free medium and inoculated with tachyzoites of T. gondii (0.5 ml/well in 24-well plates) at a multiplicity of infection (MOI) of 5. Supernatant fluids from duplicate wells of control (uninfected) and T. gondii-infected cultures were collected at various times (8, 24, 48, 72, and 96 h) postinoculation (p.i.) and frozen until used for analysis. We have selected an MOI of 5 for T. gondii inoculation studies, since it results in uniform infection of HRPE cell layers. Cultures infected at an MOI of 1 were not uniformly infected, whereas cultures infected at an MOI of 10 were rapidly destroyed.
IL-1, IL-6, GM-CSF, and ICAM-1 secretion by T. gondii-infected HRPE cells.
Culture supernatant fluids from uninfected and T. gondii-infected HRPE cells were clarified by centrifugation for 5 min at 13,000 × g in a Microfuge. Levels of interleukin 1β (IL-1β), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and intercellular adhesion molecule 1 (ICAM-1) in the culture supernatant fluids were determined by enzyme immunoassays using commercial kits (Biosource, Camarillo, Calif., and R & D Systems, Minneapolis, Minn.). Since low levels of IL-1β were detected in the media, an ultrasensitivity kit (R & D Systems) with a detection range of 0.125 to 8 pg/ml was used. Results obtained from batches of the cultures grown under similar conditions were used for the statistical analysis of the data for any given experiment.
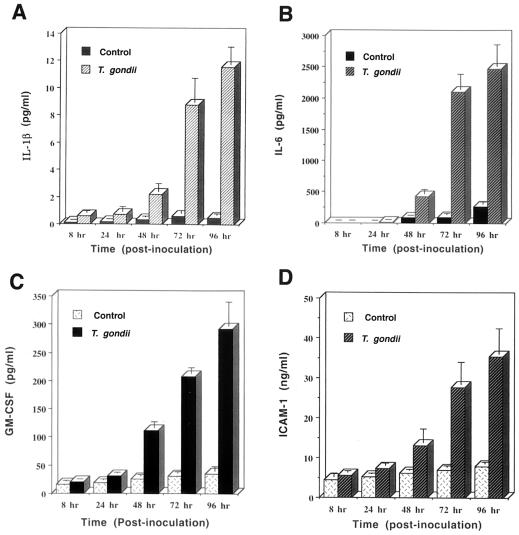
The time course of IL-1β, IL-6, GM-CSF, and ICAM-1 secretion by T. gondii-infected HRPE cells is shown in Fig. 1. T. gondii infection of HRPE cells resulted in significant enhancement (P < 0.005) in the secretion of IL-1β (control, 0.5 pg/ml, versus T. gondii, 11.5 pg/ml), IL-6 (control, 259.6 pg/ml, versus T. gondii, 2,476.3 pg/ml), GM-CSF (control, 35.4 pg/ml, versus T. gondii, 291.8 pg/ml), and ICAM-1 (control, 7.8 ng/ml, versus T. gondii, 35.3 ng/ml) at 4 days p.i. No significant changes in the levels of these molecules in HRPE cultures incubated with heat-killed T. gondii were observed (data not shown). The secretion of tumor necrosis factor alpha, IL-4, IL-10, IL-12, and IL-15 was not observed in control and T. gondii-infected HRPE cells.
FIG. 1.
Secretion of IL-β (A), IL-6 (B), GM-CSF (C), and ICAM-1 (D) by T. gondii-infected HRPE cells. Cell culture supernatant fluids were collected at indicated p.i. times, and the levels of IL-1β, IL-6, GM-CSF, and ICAM-1 were determined by enzyme-linked immunosorbent assay. The lower limits of detection were 0.13 pg/ml (IL-1β), 10 pg/ml (IL-6), 15.6 pg/ml (GM-CSF), and 1.6 ng/ml (ICAM-1). Results are the means ± standard error for six experiments, each with duplicate samples.
