Abstract
Borrelia burgdorferi-infected ticks were fed on either OspC-immunized mice or normal, nonimmunized mice. After 72 h, the ticks were detached, followed by dissection and subsequent culturing in Barbour-Stoenner-Kelley II medium of the salivary glands from each tick to determine the presence of borreliae. Forty percent (10 of 25) of salivary glands from ticks that had fed on nonimmunized mice were culture positive, while only 7.4% (2 of 27) of salivary glands from ticks that had fed on OspC-immunized mice were culture positive, thus indicating a much reduced borrelial migration from the midgut when the bloodmeal contained anti-OspC antibodies. Fluorescent antibody staining of the corresponding midguts from ticks that had fed on the OspC-immunized mice showed that borreliae were present but did not produce OspC. In contrast, borreliae in midguts from ticks that had fed on normal mice demonstrated substantial ospC expression. This study provides evidence that, during tick feeding on an OspC-immunized host, transmission of borreliae from the tick is prevented; it also suggests that OspC functions in a tick-to-host transmission mechanism.
Borrelia burgdorferi, the spirochetal agent of Lyme disease, is transmitted to a susceptible host through the bite of an infected (Ixodes) tick vector (2, 7). During the tick feeding period, borreliae residing in the tick's midgut are induced to migrate through the tick hemolymph to the salivary glands and subsequently are injected into the host (1, 5, 15). Although there may be borreliae present in the salivary glands of unfed ticks, these organisms do not appear to be infectious (8, 18). Borreliae capable of infection do not regularly reach the salivary glands until approximately 60 to 72 h postattachment (12). Interestingly, concurrent with this time period, the borreliae undergo a change in outer surface protein gene expression, as outer surface protein C (OspC) undergoes an activation in synthesis during uptake of the bloodmeal (16). It has been demonstrated that OspC immunization, active or passive, confers protective immunity to animals against infection (6, 13, 14, 17). However, it is unknown whether the protective mechanism involves (i) prevention of transmission of borreliae from tick to host or (ii) eradication of borreliae by the host's immune system following successful transmission from the tick. To determine how the normal enzootic maintenance of infection is affected by anti-OspC antibodies, this study examined tick salivary glands and midguts for the presence of borreliae at 72 h after initiation of tick feeding on OspC-immunized mice and on normal, nonimmunized mice.
Two mice were immunized with a recombinant OspC produced in an Escherichia coli lysate that had previously been shown to be protective (6). After two boosts, the mice had seroconverted against OspC, as seen by immunoblotting at a serum dilution of 1:500 (data not shown). The ticks, Ixodes scapularis, used in these experiments were derived from a colony originating in Bridgeport, Conn. They were infected with strain B31 by feeding larvae on infected mice, as previously described (11). Only feedings resulting in ≥80% infected nymphs were included in the infected colony. Ticks were held at 21°C and saturated humidity. One group of nymphal ticks was allowed to feed on the OspC-immunized mice, and a separate group of ticks fed on nonimmunized mice, for 72 h, at which time they were removed from the hosts. In the OspC-immunized group, mouse 1 harbored 8 ticks and mouse 2 harbored 19 ticks, for a total of 27. In the control, nonimmunized group, mouse 1 harbored 10 ticks, mouse 2 harbored 9 ticks, and mouse 3 harbored 6 ticks, for a total of 25. Salivary glands were dissected, washed, and placed in Barbour-Stoenner-Kelley II (BSK) medium explant cultures, as previously described (12). Midgut contents were similarly placed on glass slides, allowed to air dry, fixed in acetone, and stored at −20°C. BSK cultures were checked for the presence of borreliae by dark-field microscopy weekly for 4 weeks.
Results of salivary gland explant cultures.
Forty percent (10 of 25) of the explant cultures from ticks that fed on nonimmunized mice were positive for borrelial growth, whereas only 7.4% (2 of 27) of the cultures from ticks that fed on OspC-immunized mice were positive (P = 0.0078 [chi-square test]), a statistically significant difference (Table 1). The 40% rate of positive gland explant cultures from the control mice is lower than the 88% observed previously (12) but may simply be due to biological variability between experiments. The nonimmunized test mice were found to have been infected and the OspC-immunized test mice were not infected, as assayed by serology and ear punch biopsies 4 weeks following tick feeding.
TABLE 1.
