Abstract
Background
Although previous studies described the production of IgG antibodies in a subgroup of patients with common variable immunodeficiency (CVID) following messenger RNA vaccinations with BNT162b2 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (CVID responders), the functionality of these antibodies in terms of avidity as measured by the dissociation rate constant (kdis) and the antibody response to booster immunization has not been studied.
Objective
We sought to analyze in CVID responders and healthy individuals, the avidity of anti–SARS-CoV-2 serum antibodies and their neutralization capacity as measured by surrogate virus–neutralizing antibodies in addition to IgG-, IgM-, and IgA-antibody levels and the response of circulating (peripheral blood) follicular T-helper cells after a third vaccination with BNT162b2 SARS-CoV-2 messenger RNA vaccine.
Methods
Binding IgG, IgA, and IgM serum levels were analyzed by ELISA in patients with CVID responding to the primary vaccination (CVID responders, n = 10) and healthy controls (n = 41). The binding avidity of anti–spike antibodies was investigated using biolayer interferometry in combination with biotin-labeled receptor-binding-domain of SARS-CoV-2 spike protein and streptavidin-labeled sensors. Antigen-specific recall T-cell responses were assessed by measuring activation-induced markers by flow cytometry.
Results
After the third vaccination with BNT162b2, IgG-, IgM-, and IgA-antibody levels, surrogate virus–neutralizing antibody levels, and antibody avidity were lower in CVID responders than in healthy controls. In contrast, anti–SARS-CoV-2 spike protein avidity was comparable in CVID responders and healthy individuals following primary vaccination. Follicular T-helper cell response to booster vaccination in CVID responders was significantly reduced when compared with that in healthy individuals.
Conclusions
Impaired affinity maturation during booster response provides new insight into CVID pathophysiology.
Key words: BNT162b2 booster vaccination, CVID, antibody avidity, cTfh, biolayer interferometry
Introduction
Common variable immunodeficiency (CVID) is the most frequent symptomatic primary antibody deficiency characterized by low to absent IgG antibody production against a multitude of different antigens.1 It has been shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (BNT162b2) vaccination of patients with CVID is generally safe and that binding IgG and neutralizing antibodies develop in a substantial subset of patients.2 In 19 studies, the average percentage of a total of 809 patients with CVID with positive SARS-CoV-2 spike antibodies after primary immunization (2 doses of the coronavirus disease 2019 vaccine) was 64.92%,2 and in 4 additional studies in a total of 149 patients with CVID, this response rate increased to 78.27% after booster immunization (third vaccine dose),3, 4, 5, 6 which also included angiotensin-converting enzyme 2–blocking activity.6 Whether the antibodies produced by CVID responders (CVID R) following booster immunization with BNT162b2 are effectively comparable to those produced by healthy controls (HCs) with respect to antibody level, neutralization capacity, and binding affinity is largely unknown.
Abnormalities in both B- and T-cell response to primary vaccination with BNT162b2 have been described in CVID, raising the possibility of an extrafollicular B-cell response and the absence of proper B-cell memory following primary coronavirus disease 2019 vaccination.1 , 2 In this study, we investigated the antibody response to BNT162b2 booster vaccination (a third vaccine dose) in CVID R. To better estimate the success or failure of booster vaccination, anti–SARS-CoV-2 spike protein (αSpike)-surrogate virus–neutralizing (sVNT) antibodies and αSpike binding kinetics of serum antibodies as assessed by measuring the dissociation rate constant of the antigen-antibody complex (αSpike avidity) served as parameters of antibody functionality and were compared with IgG and IgA binding levels as measured by ELISA.