T. gondii-infected HRPE cells upregulate IL-1β, IL-6, GM-CSF, and ICAM-1 mRNA levels.
We next evaluated the steady-state levels of IL-1β, IL-6, ICAM-1, and GM-CSF mRNA in T. gondii-infected HRPE cultures to determine if the enhanced secretion of these molecules was associated with the elevated levels of mRNA. HRPE cells grown to confluence in 100-mm culture dishes were inoculated with tachyzoites of T. gondii at an MOI of 5. After 1, 2, and 3 days of incubation, total cellular RNA from the uninfected, T. gondii-infected, and incubated-with-heat-killed (dead) T. gondii HRPE cultures was prepared with extraction medium (RNA Stat-60; TEL-TEST, Friendswood, Tex.). The integrity and purity of the RNA preparations were checked by UV spectrum analysis and agarose gel electrophoresis. RNA was fractionated by electrophoresis on 1% formaldehyde agarose gels, transferred to nylon membranes, and immobilized by UV cross-linking. Human IL-6 (15), ICAM-1 (16), and GM-CSF (clone GMCSF, pCSF-1; American Type Culture Collection, Manassas, Va.) cDNA probes were labeled by the random primer method with digoxigenin-dUTP (Dig DNA labeling and detection kit; Boehringer Mannheim, Indianapolis, Ind.). Following standard protocols of hybridizations, membranes were washed twice with 2× SSC–0.1% sodium dodecyl sulfate (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature for 5 min, followed by two washes with 0.1× SSC–0.1% sodium dodecyl sulfate at 68°C for 15 min each. The hybridization signals were detected with antidigoxigenin alkaline phosphatase conjugate and the chemiluminescence substrate CSPD.
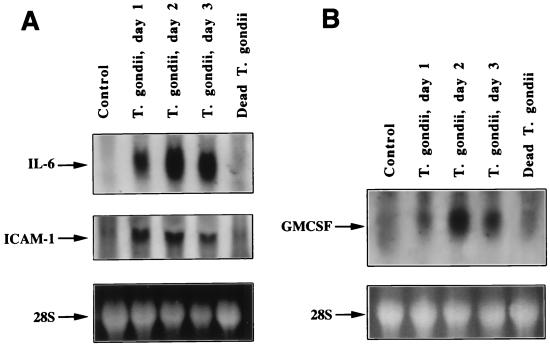
IL-6 mRNA was not detectable in untreated cells and in cells incubated with dead T. gondii (Fig. 2A, top). At day 1 p.i., an IL-6 mRNA band was clearly detected and the intensity of the band increased at days 2 and 3 p.i. The same blot was stripped and used for hybridization with a digoxigenin-labeled ICAM-1 cDNA probe. Faint bands were seen in untreated cells and cells incubated with heat-killed T. gondii (Fig. 2A, middle). ICAM-1 hybridization signals at days 1, 2, and 3 p.i. exhibit the presence of intense bands, indicating the increase in ICAM-1 mRNA. The panel showing the 28S RNA of ethidium bromide-stained RNA gel indicates that total RNA loaded in the lanes of the control and dead T. gondii are of similar intensity to that of the bands in the lanes of T. gondii, days 1, 2, and 3 p.i. Northern blot analysis for GM-CSF mRNA is shown in Fig. 2B. In untreated and dead-T. gondii-incubated cultures, a faint band is seen, indicating low background levels of GM-CSF mRNA in uninfected cells. A clear band is seen at day 1 p.i., and the intensity of the band significantly increased at days 2 and 3 p.i. The gel of 28S RNA is shown to indicate that equivalent amounts of total RNA were loaded in all the lanes of the gel.
FIG. 2.
Northern blot analyses of the expression of IL-6 and ICAM-1 (A) and GM-CSF (B) mRNA in T. gondii-infected HRPE cells. Total cellular RNA isolated from HRPE cells at indicated time points were subjected to Northern blot analysis. The arrows indicate the positions of IL-6 (1.3 kb), ICAM-1 (3.3 kb), and GM-CSF (0.8 kb) mRNA. Lanes: control, uninfected cells incubated for 3 days; T. gondii, days 1, 2, and 3, cultures infected with T. gondii and total RNA prepared 1, 2, and 3 days p.i., respectively; dead T. gondii, cells incubated for 3 days with heat-killed T. gondii. The bottom panel shows the intensity of the 28S RNA band of an ethidium bromide-stained RNA gel.
Analysis of IL-1β mRNA expression by RT-PCR.