Results of salivary gland explant cultures from ticks fed on nonimmunized and OspC-immunized mice
| Test mouse | No. of ticks/mouse | No. (%) salivary gland culture positive |
|---|---|---|
| Nonimmunized mice | ||
| Mouse 1 | 10 | 5 (50) |
| Mouse 2 | 9 | 3 (33) |
| Mouse 3 | 6 | 2 (33) |
| Total | 25 | 10 (40) |
| OspC-immunized mice | ||
| Mouse 1 | 8 | 0 (0) |
| Mouse 2 | 19 | 2 (10.5) |
| Total | 27 | 2 (7.4) |
Double immunofluorescent staining of borreliae within tick midguts.
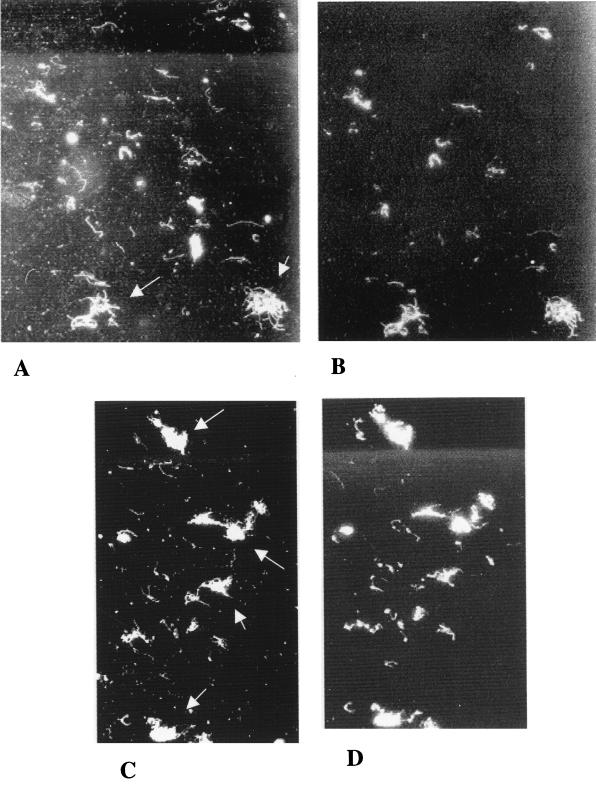
Borrelial synthesis of OspC within the tick midguts was examined by double immunofluorescent staining. The acetone-fixed midgut slides were first incubated with a 1:100 dilution of an OspC-specific murine monoclonal antibody (MAb) (9) for 1 h. Following three washes in phosphate-buffered saline, the slide was stained with a rhodamine-conjugated goat anti-mouse immunoglobulin G (IgG) and a rabbit anti-B. burgdorferi polyclonal antiserum conjugated with fluorescein isothiocyanate (FITC), both at dilutions of 1:50 for 1 h at room temperature. The double staining allowed first for the observation of total B. burgdorferi cells by FITC and subsequently, by changing to the epifluorescent rhodamine filter, for determining whether the same cells produced OspC. B. burgdorferi was observed in midguts of ticks that had fed on the nonimmunized control mice (Fig. 1A). However, only an estimated 25 to 40% of the borreliae had produced OspC during this time (72 h) (Fig. 1B). This finding corroborates earlier observations (J. Piesman and T. Schwan, personal communication) that only a subset of the borrelial cells present in the tick midgut synthesizes OspC during uptake of the bloodmeal.
FIG. 1.
Representative fields of double immunofluorescent staining of B. burgdorferi in tick midguts following tick feeding at 72 h. (A) Borreliae in ticks which had fed on a normal nonimmunized mouse stained with FITC-labeled anti-whole-cell B. burgdorferi. (B) Same field as in panel A, stained with rhodamine-labeled anti-OspC. Arrows indicate some of the borrelial cells expressing OspC in panel B with the corresponding cells in panel A. Note that not all cells seen in panel A express OspC, as seen in panel B. (C) Borreliae in ticks which had fed on an OspC-immunized mouse stained with FITC-labeled anti-whole-cell B. burgdorferi. (D) Same field as in panel C, stained with rhodamine-labeled anti-OspC.
B. burgdorferi was also observed in the midgut of ticks that had fed on the OspC-immunized mice and that were salivary gland culture negative (Fig. 1C). In no instances, however, were borrelial cells that produced OspC observed (Fig. 1D). However, two ticks that had fed on OspC-immunized mice were found by explant culture to contain B. burgdorferi in their salivary glands (Table 1). Observation of the midgut slides from these ticks revealed the presence of B. burgdorferi in one of them (no borreliae could be detected by microscopy in the other). Double immunofluorescent staining showed that some of the borrelial cells were producing OspC in this tick, exactly as seen in the nonimmunized control group (data not shown). Therefore, in this instance, OspC synthesis was not suppressed by the antibodies, and normal migration to the salivary glands occurred, thus providing an explanation for the borrelia-positive explant cultures.