From a cohort of 31 patients with CVID diagnosed according to the European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity, 15 patients (48.4%) showed a positive αSpike-IgG ELISA response of 33 relative units/mL (3 times the detection limit) or above 4 weeks after primary immunization with BNT162b2 SARS-CoV-2 messenger RNA vaccine consisting of 2 doses given 3 to 4 weeks apart.1 Ten of these 15 patients—median age in years [interquartile range] (range), 40 [37-53] (25-75), male/female: 3/7—were available for the present study (CVID R). The immune phenotype including B-cell subpopulations in CVID R and nonresponders to BNT162b2 messenger RNA coronavirus disease 2019 vaccination was included in the previous publication.1 The patients received regular subcutaneous immunoglobulin or intravenous immunoglobulin (IVIG) substitution therapy and never returned positive results for infection with SARS-CoV-2 despite repeated PCR testing of nasopharyngeal swabs; also, the HCs were repeatedly tested negative for SARS-CoV-2 infection before the study and neither the HCs nor the patients with CVID included in the study experienced signs or symptoms suspicious of SARS-CoV-2 infection before their immune response to a third vaccination was examined. The subcutaneous immunoglobulin or IVIG lots used during the study (venous blood was drawn from the patients between August 8, 2021, and January 17, 2022) were tested negative for αSpike-IgG at dilutions of IgG (1000 mg/dL, ie, immunoglobulin products diluted 1:5 or 1:10) simulating in vivo bioavailability following infusion at replacement doses. Serum was collected 41 days (median, interquartile range [IQR]: 32-46) after the third vaccine dose was given 166 days (median, IQR: 151-182) following the second dose in the 10 patients with CVID. Likewise, serum was collected 44 days (median, IQR: 29-70) after booster vaccination given 146 days (median, IQR: 140-177) after the second vaccine dose in 41 HCs (median age in years [IQR] (range): 52 [34-64] (17-82); male/female: 10/31).
αSpike-IgG, -IgA, and -sVNT responses in serum were analyzed by ELISA as previously described.1 Quantification of αSpike-IgM antibodies was measured by using a human SARS-CoV-2 Spike (trimer) IgM ELISA Kit (Cat. No.: BMS2324, Invitrogen, Lofer, Austria). For determination of binding kinetics of αSpike antibodies in serum (αSpike antibody avidity, k dis as a measure of avidity), an assay published previously was modified.7 Biolayer interferometry (manufactured by ForteBio Octet, Fremont, Calif) was used to determine the dissociation rate constant of SARS-CoV-2 antibodies in serum using a biotinylated recombinant SARS-CoV-2 Spike S1 Subunit (HEK293-derived) (R&D Systems, Minneapolis, Minn; Cat. No.: BT10569, Lot DOJW0221021) in concert with Octet Biosensor SA (Streptavidin) Sensors (Sartorius, Goettingen, Germany; Cat. No.: 18-5019) to coat the biotinylated antigen onto a sensor surface. Only the dissociation curve was used for the calculation of k dis by nonlinear regression using a 1:1 binding model and the data analysis software from ForteBio (Sartorius). To investigate whether IVIG treatment decreases anti–SARS-CoV-2 avidity, we mixed a SARS-CoV-2 monoclonal IgG antibody (final concentration 47 and 24 μg/mL) (Cat. No.: 703958, Invitrogen) with serum from infection-naive and unvaccinated HCs and IVIG without antispike IgG antibodies (IgVENA, Lot: 207607, Kedrion, Gräfelfing, Germany, final concentration 500 mg/dL). In addition, we added IVIG (final concentration 500 mg/dL) to αSpike-IgG-positive human serum (HCs after the third vaccination) and performed avidity measurements. Lastly, we determined αSpike-serum antibody avidity in vaccinated patients (after a third dose) suffering from IgG-subclass deficiency who received immunoglobulin substitution therapy. Antigen-specific recall T-cell responses were assessed by measuring activation-induced markers and proliferative responses of PBMCs after stimulation with spike peptides as previously described.1 Results are expressed as net dpm 3H-thymidine incorporation (ie, the difference in dpm between stimulated and unstimulated cells) and as the CD25+Ox40+ proportion of circulating (peripheral blood) follicular T-helper (cTfh) cells. As a measure of statistically significant correlation between 2 data sets, the P value for Spearman rank correlation coefficient was calculated; 2 study groups were compared statistically using a nonparametric 2-tailed Mann-Whitney U test (ns = not significant), and the whiskers in the box plots show minimum and maximum values.