Initial attempts to demonstrate the expression of IL-1β mRNA by Northern blot analysis were unsuccessful. This may be due to the low levels of IL-1β mRNA corresponding to the low levels of IL-1β secreted by T. gondii-infected HRPE cells. Therefore, we used the more sensitive reverse transcriptase (RT)-PCR method for the amplification of IL-1β mRNA. Total cellular RNA from the control and infected cultures was prepared as described for Northern blot analysis. An RNA PCR kit and GeneAmp PCR System 9600 (Applied Biosystems Division, Perkin-Elmer, Foster City, Calif.) were used. RNA was reverse transcribed to cDNA by incubating it with oligo(dT)16 as the RT primer and MuLV RT for 15 min at 42°C and 5 min at 99°C, followed by 5 min at 5°C. cDNAs synthesized were amplified by PCR in the presence of specific primers for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) or IL-1β (Continental Laboratory Products, San Diego, Calif.) in the presence of AmpliTaq DNA polymerase. Samples were heated for 105 s at 95°C and amplified for 30 cycles at 95°C for 15 s and 60°C for 30 s, followed by final extension for 7 min at 72°C. PCR products were separated on an ethidium bromide-containing agarose gel and photographed under UV light.
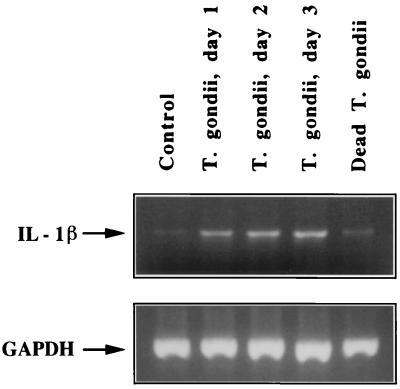
Faint bands are visible in both untreated and dead-T. gondii-incubated cultures, suggesting basal levels of IL-1β mRNA (Fig. 3). The intensity of the bands of PCR products in T. gondii-infected cultures significantly increased at days 1, 2, and 3 p.i. The lower panel shows the bands of PCR products amplified with GAPDH primers that had the same total RNA preparations as those in the upper panel.
FIG. 3.
RT-PCR analyses of IL-1β and GAPDH mRNA expression in T. gondii-infected HRPE cells. Total RNA (1 μg) was used for reverse transcription and amplification (30 cycles) in a single tube in an RNA PCR kit. Descriptions of samples loaded in the lanes are the same as those given for Fig. 2. The sizes of IL-1β and GAPDH PCR products are 331 and 600 bp, respectively.
Conclusions.
Results obtained in the present study show that HRPE cells respond to T. gondii infection by secreting IL-1, IL-6, GM-CSF, and ICAM-1. Levels of these cytokines increase progressively with time (Fig. 1) and in correlation with the number of host cells infected as well as with the intracellular parasite load. Secretion of IL-1, IL-6, GM-CSF, and ICAM-1, but not nitric oxide, by T. gondii-infected HRPE cells (17) suggest that elevated secretion of inflammatory molecules by T. gondii is not due to nonspecific injury to the cells. It should be emphasized that HRPE cells are incubated in serum-free medium during T. gondii infection studies, and therefore, no stimulating agents or serum factors are responsible for the induction of and/or interference with the cytokine secretion. Upregulation of mRNAs and the secretion of IL-1β, IL-6, GM-CSF, and ICAM-1 by T. gondii-infected HRPE cells in cultures, but not by HRPE cells incubated with heat-killed T. gondii, indicate that the stimulus for the activation of inflammatory molecule secretion is derived from the interaction between host cells and the parasite at different stages of parasite infiltration, parasite intracellular replication, and/or release of parasite secretion and degradation products (3, 18).
In vitro cell culture models of T. gondii infection have demonstrated altered cellular responses during parasite infection. Secretion of IL-1, IL-6, and GM-CSF was observed in mouse brain astrocyte and microglial cell cultures infected with virulent and avirulent T. gondii strains (7). In these experiments, heat killing of the parasites abolished the cytokine-inducing activity. However, in another study, no significant induction of IL-1α, IL-6, and tumor necrosis factor alpha mRNA was observed in human astrocytoma and macrophage cell lines infected with virulent or avirulent RH or prugniaud strains (22). The present study shows that HRPE cells contribute to the production of proinflammatory cytokines within the ocular microenvironment during T. gondii infection. Differential responses of various cell types to T. gondii infection could be due to a combination of parasite trigger and inherent properties of the host cells. Interactions of host cells and parasite may result in the activation of appropriate signal transduction pathways, resulting in transcriptional upregulation. Further studies are required to identify specific mechanisms and transcription factors involved in T. gondii-induced pathogenesis.