In vitro borreliacidal assays.
A question remained as to whether the anti-OspC antibodies effected the inhibition of borrelial migration to the salivary glands by lysis of the OspC-producing cells or by some other mechanism. In vitro borreliacidal assays to assess the killing activity of anti-OspC antibodies were performed as follows. Antiserum test samples were filter sterilized and heated to 56°C for 10 min to inactivate complement, followed by serial dilutions (starting at 1:40) made with BSK medium, of which 100 μl was added to a 96-well-round-bottom tissue culture plate. One hundred microliters containing approximately 105 to 106 B. burgdorferi B31 cells (culture passage, <8) in BSK medium was added to each serum sample well, followed by 10 μl of guinea pig complement (250 H50 U/ml) (Gibco, Gaithersburg, Md.). The plate was sealed with an adhesive cover and incubated at 34°C with 5% CO2. After 24 and 72 h of incubation, 10 μl of the samples was removed to a glass slide, air dried, fixed with acetone, and stored at −20°C until being stained. Also, at 24 and 72 h of incubation, samples were observed by dark-field microscopy for viability. Test samples were prechallenge anti-recombinant OspC serum from mice that had demonstrated protection from a tick-transmitted infection in a previous study (6). Also tested was an OspC-specific MAb that had demonstrated passive protective capability when inoculated into mice (9). Preimmunization serum from these mice served as a control. By dark-field microscopic examination, there was no evidence of any borrelial killing when in vitro-cultured infectious organisms were incubated with these antibodies compared to a rabbit anti-B. burgdorferi whole-cell antiserum that actively killed borrelial cells in this assay. Cells incubated with the anti-OspC antibodies showed normal morphology and motility and were present in numbers of >100 per field, indicating a lack of bactericidal activity. However, it may have been difficult to assess any putative in vitro bactericidal activity in this system, since not all of the cultured borrelial cells used in this assay produced OspC, as observed by indirect immunofluorescence (Fig. 2A and B). We have never observed a growth phase of the B31 strain culture in which all cells display OspC at one time. Thus, by this assay, it would have been impossible to differentiate OspC-producing cells that were killed from nonproducing cells that were growing. Therefore, to determine whether or not the viable spirochetes seen in the borreliacidal assay wells were OspC producers, samples were stained following 24 and 72 h of incubation with an anti-OspC MAb followed by FITC-conjugated anti-mouse IgG. Figure 2D shows fluorescently stained borreliae, indicating the presence of OspC on these cells. Figure 2C shows the same field under dark field, demonstrating normal morphology of the cells. This result demonstrated that the observed borrelial cells did indeed harbor OspC in the presence of anti-OspC antibodies, both polyclonal and monoclonal, and were not lysed. Presumably, since the organisms were incubated with anti-OspC serum samples in the borreliacidal assay, there would have been no need to use a primary OspC antibody in the immunofluorescent staining procedure in Fig. 2, provided that the cells were producing OspC. This was indeed the case, as the immunofluorescence was repeated without the primary OspC antibody incubation step and the cells were stained as shown in Fig. 2D.
FIG. 2.
Indirect immunofluorescence assay of B. burgdorferi incubated with anti-OspC antibodies. (A) Dark-field microscopy of input organisms cultured in vitro prior to incubation in the borreliacidal assay mixture. (B) Same field as in panel A, stained with FITC-labeled anti-OspC MAb. Note that most, but not all, borreliae express OspC. (C) Dark-field microscopy of B. burgdorferi from the borreliacidal assay incubated with anti-OspC. (D) Same field as in panel C, stained with FITC-labeled anti-OspC. Panels C and D represent fields seen whether borreliae were incubated with protected mouse polyclonal anti-OspC sera or with the OspC MAb at 24 or 72 h. Arrows in panels A and C indicate borrelial cells that have formed clumps.
Discussion.