Results and discussion
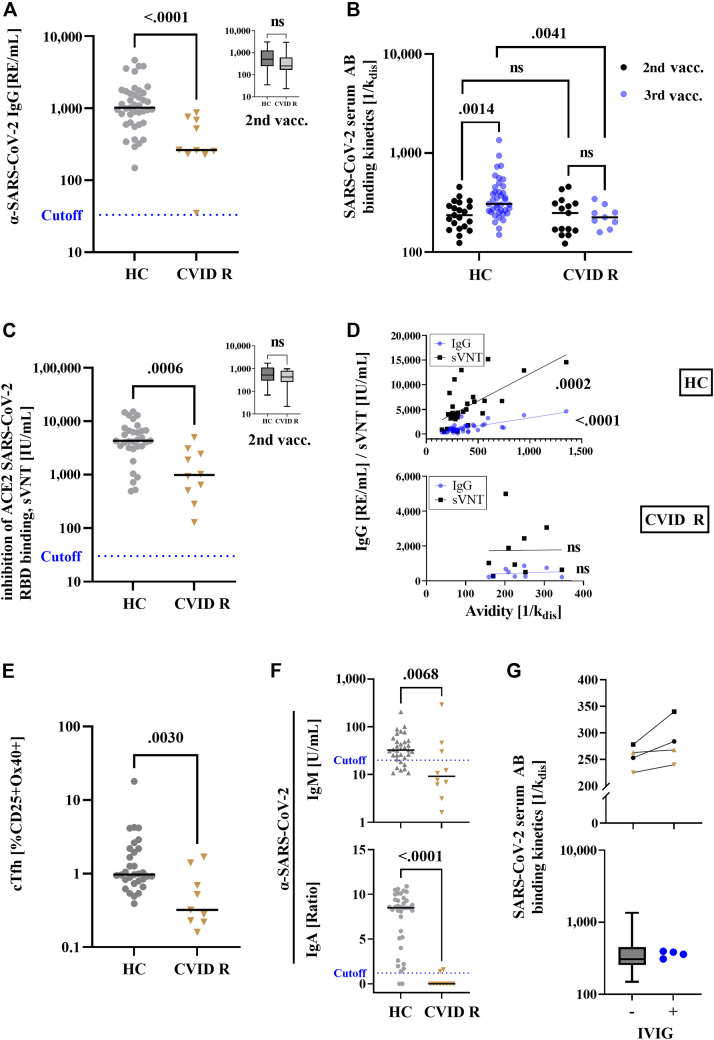
In HCs, booster vaccination against SARS-CoV-2 is characterized by an increase in αSpike-IgG and neutralizing antibodies.7 In CVID R, the concentration of αSpike-IgG and sVNT antibodies was significantly decreased after booster vaccination as compared with that in HCs (Fig 1 , A and C), whereas after primary immunization CVID R and HCs mounted comparable antibody levels (inserts to Fig 1, A and C, analysis of serum samples obtained in the course of a previous study1). Measurement of αSpike avidity following booster vaccination is especially interesting in CVID R because a possible extrafollicular generation of antibodies has been suggested.1 , 4 In healthy individuals, a rise in binding αSpike-IgG antibody levels after booster immunization is followed by a decrease over time while there is evidence that αSpike avidity progressively increases,8 which would suggest that antibody-binding kinetics represent a valuable additional information indicative of booster success. In CVID R, the avidity of αSpike antibodies was significantly decreased as compared with that in HCs after the third dose of BNT162b2 (Fig 1, B), whereas after primary immunization CVID R and HC antibody avidities were comparable (Fig 1, D, analysis of serum samples obtained in the course of a previous study1), with a significant increase in αSpike avidity following booster immunization in HCs but not in CVID R (Fig 1, B). In individual HCs, comparable IgG antibody levels or sVNT levels were associated with entirely different antibody avidities (Fig 1, D), but overall HC antibody avidities were significantly correlated with αSpike-IgG binding levels and sVNT levels (Fig 1, D, upper panel). This is in contrast to CVID R who displayed no correlation between αSpike avidity and IgG binding levels or sVNT levels (Fig 1, D, lower panel), supporting the hypothesis that different mechanisms of secondary antibody response were involved in CVID R and HCs. IgG subclass-deficient patients undergoing IgG substitution therapy with dosages comparable to those given to patients with CVID and using product lots containing no antispike IgG antibodies showed antibody avidities following the third BNT162b2 vaccination that were comparable to those of the HC group (Fig 1, G, lower panel), and IVIG added in vitro to human serum containing either postvaccination antispike IgG or an mAb against SARS-CoV-2 spike protein had no inhibitory effect on antibody avidity measurement (Fig 1, G, upper panel).
Fig 1.