The role of cytokines and adhesion molecules in uveitis and other inflammatory diseases of the eye is well documented (5, 28). Levels of IL-6 and soluble ICAM-1 are found to be elevated in the vitreous fluids of patients with uveitis and proliferative vitreoretinal disorders, respectively (1, 4). Increased expression of ICAM-1 on the RPE of uveitis patients (29) and on epiretinal membranes formed during proliferative vitreoretinopathy (10) have also been reported. Further, direct intravitreal injection of IL-1 was shown to induce uveitis in an animal model (25). Together, these observations indicate that IL-1, IL-6, and ICAM-1 play critical roles in intraocular inflammatory diseases. The secretion of IL-1, IL-6, GM-CSF, and ICAM-1 by HRPE cells in response to T. gondii infection, as shown in this study, may have multiple roles under in vivo conditions. Elevated production of these factors and other molecules by T. gondii-infected resident cells may initiate local immune reactivity during primary infections and during recurrent reactivation episodes in toxoplasma-induced retinochoroiditis.
Acknowledgments
We thank Charles Egwuagu, Kumar Srinivasan, and Laura Chesky for critical reading of the manuscript.
REFERENCES
- 1.Arocker-Mettinger E, Steurer-Georgiew L, Steurer M, Huber-Spitzy V, Hoetzl E, Grabner G, Kuchar A. Circulating ICAM-1 levels in serum of uveitis patients. Curr Eye Res. 1992;11(Suppl.):161–166. doi: 10.3109/02713689208999527. [DOI] [PubMed] [Google Scholar]
- 2.Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Investig Ophthalmol Vis Sci. 1985;26:1659–1694. [PubMed] [Google Scholar]
- 3.Cesbron-Delaw M-F. Dense-granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 4.De Boer J H, Van Hren M A C, de Vries-Knoppert W A E J, Baarsma G S, de Jong P V T M, Postema F J, Radmakers A J J M, Kijlstra A. Analysis of IL-6 levels in human vitreous fluid obtained from uveitis patients, patients with proliferative intraocular disorders. Curr Eye Res. 1992;11(Suppl.):181–186. doi: 10.3109/02713689208999530. [DOI] [PubMed] [Google Scholar]
- 5.De Vos A F, Hoekzema R, Kijlstra A. Cytokines and uveitis, a review. Curr Eye Res. 1992;11:581–597. doi: 10.3109/02713689209001814. [DOI] [PubMed] [Google Scholar]
- 6.Dutton G N, McMenamin P G, Hay J, Cameron S. The ultrastructural pathology of congenital murine toxoplasmic retinochoroiditis. Part II: the morphology of inflammatory changes. Exp Eye Res. 1986;43:545–560. doi: 10.1016/s0014-4835(86)80022-7. [DOI] [PubMed] [Google Scholar]
- 7.Fischer H-G, Nitzgen B, Reichmann G, Hadding U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur J Immunol. 1997;27:1539–1548. doi: 10.1002/eji.1830270633. [DOI] [PubMed] [Google Scholar]
- 8.Frenkel J K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988;4:273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman A H. Uveitis affecting the retina and posterior segment. In: Freeman W R, editor. Atlas of the retinal diseases and therapy. New York, N.Y: Raven Press; 1993. pp. 37–70. [Google Scholar]
- 10.Heidenkummer H P, Kampick A. Intercellular adhesion molecule-1 (ICAM-1) and leukocyte function-associated antigen-1 (LFA-1) expression in human epiretinal membranes. Graefe's Arch Clin Exp Ophthalmol. 1992;230:483–487. doi: 10.1007/BF00175938. [DOI] [PubMed] [Google Scholar]
- 11.Jabs D A. Ocular toxoplasmosis. Int Ophthalmol Clin. 1990;30:264–270. doi: 10.1097/00004397-199030040-00009. [DOI] [PubMed] [Google Scholar]
- 12.Marmor M F. Inflammations and degenerations of the retinal pigment epithelium. In: Zinn K M, Marmor M F, editors. The retinal pigment epithelium. Cambridge, Mass: Harvard University Press; 1979. pp. 454–477. [Google Scholar]
- 13.McMenamin P G, Dutton G N, Hay J, Cameron S. The ultrastructural pathology of congenital murine toxoplasmic retinochoroiditis. Part I: the localization and morphology of toxoplasma cysts in the retina. Exp Eye Res. 1986;43:529–543. doi: 10.1016/s0014-4835(86)80021-5. [DOI] [PubMed] [Google Scholar]