The results of this study are significant in helping to interpret the mechanism of protection provided by OspC immunity in the host, as well as providing evidence that OspC may be important functionally in the transmission of B. burgdorferi from tick to host. In a resting or nonfeeding tick, the outer surface of the borreliae is dominated by outer surface protein A (OspA), whereas OspC is not detectable (3, 16). Borreliae extracted from this tick stage and inoculated into test animals are noninfectious (10). When the tick begins to feed on a susceptible host, the introduction of the bloodmeal into the midgut induces changes in protein expression on the outer surface of the borreliae. OspA levels become markedly reduced (3), and OspC synthesis becomes activated during the 24 to 72 h of feeding (16). It is at approximately the 48- to 72-h stage of feeding that the borreliae migrate from the midgut to the salivary gland, to be subsequently injected into the host's skin (12).
Passive or active immunization with OspC protects laboratory animals against tick-transmitted infection of B. burgdorferi (6, 9, 17). The results of this study indicate that the OspC antibodies may effect a mechanism whereby the migration of borreliae from the midgut to the salivary glands is prevented, thereby interfering with spirochete dispersal to the host. Questions remain, however, as to how the anti-OspC antibodies may be involved in this phenomenon. A previous study from this laboratory demonstrated that, following feeding on an OspC-immunized mouse, replete ticks continue to harbor viable borreliae (6). Therefore, are the anti-OspC antibodies borreliacidal or borreliastatic against the subpopulation of OspC-displaying organisms in the tick midgut, or do they somehow signal a borrelial downregulation in the surface expression of OspC which could thereupon affect the migration? In this study, no evidence of anti-OspC borreliacidal activity from protective serum samples was seen in vitro, but that does not rule out such an activity in vivo within the tick. Therefore, we were not able to determine whether the viable borreliae remaining in the tick midgut following feeding on an OspC-immunized mouse represent (i) the remaining subset of cells which did not produce OspC (with the OspC-presenting organisms being killed) or (ii) borreliae which somehow have downregulated the surface expression of OspC in response to the anti-OspC stimulus. de Silva and colleagues recently showed that borrelial salivary gland invasion was inhibited when ospC transcription, and thus protein expression, was severely limited due to a decreased number of spirochetes in the midgut (4). That observation, plus the findings in this study, provide further evidence that OspC may be directly or indirectly involved in borrelial transmission from the tick to the host. Elucidation of the mechanisms behind these phenomena should lead to comprehension of the putative functional role of OspC in B. burgdorferi transmission, as well as a more defined understanding of which anti-OspC antibodies inhibit the deliverance of these tick-borne organisms to a susceptible host.
Acknowledgments
We thank Marc C. Dolan for producing infected ticks, Steve Sviat for growing Borrelia cultures, and M. Lamine Mbow for providing the OspC MAb and for helpful discussion and insights.
REFERENCES
- 1.Benach J L, Coleman J L, Skinner R A, Bosler E M. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987;155:1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 3.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva A M, Zeidner N S, Zhang Y, Dolan M C, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gern L, Zhu Z, Aeschlimann A. Development of Borrelia burgdorferi in Ixodes ricinus females during blood feeding. Ann Parasitol Hum Comp. 1990;65:89–93. [Google Scholar]
- 6.Gilmore R D, Jr, Kappel K J, Dolan M C, Burkot T R, Johnson B J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 8.Leuba-Garcia S, Kramer M D, Wallich R, Gern L. Characterization of Borrelia burgdorferi isolated from different organs of Ixodes ricinus ticks collected in nature. Zentbl Bakteriol. 1994;280:468–475. doi: 10.1016/s0934-8840(11)80506-2. [DOI] [PubMed] [Google Scholar]
- 9.Mbow M L, Gilmore R D, Jr, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 11.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 12.Piesman J. Dispersal of the Lyme disease spirochete Borrelia burgdorferi to salivary glands of feeding nymphal Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 1995;32:519–521. doi: 10.1093/jmedent/32.4.519. [DOI] [PubMed] [Google Scholar]
- 13.Preac-Mursic V, Wilske B, Patsouris E, Jauris S, Will G, Soutschek E, Rainhardt S, Lehnert G, Klockmann U, Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992;20:342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- 14.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro J M, Mather T N, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae) J Med Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong W, Gern L, Stehle T, Museteanu C, Kramer M, Wallich R, Simon M M. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive but not active immunization using recombinant OspC. Eur J Immunol. 1999;29:946–957. doi: 10.1002/(SICI)1521-4141(199903)29:03<946::AID-IMMU946>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Zung J L, Lewengrub S, Rudzinska M A, Spielman A, Telford S R, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zool. 1989;67:1737–1748. [Google Scholar]