Antibody response following booster immunization with BNT162b2 mRNA vaccine in patients with CVID who responded to primary vaccination (CVID R) and HCs. Anti–SARS-CoV-2 IgG (A), αSpike serum antibody (AB) avidity (B), and sVNT antibody (C) levels were assessed following booster immunization (third vaccine dose) and primary vaccination (inserts, after first 2 doses of BNT162b2 mRNA vaccine). D, Correlation of anti–SARS-CoV-2 IgG (blue dots) and sVNT (black squares) vs serum antibody avidity in HCs (upper panel) and CVID R (lower panel) after booster vaccination. E, Percent of SARS-CoV-2 spike protein–specific cTfh cells after the third vaccination (unstimulated control cells showed below 0.3% Ox40 and CD25 double-positive cells). F, αSpike-IgM (upper panel) and -IgA (lower panel) antibody concentrations in serum after booster vaccination. The dotted lines indicate the cutoff for positivity (IgG, 33 RE/mL; IgM, 20 U/mL; and IgA, a ratio of 1.2). G, αSpike serum antibody (AB) avidity was measured in serum from HCs after the third vaccination (black symbols represent 2 different individuals) and from infection-naive, unvaccinated HCs spiked with a monoclonal αSpike-IgG antibody at 2 different concentrations (triangle: 47 μg/mL, inverted triangle: 24 μg/mL) with or without addition of IVIG (upper panel, final concentration 500 mg/dL), as well as after the third vaccination in healthy individuals (box plot, n = 39) as compared with IgG subclass-deficient patients receiving immunoglobulin replacement therapy (blue dots). RBD, Receptor-binding domain; RE, relative units.
The percentage of antigen-specific cTfh cells in CVID R was also significantly reduced compared with that in HCs (Fig 1, E). αSpike IgA level was significantly reduced in CVID R after booster vaccination (Fig 1, F, lower panel) and primary vaccination1 , 2 as well. αSARS-CoV-2 spike IgM levels after the third BNT162b2 vaccination were also decreased in patients with CVID as compared with HCs (Fig 1, F, upper panel), which is in good agreement with the decreased serum IgM levels in CVID R1 and indicates that mechanisms in addition to impaired isotype switching contribute to the defective B-cell response to BNT162b2 vaccination observed in patients with CVID. Addition of IVIG to αSpike IgG-positive human serum did not significantly alter the outcome of avidity measurement (Fig 1, G, upper panel, black symbols, squares, and dots represent different individuals). Also, measurement of αSpike mAbs within human serum of infection-naive and unvaccinated HCs was not altered by addition of IVIG (Fig 1, G, lower panel, brown symbols, triangle: 46 μg/mL, inverted triangle: 24 μg/mL). Avidity of αSpike serum antibodies in IVIG-substituted patients suffering from IgG-subclass deficiency was at or above median when compared with that in HCs.
It has been described that a subset of patients with CVID displays impaired affinity maturation as determined by screening for a hypomutated V gene expressed by memory B cells.9 The present finding of impaired affinity maturation during a booster response constitutes further evidence for qualitatively different antibody responses to mRNA vaccination in CVID R and HCs,10 previously characterized by defective cTfh-cell responses1 and memory B cells with low binding capacity to spike protein.3 Antigen-specific T-cell proliferation following booster vaccination was comparable in CVID R and HCs (median [IQR] dpm 3H-thymidine incorporation of PBMCs stimulated with spike peptides: HCs: 14,663 [7,627-26,785], n = 19; CVID R: 23,220 [10,151-37,847], n = 10), indicating that SARS-CoV-2–specific T-memory generation is intact in CVID R, as previously suggested.1 , 4 A CD4 memory T-cell subset, CXCR5-positive Tfh cells, is known to be important for the formation of germinal centers, B-cell proliferation, isotype switching and affinity maturation of antibodies, and the differentiation of B cells into memory cells and antibody-secreting plasma cells. Our present findings indicate that defective cTfh-cell responses previously described in CVID after primary vaccination1 could not be corrected by an additional booster immunization and might be a relevant mechanism for the impaired antibody affinity maturation observed in patients with CVID. Taken together, our findings shed new light on the pathophysiology of CVID by showing a defect in antibody maturation following repeated encounter with an antigen; further studies are necessary to clarify whether analysis of antibody-binding kinetics provide additional clinical utility as compared with conventional ELISA to determine whether immunodeficient patients have a protective immune response following immunization.
Clinical implications.