- 14.Morris D A, Henkind P. Pathological responses of the human retinal pigment epithelium. In: Zinn K M, Marmor M F, editors. The retinal pigment epithelium. Cambridge, Mass: Harvard University Press; 1979. pp. 247–266. [Google Scholar]
- 15.Nagineni C N, Detrick B, Hooks J J. Synergistic effects of gamma interferon on inflammatory mediators that induce interleukin-6 gene expression and secretion by human retinal pigment epithelial cells. Clin Diagn Lab Immunol. 1994;1:569–577. doi: 10.1128/cdli.1.5.569-577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagineni C N, Kutty R K, Detrick B, Hooks J J. Inflammatory cytokines induce intercellular adhesion molecule-1 (ICAM-1) mRNA synthesis and protein secretion by human retinal pigment epithelial cell cultures. Cytokine. 1996;8:622–630. doi: 10.1006/cyto.1996.0083. [DOI] [PubMed] [Google Scholar]
- 17.Nagineni C N, Pardhasaradhi K, Martins M C, Detrick B, Hooks J J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols B A, Chiappino M L, O'Connor G R. Secretion from the rhoptries of Toxoplasma gondii during host cell invasion. J Ultrastruct Res. 1983;83:85–98. doi: 10.1016/s0022-5320(83)90067-9. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson D H, Wolchok E B. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch Ophthalmol. 1976;94:248–254. doi: 10.1001/archopht.1976.03910030120009. [DOI] [PubMed] [Google Scholar]
- 20.Nussenblatt R B, Whitcup S M, Palestine A G. Ocular toxoplasmosis. In: Nussenblatt R B, Whitcup S M, Palestine A G, editors. Uveitis: fundamentals and clinical practice. St. Louis, Mo: Mosby-Year Book; 1996. pp. 211–228. [Google Scholar]
- 21.Pavesio C E N, Chiappino M L, Gormeley P, Setzer P Y, Nichols B A. Acquired retinochoroiditis in hamsters inoculated with ME 49 strain Toxoplasma gondii. Investig Ophthalmol Vis Sci. 1995;36:2166–2175. [PubMed] [Google Scholar]
- 22.Pelloux H, Ricard Y, Bracchi V, Markowicz Y, Verna J M, Ambroise-Thomas P. Tumor necrosis factor alpha, interleukin 1 alpha, and interleukin 6 mRNA expressed by human astrocytoma cells after infection by three different strains of Toxoplasma gondii. Parasitol Res. 1994;80:271–276. doi: 10.1007/BF02351866. [DOI] [PubMed] [Google Scholar]
- 23.Perkins E S. Ocular toxoplasmosis. Br J Ophthalmol. 1973;57:1–17. doi: 10.1136/bjo.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao N A, Font R L. Toxoplasmic retinochoroiditis: electron-microscopic and immunofluorescence studies of formalin-fixed tissue. Arch Ophthalmol. 1977;95:273–277. doi: 10.1001/archopht.1977.04450020074012. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum J T, Samples J R, Hefeneider S H, Howes E L. Ocular inflammatory effects of intravitreal interleukin-1. Arch Ophthalmol. 1987;105:1117–1120. doi: 10.1001/archopht.1987.01060080119040. [DOI] [PubMed] [Google Scholar]
- 26.Tabbara K F. Disruption of the choroidoretinal interface by Toxoplasma. Eye. 1990;4:366–373. doi: 10.1038/eye.1990.49. [DOI] [PubMed] [Google Scholar]
- 27.Tabbara K F, Nozik R A, O'Connor G R. Clindamycin effects on experimental ocular toxoplasmosis in the rabbit. Arch Ophthalmol. 1974;92:244–247. doi: 10.1001/archopht.1974.01010010252017. [DOI] [PubMed] [Google Scholar]
- 28.Wakefield D, Lloyd A. The role of cytokines in the pathogenesis of inflammatory eye disease. Cytokine. 1992;4:1–5. doi: 10.1016/1043-4666(92)90028-p. [DOI] [PubMed] [Google Scholar]
- 29.Whitcup S M, Chan C C, Li Q, Nussenblatt R B. Expression of cell adhesion molecules in posterior uveitis. Arch Ophthalmol. 1992;110:662–666. doi: 10.1001/archopht.1992.01080170084029. [DOI] [PubMed] [Google Scholar]
- 30.Wilder H P. Toxoplasma chorioretinitis in adults. Arch Ophthalmol. 1952;48:127–136. doi: 10.1001/archopht.1952.00920010132001. [DOI] [PubMed] [Google Scholar]
- 31.Zinn K M, Marmor M F. The retinal pigment epithelium. Cambridge, Mass: Harvard University Press; 1979. [Google Scholar]