Future studies should address whether defective antibody maturation as demonstrated by analysis of antibody kinetics in CVID is associated with susceptibility to infection in other immunodeficient patients.
Acknowledgments
We thank Jakob Wallner and the entire team of the Biomolecular & Cellular Analysis (BMCA) Core Facility at BOKU, Vienna, for their advice concerning biolayer interferometry.
Footnotes
This study was supported by the Österreichische Forschungsförderungsgesellschaft mbH (grant no. 881639), and by the Jeffrey Modell Foundation, the Johns HopkinsResearch Foundation, and the Robert A. Good Endowment.
Ethics statement: The study was conducted in accordance with the Declaration of Helsinki and fulfils the guidelines of the Austrian Agency of Research Integrity (OeAWI). With respect to the patient analyses, this study was approved by the Ethics Committee of the Immunology Outpatient Clinic as a study using the biobank of residual specimen of the Immunology Outpatient Clinic. According to the Ethics Committee of the City of Vienna and the legal regulations to be applied (§15a Abs. 3a Wiener Krankenanstaltengesetz), no additional ethics committee evaluation is required for a noninterventional study using data and material collected as part of the routine medical care the patients received. The patients gave their informed consent that anonymized data collected as part of the routine medical attendance (serum antibody measurements and T-cell activation assays) could be included in a scientific publication. All patient information in this study is anonymized and deidentified. No extra intervention was carried out.
Disclosure of potential conflict of interest: K. M. T. Sauerwein and M. M. Eibl were employed by the company Biomedizinische Forschung & Bio-Produkte AG, which had no role in the design of this study or during its execution, and was not involved in the analyses, interpretation of the data, and the decision to submit the present manuscript for publication. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Sauerwein K.M.T., Geier C.B., Stemberger R.F., Akyaman H., Illes P., Fischer M.B., et al. Antigen-specific CD4+ T-cell activation in primary antibody deficiency after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2022;13:169. doi: 10.3389/fimmu.2022.827048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durkee-Shock J.R., Keller M.D. Immunizing the imperfect immune system: coronavirus disease 2019 vaccination in patients with inborn errors of immunity. Ann Allergy Asthma Immunol. 2022;129:562–571.e1. doi: 10.1016/j.anai.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields A.M., Faustini S.E., Hill H.J., Al-Taei S., Tanner C., Ashford F., et al. Increased seroprevalence and improved antibody responses following third primary SARS-CoV-2 immunisation: an update from the COV-AD study. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.912571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulvirenti F., di Cecca S., Sinibaldi M., Piano Mortari E., Terreri S., Albano C., et al. T-cell defects associated to lack of spike-specific antibodies after BNT162b2 full immunization followed by a booster dose in patients with common variable immune deficiencies. Cells. 2022;11:1918. doi: 10.3390/cells11121918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goda V., Kriván G., Kulcsár A., Gönczi M., Tasnády S., Matula Z., et al. Specific antibody and the T-cell response elicited by BNT162b2 boosting after two ChAdOx1 nCoV-19 in common variable immunodeficiency. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.907125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gernez Y., Murugesan K., Cortales C.R., Banaei N., Hoyte L., Pinsky B.A., et al. Immunogenicity of a third COVID-19 messenger RNA vaccine dose in primary immunodeficiency disorder patients with functional B-cell defects. J Allergy Clin Immunol Pract. 2022;10:1385–1388.e2. doi: 10.1016/j.jaip.2022.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho A., Muecksch F., Schaefer-Babajew D., Wang Z., Finkin S., Gaebler C., et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600:517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tauzin A., Gendron-Lepage G., Nayrac M., Anand S.P., Bourassa C., Medjahed H., et al. Evolution of anti-RBD IgG avidity following SARS-CoV-2 infection. Viruses. 2022;14:532. doi: 10.3390/v14030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonhomme D., Hammarström L., Webster D., Chapel H., Hermine O., le Deist F., et al. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J Immunol. 2000;165:4725–4730. doi: 10.4049/jimmunol.165.8.4725. [DOI] [PubMed] [Google Scholar]
- 10.Braddom A.E., Batugedara G., Bol S., Bunnik E.M. Potential functions of atypical memory B cells in Plasmodium-exposed individuals. Int J Parasitol. 2020;50:1033–1042. doi: 10.1016/j.ijpara.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]